Abstract
Spatially confined self-assembly of peptide amphiphile nanofibers inside liposomes is triggered by light
The liposome is a self-assembled structure where a lipid bilayer surrounds an aqueous compartment. With a typical volume on the order of one thousandth of a cubic micron, this interior compartment has been used to carry drugs, peptides, proteins and DNA for applications in molecular biology, pharmaceuticals, and cosmetics.1 Beyond the simple containment of molecules, the confined interior of a liposome is also an interesting space to explore supramolecular chemistry. In the literature, supramolecular structures that have been formed inside liposomes include actin fibers2 and fibril-shaped precipitates of the cancer drug doxorubicin.3 These examples, however, typically use ion injection or pH gradients that depend on the invasive diffusion of ions through the liposomal membrane in order to stimulate aggregation of molecules. In this report, we have investigated the use of light to non-invasively induce the self-assembly of encapsulated molecules into nanofiber networks inside liposomes. Bulk nanostructure formation by light has been observed in the form of photosensitive gels and liquid crystals.4 Additionally, UV irradiation has been used to change the chemical structure of molecules in order to trigger their self-assembly into nanofibers.5 However, to the best of our knowledge, no cases exist where irradiation has been used to directly create nanofibers inside liposomes by self-assembly.
In our work, peptide amphiphiles (PAs) have served as instructive models to study self-assembly inside liposomes. PAs have afforded many supramolecular structures including micelles,6 membranes7 and nanofibers.8 The PA molecules used here comprise three segments: a linear aliphatic chain, a peptide segment that promotes β-sheet formation, and a peptide terminal sequence which can be modified according to the application of interest and in some cases may be an epitope for biological signaling.8 In aqueous media, self-assembly of these PA monomers into cylindrical nanofibers can be triggered by a change in pH or salt concentration.8,9 Networks of these nanofibers can further form three-dimensional gel scaffolds resembling extracellular matrices.8 For our studies, various model PAs designed to self-assemble under acidic conditions were prepared using standard Fmoc solid-phase peptide synthesis (Scheme 1 and Supporting Information†). By sequestering PA monomers inside liposomes and subsequently adjusting the local pH in the liposomes, we propose a strategy to control self-assembly in a spatially confined environment.
Scheme 1.

Chemical structures of PAG and peptide amphiphiles 1, 2 and 3.
Encapsulation of the PA in the aqueous interior of the liposomes was achieved simply by using a PA solution to hydrate a phospholipid film. This encapsulation process was applicable to different liposome formulations including asolectin, which is a phospholipid mixture of approximately equal parts lecithin, cephalin and phosphatidylinositol, as well as controlled ratios of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-dioleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] (DOPG) and cholesterol.10 Internalization of the PA into asolectin-based liposomes was observed by confocal microscopy. In order to visualize the PA, a 2.5 mg mL-1 aqueous solution of PA 1 was doped with green-fluorescent PA 2 at a 10 : 1 molar ratio. PA 2 was designed with the alkyl tail at the carboxy-terminus of the peptide in order to expose the amine-terminus for FITC labeling. We have shown previously that PAs of different peptide directions are capable of co-assembly.11 The green-fluorescent PA was encapsulated in liposomes made from asolectin, doped with a red-fluorescent lipid (Supporting Information†) at a ≈100 : 1 molar ratio. The liposome mixture was then treated by size exclusion chromatography to remove unencapsulated PA molecules. The confocal images suggest localization of PA molecules (green) inside several liposomes (red) (Fig. 1).
Fig. 1.
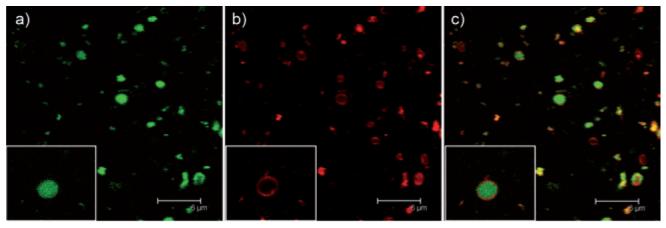
Confocal microscope images of green-fluorescent PA encapsulated by red-fluorescent asolectin liposomes. a) Green channel, b) red channel and c) overlaid images of the green and red channels. Insets show details of an individual liposome. Scale bar = 6 μm.
To enable the self-assembly of our model PAs inside the liposomes, it was necessary to use a non-invasive stimulus to increase the acidity of the liposome interior. Under acidic conditions, electrostatic repulsions among carboxylic acid groups are eliminated thus triggering self-assembly through hydrogen bonding and hydrophobic collapse.8b Photoacid generators (PAGs) were thus used here as a strategy to lower the pH by shining light on the system. PAGs have been traditionally used in the photolithographic patterning of semiconductors.12 Here, a water-soluble PAG, benzyldimethylsulfonium chloride,13a was synthesized for coencapsulation with the PA into liposomes (Scheme 1 and Supporting Information†). Photo-lysis of PAG by irradiation generates a radical cation, which reacts with water to decrease pH.13 Bulk gelation of PA 1 in the presence of PAG was indeed observed after 1 min of irradiation by 254 nm light (Fig. 2a,b). Six molar equivalents of PAG were added relative to PA 1, which has a formal charge of —4, in order to ensure protonation of carboxylic acid groups and promote self-assembly upon irradiation. As expected, TEM of the gel revealed a network of nanofibers approximately 10 nm in diameter and several microns in length (Fig. 2c).
Fig. 2.

a) 10 mg mL-1 of PA 1 with 6 equiv. PAG before irradiation and b) after irradiation at 254 nm. c) TEM image of nanofibers obtained from PA 1 gelled by irradiation in the presence of PAG.
Once behavior in bulk solution was characterized, the system was incorporated into liposomes. In a representative experiment, PAG (6 equiv.) was added to an aqueous 5 mg mL-1 PA 1 solution used to hydrate a lipid film of DPPC, DOPG and cholesterol (65 : 5 : 30). Unencapsulated PA 1 and PAG were subsequently removed by dialysis (10 000 MWCO) and the resulting mixture was irradiated with a 254 nm lamp for 1 min. The interior space of the irradiated liposomes containing PA 1 and PAG were examined using the quick-freeze/deep-etch (QFDE) technique, which produces Pt/C replicas from the fractured surface of a rapidly frozen sample.14 After fracture, the etching process sublimates water from the surface, exposing structures otherwise hidden in the ice. Replicas were transferred directly onto copper grids for imaging. Scanning electron microscopy (SEM) images of the replicas showed several larger cavities in the surface that likely result from liposomes that were fractured in the QFDE process (Fig. 3). These fractured liposomes revealed what appear to be networks of nanofiber bundles 10 to 30 nm in width, suggesting self-assembly of the PA within the liposome (Fig. 3 and Supplemental Information†). At this resolution, individual nanofibers cannot be observed by SEM as observed in the TEM of Fig. 2c. Instead one observes the typical appearance of fiber bundles at low resolution.
Fig. 3.

SEM image of a liposome containing a network of nanofibers. White arrows indicate nanostructures inside the liposome. Replicas for imaging were prepared by QFDE.
In order to obtain further evidence for the formation of nanofibers through light inside the liposomes, we used both Fourier transform infrared (FTIR) and circular dichroism (CD). FTIR spectroscopy experiments performed on lyophilized samples before and after triggering self assembly reveal an amide I stretching peak at 1630 cm-1, which corresponds to a β-sheet secondary structure.9a For the liposomes containing PA 1 and PAG, this band became slightly stronger after irradiation (Fig. 4). At the same time, the intensity of the broad shoulder at 1655–1700 cm-1 corresponding to random-coil conformations15 decreased after irradiation. The greater prevalence of β-sheet secondary structure in these PAs is a fingerprint of nanofiber formation.16
Fig. 4.

FTIR spectra in the amide I region of liposomes alone (black line) and liposomes containing PA 1 and PAG before (red line) and after irradiation at 254 nm for 1 min (blue line).
Further evidence of nanofiber formation in irradiated liposomes was obtained by CD. In order to observe a CD signal that would not overlap with background signals from the encapsulating liposomes, PA 1 was functionalized with pyrene, giving PA 3. CD spectra of pure PA 3, in the absence of liposomes, reveal the presence of a random-coil conformation for the peptide segment under basic conditions, as indicated by a minimum in the CD signal at a wavelength of 200 nm (see Supporting Information†). However, as expected when nanofibers form, there is a dramatic change to the β-sheet conformation under acidic conditions, shown by a minimum in the CD signal at 218 nm and a corresponding negative CD signal at approximately 350 nm corresponding to pyrene absorption in the chiral environment of the nanofibers. The chiral induction for pyrene is also observed after irradiation of PA 3 within liposomes, which is consistent with β-sheet formation and nanofiber self-assembly.17
A method has been developed to trigger with light the self-assembly of peptide amphiphiles into nanostructures inside liposomes. This strategy involves the use of a photoacid generator to reduce the charge of peptide amphiphiles, thus allowing their self-assembly to occur through hydrogen bonding and hydrophobic collapse. The method could enable the preparation of therapeutic nanofiber bundles with densely packed bioactive epitopes in the interior of liposomes for targeting to specific tissues, for example, using antibodies. In vivo, this would protect the fibers from untimely degradation, allowing the delivery of the PA nanofibers to specific cells while minimizing unwanted side effects. Such a system would be particularly advantageous in cancer therapy applications.
Acknowledgements
This work was supported by the Center of Cancer Nanotechnology Excellence (CCNE) initiative of the National Institutes of Health’s National Cancer Institute under Award Number U54CA119341. Any opinions, findings and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect those of the National Institutes of Health. H.-K. Lee thanks the Korea Research Foundation Grant (M01-2004-000-20166-0). We thank Dr Liam Palmer and Dr Stephany M. Standley for helpful discussions.
Footnotes
Electronic supplementary information (ESI) available: Experimental details and CD spectra. See DOI: 10.1039/b719486b
Notes and references
- 1(a).Allen TM, Cullis PR. Science. 2004;303:1818. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]; (b) Ulrich AS. Biosci. Rep. 2002;22:129. doi: 10.1023/a:1020178304031. [DOI] [PubMed] [Google Scholar]; (c) Lasic DD. Trends Biotechnol. 1998;16:307. doi: 10.1016/s0167-7799(98)01220-7. [DOI] [PubMed] [Google Scholar]
- 2(a).Cortese JD, Schwab B, Frieden C, Elson EL. Proc. Natl. Acad. Sci. U. S. A. 1989;86:5773. doi: 10.1073/pnas.86.15.5773. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Miyata H, Hotani H. Proc. Natl. Acad. Sci. U. S. A. 1992;89:11547. doi: 10.1073/pnas.89.23.11547. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Palmer AF, Wingert P, Nickels J. Biophys. J. 2003;85:1233. doi: 10.1016/S0006-3495(03)74559-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3(a).Lasic DD. Nature. 1996;380:561. doi: 10.1038/380561a0. [DOI] [PubMed] [Google Scholar]; (b) Li XG, Hirsh DJ, Cabral-Lilly D, Zirkel A, Gruner SM, Janoff AS, Perkins WR. Biochim. Biophys. Acta. 1998;1415:23. doi: 10.1016/s0005-2736(98)00175-8. [DOI] [PubMed] [Google Scholar]; (c) Abraham SA, Edwards K, Karlsson G, MacIntosh S, Mayer LD, McKenzie C, Bally MB. Biochim. Biophys. Acta. 2002;1565:41. doi: 10.1016/s0005-2736(02)00507-2. [DOI] [PubMed] [Google Scholar]
- 4(a).Ayabe M, Kishida T, Fujita N, Sada K, Shinkai S. Org. Biomol. Chem. 2003;1:2744. doi: 10.1039/b304224c. [DOI] [PubMed] [Google Scholar]; (b) Eastoe J, Vesperinas A. Soft Matter. 2005;1:338. doi: 10.1039/b510877m. [DOI] [PubMed] [Google Scholar]; (c) Geiger C, Stanescu M, Chen LH, Whitten DG. Langmuir. 1999;15:2241. [Google Scholar]; (d) Ikeda T, Nakano M, Yu YL, Tsutsumi O, Kanazawa A. Adv. Mater. 2003;15:201. [Google Scholar]; (e) Kazakov S, Kaholek M, Kudasheva D, Teraoka I, Cowman MK, Levon K. Langmuir. 2003;19:8086. [Google Scholar]; (f) Murata K, Aoki M, Suzuki T, Harada T, Kawabata H, Komori T, Ohseto F, Ueda K, Shinkai S. J. Am. Chem. Soc. 1994;116:6664. [Google Scholar]; (g) Nakano M, Yu YL, Shishido A, Tsutsumi O, Kanazawa A, Shiono T, Ikeda T. Mol. Cryst. Liq. Cryst. 2003;398:1. [Google Scholar]
- 5(a).Muraoka T, Cui H, Stupp SI. J. Am. Chem. Soc. 2008 doi: 10.1021/ja711213s. ASAP Article, DOI: 10.1021/ja711213s. [DOI] [PubMed] [Google Scholar]; (b) Santos S. Dos, Chandravarkar A, Mandal B, Mimna R, Murat K, Saucede L, Tella P, Tuchscherer G, Mutter M. J. Am. Chem. Soc. 2005;127:11888–11889. doi: 10.1021/ja052083v. [DOI] [PubMed] [Google Scholar]; (c) Taniguchi A, Sohma Y, Kimura M, Okada T, Ikeda K, Hayashi Y, Kimura T, Hirota S, Matsuzaki K, Kiso Y. J. Am. Chem. Soc. 2006;128:696–697. doi: 10.1021/ja057100v. [DOI] [PubMed] [Google Scholar]
- 6.Gore T, Dori Y, Talmon Y, Tirrell M, Bianco-Peled H. Langmuir. 2001;17:5352. [Google Scholar]
- 7(a).Kunitake T. Angew. Chem., Int. Ed. Engl. 1992;31:709. [Google Scholar]; (b) Berndt P, Fields GB, Tirrell M. J. Am. Chem. Soc. 1995;117:9515. [Google Scholar]
- 8(a).Hartgerink JD, Beniash E, Stupp SI. Proc. Natl. Acad. Sci. U. S. A. 2002;99:5133. doi: 10.1073/pnas.072699999. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hartgerink JD, Beniash E, Stupp SI. Science. 2001;294:1684. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 9(a).Niece KL, Hartgerink JD, Donners JJJM, Stupp SI. J. Am. Chem. Soc. 2003;125:7146. doi: 10.1021/ja028215r. [DOI] [PubMed] [Google Scholar]; (b) Stendahl JC, Rao MS, Guler MO, Stupp SI. Adv. Funct. Mater. 2006;16:499. [Google Scholar]
- 10.Chen HM, MacDonald RC, Li SY, Krett NL, Rosen ST, O’Halloran TV. J. Am. Chem. Soc. 2006;128:13348–13349. doi: 10.1021/ja064864h. [DOI] [PubMed] [Google Scholar]
- 11.Behanna HA, Donners JJJM, Gordon AC, Stupp SI. J. Am. Chem. Soc. 2005;127:1193–1200. doi: 10.1021/ja044863u. [DOI] [PubMed] [Google Scholar]
- 12(a).Shirai M, Tsunooka M. Prog. Polym. Sci. 1996;21:1. [Google Scholar]; (b) Crivello JV. J. Polym. Sci., Part A: Polym. Chem. 1999;37:4241. [Google Scholar]; (c) MacDonald SA, Wilson CG, Frechet JMJ. Acc. Chem. Res. 1994;27:151. [Google Scholar]
- 13(a).Akgun E, Glinski MB, Dhawan KL, Durst T. J. Org. Chem. 1981;46:2730. [Google Scholar]; (b) Sakamizu T, Shiraishi H, Ueno T. Polym. Mater. Sci. Eng. 1995;72:199. [Google Scholar]
- 14.Ruberti JW, Curcio CA, Millican CL, Menco BPM, Huang JD, Johnson M. Invest. Ophthalmol. Visual Sci. 2003;44:1753. doi: 10.1167/iovs.02-0496. [DOI] [PubMed] [Google Scholar]
- 15.Martinez G, Millhauser G. J. Struct. Biol. 1995;114:23. doi: 10.1006/jsbi.1995.1002. [DOI] [PubMed] [Google Scholar]
- 16.Jiang HZ, Guler MO, Stupp SI. Soft Matter. 2007;3:454. doi: 10.1039/b614426h. [DOI] [PubMed] [Google Scholar]
- 17.Bull SR, Guler MO, Bras RE, Meade TJ, Stupp SI. Nano Lett. 2005;5:1. doi: 10.1021/nl0484898. [DOI] [PubMed] [Google Scholar]


