Abstract
Chlamydia pneumoniae is detected by macrophages and other antigen presenting cells via Toll-like receptors (TLRs), and can exacerbate developing atherosclerotic lesions, but how that occurs is not known. Liver X receptors (LXRs) centrally control reverse cholesterol transport, but also negatively modulate TLR-mediated inflammatory pathways. We isolated peritoneal macrophages from wild type, TLR2, TLR3, TLR4, TLR2/4, MyD88, TRIF, MyD88/TRIF and IRF3 knockout mice and treated them with live or UV-killed C. pneumoniae in the presence or absence of oxidized LDL (ox-LDL), then measured foam cell formation. In some experiments, the synthetic LXR agonist GW3965 was added to macrophages infected with C. pneumoniae in the presence of ox-LDL. Both live and UV-killed C. pneumoniae induced IRF3 activation and promoted foam cell formation in wild type macrophages, while the genetic absence of TLR2, TLR4, MyD88, TRIF, or IRF3 but not TLR3 significantly reduced foam cell formation. C. pneumoniae-induced foam cell formation was significantly reduced by the LXR agonist GW3965, which in turn inhibited C. pneumoniae-induced IRF3 activation, suggesting a bidirectional cross talk. We conclude that C. pneumoniae facilitates foam cell formation via activation of both MyD88-dependent and MyD88-independent (i.e. TRIF- dependent and IRF3-dependent) pathways downstream of TLR2 and TLR4 signaling, and that TLR3 is not involved in this process. This mechanism could at least partly explain why infection with C. pneumoniae accelerates development of atherosclerotic plaque, and lends support to the proposal that LXR agonists might prove clinically useful in suppressing atherogenesis.
INTRODUCTION
Numerous studies link Chlamydia pneumoniae infection with atherosclerosis in both humans (1) and experimental animal models (2–10), but much less is understood about the cellular and molecular mechanisms involved. C. pneumoniae can infect and survive in endothelial cells, smooth muscle cells (11–13), circulating monocytes, and tissue macrophages, and can migrate from the lungs to developing plaque via circulating lymphocytes (14, 15)Macrophages are thought to be central to the pathobiology of atherosclerosis because they tend to be retained in developing atheroma, where they can express an array of proinflammatory chemokines and cytokines that promote plaque progression and plaque instability that often leads to clinical events such as myocardial infarction and stroke (16–18). In plaques, macrophages ingest necrotic cellular debris and modified lipids, which in turn triggers expression and secretion of proinflammatory molecules. Such lipid-laden macrophages are known as foam cells, owing to the foam-like appearance of their cytoplasm that is composed predominantly of accumulated lipid-filled vacuoles. Since macrophages can become infected with C. pneumoniae, one possibility is that ingested pathogen or molecules derived from dead organisms or both might accelerate atherogenesis by somehow promoting formation of macrophage foam cells.
C. pneumoniae can be detected by innate immune pattern recognition receptors such as Toll-like receptors (TLRs). Molecules derived from C. pneumoniae that are foreign to the host interact with several TLRs, including TLR2 and TLR4. Upon stimulation by ligands, these TLRs activate gene programs that involve expression and secretion of proinflammatory cytokines. Most TLRs (including TLR2 and TLR4) utilize the downstream adaptor MyD88 (Myeloid Differentiation factor 88) to transmit their signals. TLR3 relies exclusively upon TRIF (19), which is a cytoplasmic adaptor that relays signals independently of MyD88 (20, 21). TLR4 is unique among TLRs because it is the only TLR that can signal via either the MyD88-dependent or the MyD88-independent pathway that requires TRIF and TRAM (22). The MyD88-dependent pathway leads to activation of the NF-κB transcription factor, which directly controls proinflammatory genetic programs, but the primary target of the MyD88-independent pathway is IRF3 (Interferon Regulatory Factor 3), which controls genes that produce a response distinct from that of NF-κB-dependent programs.
To date, 13 murine and 11 human TLRs have been identified (23–25). Macrophages express an array of TLRs, including TLR2, TLR3, TLR4, and others (23–25). Reports from our lab (7, 26) and others (27–30) indicate that innate immune signaling via TLRs directly contributes to development of atherosclerotic plaque. Moreover, molecules derived from C. pneumoniae are detected by TLR2 and TLR4, and signaling emanating from both these receptors triggers inflammation and promotes atherosclerosis (7, 26) Genetic loss-of-function approaches indicate that both TLR2 and TLR4 are critically involved in eradication and clearance of C. pneumoniae clearance from the lung (31, 32), indicating involvement of both MyD88-dependent and -independent signaling in host defenses against C. pneumoniae. Collectively, these data suggest the possibility that C. pneumoniae infection might promote development of a proinflammatory phenotype that in turn would be expected to exacerbate atherosclerosis.
Metabolites of cholesterol bind to nuclear receptors called liver X receptors (LXRs) that play a central role in lipid metabolism, and are master regulators of cholesterol metabolism (33). LXRs centrally control reverse cholesterol metabolism can also counterbalance the proinflammatory effects of TLRs and down-modulate TLR4-mediated NF-kB activation (33, 34). TLR-mediated innate immune responses and LXR-directed regulation of cholesterol metabolic pathways influence one another bi-directionally. Stimulation of macrophages with LPS and LXR agonists reduce proinflammatory responses by a mechanism that is MyD88-dependent (34). Hence, LXRs suppress TLR signaling, conversely, TLR signaling reciprocally inhibit LXR activation (35). Indeed, stimulation of TLR3 or TLR4 by pathogen-derived ligands inhibits expression of LXR-dependent gene targets and macrophages cholesterol efflux. This effect proceeds via a MyD88-independent mechanism and involves IRF3 (35). Thus, LXR-TLR cross talk provides a potential mechanism to explain how microbial infections might interfere with cholesterol metabolism and contribute to cardiovascular disease. These results underscore the intimate association of lipid metabolic pathways with innate immune host defenses (33, 35–37), both of which are central to the pathobiology of atherosclerotic plaque development (16)
These considerations led us to hypothesize that C. pneumoniae might promote development of foam cells by triggering proinflammatory TLR-dependent signaling, but that this would be inhibited by activation of LXRs. Using a genetic loss-of-function approach, we report that in the presence of oxidized LDL (ox-LDL), C. pneumoniae promotes foam cell formation by interacting not only with TLR2 but also with TLR4 to trigger activation of NF-κB and IRF3, and can therefore utilize both the MyD88-dependent and the MyD88-independent pathways. This was confirmed using macrophages derived from TLR2−/−. TLR4−/−, TLR2−/−:TLR4−/− double KO, MyD88−/−, TRIF−/−, IRF3−/−, and MyD88−/−:TRIF−/− double KO mice. However, signaling emanating from TLR3 was not involved. We also found that C. pneumoniae-induced foam cell formation was blocked by pharmacologic LXR agonist treatment. These results therefore elucidate molecular mechanisms that link host defenses with lipid metabolic pathways, and implicate both in a potential explanation as to how C. pneumoniae might accelerate atherosclerosis on a cellular and molecular level.
MATERIALS AND METHODS
Generation of Knockout Mice
C57BL/6 wild type, TLR2−/−, and TRIFLps2/Lps2 mice were purchased from Jackson laboratories. MyD88−/− and TLR4−/− mice were kindly provided by Dr. Shizuo Akira (Osaka University, Osaka, Japan), TLR2−/−:TLR4−/− double knockout mice were obtained from Dr. Chris Wilson (University of Washington, Seattle, USA), and IRF3−/− and TLR3−/− mice were kindly provided from Dr. Genhong Cheng (University of California, Los Angeles, USA). A homogenous population of these mice was established by backcrossing onto the C57BL/6 background for at least 8 generations as previously described (26). The MyD88−/−:TRIF−/− double knockout mice were established in our laboratory by crossing the MyD88−/− with TRIFLps2/Lps2 mice. Mice were fed a standard chow diet and housed under pathogen-free conditions at Cedars-Sinai Medical Center. All animal experiments were performed under protocols that had been approved by the Institutional Animal Care and Use Committee at our facility.
Preparation of Peritoneal Macrophages
Peritoneal macrophages were isolated by injecting Hanks’ balanced salt solution (HBSS, GIBCO, USA) into the peritoneal cavity of mice immediately after euthanization. Peritoneal cells were washed and seeded in 24-well plates (2.5×105 cells/well) in RPMI 1640 medium (Cellgro, Los Angeles, CA) with 10% FBS, incubated at 37 °C, 5% CO2 for 3h, washed twice with HBSS to remove non-adherent cells, and cultured for 24 hours prior to treatment.
Infection of Macrophages
C. pneumoniae CM-1 strain (ATCC) was propagated in Hep-2 cells as previously described (38). Mycoplasma contamination was assessed by PCR(39). Inocula of C. pneumoniae were expressed as multiplicities of infection (MOIs). UV inactivation of C. pneumoniae was performed by exposure of 150 μl in a sterile tissue culture dish in a laminar flow hood to shortwave UV light at a distance of 7 cm for 25 min, with mixing after 15 min. The absence of live C. pneumoniae was confirmed infecting Hep-2 cells with the bacteria. Murine peritoneal macrophages were infected with live or UV-killed C. pneumoniae (MOI=5) overnight. Cells were washed and incubated with human ox-LDL (100 μg/ml, Biomedical Technologies, MA) for an additional 24 hours in the presence or absence of an LXR agonist GW3965 (2 μM) (40). LPS (protein-free) (10 ng/ml) or Poly (I:C) (1 μg/ml) were used as controls
Assessment of Foam Cell Formation
Foam cells were identified using Oil Red O staining. Peritoneal macrophages were washed twice with PBS, fixed and stained with 1% of Oil Red O solution (60% isopropanol; Sigma Aldrich, USA). Cells were washed three times with PBS and examined by light microscopy (x40). Designation of a macrophage as a foam cell required positive Oil Red O staining. Foam cells were quantified by cell counting and expressed as the percentage of positive Oil Red O cells to total cells. Each treatment condition was performed in triplicate.
NF-κB Activity Assay
RAW 264.7 cells were stably transfected with an NF-κB reporter plasmid (ELAM-luciferase) (41). Cells were seeded onto 96-well plates (1×105 cells/well) and treated 24 h later for 4 hours before any measurement of luciferase activity. Cells were stimulated with live or UV-killed C. pneumoniae (MOI=5), or LPS (0.01 μg/mL) with or without ox-LDL (100 μg/ml) in the presence or absence of the LXR agonist GW3965 (2 μM) (40, 42). After 4 hours, cells were washed with PBS and lysed using a lysis buffer (Promega, CA). Luciferase activity was measured by means of the dual luciferase reporter assay system (Promega, CA) according to the manufacturer’s instructions. Data were expressed as mean values of three independent experiments, with each treatment performed in triplicate.
IRF3 Activity Assay
The DNA-binding capacity of IRF3 was determined by using the TransAM™ transcription factor assay kit (Active Motif, Carlsbad, CA). The nuclear extract was subjected to binding of IRF3 to an immobilized consensus sequence in a 96-well plate. Primary and secondary antibodies were added as suggested by the manufacturer’s instructions. After the colorimetric reaction, the samples were measured in a spectrophotometer.
Statistical Analysis
Data are reported as mean ± SEM. Statistical differences were assessed by Student’s t test, and p values less than 0.05 were considered significant.
RESULTS
C. pneumoniae Promotes the Formation of Foam Cells in the Presence of ox-LDL
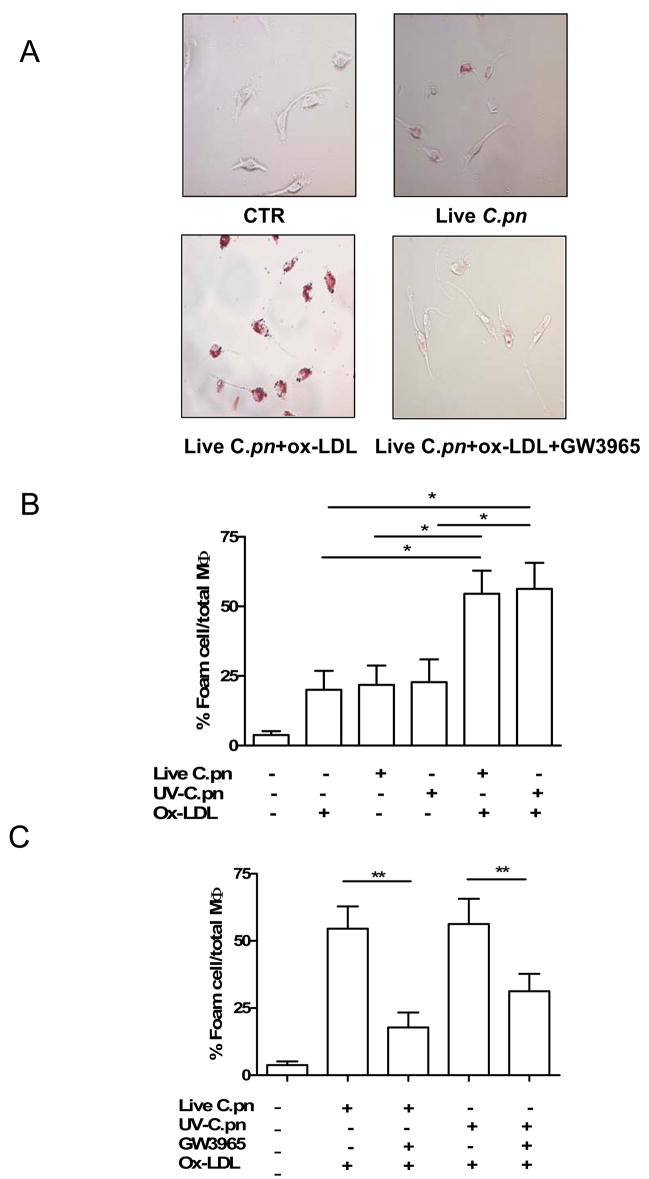
Peritoneal macrophages from wild type (C57BL/6) mice were pre-infected with live C. pneumoniae or UV-killed C. pneumoniae (MOI=5) and then treated with ox-LDL (100 μg/ml). Foam cell formation was characterized by the engulfment of positive Oil Red O lipid droplets in the macrophages. Foam cell formation was significantly induced by the presence of live C. pneumoniae (Fig. 1A, B) or UV-killed C. pneumoniae (Fig. 1B) and ox-LDL compared with the bacteria or ox-LDL alone. To evaluate the effect of LXRs on foam cell formation induced by the addition of C. pneumoniae and ox-LDL, we co-administered the LXR agonist GW3965 (2μM). GW3965 treatment significantly decreased foam cell formation induced by live or UV-killed C. pneumoniae (Fig 1C).
Fig. 1. C. pneumoniae promotes the formation of foam cells in the presence of ox-LDL from wild type-derived macrophages.
A Representative formation of foam cells following C. pneumoniae (MOI=5) or ox-LDL (100 μg/ml) alone or in combination. B. The amount of foam cells was significantly increased following live (p<0.05) or UV-killed (p<0.05) C. pneumoniae and ox-LDL administration. C. The treatment with GW3965 (2 μM) significantly reduced foam cell formation induced by the addition of ox-LDL and live (p<0.01) or UV-killed (p<0.01) C. pneumoniae. Foam cells are expressed as percentage of positive Oil Red O cells compared with total macrophages (MΦ). Data represent mean±SEM, experiments were repeated in four times in triplicates. Statistical difference was evaluated by means of Students’ t test.
C. pneumoniae Induces Foam Cell Formation via Activation of Either TLR2 or TLR4
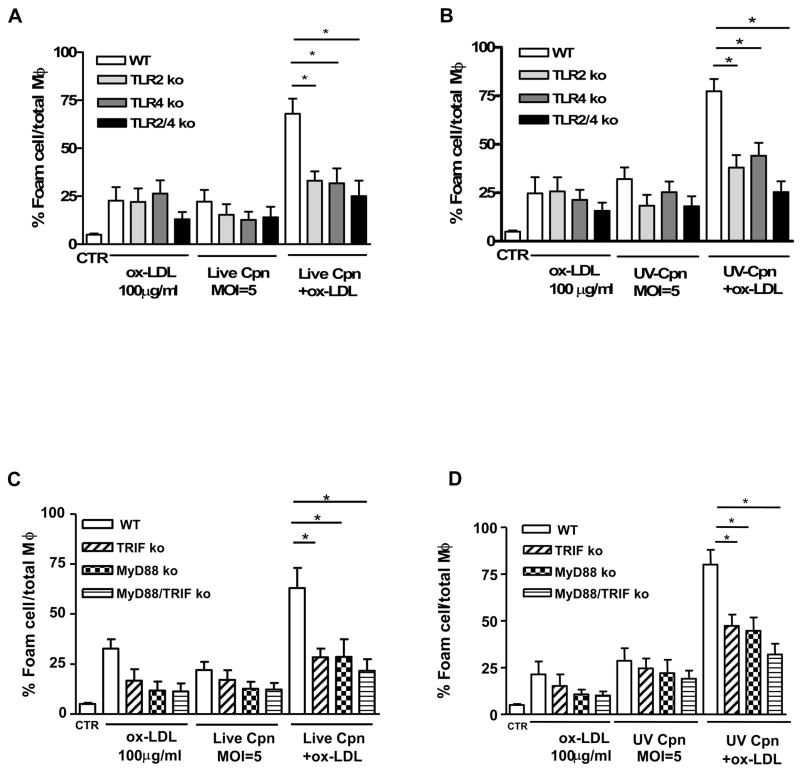
C. pneumoniae infection can trigger both TLR2- and TLR4-dependent host responses (40) (42). We therefore isolated peritoneal macrophages from TLR2−/−, TLR4−/− and TLR2−/−:TLR4−/− mice. Genetic deficiency in either TLR2 or TLR4 significantly reduced foam cell formation from macrophages treated with either live (Fig. 2A) or UV-killed C. pneumoniae (Fig. 2B) in the presence to ox-LDL. The double deficiency of TLR2 and TLR4 further reduced foam cell formation from macrophages treated with either live (Fig. 2A) or UV-killed (Fig. 2B) C. pneumoniae. These results indicate that molecules derived from C. pneumoniae can promote foam cell formation by triggering signaling through both TLR2 and TLR4.
Fig. 2. Live- or UV-killed C. pneumoniae-induced foam cell formation is TLR2- TLR4-, MyD88- and TRIF-dependent.
Peritoneal macrophages of wild type (WT), TLR2−/−, TLR4−/− and TLR2−/−:TLR4−/− mice were treated with live (A) or UV killed (B) C. pneumoniae (MOI=5) with or without ox-LDL (100 μg/ml). The same experimental protocol was applied to macrophages derived from MyD88−/−, TRIF−/− and MyD88−/−:TRIF−/− mice (C and D). Foam cells are expressed as percentage of positive Oil Red O cells compared with total M. Data represent mean±SEM, experiments were repeated three times in triplicate. Statistical difference, denoted as * for p<0.05, was evaluated by means of Students’ t test.
C. pneumoniae Promotes Foam Cell Formation by Activating Both MyD88-Dependent and MyD88-Independent Signaling Pathways
MyD88, an adaptor molecule utilized by both the TLR2 and TLR4 signaling pathways, plays an essential role in C. pneumoniae–induced infection (31). While TLR2 relies exclusively upon MyD88, TLR4 can signal through both MyD88-dependent and MyD88-independent (TRIF-IRF3) pathways. To understand whether C. pneumoniae-mediated foam cell formation was mediated by a MyD88-dependent or MyD88-independent pathway, we isolated peritoneal macrophages from MyD88−/−, TRIF−/− and MyD88−/−:TRIF−/− double knockout mice and compared them with wild type-derived peritoneal macrophages. The loss of either MyD88 or TRIF resulted in a significant reduction in the formation of foam cells induced by either live (Fig. 2C) or UV-killed (Fig. 2D) C. pneumoniae in the presence of ox-LDL. These results are most consistent with the interpretation that in the presence of ox-LDL, C. pneumoniae promotes formation of foam cells by activating both the MyD88-dependent and the MyD88-independent and TRIF/IRF3-dependent signaling pathways.
LXR Agonist (GW3965) Reduces C. pneumoniae-induced Foam Cell Formation and Diminishes NF-κB Activation
Stimulation of the LXR pathway inhibits NF-κB activation and TLR-dependent signaling and also activates cholesterol efflux by inducing the ABCA1 gene (35). We therefore predicted that treatment with the LXR agonist GW3965 would attenuate foam cell formation induced by ox-LDL and C. pneumoniae. We stably transfected the RAW264.7 murine macrophage cell line with an NF-κB luciferase reporter plasmid (RAW-NF-κB). Cells were stimulated with live or UV-killed C. pneumoniae and ox-LDL. LPS (0.01 μg/ml), TLR4 ligand, and PAM3CSK4 (1 μg/ml; a TLR2 ligand) were used as positive controls (data not shown). The activation of the NF-κB reporter ELAM-luciferase was measured. Compared to untreated cells, there was no change in NF-κB activation after treatment with ox-LDL alone (Fig. 3A, B). However, live or UV-killed C. pneumoniae infection significantly increased NF-κB activation (Fig. 3A, B). Interestingly, treatment with ox-LDL together with infection with either live or UV-killed C. pneumoniae decreased NF-κB activation (Fig. 3A, B) as was reported by others (49). As expected, the addition of GW3965 tended to further decrease NF-κB activation, although the difference did not reach statistical significance.
Fig. 3. C. pneumoniae induced NF-κB and IRF3 activation and modulation by the LXR agonist GW3965.
NF-kB activation with live (A) or UV-killed (B) C. pneumoniae in the presence of ox-LDL. The treatment with GW3965 further reduced NF-κB activation (A and B). Panel C and D represent the detection of IRF3 activation following live (C) or UV-killed (D) C. pneumoniae and ox-LDL. GW 3965 significantly reduced the formation of foam cells (C and D; p<0.001 and p<0.001, respectively). Data represent mean±SEM, experiments were repeated three times in triplicate. Statistical difference, denoted as * for p<0.05 and ** for p<0.01 as evaluated by means of Students’ t test.
C. pneumoniae-Induced Foam Cell Formation Utilizes the TRIF/IRF3 Pathway in Macrophages; Modulation of IRF3 Activity by the LXR Agonist GW3965
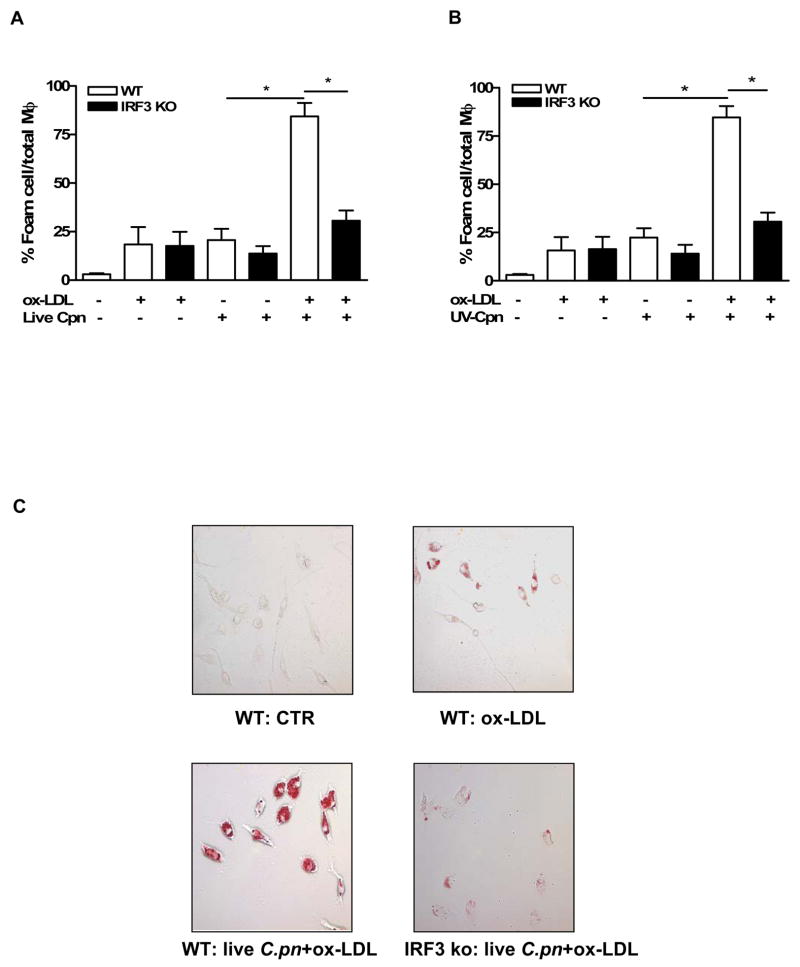
Previous reports suggest that C. trachomatis activates the IRF3 pathway in a MyD88-independent manner (i.e. TRIF-dependent pathway) (43), as well as in a MyD88-dependent manner (54). However, the role of IRF3 in C. pneumoniae-induced responses and in particular foam cell formation has not been studied. First, we investigated if C. pneumoniae induces IRF3 activity in macrophages using an in vitro IRF3 activity assay. Infection with live or stimulation with UV-killed C. pneumoniae increased significantly IRF3 activity in wild type macrophages, which was further increased in the presence of ox-LDL (Fig. 3C and 3D). Surprisingly, the addition of the LXR agonist GW3965 to live or UV-killed C. pneumoniae significantly inhibited IRF3 activation (Fig. 3C, D), suggesting a bidirectional interaction between the IRF3 and LXR pathways. To investigate the role of the IRF3 activation pathway in C. pneumoniae-induced foam cell formation we isolated IRF3−/− peritoneal macrophages and treated them in the same manner as experiments described above. IRF3 deficiency completely inhibited the formation of foam cells in response to either live (Fig. 4A) or UV-killed (Fig. 4B) C. pneumoniae in the presence of ox-LDL. Peritoneal macrophages obtained from IRF3−/− mice were resistant to C. pneumoniae-induced foam cell formation as compared to wild type macrophages as seen in a representative picture (Fig. 4C).
Fig. 4. C. pneumoniae-mediated foam cell formation requires IRF3 activation as IRF3−/− macrophages are resistant to infection-mediated foam cell production.
Peritoneal macrophages from IRF3−/− mice and wt mice were stimulated with live (A) or UV-killed (B) C. pneumoniae and ox-LDL. Panel C is a representative picture of foam cells induced in peritoneal macrophages obtained in wild type mice compared with those obtained from IRF3 −/− mice in the presence of live C. pneumoniae and ox-LDL. Data represent mean±SEM, experiments were repeated three time in triplicate. Statistical difference, denoted as * for p<0.05, was evaluated by means of Students’ t test.
C. pneumoniae-Induced Foam Cell Formation Does Not Involve TLR3
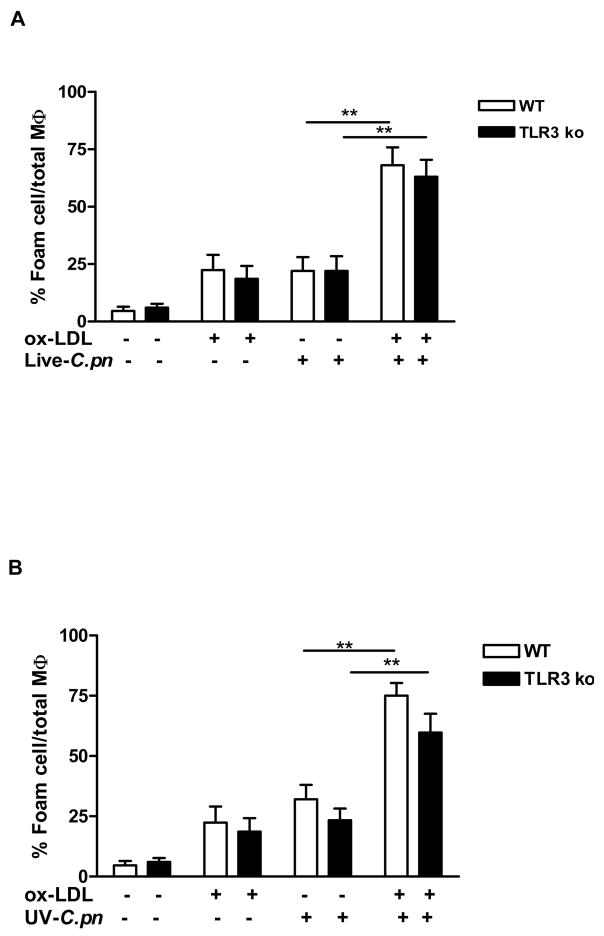
Results in the previous section demonstrate that C. pneumoniae utilizes TRIF to activate IRF3. TLR4 can signal through either MyD88 or TRIF, however TLR3 signaling is completely dependent upon TRIF (20, 44). Results summarized above indicate that TLR4 signaling was involved, but it is also possible that molecules derived from C. pneumoniae stimulated foam cell formation by triggering signaling via both TLR4 and TLR3. To clarify this issue, we isolated peritoneal macrophages from TLR3−/− mice and treated them with live or UV-killed C.pneumoniae in the presence or absence of ox-LDL. Formation of foam cells was not affected by the genetic absence of TLR3 as compared with macrophages derived from wild type mice when either live (Fig. 5A) or UV-killed (Fig. 5B) C. pneumoniae was added to ox-LDL. Furthermore, TLR3 was not essential for ox-LDL or C. pneumoniae alone or together to promote formation of Oil Red O positive foam cells. Hence, these results exclude the possibility that TLR3 is involved in formation of foam cells induced by C. pneumoniae.
Fig. 5. C. pneumoniae-mediated foam cell production does not involve TLR3 signaling.
Peritoneal macrophages derived from wild type or TLR3−/− mice were stimulated with live (A) or UV-killed (B) C. pneumoniae and ox-LDL and Oil Red O positive stained cells were calculated to measure foam cell formation. Data represent mean±SEM, experiments were repeated three times in triplicate. Statistical difference, denoted as ** for p<0.01 was evaluated by means of Students’ t test.
DISCUSSION
Numerous studies indicate that retention of lipids in developing plaques and formation of lipid-laden macrophages (i.e., foam cells) are hallmarks of the atherosclerotic process (16, 17). Clinical and experimental studies suggest that infection with Chlamydia pneumoniae exacerbates atherosclerosis (45), but the cellular and molecular signaling mechanisms involved are unclear. Here we report that accumulation of lipids in macrophages and consequent formation of foam cells is induced by infection of macrophages with C. pneumoniae or even simply treatment of macrophages with UV-killed organisms in the presence of ox-LDL. This process was mediated by signaling through either TLR2, TLR4, or both, and utilized both the MyD88-dependent and MyD88-independent (TRIF/IRF3) pathways, activated both NF-κB and IRF3, but did not involve TLR3 signaling. Foam cell formation promoted by C. pneumoniae was reversed by activation of LXR pathway by a synthetic agonist. Hence, our results provide important insights into the specific innate immune signaling mechanisms linking infection by C. pneumoniae with facilitation of plaque development, and in addition, underscore the close interconnections of host immune defenses with cholesterol metabolic pathways (33, 46) in the pathobiology of atherosclerosis.
Our results confirm and significantly extend previous work by Byrne and coworkers (3, 40, 47, 48). These investigators first reported that C. pneumoniae infection of human monocyte-derived macrophages (48) or murine RAW264.7 cells (3) in the presence of LDL causes accumulation of cholesteryl esters by macrophages and thus leads to foam cell formation, and that uptake of cholesterol occurred independently of the LDL receptor (47). Conversely, Blessing and colleagues reported that once formed, foam cells poorly support growth of C. pneumoniae, but when foam cells are exposed to C. pneumoniae secretion of proinflammatory cytokines is similar to that of macrophages that have not yet become foam cells (49). Very recent electron microscopic data indicates that oxidized lipids and C. pneumoniae co-localize in foam cells obtained from human carotid arteries, and documented amalgamation of C. pneumoniae inclusions with lipid droplets (50). Similarly, our results also show that infection of peritoneal macrophages with either the live or UV-killed pathogen induces foam cell formation. Clearly then, data reported thus far in both human and murine cell culture studies indicate that C. pneumoniae promotes foam cell formation, at least in the context of a lipid-rich environment and our data now provide the molecular signaling pathways involved in this process that may play a role in the acceleration of atherosclerosis.
More recent studies have begun to shed light on participation of innate immune signaling networks in this pro-atherogenic process. Cao et al. infected murine RAW264.7 macrophages with C. pneumoniae and also treated cells with known TLR2 or TLR4 ligands (Pam3Cys and LPS, respectively) in the presence of LDL. They reported that either treatment induced foam cell formation and experiments with TLR2- deficient cells appeared to implicate TLR2 as the primary signaling pathway that instigates C. pneumoniae-mediated foam cell formation (40). Our results with TLR2−/− and TLR4−/− primary peritoneal macrophages confirmed the involvement of TLR2, but also showed that TLR4 is involved as well. Furthermore, based on results obtained with MyD88−/−, TRIF−/−, IRF3−/− and MyD88−/−:TRIF−/− macrophages, C. pneumoniae in the presence of ox-LDL triggered signals to activate both the MyD88-NF-κB pathway and the TRIF/IRF3 downstream effectors. Although Cao et al did not find evidence for TLR4 involvement (40), it might be pertinent to note that their study utilized the GG2EE macrophage cell line derived from C3H/HeJ mice that express a mutant TLR4 containing a proline-to-histidine mutation at amino acid residue 714. Hence, C3H/HeJ mice and cells isolated from these mice do not respond normally to TLR4 ligands. However our approach was more direct, and instead used macrophages derived from homozygous TLR4 knockout mice. Of interest Kalayoglu et al reported that C. pneumoniae-derived components that induce foam cell formation include the chlamydial LPS and chlamydial HSP60 both of which are TLR4 ligands (3, 51, 52) (reviewed in (53)). The fact that TRIF−/− and IRF3−/− macrophages produced significantly diminished foam cell formation similar to that seen in TLR4−/− macrophages and that foam cell production was not altered in TLR3−/− macrophages and were similar to wt macrophages further corroborates the interpretation that TLR4 is directly involved in foam cell formation induced by C. pneumoniae. However, our studies cannot rule out any potential contribution of the intracellular TLRs (TLR7, TLR8 and TLR9) to C. pneumoniae-mediated foam cell formation.
Previous data suggest that C. trachomatis activates the IRF3 pathway in a MyD88-independent manner (i.e. TRIF-dependent), that results in upregulation of type I interferons, at least in oviduct epithelial cells, (43), as well as in MyD88-dependent manner (54). Here we show that C. pneumoniae is also able to induce IRF3 activation in macrophages. Macrophages express both TLR4 and TLR3. However, our data using TLR3−/− cells exclude the possibility that C. pneumoniae activates TLR3 and then TRIF/IRF3. TLR3 responds primarily to dsRNA and other viral ligands (55), and to date, no Chlamydia ligands for TLR3 have been identified (43). Since there is no direct evidence that C. pneumoniae stimulates TLR3, the most likely interpretation of our results is that activation of the TRIF/IRF3 pathway by the bacteria requires intact TLR4 and does not involve TLR3. On the other hand another study reported that C. pneumoniae-induced activation of NF-kB and expression of proinflammatory cytokines was TRAF6-dependent and that IRF3 and IRAK4 were redundant, and the authors did not observe increased IRF3 phosphorylation in C. pneumoniae-infected bone marrow derived macrophages (BMDMs) (56). In contrast, our results emphasize that C. pneumoniae directly stimulates IRF3 activation in peritoneal macrophages, which in turn promotes foam cell formation in the presence of ox-LDL. These differing results could be due to innate differences between the structural components of the distinct C. pneumoniae strains used between the two studies, CM-1 versus Kajaani 6, or to the use of BMDMs in the previous study versus peritoneal macrophages used in this study. Indeed, significant variations in responses to different chlamydial strains have been reported and recently discussed by Nagarajan et al (54). Besides IRF3, another key transcription factor that plays a role in type I IFN induction is IRF7, and future studies will need to investigate if IRF7 also contributes to C. pneumoniae –induced foam cell formation.
LXR activation promotes cholesterol efflux from lipid-loaded macrophages and the LXR agonist GW3965 was shown to decrease atherosclerotic lesions by 50% in hypercholesterolemic mouse models (57, 58). Cross talk between TLRs and LXR pathways converge on IRF3 signaling, as TLR3- or TLR4-TRIF mediated IRF3 signaling down regulates LXR functions as evidenced by a reduction of ABCA1 transcription and reduced cholesterol efflux from macrophages (35). Therefore, microbial agents associated with acceleration of atherosclerosis, i.e. C. pneumoniae, can trigger TLR4/TRIF/IRF3 activation, down regulate LXRs, and shift cholesterol transport toward pro-foam cell production and therefore accelerate atherogenesis. In direct support of the notion that C. pneumoniae alters processing of ingested lipids is our results demonstrating that treatment with the LXR agonist GW3965 blocked foam cell formation in the presence of the bacteria. These results were consistent with previous data (40), and underscore the intimate interconnections between cholesterol metabolism and innate immune host defenses. While LXR activation was reported to reduce TLR4-mediated NF-κB activity (33, 34), and TLR-IRF3 pathways was shown to inhibit LXR activation (35), to our knowledge, our data represent the first demonstration that an LXR agonist, in turn can down-modulate C. pneumoniae-mediated IRF3 activation in macrophages (Fig. 6- Schematic).
Fig. 6. Schematic representation of C. pneumoniae- mediated foam cell formation.
C. pneumoniae infection mediates foam cell formation through at least two signalling pathways: TLR2 and TLR4-dependent signaling through MyD88, and TLR4-TRIF-dependent signalling. The MyD88 pathway leads to activation of NF-κB, and the TRIF pathway leads to activation of IRF3. The addition of ox-LDL increases C. pneumoniae-promoted foam cell formation and activates NF-κB and IRF3 signalling pathways. LXR agonists like GW3965 reverse C. pneumoniae-induced foam cell formation by increasing cholesterol efflux via ABCA1 expression. Others have shown that TLRs that can activate TRIF and IRF3 inhibit LXR responses (35) and we show that the LXR agonist GW3965, in turn, can inhibit C. pneumoniae-induced IRF3 activation, suggesting a bi-directional cross-talk. Thus, LXR activation can negatively modulate both TLR/MyD88 and TLR/TRIF-dependent innate immune signaling networks. Abbreviations: LXR= liver-X-receptor; ABCA1= ATP-binding cassette transporter.
Collectively then, our data indicate that (Fig. 6-Schematic): 1) C. pneumoniae infection promotes foam cell formation in a TLR2- and TLR4-dependent manner through both MyD88- and TRIF-dependent signaling pathways and this process does not require TLR3 signaling; 2) LXR activation by a synthetic agonist (GW3965) reverses C. pneumoniae-mediated foam cell formation; 3) C. pneumoniae infection induces IRF3 activation in peritoneal macrophages and IRF3 in turn contributes to foam cell formation; 4) The LXR agonist GW3965, in turn reduces C. pneumoniae –induced IRF3 activation in macrophages. We therefore provide important new insights into molecular signaling mechanisms involved in the formation of foam cells that is promoted by infection with C. pneumoniae, and hence provide potential explanations for how C. pneumoniae infection might accelerate development of atherosclerosis and so contribute to adverse clinical events such as myocardial infarction and stroke.
Acknowledgments
Supported by NIH grants AI 067995 and HL66436 to MA
We are thankful to Dr. Peter Tontonoz (Department of Molecular Biology Insitute, University of California, Los Angeles, CA) who kindly provided us with the LXR agonist GW3965.
References
- 1.Saikku P, Leinonen M, Mattila K, Ekman MR, Nieminen MS, Makela PH, Huttunen JK, Valtonen V. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet. 1988;2:983–986. doi: 10.1016/s0140-6736(88)90741-6. [DOI] [PubMed] [Google Scholar]
- 2.Kaukoranta-Tolvanen SS, Ronni T, Leinonen M, Saikku P, Laitinen K. Expression of adhesion molecules on endothelial cells stimulated by Chlamydia pneumoniae. Microb Pathog. 1996;21:407–411. doi: 10.1006/mpat.1996.0071. [DOI] [PubMed] [Google Scholar]
- 3.Kalayoglu MV, Byrne GI. A Chlamydia pneumoniae component that induces macrophage foam cell formation is chlamydial lipopolysaccharide. Infect Immun. 1998;66:5067–5072. doi: 10.1128/iai.66.11.5067-5072.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiechl S, Lorenz E, Reindl M, Wiedermann CJ, Oberhollenzer F, Bonora E, Willeit J, Schwartz DA. Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med. 2002;347:185–192. doi: 10.1056/NEJMoa012673. [DOI] [PubMed] [Google Scholar]
- 5.Molestina RE, Miller RD, Ramirez JA, Summersgill JT. Infection of human endothelial cells with Chlamydia pneumoniae stimulates transendothelial migration of neutrophils and monocytes. Infect Immun. 1999;67:1323–1330. doi: 10.1128/iai.67.3.1323-1330.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coombes BK, Mahony JB. Chlamydia pneumoniae infection of human endothelial cells induces proliferation of smooth muscle cells via an endothelial cell-derived soluble factor(s) Infect Immun. 1999;67:2909–2915. doi: 10.1128/iai.67.6.2909-2915.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michelsen KS, Doherty TM, Shah PK, Arditi M. TLR signaling: an emerging bridge from innate immunity to atherogenesis. J Immunol. 2004;173:5901–5907. doi: 10.4049/jimmunol.173.10.5901. [DOI] [PubMed] [Google Scholar]
- 8.Belland RJ, Ouellette SP, Gieffers J, Byrne GI. Chlamydia pneumoniae and atherosclerosis. Cell Microbiol. 2004;6:117–127. doi: 10.1046/j.1462-5822.2003.00352.x. [DOI] [PubMed] [Google Scholar]
- 9.Rosenfeld ME, Blessing E, Lin TM, Moazed TC, Campbell LA, Kuo C. Chlamydia, inflammation, and atherogenesis. J Infect Dis. 2000;181(Suppl 3):S492–497. doi: 10.1086/315618. [DOI] [PubMed] [Google Scholar]
- 10.Campbell LA, Kuo CC. Chlamydia pneumoniae--an infectious risk factor for atherosclerosis? Nat Rev Microbiol. 2004;2:23–32. doi: 10.1038/nrmicro796. [DOI] [PubMed] [Google Scholar]
- 11.Gaydos CA, Summersgill JT, Sahney NN, Ramirez JA, Quinn TC. Replication of Chlamydia pneumoniae in vitro in human macrophages, endothelial cells, and aortic artery smooth muscle cells. Infect Immun. 1996;64:1614–1620. doi: 10.1128/iai.64.5.1614-1620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jahn HU, Krull M, Wuppermann FN, Klucken AC, Rosseau S, Seybold J, Hegemann JH, Jantos CA, Suttorp N. Infection and activation of airway epithelial cells by Chlamydia pneumoniae. J Infect Dis. 2000;182:1678–1687. doi: 10.1086/317608. [DOI] [PubMed] [Google Scholar]
- 13.Shemer-Avni Y, Lieberman D. Chlamydia pneumoniae-induced ciliostasis in ciliated bronchial epithelial cells. J Infect Dis. 1995;171:1274–1278. doi: 10.1093/infdis/171.5.1274. [DOI] [PubMed] [Google Scholar]
- 14.Haranaga S, Yamaguchi H, Friedman H, Izumi S, Yamamoto Y. Chlamydia pneumoniae infects and multiplies in lymphocytes in vitro. Infect Immun. 2001;69:7753–7759. doi: 10.1128/IAI.69.12.7753-7759.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaul R, Uphoff J, Wiedeman J, Yadlapalli S, Wenman WM. Detection of Chlamydia pneumoniae DNA in CD3+ lymphocytes from healthy blood donors and patients with coronary artery disease. Circulation. 2000;102:2341–2346. doi: 10.1161/01.cir.102.19.2341. [DOI] [PubMed] [Google Scholar]
- 16.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 17.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 18.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat Immunol. 2003;4:161–167. doi: 10.1038/ni886. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto M, Sato S, Mori K, Hoshino K, Takeuchi O, Takeda K, Akira S. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J Immunol. 2002;169:6668–6672. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto M, Sato S, Hemmi H, Uematsu S, Hoshino K, Kaisho T, Takeuchi O, Takeda K, Akira S. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol. 2003;4:1144–1150. doi: 10.1038/ni986. [DOI] [PubMed] [Google Scholar]
- 23.Beutler B, Jiang Z, Georgel P, Crozat K, Croker B, Rutschmann S, Du X, Hoebe K. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu Rev Immunol. 2006;24:353–389. doi: 10.1146/annurev.immunol.24.021605.090552. [DOI] [PubMed] [Google Scholar]
- 24.Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, Mudd S, Shamel L, Sovath S, Goode J, Alexopoulou L, Flavell RA, Beutler B. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci U S A. 2004;101:3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 26.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibson FC, 3rd, Hong C, Chou HH, Yumoto H, Chen J, Lien E, Wong J, Genco CA. Innate immune recognition of invasive bacteria accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;109:2801–2806. doi: 10.1161/01.CIR.0000129769.17895.F0. [DOI] [PubMed] [Google Scholar]
- 28.Mullick AE, Soldau K, Kiosses WB, Bell TA, 3rd, Tobias PS, Curtiss LK. Increased endothelial expression of Toll-like receptor 2 at sites of disturbed blood flow exacerbates early atherogenic events. J Exp Med. 2008;205:373–383. doi: 10.1084/jem.20071096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest. 2005;115:3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bjorkbacka H, V, Kunjathoor V, Moore KJ, Koehn S, Ordija CM, Lee MA, Means T, Halmen K, Luster AD, Golenbock DT, Freeman MW. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004;10:416–421. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- 31.Naiki Y, Michelsen KS, Schroder NW, Alsabeh R, Slepenkin A, Zhang W, Chen S, Wei B, Bulut Y, Wong MH, Peterson EM, Arditi M. MyD88 is pivotal for the early inflammatory response and subsequent bacterial clearance and survival in a mouse model of Chlamydia pneumoniae pneumonia. J Biol Chem. 2005;280:29242–29249. doi: 10.1074/jbc.M503225200. [DOI] [PubMed] [Google Scholar]
- 32.Abreu MT, Thomas LS, Arnold ET, Lukasek K, Michelsen KS, Arditi M. TLR signaling at the intestinal epithelial interface. J Endotoxin Res. 2003;9:322–330. doi: 10.1179/096805103225002593. [DOI] [PubMed] [Google Scholar]
- 33.Zelcer N, Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. J Clin Invest. 2006;116:607–614. doi: 10.1172/JCI27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9:213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 35.Castrillo A, Joseph SB, Vaidya SA, Haberland M, Fogelman AM, Cheng G, Tontonoz P. Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol Cell. 2003;12:805–816. doi: 10.1016/s1097-2765(03)00384-8. [DOI] [PubMed] [Google Scholar]
- 36.Bradley MN, Hong C, Chen M, Joseph SB, Wilpitz DC, Wang X, Lusis AJ, Collins A, Hseuh WA, Collins JL, Tangirala RK, Tontonoz P. Ligand activation of LXR beta reverses atherosclerosis and cellular cholesterol overload in mice lacking LXR alpha and apoE. J Clin Invest. 2007;117:2337–2346. doi: 10.1172/JCI31909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gordon S. Do macrophage innate immune receptors enhance atherogenesis? Dev Cell. 2003;5:666–668. doi: 10.1016/s1534-5807(03)00329-0. [DOI] [PubMed] [Google Scholar]
- 38.Peterson EM, de la Maza LM, Brade L, Brade H. Characterization of a neutralizing monoclonal antibody directed at the lipopolysaccharide of Chlamydia pneumoniae. Infect Immun. 1998;66:3848–3855. doi: 10.1128/iai.66.8.3848-3855.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pal S, Luke CJ, Barbour AG, Peterson EM, de la Maza LM. Immunization with the Chlamydia trachomatis major outer membrane protein, using the outer surface protein A of Borrelia burgdorferi as an adjuvant, can induce protection against a chlamydial genital challenge. Vaccine. 2003;21:1455–1465. doi: 10.1016/s0264-410x(02)00680-1. [DOI] [PubMed] [Google Scholar]
- 40.Cao F, Castrillo A, Tontonoz P, Re F, Byrne GI. Chlamydia pneumoniae--induced macrophage foam cell formation is mediated by Toll-like receptor 2. Infect Immun. 2007;75:753–759. doi: 10.1128/IAI.01386-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hume DA, Underhill DM, Sweet MJ, Ozinsky AO, Liew FY, Aderem A. Macrophages exposed continuously to lipopolysaccharide and other agonists that act via toll-like receptors exhibit a sustained and additive activation state. BMC Immunol. 2001;2:11. doi: 10.1186/1471-2172-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prebeck S, Kirschning C, Durr S, da Costa C, Donath B, Brand K, Redecke V, Wagner H, Miethke T. Predominant role of toll-like receptor 2 versus 4 in Chlamydia pneumoniae-induced activation of dendritic cells. J Immunol. 2001;167:3316–3323. doi: 10.4049/jimmunol.167.6.3316. [DOI] [PubMed] [Google Scholar]
- 43.Derbigny WA, Hong SC, Kerr MS, Temkit M, Johnson RM. Chlamydia muridarum infection elicits a beta interferon response in murine oviduct epithelial cells dependent on interferon regulatory factor 3 and TRIF. Infect Immun. 2007;75:1280–1290. doi: 10.1128/IAI.01525-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoebe K, Du X, Georgel P, Janssen E, Tabeta K, Kim SO, Goode J, Lin P, Mann N, Mudd S, Crozat K, Sovath S, Han J, Beutler B. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003;424:743–748. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- 45.Watson C, Alp NJ. Role of Chlamydia pneumoniae in atherosclerosis. Clin Sci (Lond) 2008;114:509–531. doi: 10.1042/CS20070298. [DOI] [PubMed] [Google Scholar]
- 46.Beaven SW, Tontonoz P. Nuclear receptors in lipid metabolism: targeting the heart of dyslipidemia. Annu Rev Med. 2006;57:313–329. doi: 10.1146/annurev.med.57.121304.131428. [DOI] [PubMed] [Google Scholar]
- 47.Kalayoglu MV, Miranpuri GS, Golenbock DT, Byrne GI. Characterization of low-density lipoprotein uptake by murine macrophages exposed to Chlamydia pneumoniae. Microbes Infect. 1999;1:409–418. doi: 10.1016/s1286-4579(99)80044-6. [DOI] [PubMed] [Google Scholar]
- 48.Kalayoglu MV, Hoerneman B, LaVerda D, Morrison SG, Morrison RP, Byrne GI. Cellular oxidation of low-density lipoprotein by Chlamydia pneumoniae. J Infect Dis. 1999;180:780–790. doi: 10.1086/314931. [DOI] [PubMed] [Google Scholar]
- 49.Blessing E, Kuo CC, Lin TM, Campbell LA, Bea F, Chesebro B, Rosenfeld ME. Foam cell formation inhibits growth of Chlamydia pneumoniae but does not attenuate Chlamydia pneumoniae-induced secretion of proinflammatory cytokines. Circulation. 2002;105:1976–1982. doi: 10.1161/01.cir.0000015062.41860.5b. [DOI] [PubMed] [Google Scholar]
- 50.Bobryshev YV, Killingsworth MC, Tran D, Lord R. Amalgamation of Chlamydia pneumoniae inclusions with lipid droplets in foam cells in human atherosclerotic plaque. Virchows Arch. 2008 doi: 10.1007/s00428-008-0629-2. [DOI] [PubMed] [Google Scholar]
- 51.Kalayoglu MV, Indrawati RP, Morrison SG, Morrison Y, Yuan Byrne GI. Chlamydial virulence determinants in atherogenesis: the role of chlamydial lipopolysaccharide and heat shock protein 60 in macrophage-lipoprotein interactions. J Infect Dis. 2000;181(Suppl 3):S483–489. doi: 10.1086/315619. [DOI] [PubMed] [Google Scholar]
- 52.Bulut Y, Faure E, Thomas L, Karahashi H, Michelsen KS, Equils O, Morrison SG, Morrison RP, Arditi M. Chlamydial heat shock protein 60 activates macrophages and endothelial cells through Toll-like receptor 4 and MD2 in a MyD88-dependent pathway. J Immunol. 2002;168:1435–1440. doi: 10.4049/jimmunol.168.3.1435. [DOI] [PubMed] [Google Scholar]
- 53.Joyee AG, Yang X. Role of toll-like receptors in immune responses to chlamydial infections. Curr Pharm Des. 2008;14:593–600. doi: 10.2174/138161208783885344. [DOI] [PubMed] [Google Scholar]
- 54.Nagarajan UM, Ojcius DM, Stahl L, Rank RG, Darville T. Chlamydia trachomatis induces expression of IFN-gamma-inducible protein 10 and IFN-beta independent of TLR2 and TLR4, but largely dependent on MyD88. J Immunol. 2005;175:450–460. doi: 10.4049/jimmunol.175.1.450. [DOI] [PubMed] [Google Scholar]
- 55.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 56.Trumstedt C, Eriksson E, Lundberg AM, Yang TB, Yan ZQ, Wigzell H, Rottenberg ME. Role of IRAK4 and IRF3 in the control of intracellular infection with Chlamydia pneumoniae. J Leukoc Biol. 2007;81:1591–1598. doi: 10.1189/jlb.0706456. [DOI] [PubMed] [Google Scholar]
- 57.Joseph SB, McKilligin E, Pei L, Watson MA, Collins AR, Laffitte BA, Chen M, Noh G, Goodman J, Hagger GN, Tran J, Tippin TK, Wang X, Lusis AJ, Hsueh WA, Law RE, Collins JL, Willson TM, Tontonoz P. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc Natl Acad Sci U S A. 2002;99:7604–7609. doi: 10.1073/pnas.112059299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Venkateswaran A, Laffitte BA, Joseph SB, Mak PA, Wilpitz DC, Edwards PA, Tontonoz P. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc Natl Acad Sci U S A. 2000;97:12097–12102. doi: 10.1073/pnas.200367697. [DOI] [PMC free article] [PubMed] [Google Scholar]