Abstract
Previous studies identified radiation therapy as a key modifier of basal cell carcinoma (BCC) risk in survivors of hematopoietic cell transplantation (HCT). In the present analysis, risk of BCC was analyzed in relation to age at transplant, attained age, race, total-body irradiation (TBI), and radiation fractionation in 6,306 patients who received HCT at ages 0–65 years after conditioning regimens with (n = 3870) or without (n = 2436) TBI, and who were followed from 100 days to 36.2 years after HCT. While age-specific BCC rates in the unirradiated patient population were higher than those reported for two non-patient populations, the general characteristics were similar; rates increased with attained age, were eightfold lower for non-white patients, and were higher in more recent birth cohorts. After adjusting for these effects, risk in unirradiated patients did not vary significantly with age at HCT. The additional BCC risk associated with radiation exposure was largest for the youngest ages at exposure to radiation, with relative risks exceeding 20 for those transplanted at ages less than 10 years, and decreased with increasing age at exposure until age 40 years, above which no excess risk was identified. Relative risk in the irradiated population did not vary significantly with attained age, dose fractionation or race. Risks per unit dose in HCT patients were similar to other populations exposed under clinical settings to similar radiation doses and were more than 10-fold lower than seen in the atomic bomb survivors, 97% of whom were exposed to doses < 1 Sv.
INTRODUCTION
Over the past three decades, the successful treatment of malignant and non-malignant diseases with hematopoietic cell transplantation (HCT) has resulted in an increasingly large cohort of long-term surviving patients. Many of these patients were treated with total-body irradiation (TBI) as conditioning in preparation for HCT. Reported risks of second malignancies in HCT patients have ranged from 3–25% with 10–15-year incidence rates of 6–11% (1, 2). A spectrum of second cancers has been identified in these patients, the most frequent one being basal cell carcinoma (BCC). In a previously published analysis (3), we investigated the risk factors for non-melanoma cancers of the skin and the mucosal surfaces. TBI was associated with increased risk for the development of BCC.
Ionizing radiation-induced skin malignancies have been reported for several exposed groups, including the atomic bomb survivors, uranium miners, radiologists and individuals treated with radiation for benign or malignant skin disorders or other conditions (4-15). Nearly all reports indicate that ionizing radiation increases the risk of BCC, as opposed to melanoma or squamous cell carcinoma. Those types of cancers are more common after exposure to ultraviolet light (16) or to chemical agents (17). Genetically determined skin pigmentation plays an important role in BCC susceptibility; light complexion is a predictor of BCC risk in exposed individuals. Age at exposure is another significant modifier of response. Individuals exposed at ages of less than 10 years have shown two to four times greater risks than older individuals (10, 11, 14). Both linear (10) and non-linear (11) dose responses have been reported.
The characteristics of BCC observed in the HCT patient population that we reported on previously (3) were similar to those reported for other radiation-exposed populations (10, 11, 14). Risks of BCC development were highest for those exposed at young ages, and risks were higher for lighter-skinned individuals. We now expand on this previous analysis and examine the risk of BCC related to radiation dose, dose fractionation, and age at time of exposure in greater detail. In the present study we analyzed risks for developing BCC in both irradiated and nonirradiated HCT patients and addressed how those risk estimates compared to BCC risks in populations not treated with HCT.
METHODS
Patient Characteristics
This study involved patients who were transplanted at the Fred Hutchinson Cancer Research Center or affiliated hospitals in Seattle before January 1, 2006. Overall 6882 patients who received HCT before 2006 and survived at least 100 days were considered (the earliest transplant was in November 1969). Of these, 300 (4.4%) were excluded due to unknown race (N = 212), age >66 years (N = 85), or both (N = 3). Another 276 patients (4.0%) of age <66 years and known race who received preparative regimens with TBI doses less than 7.5 Gy were also excluded. Most (N = 260) of these patients received only 2 Gy as part of reduced intensity conditioning regimens (18), as did 39 of the 87 irradiated patients of age >66 years. Because this is a relatively recently introduced procedure with short follow-up times (median, 1.5 years; mode, 0.4 years), we excluded these patients.
The characteristics of the 6306 patients included in the present analysis are summarized in Table 1. More than one-third of the patients (N = 2436, 38.6%) were conditioned with chemotherapy alone (non-TBI patients). The remaining 3870 patients received radiation either alone or combined with chemotherapy (TBI patients). While the age ranges at transplant were similar in the two groups, TBI patients were in general younger (mean age, 28.8 years) than non-TBI patients (mean age 38.1 years). The prescribed radiation doses ranged from 7.5 to 18.4 Gy (Table 2). Dose was calculated to the central axis. The dose to the first 1–3 mm of skin would be 90–95% of the prescribed dose.
TABLE 1.
Characteristics of 6306 Patients of Age 0–65 at HCTa and Known Race who Survived at Least 100 Days after HCT, by Inclusion of TBI in Conditioning Regimen
| No TBI (N = 2436) |
TBI (N = 3870) |
||||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Sex | |||||
| Female | 1227 | 50.4% | 1622 | 41.9% | |
| Male | 1209 | 49.6% | 2248 | 58.1% | |
| Race | |||||
| White | 2154 | 88.4% | 3512 | 90.8% | |
| Non-white | 282 | 11.6% | 358 | 9.2% | |
| Age at HCT (years) | |||||
| 0–17 | 376 | 15.4% | 1006 | 26.0% | |
| 18–44 | 1023 | 42.0% | 2194 | 56.7% | |
| 45–65 | 1037 | 42.6% | 670 | 17.3% | |
| Year of HCT | |||||
| 1969–1975 | 34 | 1.4% | 68 | 1.8% | |
| 1976–1980 | 88 | 3.6% | 308 | 8.0% | |
| 1981–1985 | 83 | 3.4% | 630 | 16.3% | |
| 1986–1990 | 226 | 9.3% | 887 | 22.9% | |
| 1991–1995 | 532 | 21.8% | 916 | 23.7% | |
| 1996–2000 | 645 | 26.5% | 693 | 17.9% | |
| 2001–2005 | 828 | 34.0% | 368 | 9.5% | |
| Cell source | |||||
| Autologous | 924 | 37.9% | 502 | 13.0% | |
| Matched related donor | 1070 | 43.9% | 1843 | 47.6% | |
| Mismatched related donor | 75 | 3.1% | 477 | 12.3% | |
| Matched unrelated donor | 322 | 13.2% | 1029 | 26.6% | |
| Unknown | 45 | 1.8% | 19 | 0.5% | |
| AGVHD | |||||
| Grade 0–1 | 1454 | 59.7% | 1274 | 32.9% | |
| Grade 2–4 | 982 | 40.3% | 2596 | 67.1% | |
| Clinical extensive CGVHD | |||||
| No | 1660 | 68.1% | 2221 | 57.4% | |
| Yes | 776 | 31.9% | 1649 | 42.6% | |
| Prior exposure to radiation therapy |
|||||
| Probably not exposed | 1041 | 42.7% | 2290 | 59.2% | |
| Possibly not exposed | 488 | 20.0% | 118 | 3.1% | |
| Possibly exposed | 347 | 14.2% | 496 | 12.8% | |
| Probably exposed | 560 | 23.0% | 966 | 25.0% | |
| Mean | Min–Max | Mean | Min–Max | ||
| Age at HCT (years) | 38.1 | 0.3–65 | 28.8 | 0.5–65 | |
| Duration of follow-up (years) | 5.8 | 0.3–33.1 | 6.6 | 0.3–36.2 | |
Abbreviations: AGVHD, acute graft-versus-host disease, CGVHD, chronic graft-versus-host disease; HCT, hematopoietic cell transplantation; TBI, transplant population irradiated as part of conditioning regimen.
TABLE 2.
Details of Radiation Exposure of 3870 HCT Patients with TBI in their Conditioning Regimens
| Dose range (Gy) | Single exposure (N = 346) |
Fractionated exposure (N = 1612) |
Hyperfractionated exposure (N = 1872) |
Total marrow irradiation (N = 40) |
All TBI patients (N = 3870) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Percentage | No. | Percentage | No. | Percentage | No. | Percentage | No. | Percentage | |
| 7.50–11.99 | 346 | 100.0% | 29 | 1.8% | 45 | 2.4% | 35 | 87.5% | 455 | 11.8% |
| 12.0–12.99 | 0 | 0.0% | 945 | 58.6% | 797 | 42.6% | 3 | 7.5% | 1745 | 45.1% |
| 13.0–14.99 | 0 | 0.0% | 65 | 4.0% | 1011 | 54.0% | 1 | 2.5% | 1077 | 27.8% |
| 15.0–18.40 | 0 | 0.0% | 573 | 35.5% | 19 | 1.0% | 1 | 2.5% | 593 | 15.3% |
| Mean | Min–Max | Mean | Min–Max | Mean | Min–Max | Mean | Min–Max | Mean | Min–Max | |
| Dose (Gy) | 10.0 | 8.0–10.0 | 13.4 | 8.0–17.5 | 12.8 | 7.5–18.4 | 9.6 | 7.5–15.0 | 12.7 | 7.5–18.4 |
| Age at HCT (years) | 18.1 | 1–60 | 27.5 | 0.7–62 | 31.6 | 0.5–65 | 47.8 | 17–65 | 28.8 | 0.5–65 |
Among the TBI patients, single exposures were more common among patients of age 0–17 years (193/1006, 19.2%) than among older patients (153/2864, 5.3%). The mean duration of follow-up was somewhat longer for TBI patients (6.6 years) than for chemotherapy patients (5.8 years), due at least in part to the fact that non-TBI regimens have been used with increasing frequency in recent years (Table 1). Age at HCT was correlated with the maximum age at risk (attained age). This was due in part to the fact that follow-up began at HCT but was also because the maximum duration of follow-up was about 36 years.
Over the time span of this study, conditioning regimens and graft-versus-host disease prophylaxis varied. Initially, conditioning consisted mostly of TBI (at doses from 9.2 Gy to 15.75 Gy) given in a single session or fractionated (total dose delivered in multiple daily treatments) in combination with cyclophosphamide. Some patients received hyperfractionated (multiple fractions/day) or “total marrow” irradiation (TBI with shielding of lungs and liver). In more recent years, many patients were conditioned with cyclophosphamide and busulfan without radiotherapy. Other combinations were used less frequently. Graft-versus-host disease prophylaxis initially consisted of single agent methotrexate given intermittently for the first 102 days after transplantation. In later years cyclosporine was given alone or combined with methotrexate. FK506 (tacrolimus), mycophenolate mofetil and other immunosuppressive agents have also been used. First-line therapy for both acute and chronic graft-versus-host disease (AGVHD, CGVHD) consisted of glucocorticoids. Other agents included cyclosporine and monoclonal or polyclonal antibodies. Additional supportive care was provided according to the standards that evolved over the observation period (3).
The Fred Hutchinson Cancer Research Center Institutional Review Board approved surveillance of patients after HCT, and patients signed informed consent for collection of long-term outcome data. Patient characteristics, HCT treatment regimens and clinical outcome data were collected prospectively and stored in the clinical database. After HCT, patients underwent a comprehensive medical evaluation prior to discharge from the Transplant Center. One year after HCT, patients were invited to return to Seattle for a follow-up medical evaluation. Both patients and their physicians were sent questionnaires each subsequent year to obtain current status data. If no response was received within 2 months, a second letter was mailed. Among surviving patients, 95% had last contact in the previous 5 years. The questionnaires inquired specifically about tumors or cancers that might have developed, and how they were treated. Whenever possible, surgery and pathology reports were obtained for verification.
Data Analysis
Each patient was followed from the day of HCT until the first diagnosis of cutaneous BCC, death from any cause, or last follow-up date, whichever occurred first. Statistical analyses were performed to analyze cutaneous BCC incidence rates. Data for these analyses consisted of a table of person-years at risk and BCC case counts cross-classified by the following: Demographic factors included age at HCT (0–4, 5–9, . . . , 54–59, 60–65 years), race (white, nonwhite), sex (female, male), and birth year (before 1951, 1951–1960, 1961–1970, 1971–1980, after 1980); categories of follow-up included year of HCT (1969–1989, 1990–1997, 1998–2005), attained age (0–17.9, 18–24.9, 25–29.9, 30–34.9, 35–39.9, 40–44.9, 45–49.9, 50–59.9, 60–74.9, 75–90), days since HCT (100–499, 500–999, 1000–1499, 1500–1999, 2000–2499, 2500–2999, 3000–3999, 4000–4999, 5000–9999, 10,000–15,000), calendar year at follow-up (1960–1969, 1970–1979, 1980–1989, 1990–1999, 2000–2007); characteristics related to the HCT procedure included TBI (no, yes), radiation dose (0, 7.5–11.99, 12.0–12.99, 13.0–14.99, 15.0–19.0 Gy), TBI type (none, single exposure, fractionated, hyperfractionated, total marrow irradiation), and cell source (autologous, HLA matched related donor, HLA mismatched related donor, HLA matched unrelated donor, or unknown). AGVHD and CGVHD were defined as time-dependent covariates defined by the presence or absence of grade 2+ AGVHD and clinically extensive CGVHD, respectively.
Records of prior radiation therapy were not available for these patients, so four categories describing the likelihood of prior exposure to radiation therapy—(1) probably exposed, (2) possibly exposed, (3) possibly not exposed, and (4) probably not exposed—were defined based on each patient's disease and status as shown in the Appendix. To address the uncertainty about prior radiation therapy, it was defined as either (1), (1)+(2), or (1)+(2)+(3), and analyses were repeated using each of the three alternatives. Results were consistent in the three sets of analyses, so only results based on (1), i.e., probable exposure to prior radiation therapy, are reported here. Covariate values assigned to each cell included indicators for categorical variables (e.g., sex = 0 for female, 1 for male; TBI = 0 for no, 1 for yes; etc.), and mean values for continuous variables such as attained age or TBI dose.
The number of BCC cases in each cell of the cross-classification was assumed to be an independent Poisson variate with mean PYi × λi, where PYi and λi are the person-years and BCC incidence rate, respectively, for cell i where i = 1, … , I indexes the cells with PYi >0. Poisson regression models for rates were used to investigate the effects on BCC incidence of such factors as TBI, age at HCT and attained age, sex, etc. Both excess relative risk (ERR) and absolute excess risk (AER) models were examined. The general ERR model was expressed as follows:
where λ0(Xi) is the background BCC incidence rate, i.e., the rate in non-TBI patients, with characteristics (age at HCT, attained age, race, sex, etc.) represented by the covariate vector Xi, and ERR(Zi, Xi) is the ERR associated with radiation exposure Zi in patients with characteristics Xi. The background rates were modeled as a loglinear function of Xi, i.e., λ0(Xi) = exp(α0 + αXi), where α0 is a constant and the parameter vector α represents the effects of the factors in Xi. The simple linear ERR model was ERR(Zi, Xi) = βZi, where Zi is an indicator of TBI, in which case 1 + β is the RR associated with TBI. Alternatively Zi can be the cell-specific mean dose, in which case β is the ERR per unit dose. More general models allowing the ERR to vary with other factors can be written ERR(Zi, Xi) = βZi exp(γXi), where γ represents the effects of the covariates in Xi on the ERR. A further generalization is ERR(Zi, Xi) = β1Zi exp(γ1Xi)IS(i) + β2Zi exp(γ2Xi)[1 − IS(i)], where IS(i) = 1 for cells in subgroup S and 0 otherwise; this model allows both the TBI effect and its modifiers to differ between subgroups.
The general AER model was expressed as
where AER(Zi, Xi) is the AER associated with radiation exposure Zi in patients with characteristics Xi and the other terms are as defined above. The analysis proceeded by first fitting a model for λ0(Xi) using only data from patients who did not receive TBI conditioning. After selection of the model for λ0(Xi), comprehensive models including AER(Zi, Xi) or ERR(Zi, Xi) were fitted to the entire data set. These analyses were performed using the AMFIT program of the Epicure package (Hirosoft, Seattle, WA).
Results were based on data available as of January 1, 2007.
RESULTS
BCC Risk in HCT Survivors not Treated with TBI
Among the 2436 non-TBI patients, 80 BCCs were observed over 13,424 person-years (PY) of follow-up. The crude BCC incidence rates increased sharply with age at HCT (Table 3). Indeed, only one BCC was observed in the 429 non-TBI patients transplanted at age <20 years, consistent with the rarity of spontaneous BCC in children and young adults. However, the apparent increase in BCC incidence with age at HCT resulted from the correlation between attained age and age at HCT. Poisson regression analysis indicated that BCC risk in non-TBI patients increased with attained age (P < 0.0001) and that the rate of age-related increase was higher for more recent birth cohorts (P = 0.0087). Also, the risk of BCC was about eightfold lower for nonwhites than for whites (RR = 0.12, 95% CI 0.02–0.85, P = 0.034) and was elevated in patients who developed CGVHD (RR = 1.90, 95% CI 1.22–2.96, P = 0.0043). After we accounted for these effects, BCC rates did not vary significantly with age at HCT (P = 0.059), sex (P = 0.24), AGVHD (P = 0.50), or probable prior therapeutic radiation (P = 0.91). The background model for the subsequent analyses of the effect of TBI therefore included attained age, birth year, race and CGVHD. The effects of attained age and birth year are illustrated for whites in Fig. 1; for nonwhites the same pattern applies, but with overall lower risks (data not shown).
TABLE 3.
Incidence Rates and Relative Risk (RR) of BCC in TBI Patients Compared to Non-TBI Patients, by Age at HCT
| Age at HCT (years) |
Non-TBI patients (N = 2436) |
TBI patients (N = 3870) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Person-years (PY) |
BCC cases |
Cases per 104 PYa |
N | Person-years (PY) |
BCC cases |
Cases per 104 PYa |
RRb | 95% CIb | P valueb,c | |
| 0–4 | 126 | 861.89 | 0 | 0.0 | 231 | 1434.53 | 3 | 20.9 | 27.7 | 6.16, 88.2 | 0.072 |
| 5–9 | 79 | 726.20 | 0 | 0.0 | 312 | 2353.85 | 11 | 46.7 | 25.8 | 11.1, 56.6 | 0.0099 |
| 10–14 | 90 | 1017.38 | 0 | 0.0 | 281 | 1965.84 | 8 | 40.7 | 11.6 | 4.65, 25.9 | 0.018 |
| 15–19 | 134 | 1251.51 | 1 | 8.0 | 328 | 2483.35 | 20 | 80.5 | 9.17 | 4.98, 16.4 | 0.0017 |
| 20–24 | 113 | 884.17 | 1 | 11.3 | 357 | 2466.40 | 18 | 73.0 | 5.75 | 3.09, 10.4 | 0.0036 |
| 25–29 | 143 | 1143.83 | 4 | 35.0 | 394 | 3048.67 | 26 | 85.3 | 3.63 | 2.17, 5.95 | 0.0024 |
| 30–34 | 186 | 1256.82 | 2 | 15.9 | 454 | 2917.30 | 23 | 78.8 | 2.11 | 1.26, 3.45 | 0.020 |
| 35–39 | 241 | 1379.96 | 7 | 50.7 | 460 | 2762.32 | 29 | 105.0 | 1.88 | 1.19, 2.89 | 0.019 |
| 40–44 | 287 | 1323.36 | 11 | 83.1 | 383 | 2081.60 | 22 | 105.7 | 1.37 | 0.83, 2.16 | 0.13 |
| 45–49 | 313 | 1313.66 | 11 | 83.7 | 334 | 1806.04 | 25 | 138.4 | 1.21 | 0.75, 1.86 | 0.23 |
| 50–54 | 288 | 1101.05 | 14 | 127.2 | 200 | 810.35 | 10 | 123.4 | 0.91 | 0.44, 1.68 | 0.62 |
| 55–59 | 270 | 863.58 | 17 | 196.9 | 109 | 443.77 | 5 | 112.7 | 0.71 | 0.24, 1.64 | 0.81 |
| 60–65 | 166 | 300.42 | 12 | 399.4 | 27 | 63.95 | 2 | 312.7 | 1.81 | 0.29, 6.17 | 0.27 |
Crude rate, calculated as 104 × (BCC Cases)/PY.
Relative risk (RR) of BCC for TBI patients, compared to non-TBI patients, and 95% confidence interval (CI) for RR estimated from ERR model as described in text.
One-tailed P value for testing RR = 1 versus RR > 1.
FIG. 1.
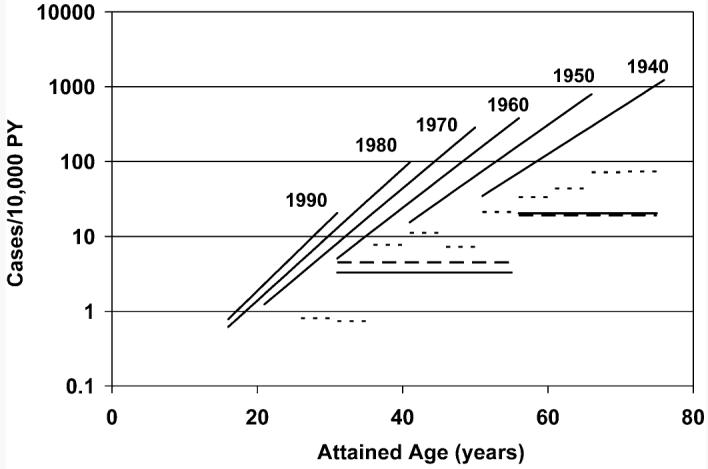
Incidence rates of cutaneous basal cell carcinoma (BCC) in nonirradiated, white hematopoietic cell transplant (HCT) patients as a function of attained age and birth year, based on the fitted background model. Rates for nonwhite nonirradiated patients are approximately eight-fold lower. Horizontal lines are age-specific standardized BCC incidence rates from population-based registries in the Netherlands (19) (males: solid lines; females: long dashed lines) and South Wales (20) (both sexes combined: short dashes).
Figure 1 also compares the fitted model for transplant patients who did not receive TBI to the standardized incidence rates for non-patient populations from the Netherlands (19) and South Wales (20). Estimated (attained-) age-specific rates for the transplant patients are shown for selected birth years (1940, 1950, etc.) by the upward-sloping lines. The horizontal lines show rates for the two non-patient populations. As indicated in this figure, the age-specific BCC incidence in non-TBI transplant patients was somewhat higher than that seen in the Northern European populations.
BCC Risk in HCT Survivors Treated with TBI
Among the 3870 TBI patients, 202 BCCs were observed over a total of 24,638 PY of follow-up. The crude BCC incidence rates in TBI patients increased with age at HCT; however, for those transplanted at younger ages the crude rates were markedly higher than the corresponding rates for non-TBI patients (Table 3). When all TBI patients were combined and compared to non-TBI patients, there was a significant radiation-related risk (P < 0.001), with TBI patients having an overall AER of 24.1 cases per 104 PY (95% CI 14.0–34.2). However, the AER decreased with age at exposure (P < 0.0001), while sharply increasing with attained age (P < 0.0001; Fig. 2). Specifically, for any given age at HCT, the AER increased by an average of 16.4% with each additional year of attained age (95% CI 12.4–20.5%), and for any given attained age the AER decreased by an average of 13.4% (95% CI 10.0–16.7%) with each 1-year increase of age at transplant. The overall AER of BCC was somewhat higher for patients who received single dose TBI (55.6 cases per 104 PY; 95% CI 24.6–86.7) compared to those who received fractionated exposures (19.2 cases per 104 PY; 95% CI 9.1–29.3). However, when attained age and age at transplant were accounted for, there were no significant differences between the effects of single-dose and fractionated TBI (Fig. 2, P = 0.53). Moreover, the effects of attained age and age at transplant did not differ significantly between single-dose and fractionated exposures (P = 0.16). Notably, only 346 (9%) of the TBI patients received single-dose exposures, and these were comparatively young patients (mean age 18.1 years; Table 2). Consequently the effects of TBI fractionation could not be estimated precisely. Also, after accounting for the effects of attained age and age at transplant, there was no significant difference in AER between whites and nonwhites (P = 0.11).
FIG. 2.
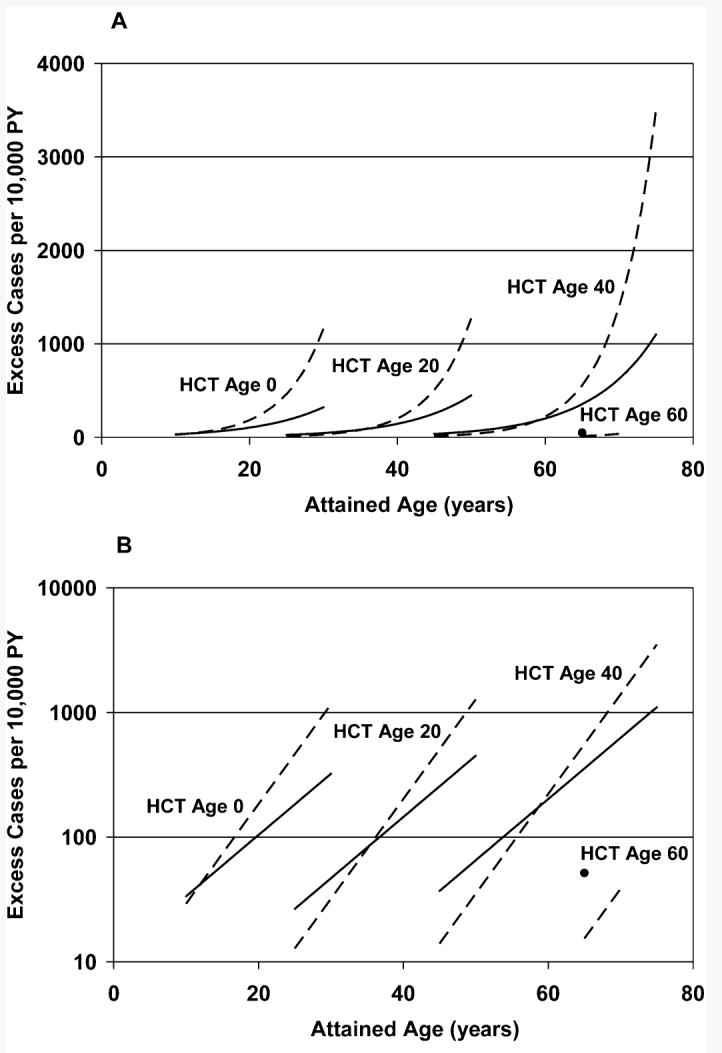
Estimated absolute excess risk (AER) as a function of attained age, age at hematopoietic cell transplant (HCT), and type of irradiation (TBI) [single dose: solid lines (solid circle for HCT Age 60); fractionated dose: dashed lines]. AER increased significantly with increasing attained age, decreased significantly with increasing age at HCT, and did not differ significantly between single and fractionated exposures. Panel A: Linear-linear; panel B: log-linear.
Since the rates of BCC in non-TBI patients and the AER in the TBI patients both increased with attained age, we turned to relative risk (RR) models to describe the radiation-related risk. Starting with the same background model, we examined RR models, allowing for effects of age at transplant, attained age, TBI fractionation, race, AGVHD, CGVHD and probable prior radiation therapy. For all ages combined, the RR associated with TBI was 1.76 (95% CI 1.36–2.30, P < 0.0001). However, the RR decreased significantly with increasing age at HCT (P < 0.0001) at an average rate of 10.9% (95% CI 8.7–13.2%) for each 1-year increase in age at HCT (Fig. 3). After we accounted for this strong effect of age at HCT, neither attained age (P = 0.99) nor TBI fractionation (P = 0.68) significantly modified the RR, and the RR did not differ significantly between whites and nonwhites (P = 0.21). Also the RR was not significantly modified by AGVHD (P = 0.69), CGVHD (P = 0.13), or probable prior radiation exposure (P = 0.62).
FIG. 3.
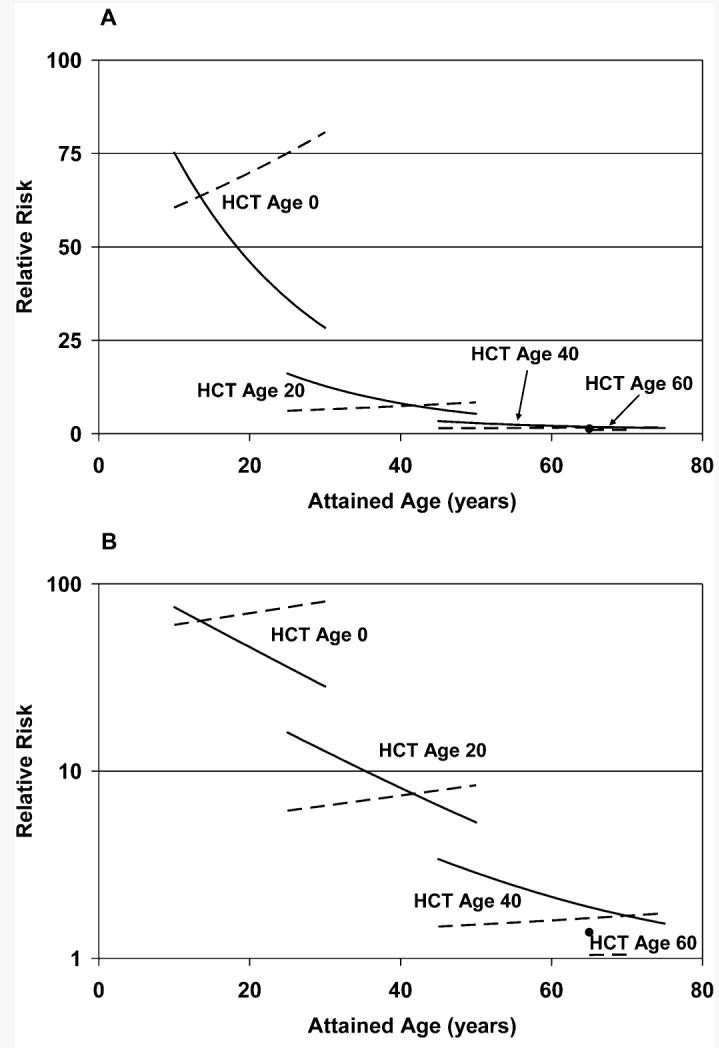
Estimated relative risk (RR) of basal cell carcinoma (BCC) in irradiated (TBI) patients as a function of attained age, age at hematopoietic cell transplant (HCT), and type of TBI [single dose: solid lines (solid circle for HCT Age 60); fractionated dose: dashed lines]. RR decreased significantly with increasing age at HCT but did not vary significantly with attained age or between single and fractionated exposures. Panel A: Linear-linear; panel B: log-linear.
As shown in Table 3, the RRs were 27.7 and 25.8 for patients who at HCT were 0–4 and 5–9 years old, respectively. The confidence intervals for their RR estimates were wide, reflecting at least in part the small numbers of BCC cases observed to date in the youngest patients; nevertheless, their BCC risks were significantly elevated. In contrast, the RRs were very estimated precisely for patients 40 to 59 years old at HCT and were not significantly greater than 1. This observation provides strong evidence that there was no increased BCC risk after TBI conditioning in patients over the age of 40 at HCT.
Age-at-HCT-dependent RRs were estimated for each of four TBI dose ranges (7.5–11.99, 12–12.99, 13–14.99 and ≥15 Gy) to assess whether the RR varied within the range of radiation doses. As shown in Table 4, the RR associated with 12–12.99 Gy was somewhat higher for patients transplanted at the youngest ages (143.5 compared to 29.1–48.2), but it decreased more steeply with increasing age at HCT (15.1%/year) compared to the remaining three dose categories (6.9%/year to 9.4%/year). However, the confidence intervals for the age-at-HCT-specific parameter estimates overlapped extensively, and as a result there was no further trend in risk across the range of radiation doses after adjusting for the increased risk associated with TBI (P = 0.28).
TABLE 4.
Incidence Rates and Relative Risk (RR) of BCC in TBI Patients Compared to Non-TBI Patients, by TBI Dose Category
| Dose range (Gy) |
Crude BCC rate |
RR (TBI compared to non-TBI) for age 0 at HCTb |
Effect of age at HCT (percentage decrease per year)c |
|||||
|---|---|---|---|---|---|---|---|---|
| N | Person-years (PY) |
BCC cases |
Cases per 104 PYa |
Estimate | 95% CI | Estimate | 95% CI | |
| 0 (non-TBI) | 2436 | 13,423.8 | 80 | 59.6 | 1.00 | — | — | — |
| 7.50–11.99 | 455 | 3,231.8 | 28 | 86.6 | 48.2 | 15.8–119.5 | 9.4 | 5.1–14.2 |
| 12.0–12.99 | 1745 | 11,949.3 | 88 | 73.6 | 143.5 | 43.9–345.7 | 15.1 | 11.0–20.5 |
| 13.0–14.99 | 1077 | 5,119.2 | 46 | 89.6 | 41.1 | 8.2–141.8 | 8.9 | 4.5–13.4 |
| 15.0–18.40 | 593 | 4,337.6 | 40 | 92.6 | 29.1 | 6.5–96.0 | 6.9 | 2.8–10.8 |
| TBI, any dose | 3870 | 24,638.0 | 202 | 82.0 | 62.7 | 28.4–109.4 | 10.9 | 8.7–13.2 |
Crude rate, calculated as 104 × (BCC Cases)/PY.
Relative risk (RR) of BCC for TBI patients compared to non-TBI patients, for age 0 years at HCT, and 95% confidence interval (CI) for RR.
Average percentage decrease in excess RR (ERR = RR − 1) of BCC, for each increase of 1 year in age at HCT.
To further investigate the possibility of a TBI dose response, we also estimated the ERR per Gy TBI administered. Since the doses were of the order of 10 Gy, the ERR/ Gy was approximately one-tenth of the ERR associated with the total dose of TBI. Specifically, the ERR/Gy for all TBI patients combined was 0.062/Gy (95% CI 0.030–0.106/Gy, P < 0.0001). However, the ERR/Gy decreased significantly with increasing age at HCT (P < 0.0001), at a rate of 10.9% for each 1-year increase in age at HCT. For comparison with results among the Japanese atomic bomb survivors, the ERR/Gy was estimated separately for ages at HCT 0–9 (ERR/Gy = 1.49, 95% CI 0.64–3.17), 10–19 (ERR/Gy = 0.55, 95% CI 0.28–1.00), 20–39 (ERR/Gy = 0.11, 95% CI 0.06–0.18), and 40+ years (ERR/Gy = 0.02, 95% CI −0.01–0.06). These estimates for the HCT patients are more than an order of magnitude smaller than the risks reported by Ron et al. (11) for the atomic bomb survivors (Table 5). Interestingly, they were similar in magnitude to risk estimates made in young patients who were irradiated for medical reasons (Table 5).
TABLE 5.
Radiation-Related BCC Risk Estimates in Atomic Bomb Survivors and in Young, Medically Irradiated Cohorts as Compared to this Study
| Studya | Age (years) at exposure |
Mean dose (Gy) |
ERR/Gy |
|
|---|---|---|---|---|
| Estimate | 95% CI | |||
| A-bomb survivors (11) | 0–9 | NAb | 21 | 4.1–73 |
| 10–19 | NAb | 6.7 | 2.1–17 | |
| 20–39 | NAb | 1.7 | 0.5–3.8 | |
| 40+ | NAb | 0.7 | −0.05–2.2 | |
| Ringworm (10) | <1–15 | 6 | 1.5 | 1.22–2.02 |
| Ringworm (14) | <1–19 | 5 | 1.49 | 1.37–1.63 |
| Lymphoid (5) | <1–49 | 16 | 1.23 | 1.13–1.59 |
| Thymus (6, 25) | <1 | 2.25 | 2.05 | 1.50–2.84 |
| Present study | 0–9 | 13.3 | 1.49 | 0.64–3.17 |
| 10–19 | 12.9 | 0.55 | 0.28–1.00 | |
| 20–39 | 12.8 | 0.11 | 0.06–0.18 | |
| 40–64 | 12.3 | 0.02 | −0.01–0.06 | |
While all groups studied young individuals, there are likely to be different distributions of ages that would affect comparisons. Also, the other studies estimated risks from sunlight-exposed regions of the body. Ours was not limited to sunlight-exposed regions.
Age-at-exposure-specific mean doses were not reported for the atomic bomb survivors; however, it was noted that 60% had estimated doses less than 0.005 Sv and only about 3% had doses of 1 Sv or more (11). These estimates were based on the DS86 dosimetry system but are likely to be very similar when based on the DS02 dosimetry system.
DISCUSSION
We reported previously that TBI given for conditioning in preparation for HCT was a risk factor for the development of BCC (1-3, 21). The characteristics of the development of BCC after TBI were similar to what had been reported for other patients treated with radiation (10, 11, 14). Therefore, we expanded our study to quantify risks in both irradiated and nonirradiated HCT populations and compared results to similar measures reported for other populations. We observed that while the general characteristics of BCC development in unirradiated patients were similar to those for non-patient populations, the rates were higher. Risks per unit dose of TBI in HCT patients were similar to other populations exposed in clinical settings to similar radiation doses but were about 10-fold lower than those seen in the atomic bomb survivors who were exposed to lower doses. Of particular concern was the observation that ERR in irradiated patients did not vary significantly with attained age, which if maintained with further follow-up predicts that radiation-related excess BCC risk for the younger part of the present population of patients may increase dramatically as those patients grow older.
In contrast to the previous analysis of this cohort (3), we explicitly modeled age-specific BCC incidence rates in the non-TBI population to determine whether either the disease history or HCT conditioning influenced the incidence. Although it was not possible to assess with confidence whether non-TBI HCT patients were at increased risk of BCC, age-specific incidence rates of BCC in white non-TBI patients were higher than those observed in population-based registry studies of BCC in the Netherlands in 1973–2000 (19) and in South Wales in 1988–1998 (20). However, such comparisons between the non-TBI patients and other populations may be confounded by several factors. Post-HCT patients almost certainly had more frequent medical examinations than did patients in the general population, and this increased surveillance may have increased their apparent BCC incidence rates. Population-based studies (19), though conducted in largely white populations, very likely included some proportions of nonwhite individuals; however, since those proportions were not reported, it is not possible to make race-adjusted comparisons. Information about skin exposures to sunlight was not available, and this parameter may also differ between the HCT cohort and other populations. Finally, this study cannot rule out the possibility that BCC risk was increased in HCT patients because they had an inherently increased risk of malignancy or as a consequence of the overall HCT procedure or other treatment components they had received. Interestingly, our analysis revealed an increased risk of developing BCC in more recent birth cohorts, a phenomenon that was also reported by de Vries et al. (19). This trend in non-patient populations has been suggested to reflect changes in lifestyle that affect the amount and intensity of sunlight exposure. The birth cohort effect observed in our non-TBI patients might similarly reflect changes in lifestyle; however, we cannot rule out the possibility that evolving changes in the non-TBI conditioning regimens used for transplant patients also influenced the BCC risk.
Comparing BCC rates in our TBI patients to those of the non-TBI patients provided estimates of the excess risk associated with radiation exposure. The AERs associated with TBI increased significantly with attained age and decreased significantly with age at HCT. This suggests that the excess risk for a given age at HCT might be proportional to the background rate. Examination of ERR models indeed showed that the ERR decreased significantly with increasing age at HCT but did not vary significantly with age at risk, type of TBI (single or fractionated), or race. The absence of a decrease in RR with attained age was particularly noteworthy: If the very high RRs observed to date in the youngest patients persist, those patients are likely to experience extremely high BCC incidence rates as they reach ages with substantially higher background rates. Whether these large RRs will in fact persist cannot be determined at this time. The present data, with a maximum follow-up of about 36 years, are not sufficient to estimate the BCC risks late in life among patients transplanted in childhood.
The effects of radiation observed in HCT patients and other medically irradiated patients are notably smaller than would be predicted from the age-at-exposure-specific dose responses that have been reported for the atomic bomb survivors (Table 5). The medical and atomic bomb exposures differ in that most medical exposures are fractionated or low-dose-rate exposures, while the atomic bomb exposures were acute. More importantly, the radiation doses used in patients irradiated for medical reasons were generally much higher than those estimated for the atomic bomb survivors. The cohort of HCT patients analyzed here did not include any patients with doses less than 7.5 Gy. It is clear from our analysis that the dose response for the atomic bomb survivors cannot be extrapolated linearly into ranges of doses received by HCT patients. The present analysis suggested that the RR of BCC did not vary within the range of doses received by patients (7.5–18.4 Gy), suggesting that the dose response, which is much steeper at the lower doses received by atomic bomb survivors, may flatten out at the much higher doses received by TBI-conditioned HCT patients.
We found no significant difference in radiation-related RR of BCC between white and nonwhite HCT patients. Based in part on the observed relationship between skin color and BCC risk, Shore (12) suggested that an interaction between ultraviolet (UV) and ionizing radiation is involved in the development of BCC. However, among atomic bomb survivors there was no evidence of higher BCC risk in body surface areas with high UV-radiation exposure (face and hands) compared to the rest of the body (11). Since our data base did not include anatomic sites of BCC, we were unable to address this issue in the present study. Also, the absence of a significant difference in RR between white and nonwhite patients must be interpreted cautiously because of the modest number and low background BCC rates in nonwhite patients in this cohort.
The radiation-related excess risk of BCC after TBI decreased significantly with increasing age at exposure, as has been observed in the Japanese atomic bomb survivors (11). In agreement with the study by Ron et al. (11), we observed no significant increase in BCC risk among patients treated after age 40 years. As mentioned above, our data in this age range had a limited follow-up time. If there was a long latency for BCC development in this age group, our conclusions might have to be modified in the future. However, over the duration of follow-up that we analyzed (Fig. 3), there was no significant change in RR with attained age, and even in children we observed increased BCC frequencies less than 10 years after exposure (Fig. 3). Relatively short minimum latencies have also been observed in individuals irradiated for clinical indications (22-24).
We were unable to detect differences between the effects of single-dose and fractionated radiation exposures. While the RR of BCC appeared to decrease with increasing attained age for single exposures and to increase with increasing attained age for fractionated exposures (Fig. 3), these differences were not statistically significant. The failure to observe a difference between single and fractionated radiation doses would suggest that the radiation-induced lesion that leads to BCC is not repairable. Alternatively, the lack of a significant effect of dose fractionation on risk might be a reflection of the very high overall doses used in both single-dose and multifractionated regimens and the fact that the single-dose exposures were delivered at relatively low dose rates. It must be emphasized that the single-exposure patients were few in number and were younger than those who received fractionated exposures, and as a result their TBI-related excess risk was not estimated with great precision. Consequently, this result did not provide strong evidence against the possibility that BCC risk differs by type of exposure.
Although risk of BCC was significantly elevated in patients with clinical extensive CGVHD, as reported previously (3), there was no evidence that the effect of TBI on risk was modified by either AGVHD or CGVHD. The present study also found no evidence that prior radiation therapy influenced the risk of BCC or modified the TBI-related risk. However, histories of prior radiation therapy were not available for these patients, and the imputation of prior exposure based on diagnosis and disease status at transplant is subject to substantial uncertainty.
In conclusion, the present analysis showed major effects of age at exposure on the development of radiation-related BCC. While BCC is generally not a fatal disease, it may be associated with considerable morbidity and emotional stress, especially in younger patients. Our analysis raises concern about potentially high BCC frequencies in patients irradiated at young ages as they grow older. Our studies also suggest that risks from low-dose conditioning regimens may yield higher relative risks of BCC. As the numbers of individuals treated with HCT for malignant or non-malignant diseases continues to grow, with most of these patients being closely followed for years after HCT, there will be an opportunity to address these two possibilities.
ACKNOWLEDGMENTS
This work was supported by HL36444, CA18029, CA15704, and CA102542 from the National Institutes of Health, Bethesda, MD and DE-FG03-003462908 from the Low Dose Radiation Research Program, Biological and Environmental Research (BER), U.S. Department of Energy.
REFERENCES
- 1.Deeg HJ, Leisenring W, Storb R, Nims J, Flowers ME, Witherspoon RP, Sanders J, Sullivan KM. Long-term outcome after marrow transplantation for severe aplastic anemia. Blood. 1998;91:3637–3645. [PubMed] [Google Scholar]
- 2.Deeg HJ, Socie G. Malignancies after hematopoietic stem cell transplantation: many questions, some answers. Blood. 1998;91:1833–1844. [PubMed] [Google Scholar]
- 3.Leisenring W, Friedman DL, Flowers ME, Schwartz JL, Deeg HJ. Nonmelanoma skin and mucosal cancers after hematopoietic cell transplantation. J. Clin. Oncol. 2006;24:1119–1126. doi: 10.1200/JCO.2005.02.7052. [DOI] [PubMed] [Google Scholar]
- 4.ICRP . Genetic Susceptibility to Cancer. Publication 79, Annals of the ICRP. Vol. 28. Elsevier; Amsterdam: 1998. pp. 1–157. [PubMed] [Google Scholar]
- 5.van Vloten WA, Hermans J, van Daal WA. Radiation-induced skin cancer and radiodermatitis of the head and neck. Cancer. 1987;59:411–414. doi: 10.1002/1097-0142(19870201)59:3<411::aid-cncr2820590310>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 6.Hildreth NG, Schneider AB, Cave WT., Jr. A comparative study between individuals receiving thymic irradiation in infancy and their nontreated siblings: clinical and laboratory thyroid abnormalities. Radiat. Res. 1987;110:458–467. [PubMed] [Google Scholar]
- 7.Karagas MR, McDonald JA, Greenberg ER, Stukel TA, Weiss JE, Baron JA, Stevens MM. Risk of basal cell and squamous cell skin cancers after ionizing radiation therapy. For The Skin Cancer Prevention Study Group. J. Natl. Cancer. Inst. 1996;88:1848–1853. doi: 10.1093/jnci/88.24.1848. [DOI] [PubMed] [Google Scholar]
- 8.Lichter MD, Karagas MR, Mott LA, Spencer SK, Stukel TA, Greenberg ER. Therapeutic ionizing radiation and the incidence of basal cell carcinoma and squamous cell carcinoma. The New Hampshire Skin Cancer Study Group. Arch. Dermatol. 2000;136:1007–1011. doi: 10.1001/archderm.136.8.1007. [DOI] [PubMed] [Google Scholar]
- 9.Puskin JS, Nelson CB. Estimates of radiogenic cancer risks. Health Phys. 1995;69:93–101. doi: 10.1097/00004032-199507000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Ron E, Modan B, Preston D, Alfandary E, Stovall M, Boice JD., Jr. Radiation-induced skin carcinomas of the head and neck. Radiat. Res. 1991;125:318–325. [PubMed] [Google Scholar]
- 11.Ron E, Preston DL, Kishikawa M, Kobuke T, Iseki M, Tokuoka S, Tokunaga M, Mabuchi K. Skin tumor risk among atomic-bomb survivors in Japan. Cancer Causes Control. 1998;9:393–401. doi: 10.1023/a:1008867617415. [DOI] [PubMed] [Google Scholar]
- 12.Shore RE. Overview of radiation-induced skin cancer in humans. Int. J. Radiat. Biol. 1990;57:809–827. doi: 10.1080/09553009014550951. [DOI] [PubMed] [Google Scholar]
- 13.Shore R, Harley N, Pasternack B, Gladstein AH. Skin cancer susceptibility among irradiated patients. J. Am. Acad. Dermatol. 1990;22:859–860. doi: 10.1016/s0190-9622(08)81190-5. [DOI] [PubMed] [Google Scholar]
- 14.Shore RE, Moseson M, Xue X, Tse Y, Harley N, Pasternack BS. Skin cancer after X-ray treatment for scalp ringworm. Radiat. Res. 2002;157:410–418. doi: 10.1667/0033-7587(2002)157[0410:scaxrt]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Perkins JL, Liu Y, Mitby PA, Neglia JP, Hammond S, Stovall M, Meadows AT, Hutchinson R, Dreyer ZE, Mertens AC. Nonmelanoma skin cancer in survivors of childhood and adolescent cancer: a report from the childhood cancer survivor study. J. Clin. Oncol. 2005;23:3733–3741. doi: 10.1200/JCO.2005.06.237. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J. Photochem. Photobiol. B. 2001;63:8–18. doi: 10.1016/s1011-1344(01)00198-1. [DOI] [PubMed] [Google Scholar]
- 17.Gallagher RP, Bajdik CD, Fincham S, Hill GB, Keefe AR, Coldman A, McLean DI. Chemical exposures, medical history, and risk of squamous and basal cell carcinoma of the skin. Cancer Epidemiol. Biomarkers Prev. 1996;5:419–424. [PubMed] [Google Scholar]
- 18.Hegenbart U, Niederwieser D, Sandmaier BM, Maris MB, Shizuru JA, Greinix H, Cordonnier C, Rio B, Gratwohl A, Storb R. Treatment for acute myelogenous leukemia by low-dose, total-body, irradiation-based conditioning and hematopoietic cell transplantation from related and unrelated donors. J. Clin. Oncol. 2006;24:444–453. doi: 10.1200/JCO.2005.03.1765. [DOI] [PubMed] [Google Scholar]
- 19.de Vries E, Louwman M, Bastiaens M, de Gruijl F, Coebergh JW. Rapid and continuous increases in incidence rates of basal cell carcinoma in the southeast Netherlands since 1973. J. Invest. Dermatol. 2004;123:634–638. doi: 10.1111/j.0022-202X.2004.23306.x. [DOI] [PubMed] [Google Scholar]
- 20.Holme SA, Malinovszky K, Roberts DL. Changing trends in non-melanoma skin cancer in South Wales, 1988–98. Br. J. Dermatol. 2000;143:1224–1229. doi: 10.1046/j.1365-2133.2000.03892.x. [DOI] [PubMed] [Google Scholar]
- 21.Curtis RE, Rowlings PA, Deeg HJ, Shriner DA, Socie G, Travis LB, Horowitz MM, Witherspoon RP, Hoover RN, Boice JD., Jr. Solid cancers after bone marrow transplantation. N. Engl. J. Med. 1997;336:897–904. doi: 10.1056/NEJM199703273361301. [DOI] [PubMed] [Google Scholar]
- 22.Dinehart SM, Anthony JL, Pollack SV. Basal cell carcinoma in young patients after irradiation for childhood malignancy. Med. Pediatr. Oncol. 1991;19:508–510. doi: 10.1002/mpo.2950190612. [DOI] [PubMed] [Google Scholar]
- 23.Marin-Gutzke M, Sanchez-Olaso A, Berenguer B, Gonzalez B, Rodriguez P, De Salamanca JE, De Prada I. Basal cell carcinoma in childhood after radiation therapy: case report and review. Ann. Plast. Surg. 2004;53:593–595. doi: 10.1097/01.sap.0000136972.23991.07. [DOI] [PubMed] [Google Scholar]
- 24.Yoshihara T, Ikuta H, Hibi S, Todo S, Imashuku S. Second cutaneous neoplasms after acute lymphoblastic leukemia in childhood. Int. J. Hematol. 1993;59:67–71. [PubMed] [Google Scholar]
- 25.Hildreth NG, Shore RE, Hempelmann LH, Rosenstein M. Risk of extrathyroid tumors following radiation treatment in infancy for thymic enlargement. Radiat. Res. 1985;102:378–391. [PubMed] [Google Scholar]


