Abstract
It has been hypothesized that cognitive mechanisms underlying lateralized complex motor actions associated with tool use in chimpanzees may have set the stage for the evolution of left-hemisphere specialization for language and speech in humans. Here we report evidence that asymmetries in the homologues to Broca’s and Wernicke’s areas are associated with handedness for tool use in chimpanzees. These results suggest that the neural substrates of tool use may have served as a preadaptation for the evolution of language and speech in modern humans.
Nearly 40 years ago, Van Lawick-Goodall (1970) described the first systematic evidence of tool use in wild chimpanzees, a finding that transformed many scientists’ views of the uniqueness of human tool making and culture. Since that time, it has been documented that chimpanzees manufacture and use tools in a wide range of ecological settings (Whiten et al., 2001), which suggests that tool use is an adaptive and flexible cognitive behavior in chimpanzees. Despite the motor and cognitive complexity underlying tool use, very little is known about the neurobiological structures associated with the expression of this complex behavior in chimpanzees. This is unfortunate because it has been suggested that the cognitive and motor functions associated with tool use may have selected for lateralized control of motor actions that served as a preadaptation for the evolution of left-hemisphere control of language and speech in humans (Bradshaw & Rogers, 1993; Greenfield, 1991). Most humans show left-hemisphere specialization for language, particularly in the inferior frontal and posterior temporal cortical regions (Binder & Frost, 1998; Cooper, 2006; Shapleske, Rossell, Woodruff, & David, 1999), although other brain regions have been implicated in language and speech processing (Lieberman, 2003; Ramnani, 2006). Moreover, a significant majority of individuals report themselves as being right-handed (Annett, 2002). Left-hemisphere specialization for language, particularly among right-handed individuals, has led some researchers to suggest that control of complex motor actions, such as tool use, may have served as a preadaptation for the emergence of these neural capacities in humans (e.g., Greenfield, 1991).
Functional imaging studies of human tool use have implicated a number of brain regions, including the left inferior frontal cortex (Broca’s area) and posterior temporal lobe (Wernicke’s area). Because these latter brain areas are also activated in linguistic tasks, these studies suggest that common neural systems may underlie language and tool use (Johnson-Frey, 2004; Lewis, 2006). Moreover, most right-handed patients with left-hemisphere damage in the inferior frontal lobe and adjacent regions show both aphasia (language deficits) and apraxia (disorders in complex movements), a finding that reinforces the view that common neural mechanisms may underlie these two distinct cognitive and motor functions (Meador et al., 1999).
In the study reported here, we examined whether handedness for tool use is associated with neuroanatomical asymmetries in chimpanzees. Behavioral data from captive and wild chimpanzees have shown evidence of population-level handedness for certain tasks, including tool use (Hopkins, 2006; Lonsdorf & Hopkins, 2005). Recent neurological studies of great apes have demonstrated population-level leftward asymmetries in two brain regions homologous to Broca’s and Wernicke’s areas (Hopkins & Cantalupo, 2004), but as yet, no functional correlates of these regions have been identified in apes. Our hypothesis was that if laterality in tool use served as a preadaptation for the emergence of left-hemisphere specialization for praxic functions and language in humans, then handedness for tool use would be associated with asymmetries in the language homologues, whereas hand use for non-tool-use actions would not.
METHOD
Magnetic resonance imaging (MRI) was used to scan 78 captive chimpanzees (44 females, 34 males) housed at the Yerkes National Primate Research Center (YNPRC), in Atlanta, Georgia. The subjects ranged in age from 6 to 42 years (M = 22.31, SD = 11.45). Within the sample, 19 chimpanzees were mother reared, 44 were nursery reared, and 15 were wild caught. Chimpanzees were classified as mother reared if they had been raised by their biological mothers for at least the first 30 days of life. Human-reared chimpanzees were those that had been removed from their biological mothers, because of medical complications or maternal neglect, within the first month of life. The wild-caught chimpanzees had been captured and brought from Africa to live in captivity before 1973, at which time importation became illegal. The exact number of subjects used in different analyses of handedness and brain asymmetries varied slightly depending on how many subjects had performed the tasks. Moreover, because the behavioral data were collected over an extended period of time, some of the apes died from natural causes between the time when they were behaviorally tested and the time when the brain scans were obtained. In these cases, the brains were scanned postmortem.
Image Collection and Procedure
Images were obtained from cadaver specimens and in vivo. Prior to scanning, the living subjects were immobilized with a telazol injection (2–6 mg/kg) and subsequently anesthetized with propofol (10 mg/kg/hr) following standard veterinary procedures used at the YNPRC. The subjects scanned in vivo remained sedated for the duration of the scans, as well as the time needed for transport between the YNPRC and the scanner location (about 1 to 1.5 hr). They were placed in the scanner chamber in a supine position with their head fitted inside the human-head coil. Scan duration ranged between 40 and 80 min.
The cadaver specimens (n = 22) were stored in a solution of water and 10% formaldehyde for intervals ranging from 1 week to 5 years and were scanned with a 4.7-T magnet (Bruker, Bio-Spec, Billerica, MA). The majority of the living subjects (n = 41) were scanned using a 1.5-T scanner (Phillips, Model 51, Amsterdam, The Netherlands). The remaining chimpanzees (n = 15) were scanned using a 3.0-T scanner (Siemens Trio, Siemens Medical Solutions USA, Inc., Malvern, PA).
For all chimpanzees scanned in vivo using the 1.5-T machine, T1-weighted images were collected in the transverse plane using a gradient echo protocol (pulse repetition = 19.0 ms, echo time = 8.5 ms, number of signals averaged 8, 256 × 256 matrix). For the postmortem scans, T2-weighted images were collected in the transverse plane using a gradient echo protocol (pulse repetition = 22.0 s, echo time = 78.0 ms, number of signals averaged 8–12, 256 × 192 matrix reconstructed to 256 × 256). For chimpanzees scanned using the 3.0-T machine, T1-weighted images were collected using a three-dimensional gradient echo sequence (pulse repetition = 2,300 ms, echo time = 4.4 ms, number of signals averaged 3, 320 × 320 matrix).
After completing the MRI procedures, the subjects scanned in vivo were temporarily housed in a single cage for 6 to 12 hr to allow the effects of the anesthesia to wear off. They were then returned to their home cage. The archived MRI data were transferred to a computer running Analyze 6.0 (Mayo Clinic, Mayo Foundation, Rochester, MN) software for postimage processing.
MRI Analysis
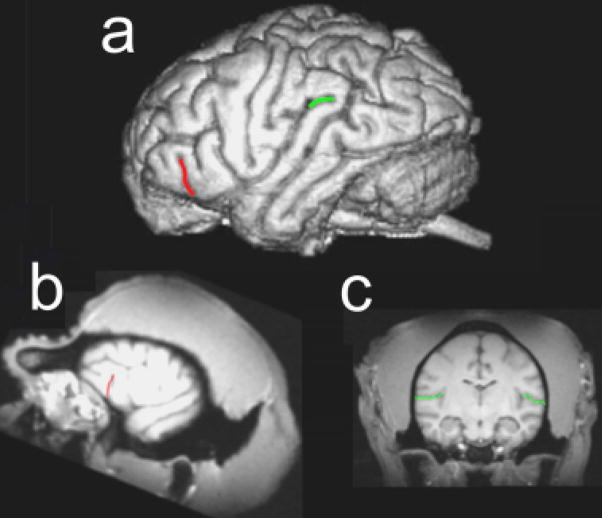
Two brain regions considered homologues to the classic language areas of the human brain were measured. These regions included the fronto-orbital sulcus (FO) and planum temporale (PT; Cantalupo & Hopkins, 2001; Cantalupo, Pilcher, & Hopkins, 2003; Gannon, Holloway, Broadfield, & Braun, 1998; see Fig. 1). Independent raters had previously established interrater reliability in the measurement of each of these brain areas (see Hopkins, Dunham, Cantalupo, & Taglialatela, 2007).
Fig. 1.
Depiction of the brain regions of interest in this study. The illustrations show (a) a three-dimensional reconstruction of a chimpanzee brain with the external sulci comprising the frontal orbital sulcus and the planum temporale highlighted in red and green, respectively; (b) a parasagittal view of a chimpanzee magnetic resonance imaging (MRI) scan with the fronto-orbital sulcus outlined in red; and (c) a coronal view of a chimpanzee MRI scan with the sylvian fissure highlighted in green. See the text for a description of the methods used to measure each brain region.
A portion of the inferior frontal gyrus where part of Broca’s area is located was measured by tracing the length of FO (see Fig. 1b). FO could be clearly seen in parasagittal (1 mm thick) MRI slices, and its length was traced from the first lateral slice where it was present up to the slice immediately preceding the opening of the insula. We measured solely FO, rather than the entire frontal operculum, because there is less variability in this sulcus than in other borders (see Sherwood, Broadfield, Holloway, Gannon, & Hof, 2003, for a discussion).
To measure the surface area of PT, we aligned the MRI scans in the coronal plane and cut them into 1-mm slices (see Fig. 1c). The anterior border of PT was defined by the most frontal slice showing Heschl’s gyrus (HG). The posterior border was defined by the most caudal slice showing the sylvian fissure. Once the anterior and posterior borders were delineated, the depth of the sylvian fissure (i.e., width of PT) on each slice was measured from the superolateral margin of the superior temporal gyrus. Depth measures were taken up to the lateral ridge of HG in all the slices where HG was present (in most cases, HG was no longer present in slices proximal to the posterior border of PT). Following a well-established procedure in the human literature, we estimated the PT surface areas (in square millimeters) as the sum of the cumulative PT depth measures within a hemisphere multiplied by the slice thickness.
Behavioral Measures
Two tasks with different motor demands were designed to simulate the termite fishing and anvil use of wild chimpanzees (see Fig. 2). In termite fishing, which involves the use of fine sensorimotor skills, chimpanzees insert small twigs into holes located on a termite mound, wait for the termites to attack and attach to the stick, and then retract the stick and digest the termites (Goodall, 1986). In contrast, anvil use involves a ballistic action—pounding hard-shelled foods on an anvil to open them (McGrew, Marchant, Wrangham, & Klein, 1999). In many ways, the motor demands of anvil use resemble those of nut cracking (Biro et al., 2003; Boesch, 1991). For comparison with the tool-use data, the subjects’ hand preferences for a simple reaching task were also obtained.
Fig. 2.
Photographs of a chimpanzee engaged in (a) simulated termite fishing and (b) simulated nut cracking.
All behavioral testing was conducted in the outside portion of the chimpanzees’ home cages. Subjects were tested on different days of the week between the hours of 10:00 a.m. and 7:00 p.m. The order of test administration was pseudorandomly determined for each subject. Individuals collecting the hand-preference data were blind to the brain-asymmetry data available for each chimpanzee. Although bouts of hand use could have been recorded for each behavior, we used frequencies of hand use as the level of analysis in determining individual handedness because this measure results in larger sample sizes and previous studies have shown significant positive correlations (r > .96) between handedness values based on bouts and handedness values based on frequency (Hopkins et al., 2001).
Simulated Termite Fishing
Handedness in simulated termite fishing was tested using a device consisting of three polyvinyl chloride (PVC) pipes (15 cm long, 4 cm in diameter) glued at 45° angles into three holes (4 cm in diameter) placed horizontally 15 cm apart on a rectangular plastic board (50 cm long × 20 cm wide). The upper end of each tube was open, to allow access to food at the other end of the tube, which was closed with a removable PVC cap. During testing, each PVC tube in the apparatus was first filled with a preferred food that had some adhesive qualities (honey or applesauce). The food filled approximately the bottom third of the length of the tube, so it was impossible for the subject to reach the food directly with its fingers. After the device was placed on a subject’s cage, a small stick or bamboo skewer (about 0.5 cm long) was handed to the subject directly. The chimpanzee had to insert this stick into a hole to extract the hidden food (see Fig. 2a). Each time the chimpanzee inserted the stick, a left- or right-hand response was recorded. For each subject, we obtained a minimum of 100 dipping responses summed across at least two test sessions.
Simulated Anvil Use
For this task, the chimpanzee was temporarily locked inside the indoor portion of its home cage, while coconuts were placed in the outside portion. The chimpanzee was then released and could obtain a coconut and attempt to open it by pounding it on the hard concrete walls and floors of the enclosure (see Fig. 2b). Each time the chimpanzee pounded the coconut on the floor or wall, a left- or right-hand response was recorded. The number of responses varied somewhat among the chimpanzees because of different success rates in opening the coconuts. A minimum of 30 hammering responses was obtained from each subject.
Simple Reaching
Simple reaching was measured by throwing raisins, one at a time, into the subject’s home cage (Hopkins, Russell, Hook, Braccini, & Schapiro, 2005). The subject had to locomote to a spot near the food, reach, and pick up the food. Hand use was recorded as right or left. A minimum of 50 responses was recorded from each subject. We ensured that hand choice on each trial was not influenced by the hand used on the previous trial by making subjects reposition themselves between trials.
Data Analysis
For the two brain regions, left-right asymmetry quotients (AQs) were calculated using the following formula: AQ = (R − L)/[(R + L) × 0.5], where R and L refer to the frequency of right- and left-hand use, respectively. Positive values indicated larger right than left hemispheres, and negative values reflected larger left than right hemispheres.
For the two tool-use handedness tasks, we calculated for each subject binomial z scores based on the total frequency of left-and right-hand use. Subjects with z scores greater than 1.95 or less than −1.95 were classified as right- and left-handed, respectively. All other subjects were classified as ambiguously handed. In addition, we derived a handedness index (HI) for each subject and task by subtracting the number of left-hand responses from the number of right-hand responses and dividing by the total number of responses. Positive values reflected right-hand preferences, and negative values reflected left-hand preferences.
For all analyses, we adopted an alpha of p < .05 as the level of significance. Significance levels are presented as prep values (probability of replication; see Killeen, 2005), and effect sizes are also provided. Post hoc tests, when necessary, were conducted using Tukey’s honestly significant difference with p < .05.
RESULTS
Descriptive Information
The mean AQ values for PT and FO in males and females are shown in Table 1. A multiple analysis of variance (ANOVA) was performed on the AQ values for PT and FO, with sex (male, female) and rearing history (mother reared, nursery reared, wild caught) as between-groups factors. No significant main effects or interactions were found. One-sample t tests on the AQ values indicated significant population-level leftward asymmetries for both PT, t(77) = 5.25, prep = .986, d = 1.21, and FO, t(77) = 2.86, prep = .979, d = 0.66. These results are consistent with previous reports based on this sample of chimpanzees, albeit with smaller sample sizes.
TABLE 1.
Mean Asymmetry Quotients for the Planum Temporale and Fronto-Orbital Sulcus for Female and Male Chimpanzees
| Brain region | Females | Males | Overall |
|---|---|---|---|
| Planum temporale | −0.125 (0.032) | −0.115 (0.036) | −0.121 (0.023) |
| Fronto-orbital sulcus | −0.157 (0.057) | −0.139 (0.063) | −0.149 (0.042) |
Note. The negative scores indicate leftward asymmetry; the magnitude of the asymmetry is indicated by the absolute value. Standard errors are given in parentheses.
Hand Preference and Brain Asymmetry
For simulated termite fishing, we had data from 54 chimpanzees, including 17 left-handed, 11 ambiguously handed, and 26 right-handed subjects. A mixed-model ANOVA of the AQ data was performed, with brain region (PT, FO) as the repeated measure and sex and hand preference as between-groups factors. A significant main effect of handedness was found, F(2, 48) = 4.17, p = .03, η2 = .153. Post hoc analysis indicated that right-handed chimpanzees (mean AQ = −0.248) had a greater leftward asymmetry compared with left-handed (mean AQ = −0.041) and ambiguously handed (mean AQ = −0.068) individuals.
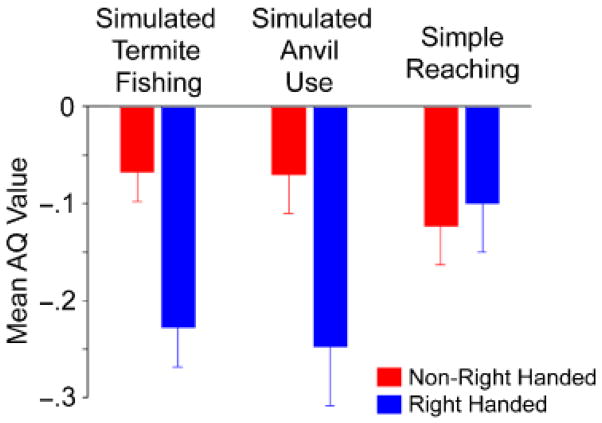
For simulated anvil use, data were available from 42 chimpanzees, including 23 left-handed, 17 right-handed, and 2 ambiguously handed chimpanzees. Because so few subjects showed no hand preference, we classified the chimpanzees as either right-handed or non-right-handed (left-handed or ambiguously handed) and compared the AQ values for these two cohorts. As for simulated termite fishing, a mixed-model ANOVA of the AQ data was performed, with brain region (PT, FO) serving as the repeated measure and handedness and sex serving as between-groups variables. A significant main effect of handedness was found, F(1, 38) = 5.16, prep = .921, d = 0.131. Right-handed subjects had a significantly greater leftward asymmetry than non-right-handed subjects (see Fig. 3).
Fig. 3.
Mean asymmetry-quotient (AQ) scores for non-right-handed and right-handed chimpanzees for each of the three handedness tasks. Error bars indicate standard errors.
To compare results for the two tool-use tasks, we classified the chimpanzees as right-handed or non-right-handed (left- and ambiguously handed) for the simulated termite-fishing task and then performed a new ANOVA on the AQ scores. This analysis also revealed a significant main effect of handedness, F(1, 48) = 8.68, prep =.986, d = 0.145. Right-handed subjects had significantly greater leftward AQ values compared with non-right-handed subjects (see Fig. 3). Among the 40 subjects with hand-preference data for both simulated nut cracking and termite fishing, the two measures were not significantly correlated with each other, r(38) =.283, prep =.786. Therefore, the observed differences in AQ scores between handedness groups cannot be attributed to the two tasks measuring the same dimension of handedness.
It could be argued that the associations between brain asymmetries and handedness for tool use simply reflect generalized hand preferences and are not due to the motor and cognitive demands of the tool-use tasks per se. To test this possibility, we reclassified the hand preferences of the chimpanzees on the basis of their preferred hand for simple reaching. For simple reaching, there were 11 left-handed, 15 right-handed, and 22 ambiguously handed individuals. The AQ scores for FO and PT showed no significant handedness effects, whether the chimpanzees were classified into three handedness groups or two (right-handed and non-right-handed; see Fig. 3). Thus, differences in AQ values between right- and non-right-handed chimpanzees were specific to hand preferences for simulated tool-use tasks.
Correlations Between Handedness and Brain Asymmetries
The results reported thus far come from ANOVAs assessing the relation between handedness and brain asymmetry. Hand preferences were based on classification criteria, but arguably, handedness can lie on a continuum from strongly left- to strongly right-handed. As an alternative means of assessing the association between brain asymmetry and handedness, Pearson product moment correlations between the HI and AQ scores were calculated. The results of these analyses are shown in Table 2. Significant negative associations were found between the HI scores for the simulated termite-fishing and anvil-use tasks and the mean AQ values for PT and FO. No significant associations were found for simple reaching.
TABLE 2.
Pearson Product-Moment Correlations Between Asymmetry Quotients and Handedness
| Handedness measure |
|||
|---|---|---|---|
| Brain region | Simulated termite fishing | Simulated anvil use | Simple reaching |
| Planum temporale | −.142 | −.269* | −.189 |
| Fronto-orbital sulcus | −.265** | −.148 | −.032 |
| Mean | −.363**** | −.322*** | −.157 |
prep = .839, d = 0.072.
prep = .878, d = 0.071.
prep = .892, d = 0.115.
prep = .953, d = 0.148.
DISCUSSION
Left- and right-handed chimpanzees, classified on the basis of their handedness for tool use, differed with respect to the lateralized organization of PT and FO. These results were consistent whether handedness was considered a continuous or discrete trait.
These findings are the first evidence of an association between handedness for tool use and neuroanatomical asymmetries in chimpanzees. Although the findings do not rule out competing theories on the evolution of lateralization and language functions, the results are consistent with the notion that tool use may have served as a preadaptation for the emergence of motor functions associated with praxic function, language and speech, or both in humans. Previous results in chimpanzees have shown that asymmetries in the inferior frontal gyrus are associated with variation in handedness for manual gestures (Taglialatela, Cantalupo, & Hopkins, 2006), and therefore it could be argued that different evolutionary scenarios for the origins of left-hemisphere specialization for language cannot be fully supported or refuted on the basis of the extant data from chimpanzees. Certainly this is a reasonable argument, but it can also be argued that the evidence of an association between asymmetries in the inferior frontal gyrus and both tool use and manual gestures may reflect similarity of the cognitive mechanisms used in the execution of these behaviors, despite the obvious differences in motor function.
Specifically, Leavens, Hopkins, and Bard (2005) have previously argued that captive chimpanzees’ use of manual gestures is, essentially, a form of social use. Manual gestures by captive chimpanzees are primarily observed when the chimpanzees are attempting to manipulate the attentional state of humans toward food items; the food items are otherwise unobtainable, so the chimpanzees must engage in communicative behaviors that draw the attention of the humans. Tool use, as described in the present article, operates in a similar manner, albeit in a non-social context. In the simulated termite-fishing and anvil-use tasks, there is a food item that the chimpanzee can obtain only by instrumental use of a stick or by utilization of a substrate as a surface on which to pound. For both tool use and manual gestures, there is an extrinsic means-ends relationship, with the food representing the goal and the tool or human (depending on the task) representing the means to achieving that goal (obtaining the food item). Thus, for captive chimpanzees, the cognitive components of tool use and of manual gestures may tap similar neural systems, notably, those that are homologous to linguistic and tool-using abilities in humans.
Alternatively, the association between handedness for tool use and neuroanatomical asymmetries in the language homologues may suggest that tool use served as a preadaptation for left-hemisphere specialization in praxic or motor functions, and that language lateralization subsequently evolved from this specialization for motor functions. This would suggest that lateralization for motor functions preceded the evolution of specialization associated with communicative functions, an interpretation at odds with the recent gestural-origins theory proposed by Corballis (2002). This interpretation is also at odds with recent studies showing evidence of population-level handedness for manual gestures in baboons, a species that has not been reported to use tools in their natural habitat (Meguerditchian & Vauclair, 2006). Indeed, these findings on lateralization in gestures in chimpanzees and baboons are more consistent with the view that lateralization in species-specific communicative behavior was selected for in evolution and that handedness for tool use and other types of manual actions postdates these early asymmetries (Bradshaw, 1997).
Finally, it might be suggested that the coassociation between asymmetries in the language homologues and handedness for tool use and manual gestures is, to some extent, dependent on different regions, particularly within the inferior frontal gyrus. On the basis of cellular organization, Broca’s area is divided into two distinct regions, Brodmann’s areas 44 and 45. A number of different cell types are located within the inferior frontal gyrus of chimpanzees (Sherwood et al., 2003), and morphology may not be a sensitive enough measure to study the roles of this brain region, and its different cell types, in behavior.
It should be emphasized that in no way are we suggesting that tool use is a necessary condition for the expression of asymmetries in chimpanzee brains. Indeed, the results reported here are consistent with the view that all handedness tasks do not necessarily tap into the same neural systems or networks. This is particularly the case for handedness tasks that differ in their sensitivity to individual differences in hand preference. In this study, the largest proportion of ambiguously handed subjects was found for simple reaching, and this measure was the least predictive of variation in AQ values for the two brain regions of interest. Arguably, tasks that more reliably induce consistent and strong individual hand preferences are more likely to be linked to neuroanatomical asymmetries. Whether different measures of hand use differ in the strength of their association with variation in brain asymmetries in humans is not clear because few studies have performed the analyses needed to answer this question. Nevertheless, recent functional MRI (fMRI) studies clearly show that left- and right-handed individuals differ in the brain regions activated for simple motor actions (Dassonville, Zhu, Ugurbil, Kim, & Ashe, 1997). Moreover, depending on the motor demands of the task (e.g., precision vs. power grasping; Ehrsson et al., 2000), or whether or not a tool is used, different brain areas are recruited in the execution of these actions. Thus, the fact that tool-use and non-tool-use actions are associated with activation of different brain areas in fMRI studies in humans may be related to the different patterns of association between handedness and morphological asymmetries in discrete brain regions that we observed in chimpanzees.
In summary, the results of this study indicate that handedness for tool use is associated with variation in neuroanatomical asymmetries in the homologues to Broca’s and Wernicke’s areas in the chimpanzee brain. These findings suggest that tool use may have served as a preadaptation for the evolution of left-hemisphere specialization in praxic functions. The results further support the view that associations between behavioral and brain asymmetries were present in the common ancestor of humans and apes, and are not unique to hominid evolution, as suggested by some researchers (e.g., Crow, 2004).
Acknowledgments
This work was supported in part by National Institutes of Health Grants RR-00165, NS-36605, NS-42867, and HD-38051. We are grateful to Hani Feeman and Leslie Dunham for their steadfast assistance in tracing brain scans and to the veterinary science department of the Yerkes National Primate Research Center for their assistance in collecting the magnetic resonance imaging scans.
References
- Annett M. Handedness and brain asymmetry: The right shift theory. Hove, England: Psychology Press; 2002. [Google Scholar]
- Binder JR, Frost JA. Functional MRI studies of language processes in the brain. Neuroscience News. 1998;1:15–23. [Google Scholar]
- Biro D, Inoue-Nakamura N, Tonooka R, Yamakoshi G, Sousa C, Matsuzawa T. Cultural innovation and transmission of tool use in wild chimpanzees: Evidence from field experiments. Animal Cognition. 2003;6:213–223. doi: 10.1007/s10071-003-0183-x. [DOI] [PubMed] [Google Scholar]
- Boesch C. Handedness in wild chimpanzees. International Journal of Primatology. 1991;6:541–558. [Google Scholar]
- Bradshaw JL. Human evolution: A neuropsychological perspective. Hove, England: Psychology Press; 1997. [Google Scholar]
- Bradshaw JL, Rogers L. The evolution of lateral asymmetries, language, tool-use and intellect. San Diego, CA: Academic Press; 1993. [Google Scholar]
- Cantalupo C, Hopkins WD. Asymmetric Broca’s area in great apes. Nature. 2001;414:505. doi: 10.1038/35107134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantalupo C, Pilcher D, Hopkins WD. Are planum temporale and sylvian fissure asymmetries directly related? A MRI study in great apes. Neuropsychologia. 2003;41:1975–1981. doi: 10.1016/s0028-3932(02)00288-9. [DOI] [PubMed] [Google Scholar]
- Cooper DL. Broca’s arrow: Evolution, prediction, and language in the brain. The Anatomical Record Part B: The New Anatomist. 2006;289B:9–24. doi: 10.1002/ar.b.20088. [DOI] [PubMed] [Google Scholar]
- Corballis MC. From hand to mouth: The origins of language. Princeton, NJ: Princeton University Press; 2002. [Google Scholar]
- Crow T. Directional asymmetry is the key to the origin of modern Homo sapiens (the Broca-Annett axiom): A reply to Rogers’ review of The speciation of modern Homo sapiens. Laterality: Asymmetries of Body, Brain and Cognition. 2004;9:233–242. [Google Scholar]
- Dassonville P, Zhu XH, Ugurbil K, Kim SG, Ashe J. Functional activation in motor cortex reflects the direction and the degree of handedness. Proceedings of the National Academy of Sciences, USA. 1997;94:14015–14018. doi: 10.1073/pnas.94.25.14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrsson HH, Fagergren A, Jonsson T, Westling G, Johansson RS, Forssberg H. Cortical activity in precision-versus power-grip tasks: An fMRI study. Journal of Neurophysiology. 2000;83:528–536. doi: 10.1152/jn.2000.83.1.528. [DOI] [PubMed] [Google Scholar]
- Gannon PJ, Holloway RL, Broadfield DC, Braun AR. Asymmetry of chimpanzee planum temporale: Humanlike pattern of Wernicke’s language area homolog. Science. 1998;279:220–222. doi: 10.1126/science.279.5348.220. [DOI] [PubMed] [Google Scholar]
- Goodall J. The chimpanzees of Gombe: Patterns of behavior. Cambridge, MA: Harvard University Press; 1986. [Google Scholar]
- Greenfield PM. Language, tools, and brain: The ontogeny and phylogeny of hierarchically organized sequential behavior. Behavioral and Brain Sciences. 1991;14:531–550. [Google Scholar]
- Hopkins WD. Comparative and familial analysis of handedness in great apes. Psychological Bulletin. 2006;132:538–559. doi: 10.1037/0033-2909.132.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Cantalupo C. Handedness in chimpanzees is associated with asymmetries in the primary motor but not with homologous language areas. Behavioral Neuroscience. 2004;118:1176–1183. doi: 10.1037/0735-7044.118.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Dunham L, Cantalupo C, Taglialatela J. The association between handedness, brain asymmetries and corpus callosum size in chimpanzees (Pan troglodytes) Cerebral Cortex. 2007;17:1757–1765. doi: 10.1093/cercor/bhl086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Fernandez-Carriba S, Wesley MJ, Hostetter A, Pilcher D, Poss S. The use of bouts and frequencies in the evaluation of hand preferences for a coordinated bimanual task in chimpanzees (Pan troglodytes): An empirical study comparing two different indices of laterality. Journal of Comparative Psychology. 2001;115:294–299. doi: 10.1037//0735-7036.115.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Russell J, Hook M, Braccini S, Schapiro S. Simple reaching is not so simple: Association between hand use and grip preferences in captive chimpanzees. International Journal of Primatology. 2005;26:259–277. doi: 10.1007/s10764-005-2924-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Frey SH. The neural basis of complex tool use in humans. Trends in Cognitive Sciences. 2004;8:71–78. doi: 10.1016/j.tics.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Killeen PR. An alternative to null-hypothesis significance tests. Psychological Science. 2005;16:345–353. doi: 10.1111/j.0956-7976.2005.01538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavens DA, Hopkins WD, Bard KA. Understanding the point of chimpanzee pointing: Epigenesis and ecological validity. Current Directions in Psychological Science. 2005;14:185–189. doi: 10.1111/j.0963-7214.2005.00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JW. Cortical networks related to human use of tools. The Neuroscientist. 2006;12:211–231. doi: 10.1177/1073858406288327. [DOI] [PubMed] [Google Scholar]
- Lieberman P. On the nature and evolution of the neural bases of human language. Yearbook of Physical Anthropology. 2003;45:36–62. doi: 10.1002/ajpa.10171. [DOI] [PubMed] [Google Scholar]
- Lonsdorf EV, Hopkins WD. Wild chimpanzees show population level handedness for tool use. Proceedings of the National Academy of Sciences, USA. 2005;102:12634–12638. doi: 10.1073/pnas.0505806102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrew WC, Marchant LF, Wrangham RW, Klein H. Manual laterality in anvil use: Wild chimpanzees cracking Strychnos fruits. Laterality. 1999;4:79–87. doi: 10.1080/03069887600760101. [DOI] [PubMed] [Google Scholar]
- Meador KJ, Loring DW, Lee K, Hughes M, Lee G, Nichols M, Heilman KM. Cerebral lateralization: Relationship of language and ideomotor apraxia. Neurology. 1999;53:2028–2031. doi: 10.1212/wnl.53.9.2028. [DOI] [PubMed] [Google Scholar]
- Meguerditchian A, Vauclair J. Baboons communicate with their right hand. Behavioural Brain Research. 2006;171:170–174. doi: 10.1016/j.bbr.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Ramnani N. The primate cortico-cerebellar system: Structure and function. Nature Reviews Neuroscience. 2006;7:511–522. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- Shapleske J, Rossell SL, Woodruff PW, David AS. The planum temporale: A systematic, quantitative review of its structural, functional and clinical significance. Brain Research Reviews. 1999;29:26–49. doi: 10.1016/s0165-0173(98)00047-2. [DOI] [PubMed] [Google Scholar]
- Sherwood CS, Broadfield DC, Holloway RL, Gannon PJ, Hof PR. Variability of Broca’s area homologue in great apes: Implication for language evolution. The Anatomical Record. 2003;217A:276–285. doi: 10.1002/ar.a.10046. [DOI] [PubMed] [Google Scholar]
- Taglialatela JP, Cantalupo C, Hopkins WD. Gesture handedness predicts asymmetry in the chimpanzee inferior frontal gyrus. NeuroReport. 2006;17:923–927. doi: 10.1097/01.wnr.0000221835.26093.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lawick-Goodall J. Tool using in primates and other vertebrates. Advances in the Study of Behavior. 1970;3:195–249. [Google Scholar]
- Whiten A, Goodall J, McGrew W, Nishida T, Reynolds V, Sugiyama Y, et al. Charting cultural variation in chimpanzees. Behaviour. 2001;138:1489–1525. [Google Scholar]