Abstract
We tested the hypothesis that apolipoprotein E allele status predicts the rate of motor decline in the elderly. 876 older participants without dementia underwent baseline and annual motor testing for up to 10 years. In a generalized estimating equation controlling for age, sex and education, motor function declined by about 0.03 unit/year. The presence of ε4 allele was associated with a two-fold increase in rate of motor decline [ε4 allele × Time: Estimate= −0.027 (S.E. 0.012, p=0.025)]. The association of ε4 allele with motor decline persisted even after controlling for cognitive status, race, BMI, vascular risk factors and diseases. Further analyses suggested that the association of ε4 with motor decline was for the most part explained by the association between ε4 allele with change in muscle strength. These results suggest that the presence of ε4 allele is a risk factor for more rapid motor decline in the elderly.
Keywords: Apolipoprotein E, ε4 allele, Aging, Motor decline, Motor function, Muscle strength
INTRODUCTION
Impaired motor function (e.g., muscle strength, motor performance) is common among older persons and associated with an increased risk of disability, loss of independence and death.1, 2 Although some risk factors for motor decline have been identified, such as reduced physical activity, its underlying biology is poorly understood.3 Identifying risk factors for motor decline is an important first step for the development of interventions to decrease the burden of motor impairment in our aging population.4 The increased availability of tests for genetic polymorphisms has shown that the presence of one or more alleles of ε4 is associated with a number of adverse health consequences, including mortality, AD, cognitive decline, cardiovascular disease and stroke.5-8 However, there are few studies about whether the presence of ε4 allele predicts motor decline and in extant studies results have been conflicting.9, 10
We used data from more than 800 older participants of the Rush Memory and Aging Project to test the hypothesis that apolipoprotein E allele status predicts the rate of motor decline in older persons. All participants underwent apolipoprotein E allele determination and annual detailed motor testing for up to 10 years.11 We also tested for interactions with demographic variables including race and examined other covariates including cognitive status which might mediate the association between ε4 allele status and motor function as well as race, body composition, vascular risk factors and diseases which might confound the association of ε4 allele status and change in motor function. Finally since motor function is not a unitary process, we examined the extent to which the association of ε4 allele with change in motor function is due to an association with measures of muscle strength or motor performance.
METHODS
Participants
All participants were from the Rush Memory and Aging Project, a community-based, longitudinal clinical-pathologic investigation of risk factors for common chronic conditions of old age Participants were recruited from more than 40 residential facilities across the metropolitan Chicago area, including subsidized senior housing facilities, retirement communities, and retirement homes, in addition to social service agencies and Church groups. Following a group presentation about the project, persons rated their interest in participating. Study personnel subsequently met with those who expressed interest and explained the project in detail. Persons then signed an informed consent agreeing to annual clinical evaluations and an anatomical gift act agreeing to donate their brain, spinal cord, and selected nerves and muscles to Rush Investigators. The study was conducted in accordance with the latest version of the Declaration of Helsinki and was approved by the Institutional Review Board of Rush University Medical Center. All evaluations were performed at the parent facility or the participants’ homes to reduce burden and enhance follow-up participation.[8].The clinical evaluation was uniform and included a medical history, complete neurological examination, and assessment of cognitive and motor function (see below). Follow-up evaluations, identical in all essential details, were performed annually by examiners blinded to previously collected data. Clinical evaluations for the study commenced in 1997 and 1121 participants had completed a baseline evaluation at the time of these analyses.
Since prior work suggests that the effect of apoE allele status might vary in persons with and without dementia, 12 eligibility for these analyses required the absence of dementia at the baseline evaluation and apolipoprotein E allele determination and in order to assess change in motor function, a valid baseline and at least one follow-up motor score. Therefore, we excluded from these analyses 85 (7.6%) persons who met criteria for dementia at the baseline (see below: Clinical Diagnoses), 20 who had died prior to the time of their follow-up motor assessment; 20 who had not been in the study for a full year and were not yet eligible for a follow-up evaluation; 43 persons with incomplete clinical data;56 who did not have a valid apolipoprotein E genotyping and 21 who showed an allele combination of apoE ε2, ε4 (see below: Apo E Allele Determination). This resulted in a final group of 876 non-demented persons for the current study whose characteristics at baseline are summarized in Table 1.
Table 1.
Demographics of the Cohort at Baseline [Mean (SD) or %]
| Variable | ε4 Yes (N=184) | ε4 No (N=692) |
|---|---|---|
| Age (years) | 79.5(6.83) | 80.7 (6.97) |
| Sex (% male) | 27.2% | 25.1% |
| Education (years) | 14.8(3.08) | 14.4(2.98) |
| Minimental Status Exam | 27.9(2.12) | 27.9(2.04) |
| BMI | 26.2(4.24) | 27.6(5.46) |
| Global Motor Function (composite)* | 0.02(0.60) | −0.08(0.57) |
| Muscle Strength (composite)* | 0.02(0.71) | −0.07(0.71) |
| Arm Abduction (lbs.) | 3.9(2.12) | 3.7(2.11) |
| Arm Flexion (lbs.) | 13.2(5.22) | 12.8(5.59) |
| Elbow Extension (lbs.) | 11.0(3.68) | 10.5(4.00) |
| Grip (lbs.) | 50.9(17.96) | 48.5(18.17) |
| Pinch (lbs.) | 11.4(5.00) | 11.4(4.89) |
| Hip Flexion (lbs.) | 10.2(4.55) | 9.8(4.40) |
| Knee Extension (lbs.) | 10.7(3.84) | 10.0(4.00) |
| Plantar Flexion (lbs.) | 14.8(4.72) | 14.3(5.09) |
| Ankle Dorsiflexion (lbs.) | 12.4(4.98) | 11.4(4.95) |
| Motor Performance (composite)* | 0.02(0.67) | −0.08(0.66) |
| Timed walk (s) | 4.0(1.47) | 4.3(3.02) |
| Steps - Timed walk | 6.4(1.65) | 6.8(2.64) |
| Turn 360° (s) | 5.9(4.53) | 5.8(4.09) |
| Steps - Turn 360° | 9.4(4.61) | 9.4(4.11) |
| Leg Stand (s) | 5.0(3.23) | 4.8(3.10) |
| Toe Stand (s) | 6.3(3.59) | 6.0(3.41) |
| Tandem Gait (errors) | 2.6(2.20) | 2.6(2.08) |
| Finger Tapping (taps/10sec) | 55.4(8.87) | 55.0(8.39) |
| Purdue Pegboard (pegs) | 10.5(2.31) | 10.1(2.50) |
| Chronic Conditions | ||
| Diabetes | 11.3% | 13.6% |
| Hypertension | 53.8% | 47.8% |
| Myocardial Infarction | 13.0% | 11.4% |
| Congestive Heart Failure | 2.8% | 5.9% |
| Claudication | 3.8% | 6.9% |
| Stroke | 10.1% | 10.3% |
| Ever Smoked | 40.8% | 39.5% |
Z scores of the composite measure of Global Motor Function is based on two subcomponents: Muscle Strength is based on nine muscle groups tested in arms and legs bilaterally; Motor Performance is based on nine motor performances in arms and legs. Composite measures are constructed so that a more positive value is consistent with a higher level of Muscle Strength or better Motor Performance and a negative value is consistent with a lower level of strength or performance.
Clinical Diagnoses
Clinical diagnoses were made using a multi-step process, as previously described.11, 13 First, participants underwent detailed cognitive function testing which included 21 performance tests administered in an approximately hour-long session as previously described.11 Participants were evaluated in person by an experienced neurologist or geriatrician this person then used all available cognitive and clinical testing results to diagnose dementia and other common neurologic conditions affecting cognitive or physical function. The diagnosis of dementia followed the criteria of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association.14 The diagnosis of mild cognitive impairment (MCI) was rendered for individuals who were found to have cognitive impairment by a neuropsychologist but who did not meet criteria for dementia as previously described.15 Persons who did not meet criteria for MCI or dementia, as determined by the clinician's review of all available data, were classified as having no cognitive impairment (NCI).13
Motor Function Testing
Muscle Strength Testing
Hand-held dynamometer (Lafayette Manual Muscle Test System, Model 01163, Lafayette, IN) was used to assess muscle strength in arm abduction, arm flexion, arm extension, hip flexion, knee extension, plantar flexion, and ankle dorsiflexion bilaterally. Grip and pinch strength were measured bilaterally using the Jamar hydraulic dynamometers (Lafayette Instruments, Lafayette, IN).
Motor Performance Testing
Time and number of steps to walk 8 feet and turn 360°. Time to stand on each leg and then on their toes for 10 seconds; We counted the number of steps off line when walking an 8 foot line in a heel to toe manner; the number of pegs that could be placed (Purdue pegboard) in 30 seconds and participants tapped an electronic tapper (Western Psychological Services, Los Angeles, CA) with their index finger as quickly as possible for 10 seconds.
Global Motor Function
A composite global motor measure was created by averaging all of the motor tests together.16, 17 Composite measures have been used effectively in other longitudinal studies of cognitive and motor function.18-20 Composite measures typically have metric properties that are more suited for longitudinal analyses than the results of individual tests. They yield more stable measures of motor function, increase power to identify risk factors as well as the adverse health consequences of motor decline in aging. Since motor function is not a unitary process and we have previously reported empiric support, using principal-components analysis and subsequent factor analysis with varimax rotation, to summarize motor function data into two subcomponents: muscle strength and motor performance.16, 17 A composite measure of strength was made by averaging the z scores for arm and leg muscle strength. A composite measure of motor performance was made by averaging the z scores for all the motor performance measures in arms and legs.
Apolipoprotein E Allele Determination
Blood was collected at each participating Memory and Aging Project site with acid citrate dextrose anticoagulant and stored at room temperature. Lymphocyte separation was performed within 24 hours of collection. DNA was extracted from approximately 2−3 million cells, and genotyping was performed by Agencourt Bioscience Corporation (Beverly, MA) utilizing high throughput sequencing of codon 112 (position 3937) and codon 158 (position 4075) of exon 4 of the apolipoprotein E gene on chromosome 19. Participants with 1 or more alleles for ε4 (i.e., ε3, ε4 [N=171] and ε4, ε4 [N=13]) were considered positive for ε4 allele for these analyses. All other participants with other allele combinations (i.e., ε2, ε2 [N=8]; ε2,ε3 [N=137]; or ε3,ε3 [N=547]) were considered negative for ε4 allele. As noted above, we excluded participants with the allele combination of ε2, ε4 [N=21] since there is some literature to suggest that the presence of ε2 may be beneficial with regards to motor disorders.21 Furthermore, all the analyses reported below were repeated and the results were unchanged when participants with the allele combination ε2, ε4 were included (results not shown).
Other Covariates
Age in years was computed from self-reported date of birth, and date of the baseline clinical examination was that at which the strength measures were first collected. Education (reported highest grade or years of education) was obtained at the time of the baseline cognitive testing. Gender, race, and ethnicity were recorded at the baseline interview. Race and ethnicity questions and categories were those used by the 1990 U.S. Census. Weight and height were measured and recorded at each visit and used to calculate body mass index (BMI). In order to assess the influence of vascular risk factor and vascular disease burden on the association of motor function and mortality, we summarized cumulative vascular risk factors and vascular disease burden.15 For the purpose of this study, smoking history, heart attack, congestive heart failure, and claudication were rated as absent or present (0 or 1) as determined by self-report; hypertension and diabetes were rated as present if the participant reported having been diagnosed with the condition or was found to be on medication for the condition, and stroke was diagnosed based on self-report plus clinical examination, as previously described.11 We computed summary scores indicating each individual's vascular risk factor (i.e. the sum of hypertension, diabetes mellitus, and smoking, resulting in a score from 0−3 for each individual, and vascular disease burden (i.e. the sum of heart attack, congestive heart failure, claudication, and stroke, resulting in a score from 0−4 for each individual. These summary scores were used as covariates in the analyses. 15
Analysis
Correlations were used to assess the relationship of global motor function with age and education, and t-tests were used to compare men and women. We used generalized estimating equation models to characterize change over time in the global measure of motor function and to test the relation of ε4 allele status with the baseline level and rate of change in global motor function.22 This model and all subsequent models controlled for age, sex and education and their interaction with Time. Age was centered at 80 years for these analyses. Sex was coded as “1” for men and “0” for women. Next we added a term for ε4 allele and its interaction with Time. We also tested whether ε4 allele status and rate of change in motor function were affected by race and examined potential higher order interactions between ε4 allele status, sex and rate of change in motor function. Next since the possession of the presence of ε2 allele genotype has been reported to be beneficial in some studies, we contrasted the affect of the presence of any ε2 allele (ε2, ε2 or ε2, ε3 genotype) and any ε4 allele (ε4,ε4 or ε3,ε4 genotype) on motor decline. For this model, participants with the ε3, ε3 allele genotype served as the reference group and those with the ε2, ε4 genotype were excluded. This model was similar to the core model described above except that we included terms for the ε2 and ε4 subgroups (each contrasted with the ε3, ε3 reference group), and the interaction of each subgroup with time. The term for time indicates the average annual rate of change in the ε3, ε3 reference group. We then examined several covariates including cognitive status, body composition based on BMI, vascular risk factors and diseases that might affect these associations. A term for each covariate was added to the previous model as well as a term for its interaction with time. Since both low and high values of BMI can be associated with adverse health consequences, we added a term for BMI and a quadratic term for BMI (BMI × BMI) as well as a term for their interaction with time. We repeated similar models with muscle strength and then with motor performance rather than global motor function as the outcome. Model assumptions of linearity, normality, independence of errors, and homoscedasticity of errors were examined graphically and analytically and were adequately met.23 Programming was done using SAS software [SAS Institute, Cary, NC].24
RESULTS
Descriptive Properties of Motor Function
Global motor function was approximately normally distributed and ranged from −1.92 to 2.15 (mean −0.06; SD=0.57), with higher scores indicating better performance. Global motor function was inversely related to age (r = −0.40. p < 0.001), positively associated with education (r=0.21, p= < 0.001), and men had higher levels of motor function than women (t [874] = 7.73, p < 0.001). Muscle strength was associated with motor performance (rho=0.35; p= < 0.001).
The Presence of ε4 allele and the Rate of Change in Motor Function
First, we examined individual differences in the rate of change in global motor function using a generalized estimating equation model controlling for age, sex and education and their interactions with Time. On average there were four measures of global motor function for each participant in these analyses (SD=2.0; range 2, 10; median= 4). On average, global motor function declined by about 0.03 units per year (Estimate=−0.034; SE, 0.005, p<0.001).
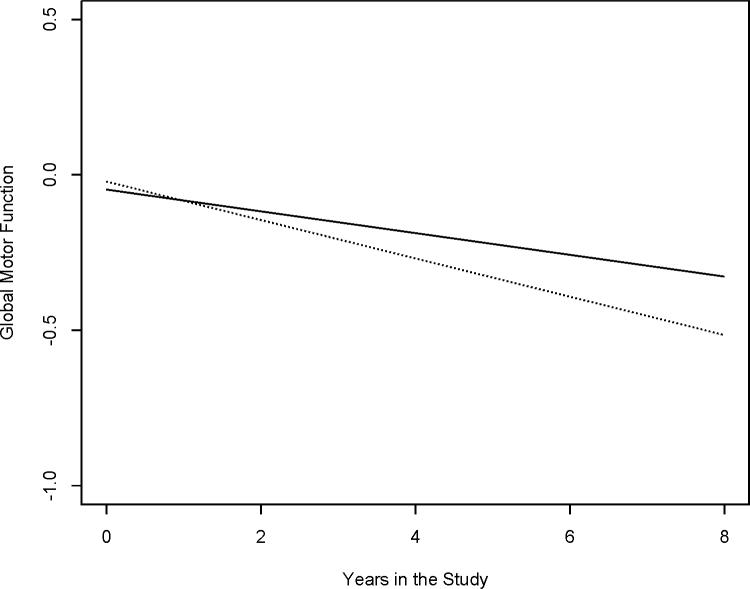
Next, to examine the relation of ε4 allele status with the level and rate of decline in global motor function, we added a term for ε4 allele status and its interaction with Time to the previous model. In this model, there was no association between the ε4 allele and the baseline level of global motor function (Term, ε4 allele status, Table 2). Global motor function declined by about 0.03 units per year. However, in this model, the presence of at least one copy of the ε4 allele was associated with a two-fold increase in the rate of motor decline (Term, ε4 allele status × Time, Table 2). Figure 1 illustrates the effect of the ε4 allele on the rate of change in global motor function for two average participants with similar age, sex and education. At baseline, there was no difference in the level of global motor function at baseline between participants with and without the ε4 allele. In contrast, the trajectory of motor decline in an average participant with at least one copy of the ε4 allele (dashed line) was 1.96 times faster than a similar participant without the ε4 allele (solid line).
Table 2.
Association of ε4 Allele Status with Level and Change in Motor Function*
| Model Terms | Estimate | S.E. | p-Value |
|---|---|---|---|
| Time | −0.028 | 0.005 | p<0.001 |
| Age | −0.031 | 0.003 | p<0.001 |
| Age × Time | −0.003 | 0.001 | p<0.001 |
| Sex | 0.324 | 0.042 | p<0.001 |
| Sex × Time | −0.028 | 0.009 | p=0.003 |
| Education | 0.029 | 0.006 | p<0.001 |
| Education × Time | 0.001 | 0.001 | p=0.469 |
| ε4 Allele Status | 0.026 | 0.041 | p=0.532 |
| ε4 Allele Status × Time | −0.027 | 0.012 | p=0.025 |
Estimated from a generalized estimating equation model with global motor function as the outcome; including a term for motor decline, Time (in years since baseline). The model terms Age at baseline (centered at 80 years), Sex (coded as “1” for men, “0” for women), Education (number of years) and ε4 allele status show the cross sectional relationships between these terms and level of global motor function at baseline. In addition, the model also included terms for age at baseline, sex, and education, ε4 allele status and their interactions with motor decline (Time). Results show that age at baseline, sex and the presence of ε4 allele are all associated with more rapid motor decline (negative estimate).
Figure 1. The Presence of ε4 Allele Is Related to Rate of Change in Motor Function.
This figures is model generated of two hypothetical participants to illustrate the trajectory of motor decline during the study in participants with similar age, sex and education but one without the ε4 allele (solid line) and the other with the ε4 allele (dashed line).
Since the rate of motor decline was more rapid in men (Term, sex × Time, Table 2), we examined whether the association between ε4 allele and the rate of change in motor function varied by sex by adding an interaction term between sex and ε4 allele as well as their interaction with time. There was no significant interaction between sex and ε4 allele status and level of motor function at baseline or in the association between sex, ε4 allele status and the rate of change in motor function (results not shown). In a final model we added a term for race. Race was not associated with change in motor function [Race × Time: Estimate =0.021 (S.E.=0.032, p=0.507)] and did not affect the association between ε4 allele status and change in motor function which remained significant [ε4 allele × Time: Estimate =−0.027 (S.E.=0.012, p=0.026)].
Age for these analyses was centered at 80 years and there was a significant interaction between age at baseline and the rate of change in global motor function such that for each year of age above 80, global motor function declined 11% more rapidly (Term, Age × Time, Table 2). Next we added an interaction term between age and ε4 allele as well as their interaction with time to determine if the association between ε4 allele and the rate of motor decline varied with increasing age. There was no interaction between age at baseline, ε4 allele status, and the level of motor function [Age × ε4 allele: Estimate 0.004 (S.E, 0.007, p=0.561)]. However, there was a significant interaction between age at baseline, ε4 allele status and the rate of change in motor function [Age × ε4 allele × Time: Estimate =−0.004 (S.E.=0.002, p=0.027)], suggesting that the presence of the ε4 allele has more of an impact with increasing age. The interaction between baseline age, ε4 allele status and the rate of motor decline is illustrated in Figure 2. Figure 2a shows the rate of motor decline for two average participants, one 80 years old (solid line) and one 85 years old (dashed line) at baseline, both of whom did not have the ε4 allele. The 85 year old participant has a lower level of global motor function at baseline and global motor function declines more rapidly than for the 80 year old. Figure 2b shows two participants of the same ages 80 years old (solid line) and one 85 years old (dashed line) at baseline both with the ε4 allele. The rate of motor decline is more rapid in both participants with the ε4 allele as compared to the participants without ε4 allele, but the rate of motor decline is even more rapid in the 85 year-old with ε4 allele as compared to the 80 year-old.
Figure 2. The Effect of ε4 Allele Status on the Rate of Change in Motor Function Varies with Age.
These figures are model generated of four hypothetical participants to illustrate the effect of ε4 allele status and age on motor function. Figure 2a shows the rate of motor decline in two participants without the ε4 allele: one age 80 years [solid line] and the other age 85 years [dashed line] at study baseline. Figure 2b shows the rate of motor decline in two participants of similar ages but who have at least one copy of the ε4 allele: age 80 years [solid line] and 85 years [dashed line] at study baseline.
In contrast to the ε4 allele, the ε2 allele may be beneficial with regards to motor disorders.21 We repeated the core model described above (Table 2) and included terms for the ε2 and ε4 subgroups (each contrasted with the ε3, ε3 reference group), and the interaction of each subgroup with time. While the presence of ε4 allele was related to a faster rate of motor decline, [ε4 × Time: Estimate =−0.024 (S.E =0.012, p=0.046)], the presence of ε2 allele was associated with a slower rate of motor decline but this was not significant, [ε2 × Time: Estimate =0.012 (S.E=0.011, p=0.250)].
The Presence of ε4 Allele, Other Covariates and the Rate of Change in Motor Function
Next, we repeated the model shown in Table 2 with the addition of terms for several covariates that might affect the association of ε4 allele and change in motor function. While we excluded those participants with dementia, the presence of ε4 allele in persons with MCI is associated with increased risk of AD.25 Therefore we added a term for baseline cognitive status (NCI vs MCI) and its interaction with Time to determine if cognitive status might mediate the association of ε4 allele with change in the rate of motor function. The addition of terms for MCI did not change the association between ε4 allele status and change in global motor function; both the presence of MCI and ε4 allele status were associated with change in motor function (Table 3, Model 1).
Table 3.
Presence of ε4 Allele, Other Covariates and the Rate of Change in Motor Function*
| Variable | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 |
|---|---|---|---|---|---|---|
| Time | −0.023 (0.006, p<0.001) | −0.029 (0.005, p<0.001) | −0.029 (0.006, p<0.001) | −0.024 (0.008, p=0.003) | −0.030 (0.006, p<0.001) | −0.022 (0.009, p=0.011) |
| ε4 Allele Status | 0.050 (0.040, p=0.221) | 0.001 (0.039, p=0.971) | 0.002 (0.038, p=0.963) | 0.014 (0.040, p=0.726) | 0.016 (0.041, p=0.695) | 0.008 (0.038, p=0.842) |
| ε4 Allele Status × Time | −0.025 (0.012, p=0.035) | −0.028 (0.012, p=0.019) | −0.028 (0.012, p=0.019) | −0.027 (0.012, p=0.026) | −0.026 (0.012, p=0.031) | −0.025 (0.012, p=0.040) |
| Cognitive Status | −0.202 (0.042, p<0.001) | −0.180 (0.037, p<0.001) | ||||
| Cognitive Status × Time | −0.021 (0.010, p=0.038) | −0.023 (0.010, p=0.025) | ||||
| BMI | −0.014 (0.003, p<0.001) | −0.011 (0.004, p=0.007) | −0.011 (0.004, p=0.007) | |||
| BMI × Time | −0.001 (0.001, p=0.192) | −0.001 (0.001, p=0.355) | −0.001 (0.001, p=0.199) | |||
| BMI*BMI | −0.000 (0.000, p=0.457) | −0.000 (0.000, p=0.574) | ||||
| BMI*BMI × Time | −0.000 (0.000, p=0.968) | 0.000 (0.000, p=0.411) | ||||
| Vascular Risks | −0.080 (0.021, p<0.001) | −0.057 (0.021, p=0.007) | ||||
| Vascular Risks × Time | −0.005 (0.005, p=0.331) | −0.007 (0.006, p=0.218) | ||||
| Vascular Diseases | −0.106 (0.027, p<0.001) | −0.076 (0.026, p=0.003) | ||||
| Vascular Diseases × Time | 0.008 (0.007, p=0.253) | 0.013 (0.007, p=0.091) |
Estimated from a generalized estimating equation model with global motor function as the outcome; including a term for motor decline, Time (in years since baseline). All models controlled for age at baseline (centered at 80 years), Sex (coded as “1” for men, “0” for women), and education (number of years) [terms not shown]. ε4 allele status and various covariates show the cross sectional relationships between these terms and level of global motor function at baseline. In addition, the model also included terms for ε4 allele status and covariates and their interactions with motor decline (Time). Results show that the presence of ε4 allele is associated with more rapid motor decline (negative estimate) even after controlling for cognitive status, body composition, vascular risk factors and diseases.
Because muscle mass is integral to motor function, we examined the extent to which body composition might affect the association of physical and motor function. The addition of these terms for body composition did not change the association between ε4 allele status and change in global motor function (Table 3, Models 2 and 3).
Because chronic diseases may contribute to motor impairment and ε4 allele is associated with an increased risk of vascular diseases, we added terms for vascular risk factors and vascular disease burden and their interactions with time to examine whether they affected the association of ε4 allele status with rate of change in motor function. Including a term for vascular risk factors and vascular disease burden did not change the extent to which ε4 allele status was associated with change in global motor function (Table 3, Models 4 and 5). Finally we repeated a single model which included terms for age, sex, education, cognitive status, BMI, and vascular risk factors and diseases, ε4 allele status and their interactions with Time and the association between ε4 allele status and change in global motor function remained significant (Table 3, Model 6). These results suggest that these covariates do not mediate the association of ε4 allele status with motor decline.
Finally, since motor function is not a unitary process, we examined the extent to which the association of ε4 allele with motor decline might vary by two main components of motor function, motor performance and muscle strength. We therefore repeated the core model described above, first replacing global motor function with its subcomponents, motor performance and then repeating the model a second time with muscle strength. The presence of the ε4 allele was not associated with the rate of change in motor performance [ε4 allele × Time: Estimate= −0.012 (S.E=0.015, p=0.439)]. However, by contrast, ε4 allele was associated with the rate of change in muscle strength [ε4 allele Status × Time: Estimate=−0.030 (S.E.=0.014, p=0.027)].
DISCUSSION
In a cohort of more than 850 older persons without dementia, we found that the presence of at least one copy of the apolipoprotein E ε4 allele was associated with an increased rate of motor decline in the elderly. This association was unchanged even after controlling body composition, cognitive status and vascular risk factors and diseases. Moreover, we found that the association of ε4 allele status and the rate of motor decline increased with age. Further analyses indicated that the association of the ε4 allele with the rate of change in global motor function was due primarily to the association of allele status with an increased rate of decline in muscle strength rather than motor performance. These findings suggest that apolipoprotein E allele status is a risk factor for age-related motor decline.
In the current study, ε4 allele status was not related to the level of motor function at baseline but was associated with a two-fold increase in the rate of motor decline which was similar in men and women. Furthermore, we found that this association was for the most part explained by the association between ε4 allele and change in muscle strength rather than motor performance. Motor systems which regulate motor planning and execution are located in multiple interconnected cortical and subcortical motor regions, which extend to the spinal cord and peripheral muscle.26-28 Thus, ε4 allele may be associated with motor decline through a number of different diseases or pathologies which can damage these widely distributed motor systems. In contrast to previous reports, in the current study the presence of ε2 allele was not related to motor decline.
The mechanism underlying the association of ε4 allele with motor decline is unknown. A number of recent studies have implicated subclinical vascular disease based on changes in white matter with loss of mobility, and ε4 allele is known to be associated with stroke.8, 29, 30 Our finding that the association between ε4 allele status and motor decline was unchanged after controlling for vascular diseases including stroke and vascular risk factors and body composition suggests that other factors may play a role in the association of allele status and motor decline. Another possible mechanism which may link the ε4 allele to the rate of motor decline is through an association with AD pathology.5 Recently there has been increasing studies which suggest that AD pathology accumulates not only in cognitive brain regions but also in regions known to subserve motor function, even among persons without dementia.31-34 Thus, ε4 allele may be linked to motor decline through the deposition of AD pathology in brain regions which subserve movement.
Finally, previous studies have suggested that the association of ε4 allele and risk of AD may decrease with increasing age.35-37 In contrast to these previous studies, in the current study, there was a significant interaction between the presence of ε4 allele and age such that those who are older are more likely to decline at a faster rate compared to those who are younger (Figure 2). This finding needs to be replicated in other cohorts and if true suggests that different factors or pathways may link ε4 allele with cognitive and motor decline.
Our study has several limitations. First, the participants in this study were a selected group of persons who agreed to post-mortem donation, so these results will need to be replicated in the general population. In addition, motor function testing, particularly muscle strength, is vulnerable to practice effects in the first years of follow-up and the duration of follow-up was relatively short in this study; practice effects therefore may account for the limited rate of change in muscle strength. If this is the case, then we may have underestimated the actual contribution of ε4 allele to decline in muscle strength and motor function overall. In addition, since we excluded persons who were demented at baseline or died before the first follow-up evaluation, these results may underestimate the magnitude of the association between ε4 allele and the rate of motor decline.
Despite these limitations, several factors increase confidence in the findings from this study. Perhaps most importantly, motor function was evaluated as part of a uniform structured clinical evaluation and incorporated many widely accepted and reliable performance measures. In addition, persons with dementia were excluded and a relatively large number of community-based older persons in the community were studied.
ACKNOWLEDGMENTS
We thank all the participants in the Rush Memory and Aging Project. We also thank Traci Colvin, and Tracey Nowakowski for project coordination; Barbara Eubeler, Mary Futrell, Karen Lowe Graham, and Pamela Smith for participant recruitment; George Dombrowski and Greg Klein for data management; and the staff of the Rush Alzheimer's Disease Center and Rush Institute for Healthy Aging.
Funding: This work was supported by National Institute on Aging grants R01AG17917, R01AG24480, and K23 AG23040, the Illinois Department of Public Health, and the Robert C. Borwell Endowment Fund.
REFERENCES
- 1.Al Snih S, Markides KS, Ray L, et al. Handgrip Strength and Mortality in Older Mexican Americans. J Am Geriatr Soc. 2002;50:1250–1256. doi: 10.1046/j.1532-5415.2002.50312.x. [DOI] [PubMed] [Google Scholar]
- 2.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-Extremity Function in Persons over the Age of 70 Years as a Predictor of Subsequent Disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warburton DE, Nicol CW, Bredin SS. Health Benefits of Physical Activity: The Evidence. Canadian Medical Association Journal. 2006;174:801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cesari M, Leeuwenburgh C, Lauretani F, et al. Frailty Syndrome and Skeletal Muscle: Results from the Invecchiare in Chianti Study. American Journal of Clinical Nutrition. 2006;83:1142–1148. doi: 10.1093/ajcn/83.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahley RW, Weisgraber KH, Huang Y. Inaugural Article: Apolipoprotein E4: A Causative Factor and Therapeutic Target in Neuropathology, Including Alzheimer's Disease. Proceedings of the National Academy of Sciences. 2006;103:5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houlden H, Greenwood R. Apolipoprotein E4 and Traumatic Brain Injury. Journal of Neurology, Neurosurgery, and Psychiatry. 2006;77:1106–1107. doi: 10.1136/jnnp.2006.095513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson RS, Schneider JA, Barnes LL, et al. The Apolipoprotein E Epsilon 4 Allele and Decline in Different Cognitive Systems During a 6-Year Period. Arch Neurol. 2002;59:1154–1160. doi: 10.1001/archneur.59.7.1154. [DOI] [PubMed] [Google Scholar]
- 8.Sudlow C, Martinez Gonzalez NA, Kim J, et al. Does Apolipoprotein E Genotype Influence the Risk of Ischemic Stroke, Intracerebral Hemorrhage, or Subarachnoid Hemorrhage?: Systematic Review and Meta-Analyses of 31 Studies among 5961 Cases and 17 965 Controls. Stroke. 2006;37:364–370. doi: 10.1161/01.STR.0000199065.12908.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melzer D, Dik MG, van Kamp GJ, et al. The Apolipoprotein E E4 Polymorphism Is Strongly Associated with Poor Mobility Performance Test Results but Not Self-Reported Limitation in Older People. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2005;60:1319–1323. doi: 10.1093/gerona/60.10.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carmelli D, DeCarli C, Swan GE, et al. The Joint Effect of Apolipoprotein E Epsilon4 and Mri Findings on Lower- Extremity Function and Decline in Cognitive Function. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2000;55:M103–109. doi: 10.1093/gerona/55.2.m103. [DOI] [PubMed] [Google Scholar]
- 11.Bennett DA, Schneider JA, Buchman AS, et al. The Rush Memory and Aging Project: Study Design and Baseline Characteristics of the Study Cohort. Neuroepidemiology. 2005;25:163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 12.Dik MG, Jonker C, Bouter LM, et al. Apoe-{Epsilon}4 Is Associated with Memory Decline in Cognitively Impaired Elderly. Neurology. 2000;54:1492–1497. doi: 10.1212/wnl.54.7.1492. [DOI] [PubMed] [Google Scholar]
- 13.Bennett DA, Schneider JA, Aggarwal NT, et al. Decision Rules Guiding the Clinical Diagnosis of Alzheimer's Disease in Two Community-Based Cohort Studies Compared to Standard Practice in a Clinic-Based Cohort Study. Neuroepidemiology. 2006;27:169–176. doi: 10.1159/000096129. [DOI] [PubMed] [Google Scholar]
- 14.McKhann G, Drachman D, Folstein M, et al. Clinical Diagnosis of Alzheimer's Disease: Report of the Nincds-Adrda Work Group under the Auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 15.Boyle PA, Wilson RS, Aggarwal NT, et al. Parkinsonian Signs in Subjects with Mild Cognitive Impairment. Neurology. 2005;65:1901–1906. doi: 10.1212/01.wnl.0000188878.81385.73. [DOI] [PubMed] [Google Scholar]
- 16.Buchman AS, Boyle PA, Wilson RS, et al. Physical Activity and Motor Decline in Older Persons. Muscle Nerve. 2007;35:354–362. doi: 10.1002/mus.20702. [DOI] [PubMed] [Google Scholar]
- 17.Buchman AS, Wilson RS, Boyle PA, et al. Motor Function and Mortality in Older Persons. Journal of the American Geriatrics Society. 2007;55:11–19. doi: 10.1111/j.1532-5415.2006.01032.x. [DOI] [PubMed] [Google Scholar]
- 18.Louis ED, Tang MX, Schupf N, et al. Functional Correlates and Prevalence of Mild Parkinsonian Signs in a Community Population of Older People. Arch Neurol. 2005;62:297–302. doi: 10.1001/archneur.62.2.297. [DOI] [PubMed] [Google Scholar]
- 19.Onder G, Penninx BW, Lapuerta P, et al. Change in Physical Performance over Time in Older Women: The Women's Health and Aging Study. J Gerontol A Biol Sci Med Sci. 2002;57:M289–293. doi: 10.1093/gerona/57.5.m289. [DOI] [PubMed] [Google Scholar]
- 20.Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and Donepezil for the Treatment of Mild Cognitive Impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 21.Bedlack RS, Strittmatter WJ, Morgenlander JC. Apolipoprotein E and Neuromuscular Disease: A Critical Review of the Literature. Arch Neurol. 2000;57:1561–1565. doi: 10.1001/archneur.57.11.1561. [DOI] [PubMed] [Google Scholar]
- 22.Zeger SL, Liang KY, Albert PS. Models for Longitudinal Data: A Generalized Estimating Equation Approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]
- 23.Collett D. 2nd Ed. Chapman & Hall; Boca Raton , Floridia: 2003. Modelling Survival Data in Medical Research. [Google Scholar]
- 24.SAS . Sas/Stat® User's Guide, Version 8. 8 Ed SAS Institute Inc; Cary, NC: 2000. [Google Scholar]
- 25.Aggarwal NT, Wilson RS, Beck TL, et al. The Apolipoprotein E Epsilon4 Allele and Incident Alzheimer's Disease in Persons with Mild Cognitive Impairment. Neurocase. 2005;11:3–7. doi: 10.1080/13554790490903038. [DOI] [PubMed] [Google Scholar]
- 26.Lehericy S, Bardinet E, Tremblay L, et al. Motor Control in Basal Ganglia Circuits Using Fmri and Brain Atlas Approaches. Cerebral Cortex. 2006;16:149–161. doi: 10.1093/cercor/bhi089. [DOI] [PubMed] [Google Scholar]
- 27.Burke RE, editor. Motor Units: Anatomy, Physiology and Functional Organization. American Physiological Society; Washington, D.C.: 1981. [Google Scholar]
- 28.Poppele R, Bosco G. Sophisticated Spinal Contributions to Motor Control. Trends Neurosci. 2003;26:269–276. doi: 10.1016/S0166-2236(03)00073-0. [DOI] [PubMed] [Google Scholar]
- 29.Schneider JA, Bienias JL, Wilson RS, et al. The Apolipoprotein E {Epsilon}4 Allele Increases the Odds of Chronic Cerebral Infarction Detected at Autopsy in Older Persons. Stroke. 2005;36:954–959. doi: 10.1161/01.STR.0000160747.27470.2a. [DOI] [PubMed] [Google Scholar]
- 30.Rosano C, Brach J, Longstreth JWT, et al. Quantitative Measures of Gait Characteristics Indicate Prevalence of Underlying Subclinical Structural Brain Abnormalities in High-Functioning Older Adults. Neuroepidemiology. 2006;26:52–60. doi: 10.1159/000089240. [DOI] [PubMed] [Google Scholar]
- 31.Schneider JA, Li JL, Li Y, et al. Substantia Nigra Tangles Are Related to Gait Impairment in Older Persons. Ann Neurol. 2006;59:166–173. doi: 10.1002/ana.20723. [DOI] [PubMed] [Google Scholar]
- 32.Wolf DS, Gearing M, Snowdon DA, et al. Progression of Regional Neuropathology in Alzheimer Disease and Normal Elderly: Findings from the Nun Study. Alzheimer Dis Assoc Disord. 1999;13:226–231. doi: 10.1097/00002093-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Burns JM, Galvin JE, Roe CM, et al. The Pathology of the Substantia Nigra in Alzheimer Disease with Extrapyramidal Signs. Neurology. 2005;64:1397–1403. doi: 10.1212/01.WNL.0000158423.05224.7F. [DOI] [PubMed] [Google Scholar]
- 34.Horoupian DS, Wasserstein PH. Alzheimer's Disease Pathology in Motor Cortex in Dementia with Lewy Bodies Clinically Mimicking Corticobasal Degeneration. Acta Neuropathol (Berl) 1999;98:317–322. doi: 10.1007/s004010051087. [DOI] [PubMed] [Google Scholar]
- 35.Formiga F, Alia P, Navarro MA, et al. Apolipoprotein E Genotypes in Nonagenarians. Journal of the American Geriatrics Society. 2006;54:1471–1473. doi: 10.1111/j.1532-5415.2006.00867.x. [DOI] [PubMed] [Google Scholar]
- 36.Bathum L, Christiansen L, Jeune B, et al. Apolipoprotein E Genotypes: Relationship to Cognitive Functioning, Cognitive Decline, and Survival in Nonagenarians. Journal of the American Geriatrics Society. 2006;54:654–658. doi: 10.1111/j.1532-5415.2005.53554.x. [DOI] [PubMed] [Google Scholar]
- 37.Asada T, Yamagata Z, Kinoshita T, et al. Prevalence of Dementia and Distribution of Apoe Alleles in Japanese Centenarians: An Almost-Complete Survey in Yamanashi Prefecture, Japan. J Am Geriatr Soc. 1996;44:151–155. doi: 10.1111/j.1532-5415.1996.tb02431.x. [DOI] [PubMed] [Google Scholar]