The ECG is one of the oldest and most versatile noninvasive cardiac diagnostic tests. It has remained in use essentially in its original form despite dramatic advances in cardiac electrophysiology. In May 1887, Augustus Desiré Waller recorded the first human Electrogram using a primitive instrument called a Libbmann capillary electrometer. It had 2 deflections corresponding to ventricular depolarization and repolarization.1 In 1903, Willem Einthoven invented the String Galvanometer—a more sophisticated voltage recording instrument and recorded an Elektrokardiogramm with 5 deflections that he named PQRST.2
Since its initial invention, the body surface ECG has become a commonly used and extremely valuable test for the diagnosis of a variety of cardiac conditions. Despite a century of prolific use and intensive investigation, the cellular basis of ECG waveforms, particularly the T wave, remains a matter of debate.
Anecdote of the T wave
The saga of the T wave began in 1856, when 2 German physiologists Kölliker and Müller attempted to explore the electric activity of the heart using frog sciatic nerve attached to gastroenemius muscle as a voltage recording instrument and observed 2 contractions (see review by Noble and Cohen3). In retrospect, the second “contraction” probably reflected a voltage gradient related to the T wave of Einthoven. In 1883, Burdon-Sanderson and Page4 were the first to demonstrate that in the frog’s heart, the wave of excitation spreads from the base to the apex of the ventricle. The record was diphasic, with the first positive (R) wave followed by a negative (T) wave. They also demonstrated that the T wave corresponds to repolarization of the ventricle. Similar series of experiments by Bayliss and Starling in 18925 in the canine heart showed that the T waves are usually upright in mammals. This was followed by Mines6 on frog’s heart using Einthoven’s String Galvanometer in 1913. Mines stated that the T wave is an electric expression of asymmetrical passing off of excitation (repolarization) in different parts of the ventricles and that the polarity of the T wave can be predicted by changing the duration of the action potential in a controlled manner. In 1931, Wilson et al7 put forward the hypothesis that the concordance of the T wave with the R wave on the surface ECG can be explained by assuming that the depolarization and the repolarization waves must travel in opposite directions at least in some parts of the ventricle. The biophysical principle behind this hypothesis requires that the cells that activate first have to repolarize last to produce a T wave of the same polarity of that of the R wave. In other words, the action potential duration (APD) in the cells that are excited first should be significantly longer than the cells that are excited last.
In an attempt to explain the positive polarity of the T wave in mammals, Noble and coworkers8 in 1976 recorded action potentials from tissue slices dissected from the “base” and the “apex” of the sheep ventricle and showed that at steady state, APD is longer at the “base” as compared with the “apex.” They concluded that such a difference could account for a positive polarity of the T wave in mammals. This series of experiments laid down the foundation of the controversial hypothesis that apico-basal difference in the time course of repolarization underlies the genesis of a positive T wave. The theory of apico-basal dispersion of repolarization as a basis of the electrocardiographic T wave dominated the medical literatures until the 1990s when the discovery of M cells9,10 and the development of the left ventricular wedge technique11–14 suggested that the transmural dispersion of repolarization may contribute importantly to the genesis of the T wave.
Second Look at History
Noble’s work is frequently cited as supporting the role of an apico-basal voltage gradient in the genesis of the T wave.8 It is important to note that the tissue slices used for recording the action potential were dissected from the endocardial surface of the base and the epicardial surface of the apex of the sheep’s ventricle. In Noble’s own words, “Small pieces of tissue were dissected from 2 regions of sheep left ventricle (LV)—the upper part (base) of the interventricular septum and the epicardial surface of the apex. The former is endocardial and the latter is epicardial.” In other words, the tissue slices from the “base” represented the endocardium and from the “apex” represented the epicardium. Hence, the voltage gradient seen in Noble’s study though frequently cited as apico-basal could very well have been transmural (between endocardium and epicardium).
According to the biophysical principle of impulse propagation, a positive T wave in sheep would require earlier activation of basal cells (longer APD) as compared with apex (shorter APD). However, mapping studies in mammals have shown that depolarization in the ventricles spreads from the apex to the base, and the base is usually the last area to be activated.15–17 Earlier activation of the apical cells combined with a late activation of the basal cells would lead to a negative T wave, which is not the usual case in mammals. Recently, Noble18 himself has acknowledged the presence of transmural repolarization gradient and its possible role in the inscription of the T wave.
Transmural Voltage Gradient: Unrecognized Historical Evidence
Although it was not the predominant hypothesis before 1990s, multiple studies had suggested the presence of a transmural voltage gradient, and its contribution to the genesis of the T wave. In 1959, Reynolds and Vander Ark19 demonstrated that in the canine heart, earlier repolarization of epicardium as compared with endocardium was associated with a positive T wave and vice versa. In 1972 Burgess et al20 showed that the ventricular functional refractory periods are longer on the endocardium than epicardium and apex as compared with the base. Using the glass microelectrode technique, Solberg et al21 in 1974 recorded transmembrane action potentials from tissue slices obtained from the canine papillary muscle. They demonstrated a transmural gradient of repolarization with the subendocardial cells having the longest duration of action potential followed by the endocardial and epicardial cells. Similar evidence that earlier repolarization occurs in the canine epicardium was obtained by Spach and Barr15 and Abildskov22 in 1975.
In an elegant experimental model of an open chest canine heart, in 1984, Higuchi and Nakaya et al23 recorded monophasic action potentials from the endocardial and the epicardial surface of the LV with a simultaneous recording of an epicardial-unipolar ECG in close proximity. They found a high degree of correlation between the transmural APD gradient and the polarity of T wave. In their experiments, warming the epicardial surface by dropping warm physiological saline solution led to abbreviation of epicardial APD with a resultant increase in transmural APD gradient, which manifested itself as an increase in the amplitude of the upright T wave on the ECG. In contrast, cooling of the epicardial surface led to prolongation of the epicardial APD with a reversal of transmural repolarization gradient, manifested as negative T waves.
This classic work was followed by a study by Franz et al24 in the human heart. He recorded monophasic action potential from the endocardial and epicardial surfaces of the human heart and showed that epicardial repolarization occurs earlier than the endocardium leading to a transmural repolarization gradient.
M Cell and the Left Ventricular Wedge Preparation
The discovery of the M cell (Masonic, Midmyocardial, Moe cell)9,11 has significantly advanced our understanding of the transmural voltage gradient. An increasing number of studies have shown that the ventricular myocardium is not a homogeneous structure, but it is comprised of 3 distinct cell types, ie, epicardial, subendocardial and endocardial cells, with clearly distinct electrophysiological and pharmacological properties. The M cells are located in deep subendocardium of the canine LV.9,10 The hallmark of M cells is the ability of its action potential to prolong more than that of the epicardium or endocardium in response to slowing of the rate or in response to agents that prolong APD.
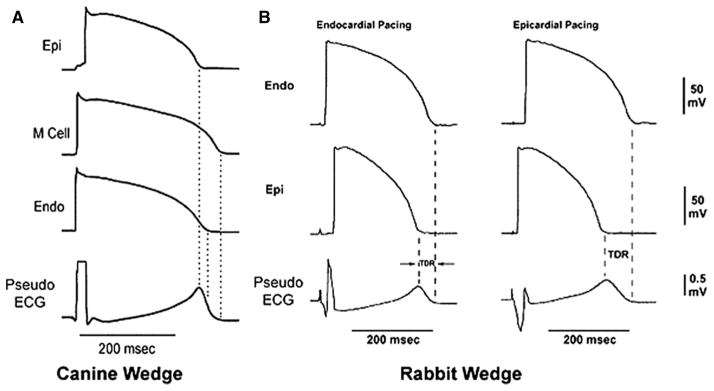
The development of the canine and rabbit left ventricular wedge models has greatly advanced our understanding the role of electric heterogeneity in the genesis of the repolarization waves of the ECG.12,13 This experimental preparation provides us with the unique ability to record transmembrane action potentials from 3 different myocardial cell types simultaneously together with a pseudo-ECG. Because the pseudo-ECG is recorded by placing 2 electrodes in the Tyrode’s solution (ie, a homogenous volume conductor) bathing the wedge preparation 1 to 1.5 cm from the epicardial and endocardial surfaces, the electric field of the preparation as a whole is measured. We use the pseudo-ECG to distinguish it from the body surface ECG, although both recordings are principally similar. The simultaneous recordings of transmembrane action potentials and the pseudo-ECG provides us with ability to correlate changes in APD with those in ECG morphology. Using the wedge preparation, we have demonstrated that differences in the time course of repolarization of the 3 predominant myocardial cell types contribute to electrocardiographic inscription of the T wave in pseudo-ECG.13 As shown in Figure 1, the different time course of repolarization of phases 2 and 3 of the 3 principle myocardial cell types gives rise to opposing voltage gradients on either side of the subendocardial M cells, leading to the inscription of the T wave. In the case of an upright T wave, the epicardial cells repolarize earliest and the M cells repolarize last. Final repolarization of the epicardial cells coincides with the peak of the T wave and final repolarization of the M cells coincides with the end of the T wave.25,26 Thus, the M cell APD approximates the QT interval and the epicardial APD approximates the QTpeak interval. The interval from the peak of T wave to the end of T wave (Tp-e) correlates closely with transmural dispersion of repolarization (TDR). Also, the polarity of the T wave can be changed by changing the APD in a controlled manner.
Figure 1.
A, The cellular basis of the T wave in the canine left ventricular wedge preparation. Simultaneous recordings of action potential with of 3 different cell type with a pseudo-ECG are shown. Note that the opposing voltage gradient on either side of the M region leads to inscription of the T wave. The peak of the T wave coincides with the end of repolarization of the epicardial cell and the end of the T wave coincides with the end of repolarization of the M cells. B, The cellular basis of the T wave in the rabbit left ventricular wedge. In the rabbit, the entire endocardium has the same electrophysiological properties as M cells. Therefore, the end of the T wave coincides with the end of repolarization of the endocardium in the rabbit. Epicardial pacing leads to earlier activation of epicardial cells with shorter APD and delays the activation of endocardial cells with a longer APD, leading to an increase in QT and Tp-e interval. Epi, Epicardium; Endo, Endocardium; AP, action potential. Modified and reproduced with permission from Circulation. 1998;98:1928–1936.
Is there an Apico-Basal Voltage Gradient in the LV?
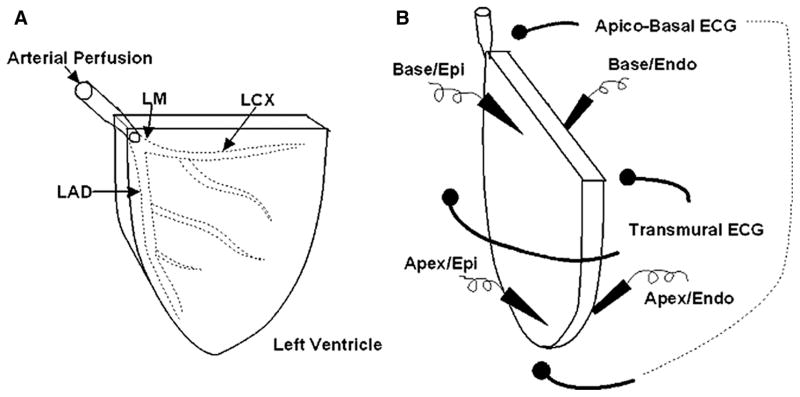
It has been argued that the canine left ventricular wedge is too small a sample and does not reflect what might happen in an intact heart.27 This prompted us to develop an experimental model where we use the intact rabbit LV instead of a wedge. In this model, we tested the relative role of the transmural versus the apico-basal voltage gradient in the genesis of the T wave. We elected to use the rabbit LV instead of the dog for several reasons. First, as shown in Figure 2A, the isolated rabbit LV is perfused by cannulating the left main coronary artery that allows us to use the complete left ventricular tissue in the experiment and it ensures that the preparation is well perfused. Because it includes the whole ventricle it allows us to record the transmural and the apico-basal voltage gradient simultaneously. Secondly, cells with electric resemblance to the M cells are located in the endocardium of the rabbit LV,28,29 so that it is technically easier to simultaneously record action potentials from the endocardium and epicardium. The action potential recordings obtained from intact epicardial and endocardial surface eliminates the possibility of injury and uncoupling due to dissection. Some have proposed that transmural dispersion of repolarization seen in the small canine wedge preparation may be related to injury and uncoupling of the cells because the action potential is recorded from a transected subendocardial surface.30,31 Realistically the cut surface constitutes only an extremely small fraction of the entire wedge preparation that should have little influence on the pseudo-ECG. In any case, the arterially-perfused rabbit LV is devoid of such a confounding factor.
Figure 2.
A, Schematic presentation of the arterially perfused rabbit LV. LM, left main coronary artery; LAD, left anterior descending coronary artery; LCD, left circumflex coronary artery. B, Schematic presentation of simultaneous recording of 4 sets of action potentials: apical epicardium, apical endocardium, basal epicardium and basal endocardium together with 2 pseudo-ECGs: transmural and apico-basal. Epi, epicardium; Endo, endocardium.
The arterially perfused rabbit LV was prepared in a similar way as the rabbit left ventricular wedge.28,32–34 To accomplish this, as shown in Figure 2B, we recorded the endocardial and the epicardial transmembrane action potentials simultaneously from the apical and the basal areas of the rabbit LV. Two pseudo-ECGs were recorded: one recorded transmurally and another across the apico-basal axis of the left ventricular wall. It is important again to emphasize that the pseudo-ECGs were recorded in the volume conductor in which the electrodes were placed at least 2 cm from the epicardial and endocardial surfaces, or from the apex and base of the isolated LV. After control recording at a basic cycle length of 1000 ms, 0.1 μmol/L cisapride at was infused to determine if it has any preferential effects on epicardial/endocardial or apical/basal cell action potential.
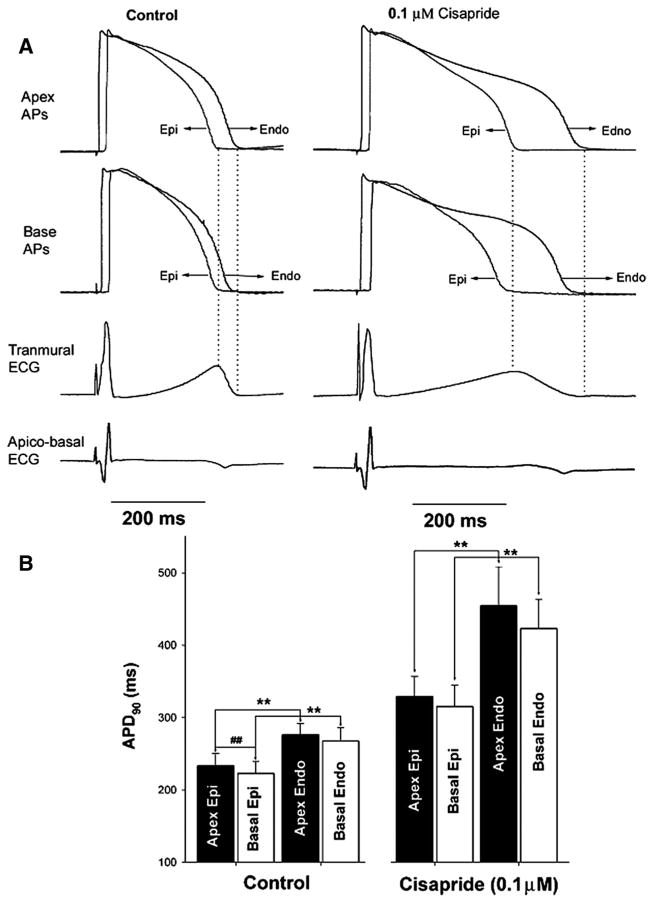
Representative recordings from these experiments are shown in Figure 3A. Under control conditions, the APD of apical cells (both endocardial and epicardial surfaces) was slightly longer than the APD of the respective basal cells; however, the difference was not statistically significant. On the other hand, both at the apical and the basal recording sites, the APD of the endocardial cells were consistently longer than the epicardial cells and the results were statistically significant. This difference in the time course of repolarization of the epicardial and endocardial cells, ie, the transmural dispersion of repolarization, caused the inscription of the T wave in the pseudo-ECG recorded across the transmural axis. In both apical and basal recording sites, the peak of the T wave was coincident with the end of repolarization of the epicardium and the end of T wave was coincident with the end of repolarization of the endocardium. In contrast, the pseudo-ECG recorded along the apico-basal axis had T waves that were either flat or negative.
Figure 3.
A, A representative recording from the rabbit LV showing well-defined T waves in pseudo-ECGs recorded across the transmural axis under control conditions and after infusion of cisapride. Note that the pseudo-ECG recorded across the apico-basal axis fails to reveal any T waves, and there is no correlation between changes in APD and ECG morphology. B, Composite data showing similar findings in 4 similar experiments. Note that APD was slightly longer at the apex as compared with the base; however, the difference is not as pronounced as seen between the endocardium and the epicardium. AP, action potential; Epi, epicardium; Endo, endo- cardium; APD90, APD at 90% repolarization. **P<0.05 compared between endocardium vs epicardium, ##P<0.05 compared between apex vs base.
After infusion of the IKr blocker cisapride, the QT interval prolonged with a preferential prolongation of APD of the endocardial cells as compared with the epicardial cells across the entire LV, leading to an increase in TDR and a corresponding increase in the duration of descending limb of the T wave, ie, the Tp-e interval. Cisapride increased the APD of the apical cells slightly more than the basal cells; however the observed difference was not statistically significant. Once again, the apico-basal pseudo-ECG showed nonspecific T wave morphologies and we were unable to identify any corelation between the morphological changes in action potential and the pseudo-ECG. Findings were reproduced in 4 similar experiments. Composite data are shown in Figure 3B.
The canine left ventricular wedge spans 5 cm of the LV across the apico-basal axis. A decade of experience with this preparation confirmed that there is no significant dispersion of repolarization across the apico-basal axis in such a small segment of LV.13 Does this hold true in an entire canine LV? This issue was elegantly addressed in recent work by Rosenbaum and coworkers35 in canine hearts using optical mapping technique. In this study investigating the physiological basis of T wave memory, 3 wedge preparations were used from anterior, lateral, and posterior surface of canine LV, respectively. Action potentials were recorded with optical mapping technique from all 3 wedge preparations. The dispersion of repolarization was calculated across the transmural axis of each wedge (ie, TDR) and also between 2 LV wedges (ie, segmental dispersion of repolarization). The transmural pseudo-ECG was calculated by subtracting the action potentials from epicardial cells and M cells. The segmental ECG was calculated by subtracting action potentials of M cells recorded from 2 different LV wedges/segments after accounting for known activation time between segments. The results showed the presence of TDR in all 3 LV wedges, but there was no significant segmental dispersion of repolarization. Both the measured and the calculated transmural Pseudo-ECGs showed upright T wave that matched the polarity of in vivo ECG. However, the calculated segmental ECG failed to register any T wave. These observations outlined above, clearly demonstrate the prominent role of TDR in the electrocardiographic inscription of the T wave, both in the rabbit and canine LV.
Clinical Evidence for Transmural Dispersion of Repolarization
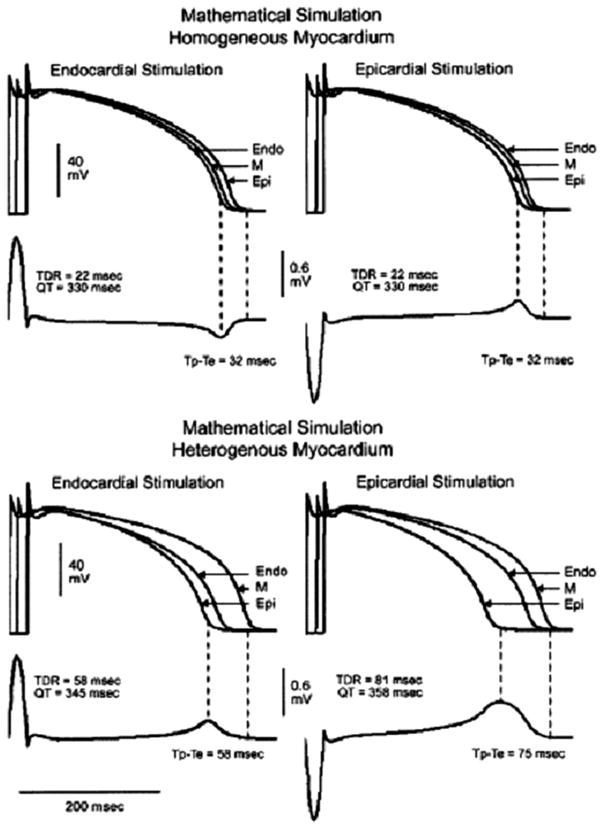
Figure 4 presents a mathematical model in which the ECG was calculated first from a transmural linear array of cells in which repolarization is assumed to be homogeneous and then from a more physiologically-relevant model in which repolarization is heterogeneous.36 In the homogeneous myocardium, the R wave and T wave would be always of opposite polarity and reversal of direction of activation does not have any effect on the QT interval, QRS duration or Tp-e interval. In such a case, the T wave is merely a reflection of the transmural conduction time. In contrast, a transmural repolarization gradient in the heterogeneous myocardium would produce a positive T wave which has the same polarity as the R wave.
Figure 4.
Mathematical modeling of homogeneous and heterogeneous myocardium. Reproduced with permission from J Am Coll Cardiol. 2005;46:2340–2347.
However, clinical observations do not agree with the result of a homogeneous model. Fish et al36 studied the effect of reversing the direction of activation of the left ventricular wall in patients with heart failure (Figure 5). The epicardium of the left ventricular free wall was paced at cycle length of 600 ms through the epicardial electrode of a resynchronization device followed by pacing from the endocardial aspect of the LV free wall by an endovascular catheter at the same cycle length. The effects of epicardial and endocardial pacing on the QT interval and Tp-e interval was measured in lead V6 on body surface ECG. Lead V6 was selected because it was closest to the site of pacing and because it can record the voltage across the transmural axis of the left ventricular wall. A representative recording from such an experiment is shown in Figure 5A. If the assumption that the myocardium is electrically homogeneous is correct, the R and the T wave should always be of opposite polarity and the reversal of direction of activation of the ventricle should not have any effect on QT and Tp-e interval. This is clearly not the case (Figure 5A)36 Reversing the direction of activation of the left ventricular wall by shifting from endocardial to epicardial stimulation caused an increase in the amplitude and width of the T wave, a prolongation of QT interval and a significant increase on Tp-e.33,37 This behavior is also observed in the canine and rabbit left ventricular wedge preparation33,37 (Figure 1) and is predicted by the mathematical model illustrated in Figure 4 to be the result of transmural heterogeneities of repolarization time course.
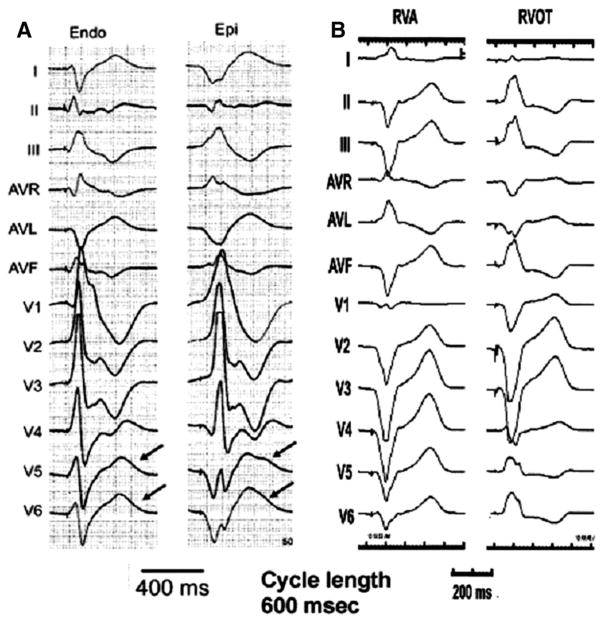
Figure 5.
Twelve lead ECGs recorded from human subjects paced from different regions of the ventricular myocardium. A, Changing the ventricular pacing site from endocardium to epicardium increases the QT and Tp-e interval (modified and reproduced with permission from J Am Coll Cardiol. 2005;46:2340–2347). B, Changing the ventricular pacing site from RVA to RVOT has no effect on QT or Tp-e interval.
Do the above findings hold true for the apico-basal voltage gradient? In other words, if apico-basal dispersion of repolarization contributes importantly to the genesis of the T wave, then pacing the ventricle from the apex followed by the base should affect the QT and the Tp-e interval in a predictable fashion. To answer this question, we analyzed the ECGs obtained during electrophysiological (EP) study in 5 patients in whom the right ventricle was paced from the apex (RVA) and the outflow tract (RVOT) at a basic cycle length of 600 ms. The QT and Tp-e intervals were calculated during RVA and RVOT pacing. We selected chest lead V2 and limb lead II for evaluation. Chest lead V2 was selected since it measures transmural gradient closest to the site of pacing, and limb lead II was selected because it samples across the apico-basal axis of the heart. As shown in Figure 5B, changing the pacing site from RVA to RVOT did not have any effect on the QT and the Tp-e interval in lead V2 or lead II. The composite data of 5 patients showed that the QT interval remained unchanged (385±16.4 RVA pacing versus 384±13.7 RVOT pacing, p=NS) as did the Tp-e interval (85±3.5 RVA pacing versus 84±3.7 RVOT pacing, p=NS). Changing the pacing site from the RVA to RVOT led to the reversal of the polarity of the T wave in the apico-basal axis in lead II. In contrast, the polarity of the T wave in transmural axis lead V2 remained unchanged.
These observations demonstrate the prominent role of TDR over apico-basal dispersion of repolarization in the genesis of T wave. It is important to emphasize that these data do not exclude the presence of apico-basal dispersion of repolarization under some conditions. Several lines of evidence support apico-basal heterogeneity of repolarization. For example, Bauer et al38 have demonstrated that in the canine heart in vivo, the effective refractory period is longer in the epicardial muscle layer of the apex then in the base and on administration of dofetilide apico-basal dispersion of repolarization becomes more pronounced with preferential increase in the refractory period at the apex. Similarly, Janse et al39 reported shortest repolarization times in anterobasal areas and longest repolarization times in posteroapical regions of intact canine LV using unipolar electrograms. Also in enzymatically isolated myocytes from rabbit heart, APD is longer in myocytes isolated from the base as compared with the apex.40
Although the apico-basal dispersion of repolarization does exist, such dispersion is much smaller as compared with transmural dispersion. Also, apico-basal dispersion occurs over a much longer distance as compared with transmural dispersion. It is the steepness of the repolarization gradient that is thought to serve as a key factor for arrhythmogenesis and not the total magnitude of dispersion. That is, a small amount of dispersion of repolarization over a long distance will yield a less steep repolarization gradient as in the case of apico-basal repolarization gradient and is less likely to be arrhythmogenic. The transmural dispersion of repolarization is not only more pronounced as compared with apico-basal dispersion, but it also occurs over a much shorter distance resulting in a very steep electric gradient that in our opinion is likely to influence not only the inscription of the T wave but also contribute to arrhythmogenesis.
Conclusions
The presence of an important transmural repolarization gradient in ventricle has been well established in a variety of species, including man. Although apico-basal voltage gradients may contribute, we believe that the transmural voltage gradient plays a predominant role in the genesis of the T wave. The wedge preparation, like any animal model, has limitations, but it has a remarkable ability to reproduce the electrocardiographic and arrhythmic manifestations of a wide variety of syndromes observed in clinical practice. This model coupled with in vivo data from humans and mathematical simulations of the myocardium provide compelling evidence in support of a predominant role for the transmural voltage gradient in the genesis of the T wave. Dispersion of repolarization, irrespective of its precise geometry, has been shown to serve as the substrate for reentry under a variety of conditions. It is clear that the T wave corresponds to repolarization of the ventricle and it is an electric manifestation of a normally asymmetrical repolarization process. An important electrocardiographic parameter obtained from body surface ECG, the Tp-e interval, has been shown to be a predictor of arrhythmias under a variety of clinical conditions.41–44 Although the exact mechanism for the genesis of the Tp-e interval on the surface ECG remains a matter of some controversy, its clinical utility as an index of arrhythmogenesis is gaining recognition.45,46
Acknowledgments
Source of Funding
Supported by the Sharpe-Strumia Research Foundation (G.-X.Y.), the Albert M. Greenfield Foundation (P.R.K. and G.-X.Y.), the W.W. Smith Charitable Trust (P.R.K.), HL47678 from NHLBI (C.A.), and Masons of New York State and Florida.
Footnotes
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association. This article is Part I of a 2-part article. Part II appears on page 89.
Disclosure
None.
References
- 1.Waller AD. A demonstration on man of electromotive changes accompanying the heart’s beat. J Physiol. 1887;8:229–234. doi: 10.1113/jphysiol.1887.sp000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Einthoven W. Die galvanometrische registerung des menschlichen elektrokardiogram: zugleich eine beurtheilung der anwendung des capillar-elektrometers in der physiologie. Pflügers Arch ges Physiol. 1903;1903:472–480. [Google Scholar]
- 3.Noble D, Cohen I. The interpretation of the T wave of the electrocardiogram. Cardiovasc Res. 1978;12:13–27. doi: 10.1093/cvr/12.1.13. [DOI] [PubMed] [Google Scholar]
- 4.Burdon-Sanderson JS, Page FJM. On the electrical phenomena of the excitatory process in the heart of the frog and of the tortoise, as investigated photographically. J Physiol. 1883;4:327–338. doi: 10.1113/jphysiol.1884.sp000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bayliss WM, Starling EH. On the electromotive phenomena of the mammalian heart. Monthly Int J Anat Physiol. 1892:256–281. [Google Scholar]
- 6.Mines GR. On functional analysis by the action of electrolytes. J Physiol. 1913;46:188–235. doi: 10.1113/jphysiol.1913.sp001588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson FN, Macleod AG, Barker PS. The T deflection of the electrocardiogram. Trans Assoc Am Physicians. 1931;46:29–38. [Google Scholar]
- 8.Cohen I, Giles W, Noble D. Cellular basis for the T wave of the electrocardiogram. Nature. 1976;262:657–661. doi: 10.1038/262657a0. [DOI] [PubMed] [Google Scholar]
- 9.Sicouri S, Antzelevitch C. A subpopulation of cells with unique electrophysiological properties in the deep subepicardium of the canine ventricle. The M cell. Circ Res. 1991;68:1729–1741. doi: 10.1161/01.res.68.6.1729. [DOI] [PubMed] [Google Scholar]
- 10.Sicouri S, Fish J, Antzelevitch C. Distribution of M cells in the canine ventricle. J Cardiovasc Electrophysiol. 1994;5:824–837. doi: 10.1111/j.1540-8167.1994.tb01121.x. [DOI] [PubMed] [Google Scholar]
- 11.Antzelevitch C, Shimizu W, Yan GX, Sicouri S, Weissenburger J, Nesterenko VV, Burashnikov A, Di DJ, Saffitz J, Thomas GP. The M cell: its contribution to the ECG and to normal and abnormal electrical function of the heart. J Cardiovasc Electrophysiol. 1999;10:1124–1152. doi: 10.1111/j.1540-8167.1999.tb00287.x. [DOI] [PubMed] [Google Scholar]
- 12.Yan GX, Antzelevitch C. Cellular basis for the electrocardiographic J wave. Circulation. 1996;93:372–379. doi: 10.1161/01.cir.93.2.372. [DOI] [PubMed] [Google Scholar]
- 13.Yan GX, Antzelevitch C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long-QT syndrome. Circulation. 1998;98:1928–1936. doi: 10.1161/01.cir.98.18.1928. [DOI] [PubMed] [Google Scholar]
- 14.Yan GX, Shimizu W, Antzelevitch C. Characteristics and distribution of M cells in arterially perfused canine left ventricular wedge preparations. Circulation. 1998;98:1921–1927. doi: 10.1161/01.cir.98.18.1921. [DOI] [PubMed] [Google Scholar]
- 15.Spach MS, Barr RC. Ventricular intramural and epicardial potential distributions during ventricular activation and repolarization in the intact dog. Circ Res. 1975;37:243–257. doi: 10.1161/01.res.37.2.243. [DOI] [PubMed] [Google Scholar]
- 16.Arisi G, Macchi E, Baruffi S, Spaggiari S, Taccardi B. Potential fields on the ventricular surface of the exposed dog heart during normal excitation. Circ Res. 1983;52:706–715. doi: 10.1161/01.res.52.6.706. [DOI] [PubMed] [Google Scholar]
- 17.Ramanathan C, Ghanem RN, Jia P, Ryu K, Rudy Y. Noninvasive electrocardiographic imaging for cardiac electrophysiology and arrhythmia. Nat Med. 2004;10:422–428. doi: 10.1038/nm1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noble D. Modeling the heart–from genes to cells to the whole organ. Science. 2002;295:1678–1682. doi: 10.1126/science.1069881. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds EW, Jr, Vander Ark CR. An experimental study on the origin of T-wave based on determination of effective refractory period from epicaradial and endocardial aspect of the ventricle. Circ Res. 1959;7:943–949. [Google Scholar]
- 20.Burgess MJ, Green LS, Millar K, Wyatt R, Abildskov JA. The sequence of normal ventricular recovery. Am Heart J. 1972;84:660–669. doi: 10.1016/0002-8703(72)90181-0. [DOI] [PubMed] [Google Scholar]
- 21.Solberg LE, Singer DH, Ten Eick RE, Duffin EG., Jr Glass microelectrode studies on intramural papillary muscle cells. Description of preparation and studies on normal dog papillary muscle. Circ Res. 1974;34:783–797. doi: 10.1161/01.res.34.6.783. [DOI] [PubMed] [Google Scholar]
- 22.Abildskov JA. The sequence of normal recovery of excitability in the dog heart. Circulation. 1975;52:442–446. doi: 10.1161/01.cir.52.3.442. [DOI] [PubMed] [Google Scholar]
- 23.Higuchi T, Nakaya Y. T wave polarity related to the repolarization process of epicardial and endocardial ventricular surfaces. Am Heart J. 1984;108:290–295. doi: 10.1016/0002-8703(84)90614-8. [DOI] [PubMed] [Google Scholar]
- 24.Franz MR, Bargheer K, Rafflenbeul W, Haverich A, Lichtlen PR. Monophasic action potential mapping in human subjects with normal electrocardiograms: direct evidence for the genesis of the T wave. Circulation. 1987;75:379–386. doi: 10.1161/01.cir.75.2.379. [DOI] [PubMed] [Google Scholar]
- 25.Antzelevitch C. The role of spatial dispersion of repolarization in inherited and acquired sudden cardiac death syndromes. Am J Physiol Heart Circ Physiol. 2007;293:H2024–H2038. doi: 10.1152/ajpheart.00355.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antzelevitch C. Heterogeneity and cardiac arrhythmias: an overview. Heart Rhythm. 2007;4:964–972. doi: 10.1016/j.hrthm.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Opthof T, Coronel R, Janse MJ, Rosen MR. A wedge is not a heart. Heart Rhythm. 2007;4:1116–1119. [Google Scholar]
- 28.Yan GX, Wu Y, Liu T, Wang J, Marinchak RA, Kowey PR. Phase 2 early afterdepolarization as a trigger of polymorphic ventricular tachycardia in acquired long-qt syndrome: direct evidence from intracellular recordings in the intact left ventricular wall. Circulation. 2001;103:2851–2856. doi: 10.1161/01.cir.103.23.2851. [DOI] [PubMed] [Google Scholar]
- 29.Yan GX, Rials SJ, Wu Y, Liu T, Xu X, Marinchak RA, Kowey PR. Ventricular hypertrophy amplifies transmural repolarization dispersion and induces early afterdepolarization. Am J Physiol Heart Circ Physiol. 2001;281:H1968–H1975. doi: 10.1152/ajpheart.2001.281.5.H1968. [DOI] [PubMed] [Google Scholar]
- 30.Anyukhovsky EP, Sosunov EA, Rosen MR. Regional differences in electrophysiological properties of epicardium, midmyocardium, and endocardium. In vitro and in vivo correlations. Circulation. 1996;94:1981–1988. doi: 10.1161/01.cir.94.8.1981. [DOI] [PubMed] [Google Scholar]
- 31.Anyukhovsky EP, Sosunov EA, Gainullin RZ, Rosen MR. The controversial M cell. J Cardiovasc Electrophysiol. 1999;10:244–260. doi: 10.1111/j.1540-8167.1999.tb00667.x. [DOI] [PubMed] [Google Scholar]
- 32.Liu T, Brown BS, Wu Y, Antzelevitch C, Kowey PR, Yan GX. Blinded validation of the isolated arterially perfused rabbit ventricular wedge in preclinical assessment of drug-induced proarrhythmias. Heart Rhythm. 2006;3:948–956. doi: 10.1016/j.hrthm.2006.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medina-Ravell VA, Lankipalli RS, Yan GX, Antzelevitch C, Medina-Malpica NA, Medina-Malpica OA, Droogan C, Kowey PR. Effect of epicardial or biventricular pacing to prolong QT interval and increase transmural dispersion of repolarization. Does resynchronization therapy pose a risk for patients predisposed to long QT or torsade de pointes? Circulation. 2003;107:740–746. doi: 10.1161/01.cir.0000048126.07819.37. [DOI] [PubMed] [Google Scholar]
- 34.Wang D, Patel C, Cui C, Yan GX. Preclinical assessment of drug-induced proarrhythmias: role of the arterially perfused rabbit left ventricular wedge preparation. Pharmacol Ther. 2008;119:141–151. doi: 10.1016/j.pharmthera.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Jeyaraj D, Wilson LD, Zhong J, Flask C, Saffitz JE, Deschenes I, Yu X, Rosenbaum DS. Mechanoelectrical feedback as novel mechanism of cardiac electrical remodeling. Circulation. 2007;115:3145–3155. doi: 10.1161/CIRCULATIONAHA.107.688317. [DOI] [PubMed] [Google Scholar]
- 36.Fish JM, Brugada J, Antzelevitch C. Potential proarrhythmic effects of biventricular pacing. J Am Coll Cardiol. 2005;46:2340–2347. doi: 10.1016/j.jacc.2005.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fish JM, Di Diego JM, Nesterenko VV, Antzelevitch C. Epicardial activation of left ventricular wall prolongs QT interval and transmural dispersion of repolarization: implications for biventricular pacing. Circulation. 2004;109:2136–2142. doi: 10.1161/01.CIR.0000127423.75608.A4. [DOI] [PubMed] [Google Scholar]
- 38.Bauer A, Becker R, Karle C, Schreiner KD, Senges JC, Voss F, Kraft P, Kuebler W, Schoels W. Effects of the I(Kr)-blocking agent dofetilide and of the I(Ks)-blocking agent chromanol 293b on regional disparity of left ventricular repolarization in the intact canine heart. J Cardiovasc Pharmacol. 2002;39:460–467. doi: 10.1097/00005344-200203000-00018. [DOI] [PubMed] [Google Scholar]
- 39.Janse MJ, Sosunov EA, Coronel R, Opthof T, Anyukhovsky EP, de Bakker JM, Plotnikov AN, Shlapakova IN, Danilo P, Jr, Tijssen JG, Rosen MR. Repolarization gradients in the canine left ventricle before and after induction of short-term cardiac memory. Circulation. 2005;112:1711–1718. doi: 10.1161/CIRCULATIONAHA.104.516583. [DOI] [PubMed] [Google Scholar]
- 40.Cheng J, Kamiya K, Liu W, Tsuji Y, Toyama J, Kodama I. Heterogeneous distribution of the two components of delayed rectifier K+ current: a potential mechanism of the proarrhythmic effects of methanesulfonanilide class III agents. Cardiovasc Res. 1999;43:135–147. doi: 10.1016/s0008-6363(99)00061-9. [DOI] [PubMed] [Google Scholar]
- 41.Shimizu M, Ino H, Okeie K, Yamaguchi M, Nagata M, Hayashi K, Itoh H, Iwaki T, Oe K, Konno T, Mabuchi H. T-peak to T-end interval may be a better predictor of high-risk patients with hypertrophic cardiomyopathy associated with a cardiac troponin I mutation than QT dispersion. Clin Cardiol. 2002;25:335–339. doi: 10.1002/clc.4950250706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Topilski I, Rogowski O, Rosso R, Justo D, Copperman Y, Glikson M, Belhassen B, Hochenberg M, Viskin S. The morphology of the QT interval predicts torsade de pointes during acquired bradyarrhythmias. J Am Coll Cardiol. 2007;49:320–328. doi: 10.1016/j.jacc.2006.08.058. [DOI] [PubMed] [Google Scholar]
- 43.Watanabe N, Kobayashi Y, Tanno K, Miyoshi F, Asano T, Kawamura M, Mikami Y, Adachi T, Ryu S, Miyata A, Katagiri T. Transmural dispersion of repolarization and ventricular tachyarrhythmias. J Electrocardiol. 2004;37:191–200. doi: 10.1016/j.jelectrocard.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Yamaguchi M, Shimizu M, Ino H, Terai H, Uchiyama K, Oe K, Mabuchi T, Konno T, Kaneda T, Mabuchi H. T wave peak-to-end interval and QT dispersion in acquired long QT syndrome: a new index for arrhythmogenicity. Clin Sci (Lond) 2003;105:671–676. doi: 10.1042/CS20030010. [DOI] [PubMed] [Google Scholar]
- 45.Antzelevitch C. Role of spatial dispersion of repolarization in inherited and acquired sudden cardiac death syndromes. Am J Physiol Heart Circ Physiol. 2007;293:H2024–H2038. doi: 10.1152/ajpheart.00355.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gupta P, Patel C, Patel H, Narayanaswamy S, Malhotra B, Green JT, Yan GX. Tp-e/QT ratio as an index of arrhythmogenesis. J Electrocardiol. 2008;41:567–574. doi: 10.1016/j.jelectrocard.2008.07.016. [DOI] [PubMed] [Google Scholar]