Abstract
Copper is an essential metal that is able to produce reactive oxygen species and induce intracellular oxidative stress. Several studies have examined the effects of excessive copper and oxidative stress in various organisms and tissues, but few address the molecular mechanisms by which copper affects transcription. Our results demonstrated that in COS-7 cells, copper treatment caused an increase in the binding of nuclear proteins to AP-1 and antioxidant response elements. The level of copper-inducible nuclear protein binding was modulated by increasing or decreasing the level of intracellular oxidative stress. Copper exposure also led to an increase in the steady state levels of c-fos, c-jun, and c-myc mRNAs. Exposure to copper resulted in an increase in the levels of phosphorylation and activation of the c-Jun N-terminal kinase/stress-activated protein kinase and p38 pathways. The activation of these pathways resulted in a concomitant increase in c-Jun phosphorylation. We investigated the hypothesis that copper-induced oxidative stress leads to the formation of stable lipid peroxidation by-products, which activate MAPK pathways; ultimately affecting transcription. While exposure did result in the production of HNE, the timing of the increased levels of proto-oncogene mRNA, phosphorylation of c-jun, and phosphorylation and activation of MAPKs, as well as the inability of the lipophilic antioxidant vitamin E to abrogate MAPK phosphorylation suggests that the formation of stable lipid peroxidation by-products may not be the primary mechanism by which copper activates MAKKs. These results further elucidate the effects of copper on signal transduction pathways to alter gene expression.
Keywords: copper, transcription, MAPK, signal transduction, reactive oxygen species, p38, JNK/SAPK, AP-1
INTRODUCTION
The transition metal copper is an essential trace element that functions as a catalytic co-factor in a number of enzymes due to its redox properties. The same redox properties that contribute to copper’s biological activity also make it cytotoxic; when present in excess of cellular requirements. The intracellular, cytotoxic effects associated with copper exposure are consistent with oxidative damage of lipids, proteins and nucleic acids. Copper, via Fenton-like reactions, is able to catalyze the formation of reactive oxygen species (ROS) 1, which results in intracellular oxidative stress. Oxidative stress induces the transcription of a variety of genes involved in the detoxification of ROS, or in the repair of ROS induced damage 1; 2; 3; 4; 5. The transcription of these genes is regulated in part by antioxidant response elements 6. The ARE sequence is similar to the consensus sequence of the activating protein-1 (AP-1) binding site 6. AP-1 is a group of related, heterodimers of basic-leucine zipper proteins consisting of Jun, Fos and ATF family proteins 7. Genes regulated by AP-1, or via AREs, are in turn controlled by mitogen-activated protein kinase (MAPK) signaling cascades. The three MAPK pathways; ERK, JNK/SAPK and p38; transduce intra-and extra-cellular stimuli, which lead to diverse cellular responses 8; 9; 10; 11; 12. The ERK pathway typically responds to growth factor signals, which leads to cell differentiation or proliferation 13. In contrast, the JNK/SAPK and p38 pathways respond to cytokines and cellular stress; resulting in the transcriptional activation of genes involved in stress responses, growth arrest or apoptosis 12; 14; 15; 16.
Exposure to metals has been shown to activate components of MAPK signaling cascades 17. Once activated, MAPKs can phosphorylate a number of proteins involved in regulating the transcription of a multitude of genes. Increases in AP-1 binding, c-jun and c-fos mRNA expression, and c-Jun phosphorylation have been observed in cells exposed to arsenic, vanadium, and chromium 18; 19; 20; 21; 22; 23. The transcriptional activities of AP-1 or ARE binding proteins are also affected by a other stressors 24; 25; 26. Exposure of cells to HNE, a toxic by-product of lipid peroxidation, results in an increase in JNK/SAPK and p38 phosphorylation, AP-1 activity, and c-jun expression 27.
Copper has the potential to mediate several important biological processes through multiple signal transduction pathways; however the cellular and molecular mechanisms underlying copper-regulated gene expression are poorly understood. We have previously shown; using metallothionein-1 and rat NAD(P)H:oxidoreductase 1-based reporter genes; that copper activates transcription via both a metal and antioxidant responsive pathways 28. Modulation of ROS levels, either by depleting glutathione or by exposing cells to oxygen scavengers (aspirin, vitamin E) enhanced or attenuated the level of copper inducible transcription, respectively. Studies using Protein Kinase C and mitogen-activated protein kinase inhibitors and MTF-1 null cells clearly demonstrated independent roles for both metals and ROS in the regulation of copper-inducible transcription. Here we report that copper is capable of activating MAPK signal transduction pathways and increasing proto-oncogene expression.
RESULTS
Effect of Copper on Proto-oncogene and MT mRNA Levels
Real time quantitative RT-PCR was used to measure changes in the steady state level of MT-1, c-jun, c-fos, and c-myc mRNAs in response to copper exposure (Table 1). The levels of MT, c-jun, c-fos, and c-myc mRNAs significantly increased after the 4 h exposure (p < 0.004, < 0.001, < 0.001, and < 0.001, respectively). In contrast, the levels of expression were not significantly different from that of the non-treated, control cells for any of the genes after 24 h (p = 0.12, 0.74, 0.059, and 0.12, respectively).
TABLE 1.
Effect of copper on the steady-state levels of MT and proto-oncogene mRNAs.
| Exposure Time (h) |
|||
|---|---|---|---|
| Gene | 0 | 4 | 24 |
| MT-1 | 1.00 +/− 0.62 | 3.51 +/− 0.53* | 1.73 +/− 0.60 |
| c-fos | 1.00 +/− 0.96 | 28.84 +/− 7.06* | 2.08 +/− 0.91 |
| c-jun | 1.00 +/− 0.46 | 30.48 +/− 5.73* | 8.82 +/− 2.09 |
| c-myc | 1.00 +/− 0.66 | 16.22 +/− 2.02* | 2.06 +/− 0.53 |
COS-7 cells were exposed to 400 ìM copper for 0, 4 or 24 h. Differences in mRNA expression were detected by Real Time RT-PCR.
indicates significantly different from untreated cells by ANOVA, p<0.05, n=3 observations.
Effect of Copper on AP-1 Binding Activity
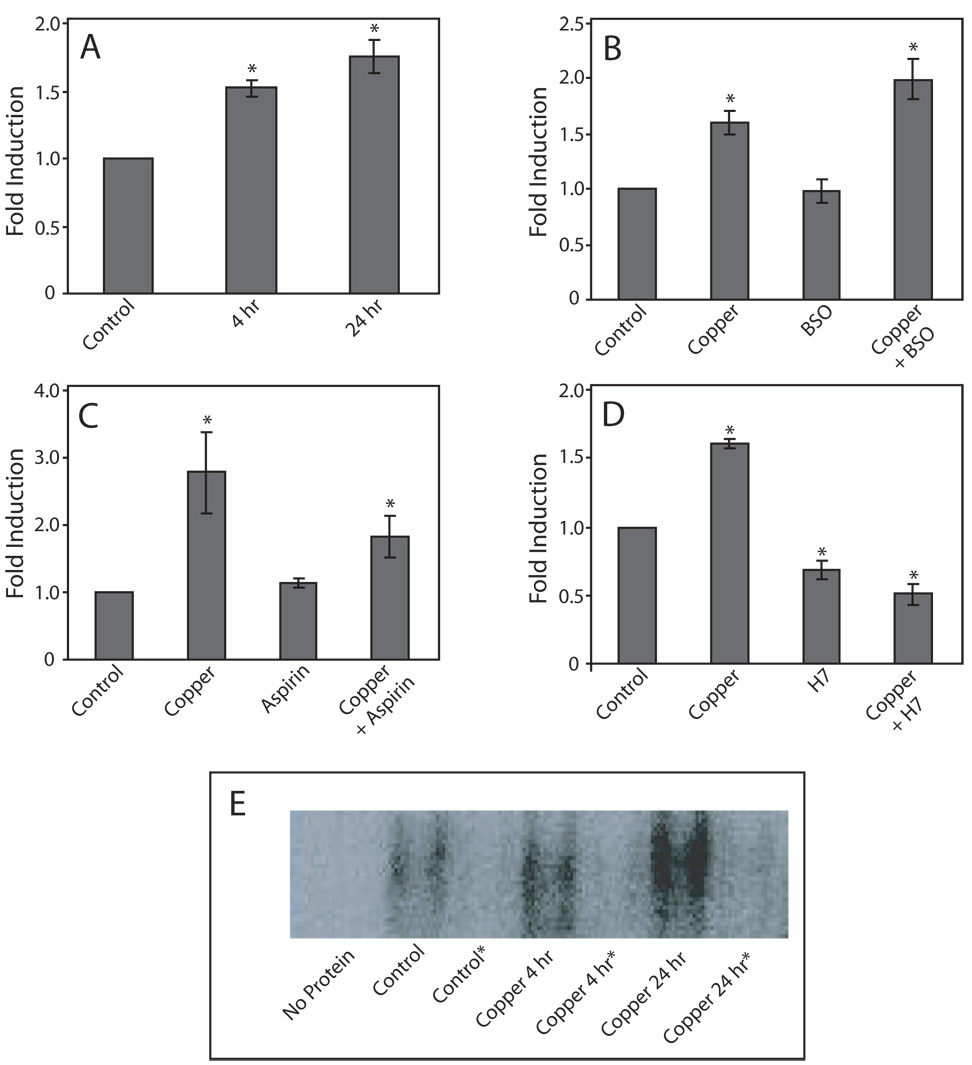
Binding of nuclear proteins to the AP-1 consensus sequence in response to copper exposure significantly increased after treatment with 400 µM copper for 4 h and 24 h (p < 0.002 and p < 0.0001, respectively), compared to untreated control cells (Fig. 1A). AP-1 binding after 24 h was not significantly different than that seen after 4 h.
Figure 1. Effect of copper on protein binding to AP-1 elements.
COS-7 cells were exposed to 400 µM copper 4 or 24 h (panel A). To increase intracellular oxidative stress, cells were pretreated for 8 h with BSO prior to exposure to copper (panel B). To decrease the levels of intracellular oxidative stress, cells were pre-treated for 6 h with aspirin prior to copper treatment (panel C). In PKC inhibition experiments, cells were treated with H7 for 30 min prior to exposure to copper (panel D). Data are expressed as mean fold induction +/− S.E.M in comparison to untreated controls. * indicates significantly different from untreated controls by ANOVA, p < 0.05, n = 3 observations. Panel E, Representative EMSA of the data presented in panel A, *indicates reactions that contained excess non-radiolabeled AP-1 oligonucleotide.
To increase the level of copper-inducible, intracellular oxidative stress, glutathione was depleted by pre-treating cells with BSO 28. The level of protein binding to the AP-1 consensus sequence, induced by a 24-h exposure to copper was significantly greater when cells were treated with BSO (p < 0.02), compared to cells exposed to copper alone (Fig. 1B). Treatment with BSO alone did not cause a significant change in binding, compared to controls (p = 0.89).
To protect cells from oxidative stress, aspirin was added to cells prior to copper exposure 29. Aspirin treatment caused a significant decrease in the level of copper-inducible protein binding, compared to cells exposed to only copper (Fig. 1C; p < 0.03). However, the level of protein binding was still significantly greater that that observed in non-treated cells (p < 0.03). Treatment with aspirin alone did not cause a significant change in AP-1 binding (p = 0.70).
Pretreatment with the PKC inhibitor H7 completely blocked copper-induced AP-1 binding (Fig. 1D; p < 0.0001). Treatment with H7 alone, or H7 plus copper resulted in AP-1 binding that was significantly lower than that observed in untreated cells (p < 0.0001). In all studies, the addition of a 50-fold molar excess non-labeled AP-1 oligonucleotide to the reaction mixtures blocked protein binding to the 32P-abeled, AP-1-containing oligonucleotide (Fig. 1E)
Effect of Copper on Antioxidant Response Element Binding Activity
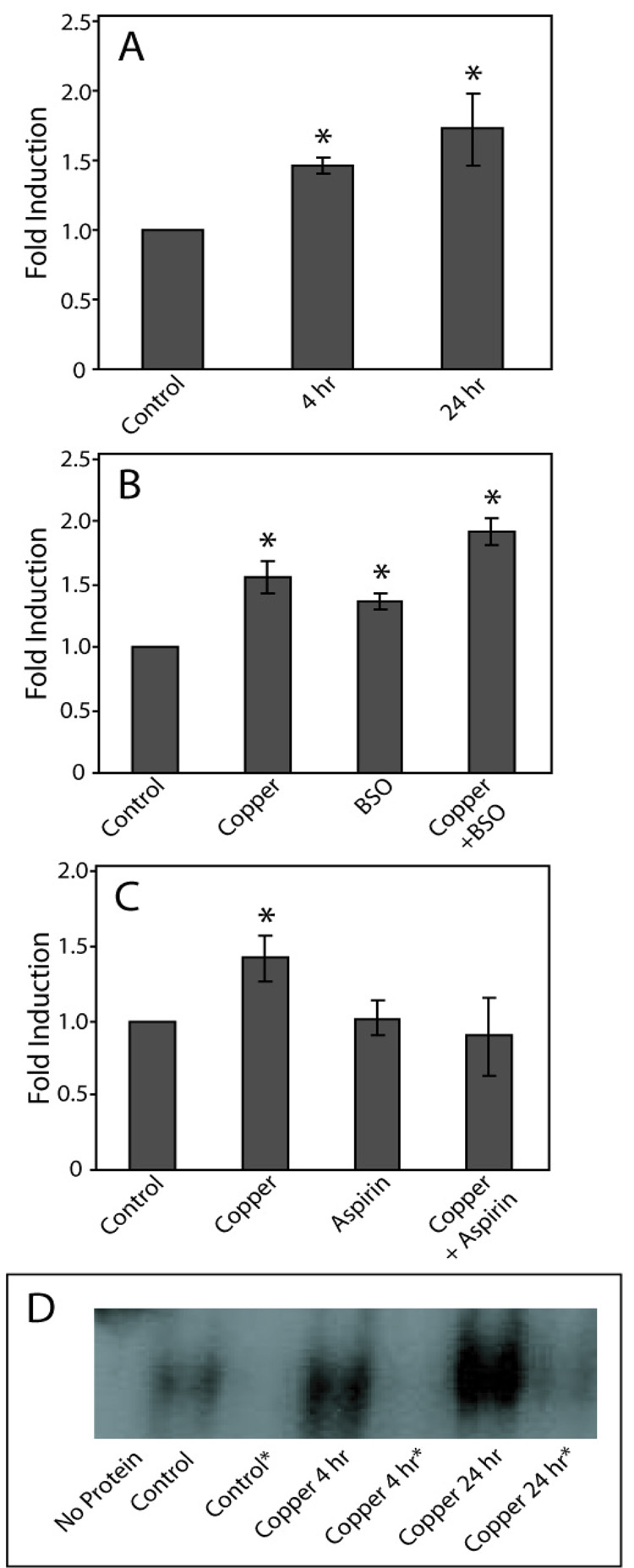
Protein binding to the NAD(P)H:quinone oxidoreductase 1 ARE sequence by nuclear proteins significantly increased after treatment with 400 µM copper for 4 h (p < 0.05) and 24 h (p < 0.005), compared to untreated cells (Fig. 2A). The level of binding between cells exposed to copper for 4 h and 24 h was not significantly different. Following glutathione depletion, ARE binding induced by a 24 h exposure to copper significantly increased, compared to that observed with copper alone (Fig. 2B; p < 0.02). Treatment with BSO alone also caused a significant increase in protein binding to the ARE (p < 0.02). However, the level of binding was significantly lower than that observed following treatment with copper plus BSO (p < 0.001). Treatment with aspirin prior to copper exposure caused a significant decrease in the level of copper-inducible protein binding to AREs, compared to treatment with copper alone (Fig. 2C; p < 0.03). In contrast to what was observed using the AP-1 containing oligonucleotide, aspirin reduced ARE binding to a level that was not significantly different from untreated cells (p = 0.94). Treatment with aspirin did not cause a significant change in AP-1 binding, compared to untreated cells (p = 0.70). In all studies, the addition of 50-fold molar excess non-labeled ARE containing oligonucleotide to the reaction mixtures blocked protein binding to the 32P-labeled probe (Fig. 2D).
Figure 2. Effect of copper on binding to AREs.
COS-7 cells were exposed to 400 µM copper 4 or 24 h, and EMSA performed as described (panel A). In BSO experiments cells were pretreated with BSO prior to the addition of copper (panel B). In aspirin experiments, cells were pre-treated with aspirin prior to copper exposure (panel C). Data are expressed as mean fold induction +/− S.E.M. in comparison to untreated controls. * indicates significantly different from untreated controls by ANOVA, p < 0.05, n = 3 observations. Panel D, Representative EMSA of the data presented in panel A, *indicates reactions that contained excess non-radiolabeled ARE-containing oligonucleotide.
Effect of Copper on the Levels of c-Jun Phosphorylation
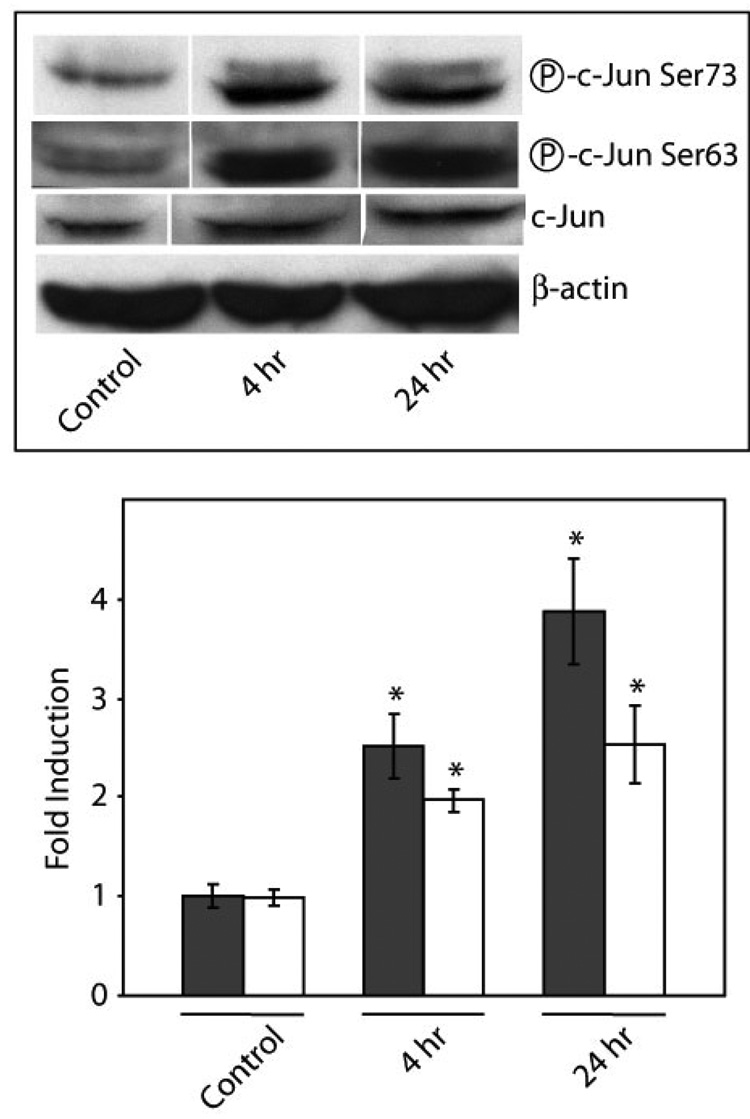
Exposure of COS-7 cells to 400 µM copper for 4 h and 24 h resulted in significant increases in the levels of c-Jun phosphorylation at Ser63 and Ser73 (Fig. 3). Phosphorylation at Ser63 increased 2.5 fold (p < 0.03) and 3.9 fold (p < 0.002) after exposure to copper for 4 h and 24 h, respectively. Phosphorylation at Ser73 increased by 2.0-fold (p < 0.04) and 2.6-fold (p < 0.005) after 4 h and 24 h exposures, respectively.
Figure 3. Effect of copper on c-Jun phosphorylation.
COS-7 cells were exposed to 400 µM copper 4 h or 24 h. Upper Panel; representative Western immunoblots of phosphorylated and non-phosphorylated c-Jun. Lower Panel; the levels of c-Jun phosphorylation at Ser63 (solid bars) and Ser73 (open bars) was measured using anti-phospho-Ser63 and anti-phospho-Ser73 c-Jun antibodies, respectively. Data are expressed as mean fold induction +/− S.E.M. in comparison to untreated controls. * indicates significantly different from untreated controls by ANOVA, p < 0.05, n = 3 observations.
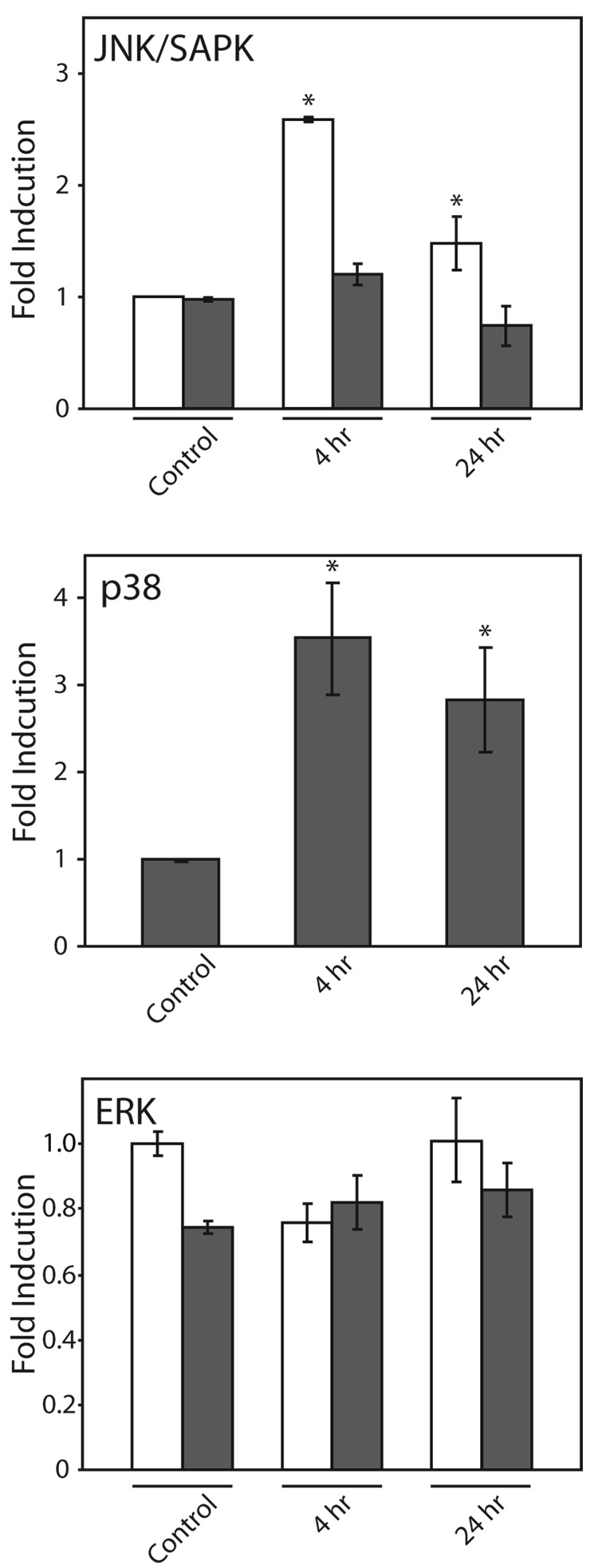
Effect of Copper on the Levels of JNK/SAPK, p38, and ERK Phosphorylation
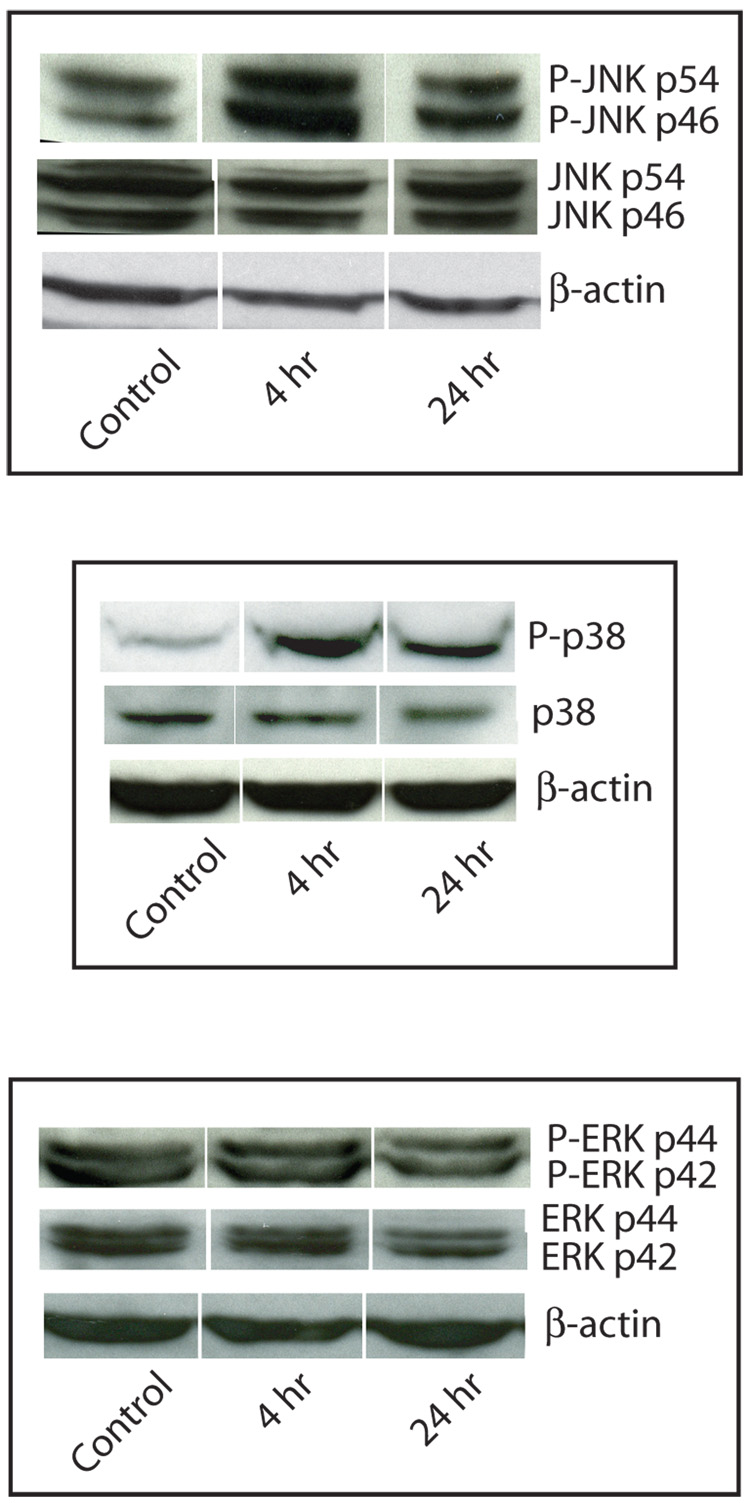
Phosphorylation of the p54 isoform of JNK/SAPK significantly increased: 2.6-fold (p < 0.001) after 4 h exposure, and 1.5-fold after 24 h exposure to copper (p < 0.05) (Fig. 4A and 4B). The level of phosphorylation of the JNK/SAPK p46 isoform did not significantly change in response to copper. Phosphorylation of p38 significantly increased after 4 h and 24 h exposure to 400 µM copper: 3.5-fold (p < 0.004) and 2.8-fold (p < 0.03), respectively (Fig. 4 A and 4B). The levels of phosphorylation of the two isoforms of ERK, p42 and p44, did not significantly change following copper treatment (Fig. 4A and 4B).
Figure 4. Effect of copper on MAP kinase phosphorylation.
COS-7 cells were exposed to 400 µM copper 4 h or 24 h. A. Representative Western immunoblots of phosphorylated and non-phosphorylated JNK/SAPK (upper panel), p38 (middle panel), and ERK(lower panel). B. Changes in the phosphorylation of JNK/SAPK (upper panel) p46 (solid bars) and p54 (open bars) isoforms, p38 (middle panel), and ERK(lower panel); p42 (solid bars) and p44 (open bars) isoforms were measured by Western immunoblot analysis. Data are expressed as mean fold induction +/− S.E.M. in comparison to untreated controls. * indicates significantly different from untreated cells by ANOVA, p < 0.05, n = 3 observations.
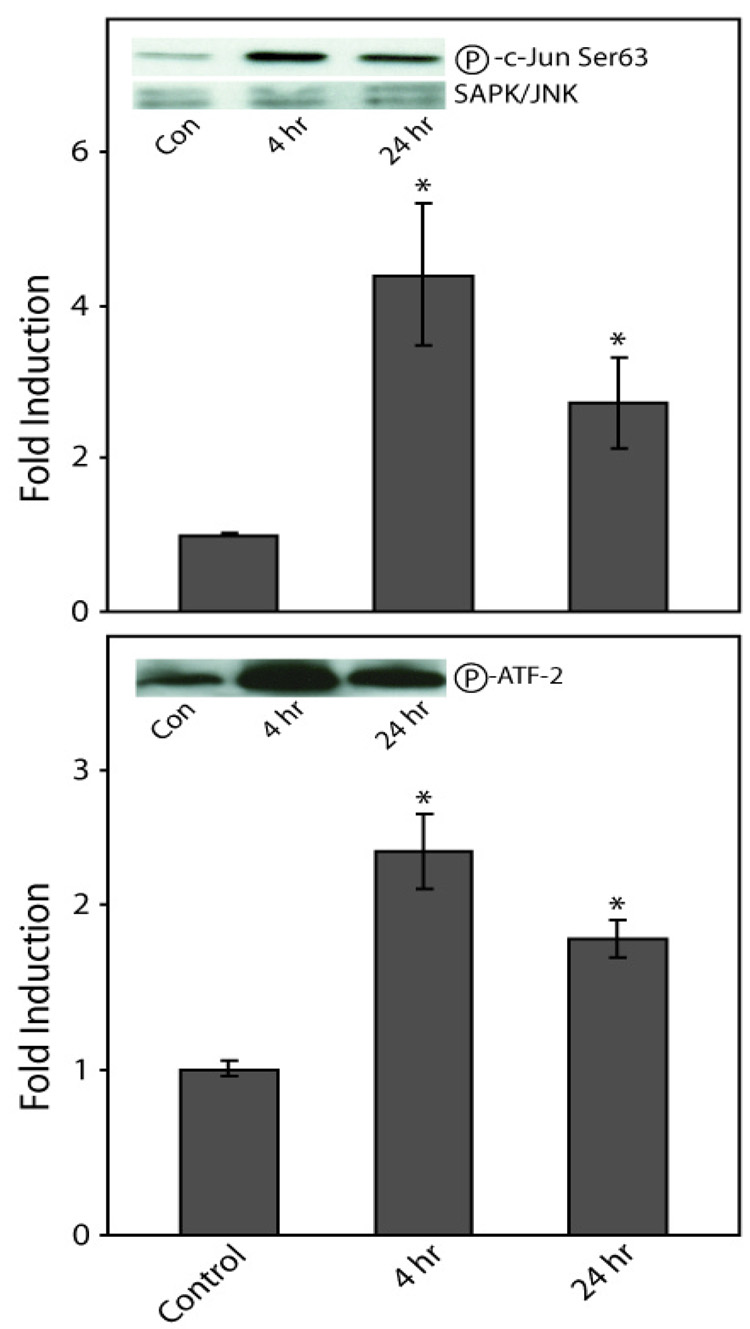
Effect of Copper on MAPK Activity
To assess the effect of copper on the activities of MAPKs, JNK/SAPK and p38 kinase, activity assays were performed. JNK/SAPK activity, as determined by changes in the levels of phosphorylation of c-Jun at Ser63, significantly increased following 4 h and 24 h exposures to copper: 4.4-fold (p < 0.03) and 2.7-fold (p < 0.02), respectively (Fig. 5). p38 kinase activity, as determined by changes in the levels of ATF-2 phosphorylation, significantly increased; 2.3-fold (p < 0.001) after 4 h and by 1.8-fold (p < 0.02) after 24 h (Fig. 5). The decrease in JNK/SAPK and p38 kinase activity between 4 h and 24 h exposures to copper are consistent with observed differences in the levels of JNK/SAPK and p38 phosphorylation observed above (Fig. 4).
Figure 5. Effect of copper on MAP kinase activity.
COS-7 cells were exposed to 400 µM copper for 4 h or 24 h. Changes in kinase activity were measured by the time dependent changes in the level of phosphorylation for target substrates c-Jun (Ser63) for JNK/SAPK (upper panel) and ATF-2 for p38 (lower panel). Data are expressed as mean fold induction +/− S.E.M. in comparison to untreated controls. * indicates significantly different from untreated cells by ANOVA, p < 0.05, n = 3 observations. Insets, representative Western immunoblots of target substrates for SAPK/JNK and P38.
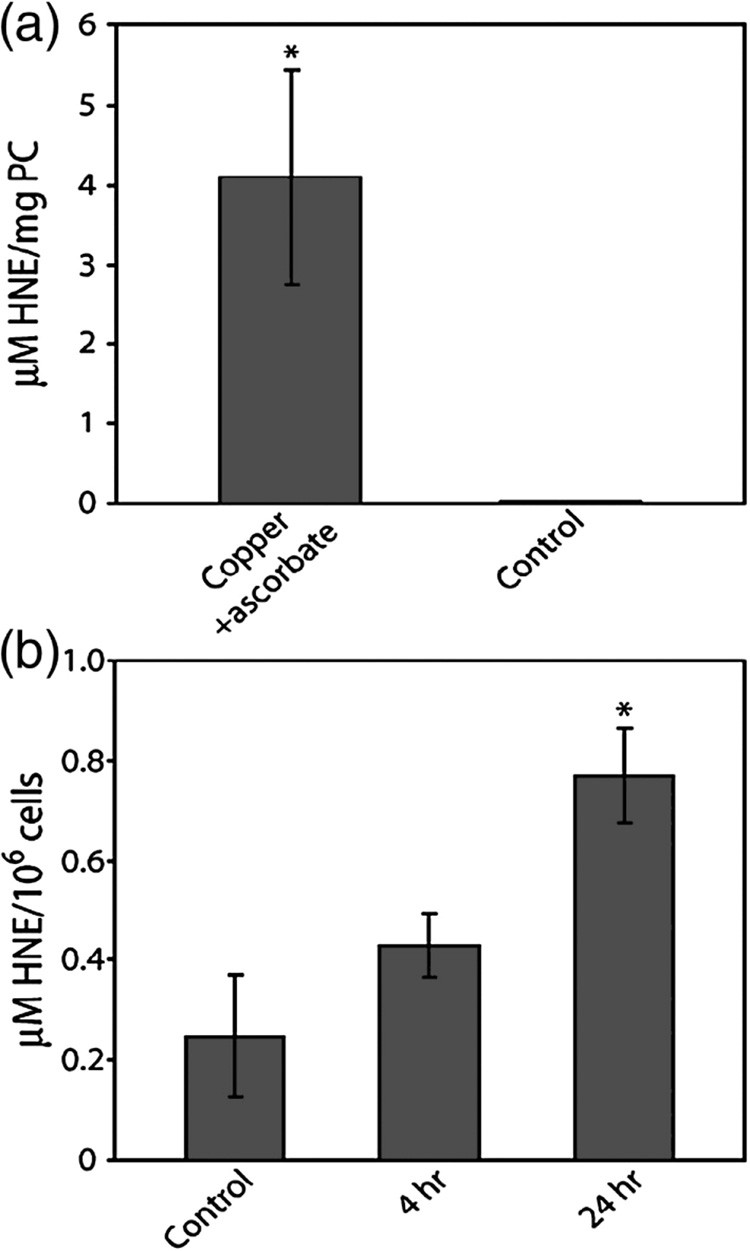
Effect of Copper on Lipid Peroxidation in Cells
Experiments were performed to evaluate the ability of copper to produce HNE as a result of lipid peroxidation. In control experiments using phosphatidylcholine vesicles, a 24 h exposure to copper resulted in significant increases in HNE (p < 0.001) (Fig. 6A). The amount of HNE produced in cells exposed to copper for 24 h was greater than controls (Fig 6B). The increase in HNE observed after 4 h exposure was not significantly different than the untreated controls. In cells exposed to copper, the amount of MDA was not significantly different than the level observed in untreated cells (data not shown)
Figure 6. Copper-induced 4-hydroxy-2-nonenal production.
The amount of HNE produced in a reaction containing phosphatidylcholine vesicles (1 mg/ml) that were incubated for 24 h with 100 µM copper and 1 mM ascorbate (upper panel). COS-7 cells were incubated for 4 or 24 h with 400 µM copper and the levels of HNE determined (lower panel). Data are expressed as mean fold induction +/− S.E.M. in comparison to untreated controls. * indicates significantly different from untreated cells by ANOVA, p < 0.05, n = 3 observations.
Effect of Vitamin E on Copper-Induced Increases in c-Jun and MAPK Phosphorylation
Copper exposure increased the levels of HNE in COS-7 cells. This suggests that copper may activate JNK/SAPK and p38 through the formation of HNE. We tested whether a 2 h pre-treatment with the lipophillic antioxidant vitamin E followed by a 4 or 24 h co-exposure with 400 µM copper could abrogate phosphorylation of c-Jun, JNK/SAPK or p38. In all cases there was a slight decrease in the level of phosphorylation associated with vitamin E-co-exposures (Table 2). To test the level of significance for these differences, ANOVA on all of the data points (n=100) were performed. The p-value for a difference in fold change in vitamin E treated cells vs. non-treated samples was 0.07. Thus, vitamin E may not significantly affect the level of phosphorylation of c-Jun or MAPKs at either 4 or 24 h.
TABLE 2.
Effect of vitamin E on copper-induced changes in c-Jun and MAPK phosphorylation
| Exposure Time (h) | Vitamin E | Fold Change Average a | |
|---|---|---|---|
| c-jun-63 | 4 | − | 7.28 ± 3.10 b |
| + | 4.77 ±1.66 | ||
| 24 | − | 3.66 ±1.91 | |
| + | 2.94 ±1.50 | ||
| c-jun-73 | 4 | − | 2.04 ±0.25 |
| + | 1.77 ±0.21 | ||
| 24 | − | 1.62 ±0.20 | |
| + | 1.72 ±0.77 | ||
| ERK-42 | 4 | − | 1.31 ±0.17 |
| + | 1.37 ±0.31 | ||
| 24 | − | 1.27 ±0.28 | |
| + | 1.36 ±0.38 | ||
| ERK-44 | 4 | − | 1.80 ±0.34 |
| + | 1.92 ±0.53 | ||
| 24 | − | 1.28 ±0.28 | |
| + | 1.30 ±0.43 | ||
| JNK-46 | 4 | − | 6.27 ±2.76 |
| + | 6.04 ±2.71 | ||
| 24 | − | 2.07 ±0.38 | |
| + | 1.65 ±0.60 | ||
| JNK-54 | 4 | − | 4.09 ±0.62 |
| + | 3.46 ±0.67 | ||
| 24 | − | 1.90 ±0.35 | |
| + | 1.82 ±0.63 | ||
| p38 | 4 | − | 3.03 ±0.82 |
| + | 2.39 ±0.35 | ||
| 24 | − | 1.54 ±0.34 | |
| + | 1.28 ±0.53 |
Relative to treatment-matched controls
Mean ± standard mean error
DISCUSSION
Copper is a persistent environmental toxicant, and concentrations of copper in excess of 1 mM 30 and as high as 90 mM have been found in surface waters 31. In aquatic and invertebrate species copper is extremely toxic 32; 33; 34; 35; 36; 37; 38; 39. Exposing invertebrate larva to elevated levels of copper has also been shown to affect development 40; 41; 42. Indian childhood cirrhosis is hepatic disorder that has an undetermined genetic component, but is believed to be caused by the consumption of milk boiled in copper kettles. There are reported cases of hepatic copper concentrations in excess of 6 mg/g dry weight in children with this disorder 43.
Although a variety of pathophysiological changes are associated with copper exposure, the mechanism(s) by which this metal induces these changes is not well understood. In this report a model is proposed in which copper induces intracellular oxidative stress that subsequently activates MAPK signaling pathways. The role of ROS in mediating the ability of copper to activate MAPK signaling pathways was previously demonstrated using Protein Kinase C, p38, ERK and JNK inhibitors 28. In addition, modulating ROS levels affects copper toxicity and its ability to activate transcription 28; 29. The current observations that modulating the levels of intracellular copper-induced oxidative stress alters the levels of protein binding to AP-1 and ARE (Fig. 1 and Fig 2) further demonstrates a role for the ROS-mediated activation of MAPK signaling pathways. Similar effects have been observed in cells exposed to other redox-active metals, and agents that cause intracellular oxidative stress 20; 23. For example, hydrogen peroxide can induce the expression of c-jun, c-fos, c-myc and MT-1, as well as AP-1 reporter gene activity 4; 44.
Copper exposure causes an increase in the phosphorylation and activation of both p38 and JNK/SAPK in COS-7 cells, but has no effect on ERK1/2 (Fig. 4). Activation of JNK and p38 was not surprising since these two pathways are known to be sensitive to oxidative stress. The specific MAPK that is activated depends on the type of metal and cell. For example, chromium activates p38 and JNK/SAPK in a non-small-cell lung carcinoma cell line, while arsenic affects p38, JNK/SAPK and ERK 17; 23; 45.
The phosphorylation and activation of JNK and p38 by copper is correlated with a sustained increase in c-Jun phosphorylation and AP-1 activity (Fig. 3 and 5). Similar increases in c-Jun phosphorylation have been observed in BEAS cells treated with a variety of metals 17. The increased c-Jun phosphorylation is likely to have contributed to the observed increase in protein binding to AP-1 and ARE sequences associated with copper exposure (Fig. 1 and Fig 2). Increased AP-1 binding and AP-1-regulated transcription have been observed in response to several metals 18; 20; 23; 46; 47; 48.
AP-1 and ARE sequences control the expression of a number of genes, including proto-oncogenes. Exposure to copper led to increases in c-jun, c-fos, and c-myc expression. The steady state levels of the mRNAs for these proto-oncogenes were greater following a 4 h exposure, compared to the levels observed after a 24 h exposure (Table 1). Similar increases in c-fos, c-jun and c-myc have been observed in response to arsenic and cadmium 20; 49. The temporal expression of these proto-oncogenes in response to copper is similar to the response to cadmium, and is also consistent with the transient increases in JNK and p38 phosphorylation and activation.
There are several mechanisms by which ROS can affect signal transduction pathways. Copper-induced ROS can cause lipid peroxidation, which leads to an increase in the levels of HNE (Fig 6). It has been proposed that HNE acts as a second messenger to activate JNK and p38 signaling transduction pathways. Correlated with HNE production is an increase in AP-1 activity and the expression of several genes including collagen type I, TGFβ1, γ -glutamylcysteine synthetase and c-myc 50; 51; 52; 53; 54. In addition, HNE is capable of increasing c-Jun expression, and activating PKC and JNK/SAPK 27; 55; 56; 57. Copper is able to catalyze the formation of HNE in test tube and copper exposure is correlated with elevated levels of HNE (Fig. 6). Vitamin E co-exposure, however, did not significantly affect the levels of c-jun, p38, and JNK/SAPK phosphorylation. This suggests that HNE may not mediate copper activation of MAPK signaling.
Although the results presented above support the ROS -MAPK-mediated mechanism for the activation of copper-inducible transcription, additional processes may also contribute. For example, additional signal transduction pathways may be activated by copper 58; the generation of reactive nitrogen species59; 60; or the metal may disrupt the cellular redox potential 61; 62. In addition, copper can affect transcription independent of oxidative stress 28. In summary, a mechanistic model is proposed in which copper exposure causes an increase in the intracellular level of ROS, which activates MAPK signaling pathways to cause an increase in the levels of phosphorylation and cognate activities of JNK/SAPK and p38. The activation of these kinases leads to increased phosphorylation of c-jun and a concomitant increase in transcription factor binding to AP-1 and ARE sequences.
EXPERIMENTAL PROCEDURES
Cell Culture
COS-7 cells (ATCC CRL-1651) were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 units/ml penicillin, 100 µg/ml streptomycin, and 100 µM nonessential amino acids. Where indicated, copper was added as cupric sulfate. Cells were maintained in a humidified incubator at 37°C in 5% CO2.
For buthionine sulfoximine (BSO) exposures, cells were pre-treated with 500 µM BSO for 12 h prior to the addition of copper. When cells were exposed to aspirin, cells were pretreated with 20 µM aspirin for 6 h prior to the addition of copper. In cases where cells were treated with the PKC inhibitor 1-(5-isoquinolinesulfonyl)-2-methylpiperazine (H7), 100 µM H7 was added 30 min prior to the addition of copper.
Real Time Quantitative RT-PCR
Total RNA was isolated from cells exposed to 400 µM copper for 4 and 24 h, and non-treated, control cells, using the SV Total RNA Isolation System according to the manufacturer’s instructions (Promega, Madison, WI). Reverse transcriptase real time quantitative PCR was performed using an ABI Prism 7000 thermal cycler (Applied Biosystems, Foster City, CA). RT-PCR reaction mixtures contained 0.5 µg of total RNA, 200 nM each of the forward and reverse primers. Primers were designed to amplify monkey β-actin (forward: GGAAATCGTGCGTGACATTAAG, reverse: TCAGGCAGCTCGTAGCTCTTCT), MT-1 (forward: GCTCCCTTGTTCAGGTATCCA, reverse: TAATTCCCCTGAGGATGGTGG), c-fos (forward: ATTCCGTGGCAGGATCGTTT, reverse: TCATGGTCTTCACAACGCCAG), c-jun (forward: GAGCATTACCTCATCCCGTGA, reverse: CACCCCTAAAAATAGCCCATG), and c-my (forward: GGCAGGCTCCTGGCAAAAGGT, reverse: CTCCTTCCGTGTGGAGGGAGG). Reaction mixtures also contained HotStart Taq DNA polymerase, DNA double-strand specific SYBR Green I dye, Omniscript and Sensiscript reverse transcriptases, ROX passive dye, and nucleotides (Quantitect SYBR Green RT-PCR kit; Qiagen, Valencia, CA). Amplification was achieved following an initial incubation at 50° C for 30 min, followed by denaturation at 95° C for 15 min, and then 40 cycles of 94° C for 15 s, 60° C for 30 s, and 72° C for 30 s. At the end of the 40 cycles, samples were subjected to melting analyses to confirm specificity of the PCR products. The fold induction of MT, c-fos, c-jun, and c-myc mRNAs was determined by the ΔΔCt relative quantification method (Applied Biosystems). The amount of mRNA in each sample was normalized to the level of β-actin mRNA in cognate samples.
Electrophoretic Mobility Shift Assays
COS-7 cells were exposed to copper for 4 or 24 h. After the treatments the medium was removed, and the cells rinsed with ice-cold phosphate-buffered saline. Cells were collected, nuclei isolated, and nuclear extracts prepared as described 63. The final nuclear protein extract was dialyzed against 20 mM HEPES, pH 7.9 containing 20% glycerol, 100 mM KCl, 0.2 mM EDTA, 0.2 mM phenyl methyl sulfonyl fluoride, 0.5 mM dithiothreitol for 1 h at 4°C. Protein concentrations of the nuclear extracts were determined by Bradford assays using bovine serum albumin as a standard 64.
Nuclear protein binding to 5’ 32P-end-labeled double-stranded oligonucleotide probes was measured by EMSA. An oligonucleotide containing the AP-1 consensus sequence was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The oligonucleotide for the human NAD(P)H:quinone oxidoreductase 1 ARE sequence was synthesized at the Duke University Sequencing Facility. EMSAs were performed by incubating 10 µg of nuclear extract with 1 µg poly dI-dC for 15 min at 4°C in incubation buffer (10 mM Tris, 100 mM NaCl, 1 mM dithiothreitol, 1 mM EDTA, 4% glycerol, 60 µg/µl bovine serum albumin); and where applicable, a 50-fold excess of non-labeled oligonucleotide competitor. Subsequently, 32P-labeled probe was added (100,000 cpm), and the mixture incubated for an additional 20 min at room temperature. DNA-protein complexes were resolved on 6% polyacrylamide gels. Gels were dried and DNA protein complexes visualized following PhosphorImager analysis. Densitometry was performed using ImageQuant software (Molecular Dynamics, Valencia, CA). The amount of protein bound to the oligonucleotide is expressed as fold-induction of treated samples compared to untreated controls.
Western Immunoblot Analysis
Western immunoblot analysis was used to measure the effects of copper on the levels of c-Jun, ERK, p38, and SAPK/JNK phosphorylation. COS-7 cells were treated with 400 µM copper for 4 or 24 h. In cases where cells were treated with vitamin E, 100 µM was added 2 h prior to copper exposure. After the incubation, phosphate-buffered saline washed cells were lysed by adding Laemmli sample buffer 63, sonicated, and then heated for 5 min at 95°C. Proteins were resolved by SDS-PAGE, and then transferred to polyvinylidene difluoride membranes.
Levels of protein phosphorylation were measured using commercial antibodies (Cell Signaling Technology, Beverley, MA). The Phospho Plus® c-Jun (Ser63) and c-Jun (Ser73) Antibody Kit includes antibodies that recognize c-Jun which is phosphorylated at Ser63 or Ser73, as well as an antibody for the non-phosphorylated form of c-Jun. The Phospho-MAPK Antibody Sampler Kit contains antibodies to Phospho-p44/42 MAPK, Phospho-SAPK/JNK and Phospho-p38 MAPK, which recognize the phosphorylated forms of p44/42 MAPK, SAPK/JNK and p38 MAPK, respectively. A MAPK Family Antibody Sampler Kit was used to determine the levels of non-phosphorylated ERK, p38, and SAPK/JNK. The level of β-actin was determined as a loading control, using anti-mouse or human actin antibodies (Abcam, Cambridge, UK or Santa Cruz Biotechnology, Inc, Santa Cruz, CA)
To visualize the phosphorylated and non-phosphorylated kinases, membranes were incubated with the appropriate antibody according to the manufacturer’s instructions. Locations of antigen-antibody complexes were visualized by chemiluminescence using cognate, species specific, horseradish peroxidase-conjugated secondary antibodies (ECL, Amersham, Piscataway, NJ). Quantification of the proteins was performed using Scion Image software (Scion Corporation, Frederick, MD) or using a STORM 860 Molecular Imager (Amersham Biosciences, Piscataway, NJ) to visualize and ImageQuant software (Molecular Dynamics, Valencia, CA) to quantify the protein levels. The data are expressed as fold-induction of actin-normalized treated samples over that of the actin-normalized untreated controls.
Kinase Activity Assays
Kinase activity assays were performed to determine the effects of copper on p38, and SAPK/JNK activity. COS-7 cells were treated with 400 µM copper for 4 or 24 h. After the incubation, phosphate-buffered saline washed cells were lysed by adding 1.5 ml of cell lysis buffer containing 1 mM phenyl methyl sulfonyl fluoride, and then sonicated four times for 5 s. The lysate was centrifuged and supernatants collected. Kinase activity was measured then using Cell Signaling Technology kinase assay kits according to the manufacturer’s instructions.
To measure SAPK/JNK activity, the kinase was collected using an N-terminal c-Jun (1–89) fusion protein bound to glutathione-Sepharose beads. After collecting JNK/SAPK, kinase reactions were performed using Jun as a substrate. The level of c-Jun phosphorylation was measured using an antibody that specifically binds to phospho-Ser63 c-Jun.
p38 kinase was collected using a monoclonal antibody that binds to phospho-Thr180/Tyr182 p38. To measure p38 kinase activity, the immunoprecipitate was incubated with ATF-2 fusion protein. The level of ATF-2 phosphorylation was measured by Western immunoblotting using a phospho-Thr71 ATF-2 antibody. Western immunoblot analysis was performed as described above. As loading controls, the levels of the non-phosphorylated forms of p38 and SAPK/JNK were determined. The data are expressed as fold-induction of treated samples over that of the untreated controls.
Detection of 4-Hydroxynonenal and Malondialdehyde
The level of HNE and MDA in copper-treated cells was measured using a commercially available lipid peroxidation assay kit (Calbiochem, San Diego, CA). As a positive control, HNE and MDA were measured in a reaction mixture that contained 100 µM copper and 1 mM ascorbate with 1 mg/ml of phosphatidylcholine vesicles. Following 24 h incubation, the reaction was stopped following the addition of 110 µM EDTA, which chelated the copper. To measure HNE and MDA in COS-7 cells, cells were treated with 400 µM copper for 4 and 24 h. Cells were washed with phosphate buffered saline and diluted to a final concentration of 3 × 107cells/ml. HNE and MDA levels were measured according to the manufacture’s instructions.
Transient Transfection and Reporter Gene Assays
Cells were plated at a density of 8.0 × 104cells/well in 24 well culture plates, and allowed to grow for 18–24 h before transfection. Cells were transfected with chloramphenicol acetyltransferase-containing reporter genes containing regions of the mouse metallothionein-1 promoter (−42/+63-CAT, −153/+62-CAT, MREd5-CAT, and ARE4-CAT) as previously described 4. Following transfection, the cells were allowed to recover overnight. Cells were subsequently treated with 5 µM HNE for 24 h. The levels of reporter gene activity were measured as previously described 28.
Statistical Analysis
Statistical analysis was performed using StatView software (JMP software, Cary, NC). The results are presented as the mean ± standard mean error. The significance of mean differences was determined by analysis of variance (ANOVA) followed by Fisher’s Protected Least Squares Difference post hoc test for individual comparisons. The criterion for statistical significance was set at p < 0.05.
ACKNOWLEDGEMENTS
We thank Dr. G. Andrews (University of Kansas Medical Center) for the generous gift of the MT-1-based reporter genes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was supported in part by National Institute of Environmental Health Sciences Grants ES-10356 and 5T32-ES-07031-22, and by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
The abbreviations used are: MAPK, mitogen-activated protein kinase; AP-1, activating protein-1; HNE, 4-hydroxynonenal; JNK/SAPK, c-Jun N-terminal kinase/stress activated protein kinase; ARE, antioxidant response element; ROS, reactive oxygen species; ERK, extracellularly regulated kinase; ATF-2, activating transcription factor 2; MDA, malondialdehyde; BSO, buthionine sulfoximine; PKC, Protein Kinase C; MT, metallothionein.
REFERENCES
- 1.Camhi SL, Alam J, Otterbein L, Sylvester SL, Choi AM. Induction of heme oxygenase-1 gene expression by lipopolysaccharide is mediated by AP-1 activation. Am J Respir Cell Mol Biol. 1995;13:387–398. doi: 10.1165/ajrcmb.13.4.7546768. [DOI] [PubMed] [Google Scholar]
- 2.Daniel V. Glutathione S-transferases: gene structure and regulation of expression. Crit Rev Biochem Mol Biol. 1993;28:173–207. doi: 10.3109/10409239309086794. [DOI] [PubMed] [Google Scholar]
- 3.Radi AA, Matkovics B. Effects of metal ions on the antioxidant enzyme activities, protein contents and lipid peroxidation of carp tissues. Comp Biochem Physiol C. 1988;90:69–72. doi: 10.1016/0742-8413(88)90099-0. [DOI] [PubMed] [Google Scholar]
- 4.Dalton T, Palmiter RD, Andrews GK. Transcriptional induction of the mouse metallothionein-I gene in hydrogen peroxide-treated Hepa cells involves a composite major late transcription factor/antioxidant response element and metal response promoter elements. Nucleic Acids Res. 1994;22:5016–5023. doi: 10.1093/nar/22.23.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz JL, Antoniades DZ, Zhao S. Molecular and biochemical reprogramming of oncogenesis through the activity of prooxidants and antioxidants. Ann N Y Acad Sci. 1993;686:262–278. doi: 10.1111/j.1749-6632.1993.tb39185.x. discussion 278–279. [DOI] [PubMed] [Google Scholar]
- 6.Jaiswal AK. Antioxidant response element. Biochem Pharmacol. 1994;48:439–444. doi: 10.1016/0006-2952(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 7.Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Brain Res Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- 8.Geilen CC, Wieprecht M, Orfanos CE. The mitogen-activated protein kinases system (MAP kinase cascade): its role in skin signal transduction. A review. J Dermatol Sci. 1996;12:255–262. doi: 10.1016/0923-1811(95)00481-5. [DOI] [PubMed] [Google Scholar]
- 9.Kortenjann M, Shaw PE. The growing family of MAP kinases: regulation and specificity. Crit Rev Oncog. 1995;6:99–115. [PubMed] [Google Scholar]
- 10.Moriguchi T, Gotoh Y, Nishida E. Roles of the MAP kinase cascade in vertebrates. Adv Pharmacol. 1996;36:121–137. doi: 10.1016/s1054-3589(08)60579-7. [DOI] [PubMed] [Google Scholar]
- 11.Su B, Karin M. Mitogen-activated protein kinase cascades and regulation of gene expression. Curr Opin Immunol. 1996;8:402–411. doi: 10.1016/s0952-7915(96)80131-2. [DOI] [PubMed] [Google Scholar]
- 12.Waskiewicz AJ, Cooper JA. Mitogen and stress response pathways: MAP kinase cascades and phosphatase regulation in mammals and yeast. Curr Opin Cell Biol. 1995;7:798–805. doi: 10.1016/0955-0674(95)80063-8. [DOI] [PubMed] [Google Scholar]
- 13.Marais R, Marshall CJ. Control of the ERK MAP kinase cascade by Ras and Raf. Cancer Surv. 1996;27:101–125. [PubMed] [Google Scholar]
- 14.Kyriakis JM, Avruch J. Protein kinase cascades activated by stress and inflammatory cytokines. Bioessays. 1996;18:567–577. doi: 10.1002/bies.950180708. [DOI] [PubMed] [Google Scholar]
- 15.Beyaert R, Cuenda A, Vanden Berghe W, Plaisance S, Lee JC, Haegeman G, Cohen P, Fiers W. The p38/RK mitogen-activated protein kinase pathway regulates interleukin-6 synthesis response to tumor necrosis factor. Embo J. 1996;15:1914–1923. [PMC free article] [PubMed] [Google Scholar]
- 16.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 17.Samet JM, Graves LM, Quay J, Dailey LA, Devlin RB, Ghio AJ, Wu W, Bromberg PA, Reed W. Activation of MAPKs in human bronchial epithelial cells exposed to metals. Am J Physiol. 1998;275:L551–L558. doi: 10.1152/ajplung.1998.275.3.L551. [DOI] [PubMed] [Google Scholar]
- 18.Ding M, Li JJ, Leonard SS, Ye JP, Shi X, Colburn NH, Castranova V, Vallyathan V. Vanadate-induced activation of activator protein-1: role of reactive oxygen species. Carcinogenesis. 1999;20:663–668. doi: 10.1093/carcin/20.4.663. [DOI] [PubMed] [Google Scholar]
- 19.Hu Y, Jin X, Snow ET. Effect of arsenic on transcription factor AP-1 and NF-kappaB DNA binding activity and related gene expression. Toxicol Lett. 2002;133:33–45. doi: 10.1016/s0378-4274(02)00083-8. [DOI] [PubMed] [Google Scholar]
- 20.Li M, Cai JF, Chiu JF. Arsenic induces oxidative stress and activates stress gene expressions in cultured lung epithelial cells. J Cell Biochem. 2002;87:29–38. doi: 10.1002/jcb.10269. [DOI] [PubMed] [Google Scholar]
- 21.Buzard GS, Kasprzak KS. Possible roles of nitric oxide and redox cell signaling in metal-induced toxicity and carcinogenesis: a review. J Environ Pathol Toxicol Oncol. 2000;19:179–199. [PubMed] [Google Scholar]
- 22.Kaltreider RC, Pesce CA, Ihnat MA, Lariviere JP, Hamilton JW. Differential effects of arsenic(III) and chromium(VI) on nuclear transcription factor binding. Mol Carcinog. 1999;25:219–229. [PubMed] [Google Scholar]
- 23.Chen NY, Ma WY, Huang C, Ding M, Dong Z. Activation of PKC is required for arsenite-induced signal transduction. J Environ Pathol Toxicol Oncol. 2000;19:297–305. [PubMed] [Google Scholar]
- 24.Herdegen T, Skene P, Bahr M. The c-Jun transcription factor-bipotential mediator of neuronal death, survival and regeneration. Trends Neurosci. 1997;20:227–231. doi: 10.1016/s0166-2236(96)01000-4. [DOI] [PubMed] [Google Scholar]
- 25.Mendelson KG, Contois LR, Tevosian SG, Davis RJ, Paulson KE. Independent regulation of JNK/p38 mitogen-activated protein kinases by metabolic oxidative stress in the liver. Proc Natl Acad Sci U S A. 1996;93:12908–12913. doi: 10.1073/pnas.93.23.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilhelm D, van Dam H, Herr I, Baumann B, Herrlich P, Angel P. Both ATF-2 and c-Jun are phosphorylated by stress-activated protein kinases in response to UV irradiation. Immunobiology. 1995;193:143–148. doi: 10.1016/S0171-2985(11)80537-1. [DOI] [PubMed] [Google Scholar]
- 27.Uchida K, Shiraishi M, Naito Y, Torii Y, Nakamura Y, Osawa T. Activation of stress signaling pathways by the end product of lipid peroxidation. 4-hydroxy-2-nonenal is a potential inducer of intracellular peroxide production. J Biol Chem. 1999;274:2234–2242. doi: 10.1074/jbc.274.4.2234. [DOI] [PubMed] [Google Scholar]
- 28.Mattie MD, Freedman JH. Copper-inducible transcription: regulation by metal- and oxidative stress-responsive pathways. Am J Physiol Cell Physiol. 2004;286:C293–C301. doi: 10.1152/ajpcell.00293.2003. [DOI] [PubMed] [Google Scholar]
- 29.Mattie MD, Freedman JH. Protective effects of aspirin and vitamin E (alpha-tocopherol) against copper- and cadmium-induced toxicity. Biochem Biophys ResCommun. 2001;285:921–925. doi: 10.1006/bbrc.2001.5259. [DOI] [PubMed] [Google Scholar]
- 30.Rösner U. Effects of historical mining activities on surface water and groundwater - an example from northwest Arizona. Environmental Geology. 1998;33:224–230. [Google Scholar]
- 31.Service UPH, editor. Agency for Toxic Substances and Disease Registry. Toxicological Profile for Copper. Atlanta: US Public Health Service; 1990. TP-90--08 edit. [Google Scholar]
- 32.Torres P, Tort L, Flos R. Acute toxicity of copper to mediterranean dogfish. Comp Biochem Physiol C. 1987;86:169–171. doi: 10.1016/0742-8413(87)90161-7. [DOI] [PubMed] [Google Scholar]
- 33.McKim JM, Eaton JG, Holcombe GW. Metal toxicity to embryos and larvae of eight species of freshwater fish-II: copper. Bull Environ Contam Toxicol. 1978;19:608–616. doi: 10.1007/BF01685847. [DOI] [PubMed] [Google Scholar]
- 34.Hamilton SJ, Buhl KJ. Safety assessment of selected inorganic elements to fry of chinook salmon (Oncorhynchus tshawytscha) Ecotoxicol Environ Saf. 1990;20:307–324. doi: 10.1016/0147-6513(90)90009-t. [DOI] [PubMed] [Google Scholar]
- 35.Wu SM, Jong KJ, Kuo SY. Effects of copper sulfate on ion balance and growth in tilapia larvae (Oreochromis mossambicus) Arch Environ Contam Toxicol. 2003;45:357–363. doi: 10.1007/s00244-003-0122-5. [DOI] [PubMed] [Google Scholar]
- 36.Khangarot BS. Copper-induced hepatic ultrastructural alterations in the snake-headed fish, Channa punctatus. Ecotoxicol Environ Saf. 1992;23:282–293. doi: 10.1016/0147-6513(92)90078-h. [DOI] [PubMed] [Google Scholar]
- 37.Karan V, Vitorovic S, Tutundzic V, Poleksic V. Functional enzymes activity and gill histology of carp after copper sulfate exposure and recovery. Ecotoxicol Environ Saf. 1998;40:49–55. doi: 10.1006/eesa.1998.1641. [DOI] [PubMed] [Google Scholar]
- 38.Boyd WA, Williams PL. Comparison of the sensitivity of three nematode species to copper and their utility in aquatic and soil toxicity tests. Environ Toxicol Chem. 2003;22:2768–2774. doi: 10.1897/02-573. [DOI] [PubMed] [Google Scholar]
- 39.Milani D, Reynoldson TB, Borgmann U, Kolasa J. The relative sensitivity of four benthic invertebrates to metals in spiked-sediment exposures and application to contaminated field sediment. Environ Toxicol Chem. 2003;22:845–854. [PubMed] [Google Scholar]
- 40.Rayms-Keller A, Olson KE, McGaw M, Oray C, Carlson JO, Beaty BJ. Effect of heavy metals on Aedes aegypti (Diptera: Culicidae) larvae. Ecotoxicol Environ Saf. 1998;39:41–47. doi: 10.1006/eesa.1997.1605. [DOI] [PubMed] [Google Scholar]
- 41.Ramachandran S, Patel TR, Colbo MH. Effect of copper and cadmium on three Malaysian tropical estuarine invertebrate larvae. Ecotoxicol Environ Saf. 1997;36:183–188. doi: 10.1006/eesa.1996.1508. [DOI] [PubMed] [Google Scholar]
- 42.Bellas J, Beiras R, Vazquez E. Sublethal effects of trace metals (Cd, Cr, Cu,Hg) on embryogenesis and larval settlement of the ascidian Ciona intestinalis. Arch Environ Contam Toxicol. 2004;46:61–66. doi: 10.1007/s00244-003-0238-7. [DOI] [PubMed] [Google Scholar]
- 43.Bhave SA, Pandit AN, Pradhan AM, Sidhaye DG, Kantarjian A, Williams A, Talbot IC, Tanner MS. Liver disease in India. Arch Dis Child. 1982;57:922–928. doi: 10.1136/adc.57.12.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tatli MM, Vural H, Koc A, Kosecik M, Atas A. Altered anti-oxidant status and increased lipid peroxidation in marasmic children. Pediatr Int. 2000;42:289–292. doi: 10.1046/j.1442-200x.2000.01217.x. [DOI] [PubMed] [Google Scholar]
- 45.Chuang SM, Yang JL. Comparison of roles of three mitogen-activated protein kinases induced by chromium(VI) and cadmium in non-small-cell lung carcinoma cells. Mol Cell Biochem. 2001;222:85–95. [PubMed] [Google Scholar]
- 46.Liu J, Kadiiska MB, Liu Y, Lu T, Qu W, Waalkes MP. Stress-related gene expression in mice treated with inorganic arsenicals. Toxicol Sci. 2001;61:314–320. doi: 10.1093/toxsci/61.2.314. [DOI] [PubMed] [Google Scholar]
- 47.Wijeweera JB, Gandolfi AJ, Parrish A, Lantz RC. Sodium arsenite enhances AP-1 and NFkappaB DNA binding and induces stress protein expression in precision-cut rat lung slices. Toxicol Sci. 2001;61:283–294. doi: 10.1093/toxsci/61.2.283. [DOI] [PubMed] [Google Scholar]
- 48.Huang C, Bode AM, Chen NY, Ma WY, Li J, Nomura M, Dong Z. Transactivation of AP-1 in AP-1-luciferase reporter transgenic mice by arsenite and arsenate. Anticancer Res. 2001;21:261–267. [PubMed] [Google Scholar]
- 49.Joseph P, Muchnok TK, Klishis ML, Roberts JR, Antonini JM, Whong WZ, Ong T. Cadmium-induced cell transformation and tumorigenesis are associated with transcriptional activation of c-fos, c-jun, and c-myc proto-oncogenes: role of cellular calcium and reactive oxygen species. Toxicol Sci. 2001;61:295–303. doi: 10.1093/toxsci/61.2.295. [DOI] [PubMed] [Google Scholar]
- 50.Parola M, Pinzani M, Casini A, Albano E, Poli G, Gentilini A, Gentilini P, Dianzani MU. Stimulation of lipid peroxidation or 4-hydroxynonenal treatment increases procollagen alpha 1 (I) gene expression in human liver fat-storing cells. Biochem Biophys Res Commun. 1993;194:1044–1050. doi: 10.1006/bbrc.1993.1927. [DOI] [PubMed] [Google Scholar]
- 51.Leonarduzzi G, Scavazza A, Biasi F, Chiarpotto E, Camandola S, Vogel S, Dargel R, Poli G. The lipid peroxidation end product 4-hydroxy-2,3-nonenal up-regulates transforming growth factor beta1 expression in the macrophage lineage: a link between oxidative injury and fibrosclerosis. Faseb J. 1997;11:851–857. doi: 10.1096/fasebj.11.11.9285483. [DOI] [PubMed] [Google Scholar]
- 52.Liu RM, Gao L, Choi J, Forman HJ. gamma-glutamylcysteine synthetase: mRNA stabilization and independent subunit transcription by 4-hydroxy-2-nonenal. Am J Physiol. 1998;275:L861–L869. doi: 10.1152/ajplung.1998.275.5.L861. [DOI] [PubMed] [Google Scholar]
- 53.Fazio VM, Barrera G, Martinotti S, Farace MG, Giglioni B, Frati L, Manzari V, Dianzani MU. 4-Hydroxynonenal, a product of cellular lipid peroxidation, which modulates c-myc and globin gene expression in K562 erythroleukemic cells. Cancer Res. 1992;52:4866–4871. [PubMed] [Google Scholar]
- 54.Leonarduzzi G, Arkan MC, Basaga H, Chiarpotto E, Sevanian A, Poli G. Lipid oxidation products in cell signaling. Free Radic Biol Med. 2000;28:1370–1378. doi: 10.1016/s0891-5849(00)00216-1. [DOI] [PubMed] [Google Scholar]
- 55.Chiarpotto E, Domenicotti C, Paola D, Vitali A, Nitti M, Pronzato MA, Biasi F, Cottalasso D, Marinari UM, Dragonetti A, Cesaro P, Isidoro C, Poli G. Regulation of rat hepatocyte protein kinase C beta isoenzymes by the lipid peroxidation product 4-hydroxy-2,3-nonenal: A signaling pathway to modulate vesicular transport of glycoproteins. Hepatology. 1999;29:1565–1572. doi: 10.1002/hep.510290510. [DOI] [PubMed] [Google Scholar]
- 56.Parola M, Robino G, Marra F, Pinzani M, Bellomo G, Leonarduzzi G, Chiarugi P, Camandola S, Poli G, Waeg G, Gentilini P, Dianzani MU. HNE interacts directly with JNK isoforms in human hepatic stellate cells. J Clin Invest. 1998;102:1942–1950. doi: 10.1172/JCI1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Camandola S, Poli G, Mattson MP. The lipid peroxidation product 4-hydroxy-2,3-nonenal increases AP-1-binding activity through caspase activation in neurons. J Neurochem. 2000;74:159–168. doi: 10.1046/j.1471-4159.2000.0740159.x. [DOI] [PubMed] [Google Scholar]
- 58.Maziere C, Auclair M, Djavaheri-Mergny M, Packer L, Maziere JC. Oxidized low density lipoprotein induces activation of the transcription factor NF kappa B in fibroblasts, endothelial and smooth muscle cells. Biochem Mol Biol Int. 1996;39:1201–1207. doi: 10.1080/15216549600201392. [DOI] [PubMed] [Google Scholar]
- 59.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals,metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006 doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 60.Videla LA, Fernandez V, Tapia G, Varela P. Oxidative stress-mediated hepatotoxicity of iron and copper: role of Kupffer cells. Biometals. 2003;16:103–111. doi: 10.1023/a:1020707811707. [DOI] [PubMed] [Google Scholar]
- 61.Rahman I, Biswas SK, Jimenez LA, Torres M, Forman HJ. Glutathione, stress responses, and redox signaling in lung inflammation. Antioxid Redox Signal. 2005;7:42–59. doi: 10.1089/ars.2005.7.42. [DOI] [PubMed] [Google Scholar]
- 62.Filomeni G, Aquilano K, Rotilio G, Ciriolo MR. Reactive oxygen species-dependent c-Jun NH2-terminal kinase/c-Jun signaling cascade mediates neuroblastoma cell death induced by diallyl disulfide. Cancer Res. 2003;63:5940–5949. [PubMed] [Google Scholar]
- 63.Ausubel FM. Current Protocols in Molecular Biology on CD-ROM. New York, NY: John Wiley & Sons; 2003. [Google Scholar]
- 64.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]