Abstract
Invariant Natural Killer T cell (iNK Tcell) have an innate immunity-like rapidity of response and the capacity to modulate effector functions of other cells. We show that the BTB-ZF transcriptional regulator, PLZF, is specifically expressed in iNKT cells. iNKT cells develop in the absence of PLZF, but lack many innate T cell features. PLZF deficient iNKT cells accumulate in the lymph nodes rather than in the liver and do not have an activated phenotype or express NK markers. PLZF deficient iNKT cells do not secrete high levels of IL-4 and IFNγ upon activation, however some cells produce either IL-4 or IFNγ, but not both. PLZF, therefore, is an iNKT cell specific transcription factor that is necessary for full functionality.
Nearly all hematopoietic cells mature in the bone marrow. In contrast, multipotent progenitor T cells leave the bone marrow and home to the thymus, where signals from stromal cells are required for commitment to the T lineage1. Once directed into the T lineage, the cells undergo a rigorous selection process that eliminates more than 95% of the candidate T cells. Full maturation requires the expression of a T cell receptor (TCR) that binds self-peptide:self-MHC complexes with sufficient avidity. At some point during development, T cells are directed into one of several distinct T cell lineages such as CD4 single positive “helper” cells, CD8 single positive “killer” cells or CD4+CD25+ regulatory cells. Commitment to these various lineages defines the specialized functions of the cell, which is critical since each cell type plays an essential and distinct role for host defense. The genes responsible for directing multipotent T cell progenitors into the various lineages are largely unknown 2.
Among the various lineages of T cells, invariant Natural Killer T cells (iNKT cells) have several unique phenotypic traits such as the expression of receptors typically associated with Natural Killer cells (NK cells), the constitutive expression of activation markers and extremely restricted TCR diversity3. iNKT cells express an identical TCRα chain and most use a TCRβ chain that utilizes the Vβ8.2 gene segment. This TCR confers specificity to the non-MHC encoded self-molecule, CD1d, which binds and presents glycolipids rather than the typical peptide cargo presented by conventional MHC molecules.
iNKT cells are also functionally distinct. Of particular interest is their ability to secrete large quantities of a variety of cytokines only minutes after activation via the TCR3. The rapid response of these cells, the conserved nature of the TCR and their indirect ability to modulate the function of many different cell types of the immune system has led to the appreciation that iNKT cells lay at a functional cusp between the innate and adaptive immune systems4. The broad range of cytokines released by iNKT cells results in their potential to regulate seemingly opposing immune responses. For example, iNKT cells have been shown to enhance immune responses against tumors, but they have also been shown to prevent autoimmunity by diminishing self-reactive T cell responses5.
The expression of a TCR specific for the CD1d ligand loaded with an appropriate glycolipid is the only unique identifier of iNKT cells. This highly conserved TCR also allows for the direct detection of iNKT cells by the use of a tetramerized version of the CD1d molecule loaded with a glycolipid, referred to as α- galactosylceramide (α-GalCer)6. This reagent allows for the unambiguous detection of iNKT cells, which has made these cells much more amenable to genetic studies than the extremely diverse conventional T cell populations. As a result, several genes that influence iNKT cell development have been identified. Loss of any one of a handful of genes, such as T-bet or CSF-2, impacts events late in development and typically results in altered iNKT cell function7. Loss of Fyn expression dramatically affects development, presumably due to a decrease in signaling via the TCR or other receptors, for example SLAM8-10. Indeed, loss of the SLAM family adapter protein, SAP, also eliminates iNKT cell development11-13. The Runx1 transcription factor has also been shown to be required for iNKT cell development14. Loss of Runx1 expression, however, has severe and pleiotropic affects on thymocyte development. Indeed, none of the genes that have been identified to affect iNKT cell development are specifically expressed in iNKT cells.
The BTB/POZ-ZF [Broad complex, Tramtrack, Bric-à-brac (BTB) or poxvirus and zinc finger (POZ)-zinc finger] protein family of transcription factors has been found to control a wide variety of biological processes15. These BTB-zinc finger (BTB-ZF) family members are defined by the presence of an N-terminal protein-protein interaction domain (BTB/POZ) and C-terminal C2H2 zinc finger domains. Several members of this gene family have been shown to have transcriptional repressor activity. BTB-ZF genes have also been shown to control several fundamental aspects of immune system function. For example, BCL6 controls the germinal center reaction that is required for affinity maturation of B cells16. In T cells, it has been shown that Th-POK is the master regulator of the CD4 versus CD8 lineage commitment step in the thymus17, 18,which is fundamental for T cell function. And, most recently, the T versus B cell fate decision was also shown to be controlled by a BTB-ZF gene called LRF19. Here we report that PLZF, also a member of the BTB-ZF gene family, is necessary for the development of iNKT cells.
Results
Among lymphoid cells, PLZF expression is highly restricted to iNKT cells
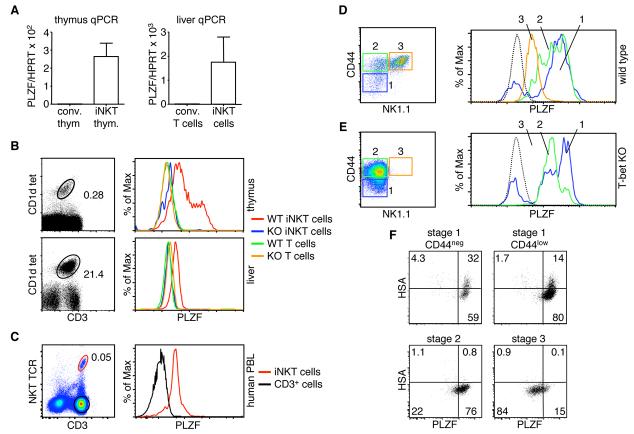
Recognizing the increasing number of BTB-ZF finger genes involved in the function of the immune system, we screened various thymocyte populations for the expression of several different family members. Many of the BTB-ZF genes we analyzed were expressed in all thymic subpopulations (data not shown). One, however, the promyelocytic leukemia zinc finger gene (PLZF)20, was highly expressed in iNKT cells, as compared to conventional T cells. The expression of PLZF in iNKT cells was apparent in thymocytes and also in lymphocytes isolated from the liver of mice (Fig. 1A). To further analyze PLZF expression patterns, we used a new monoclonal antibody directed against the hinge region of the protein. CD1d:αGalCer tetramers6 were used to specifically identify iNKT cells (Fig. 1B, left). Intracellular staining for PLZF in iNKT cells and conventional T cells clearly showed that the transcription factor was expressed at variable levels in thymic iNKT cells and at moderate levels in liver iNKT cells (Fig. 1B, right). PLZF was not detected, however, in conventional thymocytes or conventional liver T cells (Fig. 1B). T cells from a PLZF deficient mouse21 (see below) were used to confirm the specificity of the antibody (Fig. 1B).
Fig. 1. PLZF is highly expressed in iNKT cells.
(A) Relative PLZF mRNA expression measured by quantitative real time PCR of cDNA derived from CD1d tetramer positive iNKT cells and tetramer negative conventional T cells isolated by cell sorting from the thymus and the liver. HPRT cDNA levels were used to normalize samples. Average of two experiments is shown with standard deviations. (B) FACS analysis of thymocytes and percol gradient enriched, liver lymphocytes. iNKT cells were detected with the CD1d:α-GalCer tetramer and anti-CD3. Histograms show PLZF expression in permeabilized iNKT cells gated as shown in dot plots. Specificity of the anti-PLZF antibody was confirmed by staining T cells and iNKT cells from PLZF deficient mice. Data are representative of more than ten experiments. (C) Peripheral blood was collected from healthy human volunteers. Lymphocytes were isolated by ficoll separation and stained with the indicated antibodies. FACS was repeated on five different individuals with similar results. Thymocytes from (D) wild type and (E) T-bet deficient mice were stained with antibodies against CD44, NK1.1, PLZF and the CD1d tetramer. Only tetramer positive cells are shown. The stage of development is indicated (stage 1 – 3) on the two-color dot plot. PLZF levels within a particular stage of development are shown in the histogram to the right. T-bet deficient iNKT cells do not mature to stage 3. Data from one of two similar experiments is shown. (F) The thymuses from pooled 10-13 day old wild type mice were depleted of CD8 positive cells and then stained with the antibodies against CD3, HSA, CD44, NK1.1, PLZF and the CD1d tetramer. Staining of the same cell preparations with empty tetramer confirmed specificity. Empty tetramer labeled with a different fluorochrome was also used in some experiments to further eliminate non-specific events. For FACS analysis, single cell suspensions were first incubated with normal mouse serum, unlabeled streptavidin and anti-FC receptor blocking antibody. Cells were stained with antibody and tetramer on ice, permeabilized with the eBioscience intracellular staining buffer set followed by staining with the anti-PLZF monoclonal antibody. Lines in histograms are matched to the color of the gates in the dot plots. The black, dotted line shows CD4 single positive thymocytes, which do not express PLZF.
Peripheral blood lymphocytes from humans were collected and examined by FACS for PLZF expression. iNKT cells were identified with the 6B11 monoclonal antibody, which recognizes a unique determinant within the TCR CDR3 region, in combination with an antibody against CD3 (Fig. 1C). PLZF was detected by intracellular staining with our anti-mouse antibody, which crossreacts with the human protein. Human iNKT cells clearly express PLZF, while CD3+ T cells express little or no PLZF (Fig. 1C).
iNKT cell development is typically divided into three stages22, which are primarily identified by the cell surface upregulation of CD44, followed by NK1.1 (Fig. 1D). Nearly all stage 1 cells express high levels of PLZF. The extremely rare PLZF negative events (∼0.003%) seen among the stage 1 cells could not definitively be determined to be real iNKT cell events since staining with empty tetramer resulted in the detection of similar events (data not shown). Consistent with the finding that PLZF is downregulated in other cell types during differentiation, including CD34+ human stem cells23, 24, we find that the transcription factor is downregulated in the iNKT cells found within the more mature stage 2 and 3 populations (Fig. 1D). PLZF expression at stage 3 is equivalent to expression in mature, liver iNKT cells.
T-bet deficient iNKT cells are blocked at stage 2 of development25 (Fig. 1E). These genetically blocked iNKT cells did not downregulate PLZF to the mature stage 3 levels. The levels of PLZF in stage 1 and 2, however, were similar to wild type cells (Fig. 1E). Therefore, regulation of PLZF expression is tightly linked to the stage of iNKT cell development.
Young mice have a much greater number of immature, developing iNKT cells than adult mice9. Therefore, we examined 10-13 days old mice to determine the expression of PLZF during early subsets of cells typically not detectable in adult mice. CD44neg, HSAhi iNKT cells, which are thought to represent the most immature iNKT cell population in the thymus, clearly express high levels of PLZF (Fig. 1F). As the developing iNKT cells begin to express CD44 (Stage 1 CD44lo), HSA starts to be downregulated, while PLZF remains high (Fig. 1F). As seen in adult mice (Fig. 1D), PLZF expression continued to downregulate as the cells mature.
Lack of PLZF expression does not affect lymphocyte development
To further examine the expression of PLZF in the lymphoid compartment, we examined B cells and NK cells by FACS. PLZF was not detected in either of these cell types (Fig. 2A). PLZF was also not detected in eosinophils, neutrophils or macrophages (data not shown).
Fig. 2. PLZF expression is limited to iNKT cells and does not affect the bulk of lymphocyte development.
(A) Intracellular staining for PLZF expression in B cells and NK cells was done and compared to the same cell types from PLZF deficient mice. Expression of PLZF in iNKT cells is shown as a positive control. (B) Single cell suspensions of thymus and lymph node cells were stained with the indicated monoclonal antibodies. The percentage and phenotype of the major subpopulations of thymocytes and lymphocytes were similar in wild type and PLZF deficient mice, based on staining with CD4, CD8 and TCR. Subtle variations in thymocyte numbers were not reproducible. Only TCR+ lymph node cells are shown. Experiment was repeated approximately 10 times. (C) Frozen thymus sections were stained with Dec205 and with UEA-1 to identify the thymic cortex and medulla. No structural changes to the organ were observed. (D) The frequency of NK cells, DX5+ T cells, and γδ T cells is not affected by the loss of PLZF expression. Data are representative of three independent experiments.
PLZF deficient mice were previously shown to have disrupted patterning of the hind limbs21 and a progressive loss of spermatogonia26. Development of the hematopoietic system, however, was not overtly perturbed in PLZF deficient mice (Fig. 2B). For example, normal percentages of all the thymic subpopulations were found, although the size of the thymus from PLZF deficient mice was consistently decreased by ∼25%. Nonetheless, normal thymic structure was observed (Fig. 2C). Also, thymocytes expressing an MHC class II restricted TCR transgene developed normally in the absence of PLZF (data not shown). Lymph node T cells also appeared normal in the PLZF deficient mice (Fig. 2B) as well as in the spleen (data not shown). DX5+ T cells, which largely represent non-CD1d restricted NKT cells27, NK cells and γδ T cells also appeared normal (Fig. 2D). Finally, no obvious differences were seen in the B cell compartment (data not shown). Therefore, consistent with the restricted expression pattern of PLZF, the bulk of the lymphoid compartment is intact. The alteration in thymic cellularity appears to be secondary to any affects that PLZF may have on T cell development and potentially is simply a consequence of the stress caused by the malformed hind limbs.
Altered iNKT cell development in PLZF deficient mice
Although conventional T cell development was not perturbed in the absence of PLZF, the frequency of CD1d tetramer positive iNKT cells in the thymus was dramatically reduced (∼9 fold) as compared to wild type mice (Fig. 3A). The specificity of the FACS staining of the iNKT cells is made clear by comparison to CD1d deficient mice28, which are devoid of iNKT cells due to the lack of the selecting ligand and by staining with empty CD1d tetramer (data not shown). The detection of some tetramer positive cells was important for several reasons. First, it proved that the invariant TCRα chain can be generated, which is different than the situation in RORγ-T deficient mice14. Generation of the iNKT TCRα chain was also confirmed by rt-PCR followed by DNA sequencing (data not shown). Secondly, the iNKT cells in the PLZF deficient mice must have undergone positive selection. Random V(D)J recombination of the TCR locus is estimated to produce at least 1015 different TCR combinations29. Therefore, the frequency of 1 tetramer positive cell per ∼2500 thymocytes (∼0.04%) that we find in the PLZF deficient mice (Fig. 3A) must be a consequence of positive selection that results in the enrichment of thymocytes expressing this TCR. Thirdly, the presence of tetramer positive cells suggested that we could directly examine the phenotype and function of iNKT cells in the absence of PLZF.
Fig. 3. Altered iNKT cell development in PLZF deficient mice.

Thymocytes from the indicated mouse lines were stained with CD1d:α-GalCer tetramers and antibodies against CD3, CD44, CD69, CD4 and CD8. (A) PLZF deficient mice have an approximately 9-fold decrease in the number of iNKT cells. Numbers indicate the percentage of cells within the gate. The frequency of iNKT cells in 13 wild type and 14 PLZF deficient mice is shown in the scatter plot. Analysis was done by determining the percentage of tetramer positive cells among the CD3hi thymocytes. Percentages were used rather than absolute numbers to normalize for differences in the total cellularity of the thymus due to the age or sex of the mice. P values are indicated in the figure. (B) iNKT cells from PLZF deficient mice do not upregulate CD44. Both WT and KO iNKT cells upregulate CD69. (C) BrdU incorporation into iNKT cells in the thymus 16-hours after injection into 10-13 day old mice. Both total iNKT cells and only CD44lo iNKT cells are shown. Experiment was repeated twice. (D) iNKT cells from PLZF deficient mice are skewed towards CD4+. Only iNKT cells are shown in the FACS plot. Numbers indicate the percentage of cells in the region. The increase in the percentage of CD4+ iNKT cells and the corresponding decrease in the percentage of CD4− iNKTs in the in PLZF deficient mice in 8 wild type and 9 PLZF deficient mice are shown as a scatter plots. Data set comparisons and P values are indicated in the figure. (E) PLZF deficient iNKT cells have reduced expression of many cell surface markers typical of iNKT cells such as NK1.1, DX5, NKG2D, 2B4 (CD244) and CD122. Change in expression of each marker was confirmed in at least three independent experiments. P values were derived from two-tailed, unpaired Student's t-test. Choices of statistical tests are explained in the methods section.
CD44, which is an activation marker for conventional T cells, is upregulated during development of all iNKT cells in the thymus and is constitutively expressed by all mature iNKT. The tetramer positive iNKT cells found in the thymus of PLZF deficient mice, however, had not upregulated CD44 (Fig. 3B). Nearly half of the PLZF deficient iNKT cells had upregulated CD69, which, like CD44, is a marker of T cell activation that is expressed by most mature iNKT cells. In the thymus, CD69 also serves as a marker for thymocytes that have productively interacted with self-MHC molecules and, therefore, upregulation of CD69 suggests that the PLZF deficient iNKT cells have been signaled through the TCR. The requirement for PLZF is intrinsic to iNKT cells since PLZF deficient bone marrow transferred into irradiated C57Bl/6 hosts resulted in the development of few CD44lo, iNKT cells (Fig. S1). Transfer of bone marrow from wild type littermates into C57Bl/6 hosts resulted in the expected frequencies of iNKT cells that had fully matured to the CD44hiCD69+ stage. Additionally, FACS analysis showed that CD1d expression is not altered in PLZF deficient mice (Fig. S2).
iNKT cells are known to proliferate during the early stages (HSAlo, CD44lo)of thymic development. This expansion appears to be subsequent to positive selection30. Division of iNKT cells in wild type and PLZF deficient mice was tested by analyzing BrdU incorporation into iNKT cells. Young mice were injected with BrdU and sacrificed sixteen hours later. iNKT cells were stained with CD44, CD69, CD1d tetramer, CD3 and then permeabilzed and stained with anti-BrdU. Incorporation of BrdU into CD4−CD8− thymocytes was tested as a positive control (data not shown). The percentage of BrdU positive iNKT cells in the absence of PLZF was found to be similar to wild type (Fig. 3C). Similar results were obtained when either total iNKT cells were compared or when just the CD44lo cells were compared.
Wild type iNKT cells transit through the CD4+CD8+ double positive stage in the thymus9, 14, 31, then downregulate CD8 to become CD4 single positive. Of these CD4 single positive cells, nearly half also downregulate CD43. The iNKT cell population, therefore, always consist of high percentages of both CD4+ and CD4− cells (Fig. 3D). In humans, CD4+ and CD4− iNKT cells have been shown to be functionally distinct32. Intriguingly, in PLZF deficient mice, this ratio is skewed towards the CD4+ iNKT cells (Fig. 3D). NK1.1, DX5, NKG2D, 2B4 (CD244) and CD122, markers that are typically expressed by iNKT cells33, were found to be expressed at substantially reduced levels on PLZF deficient iNKT cells (3E). Finally, nearly all of the PLZF deficient iNKT cells in the thymus downregulated CD24 (Fig. 3E).
The reduction of iNKT cells in the thymus of PLZF deficient mice was not due to increased apoptosis since the cells were negative for Annexin V staining (Fig. S3) and, moreover, because iNKT cells left the thymus and could be detected in the livers of PLZF deficient mice (Fig. 4A). Like in the thymus, the frequency of iNKT cells in the liver was greatly reduced (∼40 fold decrease, Fig. 4A). Also as in the thymus, the PLZF deficient liver iNKT cells were largely CD44lo (Fig. 4B) and were skewed towards being CD4+ (Fig. 4C).
Fig. 4. Reduced frequency and altered phenotype of liver iNKT cells in PLZF deficient mice.

(A) Percoll enriched liver lymphocytes were stained and analyzed as above. PLZF deficient mice had approximately 40-fold fewer iNKT cells. The percentage of iNKT cells among CD3+ cells was used to compare 10 WT and 10 KO mice. Percentages were used rather than absolute numbers to correct for differences in extraction of lymphocytes and potential variations of other cell populations. The P values are indicated in the figure. (B) PLZF deficient liver iNKT cells were mostly CD44lo and CD69lo as compared to wild type iNKT cells. (C) As in the thymus, PLZF deficient iNKT cells are skewed towards expressing CD4. Data from 5 WT and 5 KO mice are shown in the scatter plot along with P values. (D) Liver iNKT cells harvested from older (∼6 months) Fyn deficient mice expressed wild type levels of PLZF. This finding was confirmed in three independent experiments. P values were derived from two-tailed, unpaired Student's t-test. Choices of statistical tests are explained in the methods section.
Although iNKT cell development is severely impaired in the absence of the Src-family kinase Fyn, some iNKT cells do accumulate in the livers of older mice. We analyzed these cells to determine if PLZF expression was dependent on Fyn-mediated signaling events. SLAM family receptor signaling, which is mediated by the adapter protein SAP and dependent on Fyn, is required for the development of iNKT cells. As shown in Fig. 4D, PLZF expression in Fyn deficient iNKT cells was essentially identical to that in wild type iNKT cells, suggesting that SLAM family mediated signals are not required for the expression of PLZF.
PLZF deficient iNKT cells accumulate in the lymph nodes and spleen
Remarkably, in contrast to the dramatic reduction of PLZF deficient iNKT cells in the thymus and liver, PLZF deficient mice actually had approximately 2-fold more iNKT cells in the lymph nodes as compared to wild type mice (Fig. 5A). iNKT cells were also found among the spleen cells of PLZF deficient mice (Fig. 5A), although the frequency was reduced as compared to iNKT cells from wild type spleens (Fig. 5A). PLZF deficient iNKT cells in the lymph nodes and spleen were CD44lo and were mostly CD69neg (Fig.5B). The lymph node PLZF deficient iNKT cells also had a much higher ratio of CD4+ to CD4− cells, as compared to wild type iNKT cells (Fig. 5C). Similarly to PLZF deficient thymic iNKT cells, the spleen and lymph node iNKT cells in PLZF deficient mice expressed greatly reduced levels of NK1.1, DX5, NKG2D, 2B4 and CD122 (Fig. 5D and data not shown). The frequency of NK1.1 positive NKT cells varied in the lymph nodes and spleens of wild type mice, but on average was in agreement with previous reports6. Furthermore, resting PLZF deficient iNKT cells expressed markedly reduced levels of granzyme B (Fig. S4). FasL (Fig. S4) and perforin (data not shown) were also tested, but were not detected on either wild type or PLZF deficient iNKT cells. Finally, RT-PCR for IL-4 expression was done on cDNA made from sorted wild type and PLZF deficient iNKT cells (Fig. S5). “Pre-formed” IL-4 message, which is a characteristic of iNKT cells34, was not detected in PLZF deficient iNKT cells.
Fig. 5. PLZF deficient iNKTs accumulate in the lymph nodes and spleen.
Lymph node (axillary, brachial, inguinal and mesenteric) and spleen cells from the indicated mouse lines were stained with CD1d:α-GalCer tetramers and antibodies against CD3, CD44, CD69, CD4 and CD8. (A) PLZF deficient mice have approximately 2-fold more lymph node iNKT cells and approximately 5-fold fewer spleen iNKT cells. Numbers indicate the percentage of cells within the gate. The frequency of iNKT cells among lymph node cells from 6 wild type and 8 PLZF deficient mice and spleen cells from 8 wild type and 10 PLZF deficient mice are shown in the scatter plots. Analysis was done by determining the percentage of tetramer positive cells among the total CD3+ T cells. Percentages were used rather than absolute numbers to normalize for differences in the total cellularity of the tissues due to the age of the mice. Cellularity of the lymph nodes and the spleens from age matched WT and PLZF KO mice were, however, nearly identical. Data set comparisons and P values are indicated in the figure. (B) iNKT cells from the lymph nodes and spleens of PLZF deficient mice do not upregulate CD44 and tend to be CD69lo. Data is representative of at least five independent experiments. (C) Lymph node PLZF deficient iNKT cells are skewed towards expressing CD4. The increase in the percentage of CD4+ iNKT cells and the corresponding decrease in the percentage of CD4− iNKTs in the lymph nodes and spleens from wild type and PLZF deficient mice are shown in the scatter plots. P values are shown in the figure. (D) PLZF deficient iNKT cells have markedly reduced expression of several markers typically found on wild type iNKT cells from the spleen. P values were derived from two-tailed, unpaired Student's t-test. Choices of statistical tests are explained in the methods section.
Functional analysis of PLZF deficient iNKT cells
iNKT cells secrete copious amounts of various cytokines, including IL-4 and IFNγ, shortly after activation3. To test if PLZF deficient iNKT cells had these critical effector functions, we immunized wild type and PLZF deficient mice with the iNKT cell antigen α-GalCer. Cytokine secretion by the iNKT cells subsequently leads to activation of many other cells of the immune system3. As shown in Fig. 6A, within six hours of iNKT cell activation, B cells, T cells, NK cells and macrophages all upregulated the activation marker CD69 in wild type mice. There is no measurable activation of these cell types, however, following immunization of PLZF deficient mice, similar to the negative control, CD1d deficient mice. IL-2, IL-4 and IFNγ were easily detectable in the serum of wild type animals following immunization, however, these cytokines could not be detected in the PLZF deficient or the control CD1d deficient mice (Fig. 6B).
Fig. 6. PLZF deficient iNKT cells do not rapidly produce cytokines.

(A) Mice (wild type, PLZF deficient, CD1d deficient) were I.P. immunized with 4 gof α-GalCer. Six hours later the spleen was removed, dissociated and stained with the indicated antibodies. Activation of the indicated cell type was measured by upregulation of CD69. (B) Sera were collected from the immunized mice six hours after immunization, diluted 10-fold and ELISAs were done to detect the cytokines IL-4, IFNγ and IL-2. These cytokines were not detected (n.d.) in the PLZF deficient mice or in the CD1d deficient mice. Experiments were done with three animals per group and were repeated twice with nearly identical results. ELISA data represent the means, with standard deviations for the two independent experiments.
The failure to detect cytokines in immunized PLZF deficient mice might be due to the decreased frequency of iNKT cells. Therefore, to further test iNKT cell function, tetramer positive iNKT cells were sorted from the spleen and lymph nodes of wild type and PLZF deficient mice. Sorted cells were activated with PMA/ionomycin for a total of five hours; Brefeldin A was added for the final four hours of the culture. The activated cells were then analyzed for cytokine production by intracellular staining followed by FACS. As expected a large percentage of wild type iNKT cells produced cytokine under these conditions; with approximately half of the activated cells expressing both IL-4 and IFN (Fig. 7A). Remarkably, far fewer PLZF deficient iNKT cells expressed cytokine (Fig. 7A). Of particular interest was the complete loss of the capacity to simultaneously produce both IL-4 and IFNγ. CD4 T cells from wild type and PLZF deficient mice produced some IFNγ and little IL-4 (Fig. 7A).
Fig. 7. PLZF deficient iNKT present an altered cytokine secretion pattern.
(A) Cells were collected by cell sorting and then activated with 50 ng/ml PMA and 500 ng/ml ionomycin for a total of five hours. Brefeldin A was added for the last four hours of the incubation. Cells were then fixed and permeabilized with BD's perm/fix reagents. Permeabilized cells were then stained with antibodies against IL-4 and IFNγ. (B) Single cell suspensions of combined lymph node and spleen cells were depleted of CD8 and MHC class II expressing cells and then stained with the CD1d tetramer and an antibody against TCR Cβ. Tetramer+,TCR Cβ+ cells then were collected by FACS. Approximately 1×104 of each T cell type was activated with 5 μg/ml plate bound anti-CD3 and 5 μg/ml soluble anti-CD28 in the presence of IL-2 and IL-15. Supernatants from the primary activation were collected after 24 hours. Cells were then fed with fresh media containing IL-2 and IL-15. After a total of five days, supernatant was removed and discarded. The cells, in fresh media, were reactivated with plate bound anti-CD3 and soluble anti-CD28. Supernatants were again collected after 24 hours. Supernatants were analyzed for the presence of IL-4 and IFNγ by the use of BD Biosciences Cytokine Bead Arrays. Supernatants were collected and analyzed as described above from sorted T cells that were (C) activated and then (D) reactivated as described above. Each T cell type was set up in duplicate or triplicate depending upon the number of cells collected. Repeats from each experiment were averaged. A total of five independent experiments were done. Means and standard deviations for the five experiments are shown. The value for the PLZF deficient iNKTs (KO iNKT) was compared to the other three cell types. P values are marked as * <0.05; ** <0.005. P values were derived using a two-tailed, unparied Mann-Whitney U-test. Choices of statistical tests are explained in the methods section.
To further analyze the functionality of PZLF deficient iNKT cells, sorted cells were activated with plate bound anti-CD3 and soluble anti-CD28 in the presence of IL-2 and IL-15. The addition of IL-15 was required for the in vitro culture of the wild type iNKT cells. As a control and for comparison, wild type and PLZF deficient CD4 T cells were also collected and grown in culture under the same conditions. Supernatants were removed after twenty-four hours and replaced with fresh media. Five days after the initial activation the T cells were reactivated with plate bound anti-CD3 and soluble anti-CD28. Supernatants were again collected after twenty-four hours.
The supernatants from the primary and secondary activations were analyzed by with BD Biosciences Cytokine Bead Arrays for the presence of a variety of cytokines. As suggested by the experiments in Fig. 6 and Fig. 7A, PLZF deficient iNKT cells produced little IL-4 and IFNγ upon primary activation (Fig. 7B). Failure to produce cytokines was not a general defect in the cells, however, since upon secondary activation, PLZF deficient iNKT cells produced robust amounts of the two cytokines (Fig. 7B). The limited capacity of PLZF deficient iNKT cells to produce IL-4 and IFNγ upon primary activation was in stark contrast to wild type iNKT cells (Fig. 7C). As a comparison, the capacity of sorted CD4 T cells from wild type and PLZF deficient CD4 T cells is also shown (Fig. 7D). Wild type iNKT cells also produced more IL-4 and IFNγ upon secondary activation than PLZF deficient iNKT cells (Fig. 7D).
iNKT cells are known to produce multiple cytokines upon primary activation including IL-3, IL-5, IL-9, IL-10, IL-12p70, IL-13, GMCSF and TNF5. Therefore, we further tested supernatants for the presence of these cytokines (Fig. 8). Wild type iNKT cells produced significantly more IL-13, GMCSF, IL-10 and IL-3 and TNF than PLZF deficient iNKT cells, upon primary activation. IL-5, IL-9 and IL-12p70 were not detected under these activating conditions. Sorted CD4 T cells from wild type and PLZF deficient cells were included as controls.
Fig. 8. Expression of Multiple cytokines are affected by the loss of PLZF expression.
Supernatants generated and collected as for Fig. 7 were analyzed for TNF, IL-13, GMCSF, IL-3, and IL-10 by BD Biosciences Cytokine Bead Arrays. Supernatants were also tested for IL-5, IL-9 and IL-12p70, but these cytokines were not detected under these conditions. Five independent experiments were done. Triplicates from each experiment were averaged and considered as a single data point. Means and standard deviations for the five experiments are shown. The value for the PLZF deficient iNKTs (KO iNKT) was compared to the other three cell types. P values are marked as * <0.05; ** <0.005. P values were derived using a two-tailed, unparied Mann-Whitney U-test. Choices of statistical tests are explained in the methods section.
Secondary activation of the cells showed that the PLZF deficient iNKT cells were capable of producing all of these cytokines (Fig. 8). Wild type iNKT cells produced significantly more IL-13 and IL-10 upon secondary activation. More TNF was produced by the wild type cells than was produced by the PLZF deficient iNKT cells, as well, but this difference did not reach statistical significance. The results of the secondary activation of wild type and PLZF deficient CD4 T cells are included as a control.
Discussion
We show that in the absence of the BTB-ZF gene, PLZF, iNKT cells develop, but fail to acquire many of the salient features characteristic to these cells. In particular, the frequency of PLZF deficient iNKT cells is reduced, the cells do not express high levels of cell surface markers such as NK1.1, DX5, 2B4, CD122 and NKG2D and the cells fail to make numerous cytokines upon primary activation. PLZF deficient cells also expressed reduced levels of Granzyme B and do not have the pre-formed IL-4 mRNA that is characteristic of wild type iNKT cells. Furthermore, PLZF deficient iNKT cells accumulate in the lymph nodes and spleen rather than in the thymus and liver like wild type iNKT cells. Finally, among cells of the immune system, PLZF expression appears to be largely limited to iNKT cells. Together, these data show that PLZF is a critical regulator of iNKT cell differentiation. PLZF is also expressed in human iNKT cells where this highly conserved protein is likely to have a similar function.
PLZF is likely not involved in positive selection of iNKT cells since PLZF deficient mice do develop substantial numbers of T cells expressing the canonical TCR. PLZF is, however, expressed at high levels even in HSAhi, CD44neg iNKT cells, which are the most immature cells that have been described30. Therefore, PLZF expression must occur immediately after or perhaps even during positive selection of iNKT cells.
It is not clear why there is a reduced number of iNKT cells in the thymus and liver and spleen of PLZF deficient mice. The decreased frequency of iNKT cells is not directly a consequence of altered proliferative capacity in the thymus since an equal percentage of the PLZF deficient iNKT cells were labeled with BrdU over a sixteen-hour period. TCRβ thymocytes at the double negative stage of development have been shown to undergo a ∼500-fold expansion within four days35. Each TCRβ clone divides, on average, more than three times a day. It is speculated, however, that different clones may expand with different kinetics. Therefore, it is possible that wild type iNKT cells, like some double negative thymocytes, are undergoing more rounds of division than the PLZF deficient iNKT cells. It is not possible to make conclusions about the total number of cell divisions, since BrudU labeling does not differentiate between cells that have undergone one round or several rounds of proliferation.
There is no indication that PLZF deficient iNKT cells are dying. The cells in the thymus are not annexin V positive and clearly can migrate from the thymus and populate peripheral tissues. Apoptotic thymocytes are rapidly cleared from the thymus, however, so increased cell death remains a formal possibility. It is also possible that PLZF deficient iNKT cells simply do not accumulate in the thymus and liver because they fail to express chemokine receptors necessary for their retention in these tissues. Furthermore, it is known that NK1.1 negative iNKT cells more readily emigrate from the thymus than NK1.1 positive iNKT cells36. Since PLZF deficient iNKT cells do not upregulate NK1.1 it is possible that these cells more efficiently leave the thymus.
It is not known why PLZF is specifically expressed in iNKT cells. It is tempting to speculate that the engagement of SLAM family receptor signals via Fyn results in the expression of PLZF since the SLAM family adapter protein, SAP is required for iNKT cell development13, 37. Furthermore, the SLAM family receptors Slamf1 and Slamf6 were recently shown to be critical for iNKT cell development38. SAP is thought to signal via the SRC family protein tyrosine kinase Fyn and, in the absence of Fyn, there is a dramatic reduction in the frequency of iNKT cells8, 10. PLZF is, however, expressed at wild type levels in Fyn deficient iNKT cells. Preliminary data also shows that the rare CD1d tetramer positive cells found in the thymus of SAP deficient mice express PLZF (data not shown). These data would suggest that SLAM mediated signaling is not essential for PLZF expression. Alternatively, the few iNKT cells that develop in these SAP and Fyn deficient mice might be “escapees” that have managed to express PLZF without appropriate signaling events.
Several genes have been shown to impact the development and/or function of iNKT cells. For example, T-Bet deficient mice have a profound block at stage 2 of iNKT cell development25. RORγt deficient mice are devoid of iNKT cells as a result of a failure to produce the invariant Vα14 TCR due to the short lifespan of CD4+CD8+ thymocytes14. This same report showed that Runx1 is necessary for the very early stages of iNKT cell development. GMCSF (CSF-2) has also been shown to be important for iNKT cells. iNKT cells in GMCSF deficient mice develop normally, but fail to secrete cytokines due to a defect in secretory vesicles39. It was recently shown that mice deficient in the Tec family tyrosine kinase, ITK, have defects in iNKT cell development40, 41. The defects in iNKT cell function and phenotype, while seemingly related, were far milder than the changes in PLZF deficient iNKT cells. CD4 and CD8 T cells in IKT deficient mice have also been shown to have innate-like features42-46. However, the innate-like T cells in ITK deficient mice do not appear to express PLZF (data not shown).
BTB-ZF proteins have typically been found to be transcriptional repressors. For example, BCL6 represses p53 expression in germinal center B cells presumably to prevent the apoptotic cell death that the double stranded DNA breaks associated with affinity maturation should cause47. A second example is LRF, which is thought to suppress Notch mediated signals in hematopoietic stem cells, thereby promoting T cell over B cell commitment19. Gene targets of PLZF include HoxD gene during limb bud formation48 and kit during spermatogonial differentiation49. Neither of these genes, however, has been reported to have a function in iNKT cells. Cell line based studies also suggest that Pbx150,VLA-451, c-myc52 and BID53 are transcriptionally repressed by expression of PLZF. Preliminary gene array data shows that expression of these genes is not altered in PLZF deficient iNKT cells. Expression of Th-POK17, another BTB-ZF gene known to be expressed in iNKT cells, is also not affected by the loss of PLZF expression (data not shown). Perhaps PLZF suppresses unknown suppressors of transcription factors required for cytokine production such as T-Bet54 or RORγt55. Indeed both T-Bet and RORγt have been shown to be essential for iNKT cell development14, 25.
Finally it is interesting to note that PLZF deficient iNKT cells do not have an activated phenotype. It has been generally accepted that iNKT cells are not tolerant to self and that this is likely the reason that the cells maintain an activated phenotype7, 56. Reactivity to self has been proposed to be a consequence of the unique and highly restricted TCRs that are expressed by iNKT cells. The failure of PLZF deficient iNKT cells to maintain the activated phenotype strongly implies that high affinity interactions of the TCR with self-antigens do not play a role in maintaining the activated phenotype of iNKT cells. Rather, it would appear, that expression of PLZF controls this aspect of iNKT cell biology.
Overall, our findings offer fundamental insight into the mechanisms of iNKT cell development. This work also highlights the fundamental role that BTB-ZF genes play in immune system development and function.
Methods
Mice
PLZF deficient mice have been previously described21. PLZF deficient mice were generated in the 129 ES cells. The resulting mice have been backcrossed to the C57Bl/6 for four generations. T-bet deficient mice were purchased from The Jackson Labs, Bar Harbor, ME. All animal work was approved and done in compliance with MSKCC's IACUC guidelines.
Flow cytometry and cell sorting
Cells were prepared and analyzed by standard methods. Antibodies were obtained from BD Biosciences. CD1d:α-GalCer tetramers were prepared according to published protocols57. CD1d:PBS57 tetramers, used for some experiments, were obtained from the NIH Tetramer Core Facility. The Armenian hamster anti-PLZF monoclonal antibody was generated by MSKCC's Monoclonal Antibody Core Facility using standard approaches by immunizing animals with a combination of peptides covering the amino, carboxyl and hinge regions of the protein. During FACS analysis, dead cells were excluded from analysis by the use of the DNA specific dye, DAPI. Cell doublets were excluded by comparing side scatter width to forward scatter area. For iNKT cell analysis, irrelevant cells that were MHC class II positive and CD8 positive were excluded from analysis when possible. Samples were collected on either a Dako CyAn ADP or a BD LSR II. Cell sorts were done by MSKCC's Flow Cytometry Core Facility on a Dako MoFlo, a BD FACS Vantage or a BD FACS ARIA. Post sort analysis was done for all samples to confirm that cell purity was greater than 97%. BrdU labeling of T cells and detection by FACS was done as previously described31.
In Vitro T cell activation
T cells were activated with 5 μg/ml anti-CD3 and 5 μg/ml anti-CD28 in round bottom 96-well plates in the presence of IL-2 and IL-15. Secreted cytokines were detected with BD Biosciences cytokine bead arrays.
Histology
6 μm serial sections were cut and prepared by standard methods. Sections were stained with Dec-205 biotin and UEA-1 – Fitc, followed by streptavidin-PE.
Statistics
Statistical validation of cell number and frequency comparisons (Figs. 3, 4 and 5) was done with unpaired, two-tailed Student's t-test. The use t-tests for samples of this size (approximately 5-10 samples per group) requires an assumption of normality and homogeneity of variance58. These assumptions were valid for these samples. Statistical validation of cytokine data (Figs. 7 and 8) was done with the Mann-Whitney U test, which is a nonparametric alternative to the Student's t-test that is more robust for small sample sizes (4-5 repeats of each experiment). One important difference is that this test does not assume a Gaussian distribution of the data. A second independent test (not shown) was use to confirm the validity of the results. The log value of each data point was calculated prior to applying unpaired, two-tailed Student's t-test. Transforming the data in this way enhances the validity of the t-test since the data take on a more Gaussian distribution. All differences determined to be significant or not significant with the Mann-Whitney U-test were confirmed by this second, independent test. The validity of these tests was discussed and confirmed with MSKCC's Biostatistics Department.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge T. Martillotti for technical assistance; L. Denzin, J. Chaudhuri, E. Pamer, C.-H. Chang for critical reading of the manuscript and excellent advice. We also thank MSKCC's Monoclonal Antibody and Flow Cytometry Core Facilities. This work was partially supported by NIH grant AI059739 and funds from MSKCC. D.K was supported by NIH T32 training grant CA009149.
References
- 1.Petrie HT, Zuniga-Pflucker JC. Zoned out: functional mapping of stromal signaling microenvironments in the thymus. Annu Rev Immunol. 2007;25:649–679. doi: 10.1146/annurev.immunol.23.021704.115715. [DOI] [PubMed] [Google Scholar]
- 2.Rothenberg EV, Anderson MK. Elements of transcription factor network design for T-lineage specification. Dev Biol. 2002;246:29–44. doi: 10.1006/dbio.2002.0667. [DOI] [PubMed] [Google Scholar]
- 3.Bendelac A, Savage PB, Teyton L. The Biology of NKT Cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 4.Bendelac A, Medzhitov R. Adjuvants of immunity: harnessing innate immunity to promote adaptive immunity. J Exp Med. 2002;195:F19–23. doi: 10.1084/jem.20020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 6.Matsuda JL, et al. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med. 2000;192:741–754. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kronenberg M, Engel I. On the road: progress in finding the unique pathway of invariant NKT cell differentiation. Curr Opin Immunol. 2007;19:186–193. doi: 10.1016/j.coi.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Gadue P, Morton N, Stein PL. The Src family tyrosine kinase Fyn regulates natural killer T cell development. J Exp Med. 1999;190:1189–1196. doi: 10.1084/jem.190.8.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dao T, et al. Development of CD1d-restricted NKT cells in the mouse thymus. Eur J Immunol. 2004;34:3542–3552. doi: 10.1002/eji.200425546. [DOI] [PubMed] [Google Scholar]
- 10.Eberl G, Lowin-Kropf B, MacDonald HR. Cutting edge: NKT cell development is selectively impaired in Fyn- deficient mice. J Immunol. 1999;163:4091–4094. [PubMed] [Google Scholar]
- 11.Borowski C, Bendelac A. Signaling for NKT cell development: the SAP-FynT connection. J Exp Med. 2005;201:833–836. doi: 10.1084/jem.20050339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung B, Aoukaty A, Dutz J, Terhorst C, Tan R. Signaling lymphocytic activation molecule-associated protein controls NKT cell functions. J Immunol. 2005;174:3153–3157. doi: 10.4049/jimmunol.174.6.3153. [DOI] [PubMed] [Google Scholar]
- 13.Pasquier B, et al. Defective NKT cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J Exp Med. 2005;201:695–701. doi: 10.1084/jem.20042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egawa T, et al. Genetic evidence supporting selection of the Valpha14i NKT cell lineage from double-positive thymocyte precursors. Immunity. 2005;22:705–716. doi: 10.1016/j.immuni.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Kelly KF, Daniel JM. POZ for effect--POZ-ZF transcription factors in cancer and development. Trends Cell Biol. 2006;16:578–587. doi: 10.1016/j.tcb.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Dent AL, Shaffer AL, Yu X, Allman D, Staudt LM. Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science. 1997;276:589–592. doi: 10.1126/science.276.5312.589. [DOI] [PubMed] [Google Scholar]
- 17.He X, et al. The zinc finger transcription factor Th-POK regulates CD4 versus CD8 T-cell lineage commitment. Nature. 2005;433:826–833. doi: 10.1038/nature03338. [DOI] [PubMed] [Google Scholar]
- 18.Sun G, et al. The zinc finger protein cKrox directs CD4 lineage differentiation during intrathymic T cell positive selection. Nat Immunol. 2005;6:373–381. doi: 10.1038/ni1183. [DOI] [PubMed] [Google Scholar]
- 19.Maeda T, et al. Regulation of B versus T lymphoid lineage fate decision by the proto-oncogene LRF. Science. 2007;316:860–866. doi: 10.1126/science.1140881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McConnell MJ, Licht JD. The PLZF gene of t (11;17)-associated APL. Curr Top Microbiol Immunol. 2007;313:31–48. doi: 10.1007/978-3-540-34594-7_3. [DOI] [PubMed] [Google Scholar]
- 21.Barna M, Hawe N, Niswander L, Pandolfi PP. Plzf regulates limb and axial skeletal patterning. Nat Genet. 2000;25:166–172. doi: 10.1038/76014. [DOI] [PubMed] [Google Scholar]
- 22.Matsuda JL, Gapin L. Developmental program of mouse Valpha14i NKT cells. Curr Opin Immunol. 2005;17:122–130. doi: 10.1016/j.coi.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Zelent A, Guidez F, Melnick A, Waxman S, Licht JD. Translocations of the RARalpha gene in acute promyelocytic leukemia. Oncogene. 2001;20:7186–7203. doi: 10.1038/sj.onc.1204766. [DOI] [PubMed] [Google Scholar]
- 24.Koken MH, et al. Leukemia-associated retinoic acid receptor alpha fusion partners, PML and PLZF, heterodimerize and colocalize to nuclear bodies. Proc Natl Acad Sci U S A. 1997;94:10255–10260. doi: 10.1073/pnas.94.19.10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Townsend MJ, et al. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 2004;20:477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 26.Costoya JA, et al. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet. 2004;36:653–659. doi: 10.1038/ng1367. [DOI] [PubMed] [Google Scholar]
- 27.Pellicci DG, et al. DX5/CD49b-positive T cells are not synonymous with CD1d-dependent NKT cells. J Immunol. 2005;175:4416–4425. doi: 10.4049/jimmunol.175.7.4416. [DOI] [PubMed] [Google Scholar]
- 28.Mendiratta SK, et al. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity. 1997;6:469–477. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- 29.Slansky JE. Antigen-specific T cells: analyses of the needles in the haystack. PLoS Biol. 2003;1:E78. doi: 10.1371/journal.pbio.0000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benlagha K, Wei DG, Veiga J, Teyton L, Bendelac A. Characterization of the early stages of thymic NKT cell development. J Exp Med. 2005;202:485–492. doi: 10.1084/jem.20050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gapin L, Matsuda JL, Surh CD, Kronenberg M. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat Immunol. 2001;2:971–978. doi: 10.1038/ni710. [DOI] [PubMed] [Google Scholar]
- 32.Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. 2002;195:625–636. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammond KJ, et al. NKT cells are phenotypically and functionally diverse. Eur J Immunol. 1999;29:3768–3781. doi: 10.1002/(SICI)1521-4141(199911)29:11<3768::AID-IMMU3768>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 34.Stetson DB, et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198:1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falk I, Potocnik AJ, Barthlott T, Levelt CN, Eichmann K. Immature T cells in peripheral lymphoid organs of recombinase-activating gene-1/-2-deficient mice. Thymus dependence and responsiveness to anti-CD3 epsilon antibody. J Immunol. 1996;156:1362–1368. [PubMed] [Google Scholar]
- 36.Pellicci DG, et al. A natural killer T (NKT) cell developmental pathway iInvolving a thymus-dependent NK1.1(−)CD4(+) CD1d-dependent precursor stage. J Exp Med. 2002;195:835–844. doi: 10.1084/jem.20011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nichols KE, et al. Regulation of NKT cell development by SAP, the protein defective in XLP. Nat Med. 2005;11:340–345. doi: 10.1038/nm1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Griewank K, et al. Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity. 2007;27:751–762. doi: 10.1016/j.immuni.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bezbradica JS, et al. Granulocyte-macrophage colony-stimulating factor regulates effector differentiation of invariant natural killer T cells during thymic ontogeny. Immunity. 2006;25:487–497. doi: 10.1016/j.immuni.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 40.Felices M, Berg LJ. The tec kinases itk and rlk regulate NKT cell maturation, cytokine production, and survival. J Immunol. 2008;180:3007–3018. doi: 10.4049/jimmunol.180.5.3007. [DOI] [PubMed] [Google Scholar]
- 41.Au-Yeung BB, Fowell DJ. A key role for Itk in both IFN gamma and IL-4 production by NKT cells. J Immunol. 2007;179:111–119. doi: 10.4049/jimmunol.179.1.111. [DOI] [PubMed] [Google Scholar]
- 42.Broussard C, et al. Altered development of CD8+ T cell lineages in mice deficient for the Tec kinases Itk and Rlk. Immunity. 2006;25:93–104. doi: 10.1016/j.immuni.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 43.Horai R, et al. Requirements for selection of conventional and innate T lymphocyte lineages. Immunity. 2007;27:775–785. doi: 10.1016/j.immuni.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu J, Sahu N, Walsh E, August A. Memory phenotype CD8+ T cells with innate function selectively develop in the absence of active Itk. Eur J Immunol. 2007;37:2892–2899. doi: 10.1002/eji.200737311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berg LJ. Signalling through TEC kinases regulates conventional versus innate CD8(+) T-cell development. Nat Rev Immunol. 2007;7:479–485. doi: 10.1038/nri2091. [DOI] [PubMed] [Google Scholar]
- 46.Hu J, August A. Naive and innate memory phenotype CD4+ T cells have different requirements for active Itk for their development. J Immunol. 2008;180:6544–6552. doi: 10.4049/jimmunol.180.10.6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phan RT, Dalla-Favera R. The BCL6 proto-oncogene suppresses p53 expression in germinal-centre B cells. Nature. 2004;432:635–639. doi: 10.1038/nature03147. [DOI] [PubMed] [Google Scholar]
- 48.Barna M, et al. Plzf mediates transcriptional repression of HoxD gene expression through chromatin remodeling. Dev Cell. 2002;3:499–510. doi: 10.1016/s1534-5807(02)00289-7. [DOI] [PubMed] [Google Scholar]
- 49.Filipponi D, et al. Repression of kit expression by Plzf in germ cells. Mol Cell Biol. 2007;27:6770–6781. doi: 10.1128/MCB.00479-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shiraishi K, et al. Pre-B-cell leukemia transcription factor 1 is a major target of promyelocytic leukemia zinc-finger-mediated melanoma cell growth suppression. Oncogene. 2007;26:339–348. doi: 10.1038/sj.onc.1209800. [DOI] [PubMed] [Google Scholar]
- 51.Quaranta MT, et al. PLZF-mediated control on VLA-4 expression in normal and leukemic myeloid cells. Oncogene. 2006;25:399–408. doi: 10.1038/sj.onc.1209060. [DOI] [PubMed] [Google Scholar]
- 52.McConnell MJ, et al. Growth suppression by acute promyelocytic leukemia-associated protein PLZF is mediated by repression of c-myc expression. Mol Cell Biol. 2003;23:9375–9388. doi: 10.1128/MCB.23.24.9375-9388.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parrado A, et al. The promyelocytic leukemia zinc finger protein down-regulates apoptosis and expression of the proapoptotic BID protein in lymphocytes. Proc Natl Acad Sci U S A. 2004;101:1898–1903. doi: 10.1073/pnas.0308358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Szabo SJ, et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 55.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 56.Kronenberg M, Rudensky A. Regulation of immunity by self-reactive T cells. Nature. 2005;435:598–604. doi: 10.1038/nature03725. [DOI] [PubMed] [Google Scholar]
- 57.Yu KO, et al. Production and characterization of monoclonal antibodies against complexes of the NKT cell ligand alpha-galactosylceramide bound to mouse CD1d. J Immunol Methods. 2007;323:11–23. doi: 10.1016/j.jim.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lamb TJ, Graham AL, Petrie A. T testing the immune system. Immunity. 2008;28:288–292. doi: 10.1016/j.immuni.2008.02.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.