Abstract
Endothelin (ET)-1 is a chemical mediator released by the body at sites of injury and disease and is involved in various painful states. This study examined whether ET-1 exposure in the neonatal period alters subsequent ET-1 induced nociception and expression of the ETB receptor. ET-1 or saline was administered to postnatal day 7 rats. On postnatal day 11, ET -1 or saline was administered; a first exposure to ET-1 for one group, and a second exposure to ET-1 for another group. A statistically significant increase in ET-1 induced paw flinching was observed in postnatal day 11 male rats exposed to ET-1 for the second time as compared to male rats exposed to ET-1 for the first time. In contrast, a statistically significant decrease in ET-1 induced paw flinching was observed in postnatal day 11 female rats exposed to ET-1 for the second time as compared to female rats exposed to ET-1 for the first time. Furthermore, in males a positive correlation was found between ET-1 induced paw flinching on postnatal day 7 versus 11. In contrast, in females a negative correlation was found between ET-1 induced paw flinching on postnatal day 7 versus 11. Changes in behavioral sensitivity to ET-1 were accompanied by sex-specific ET-1 induced changes in expression of the ETB receptor on postnatal day 11 in the plantar hindpaw with a statistically significant decrease and increase in ETB receptor expression in males and females, respectively. These findings suggest that ET-1 exposure in the neonatal period sex-specifically alters expression of the ETB receptor and behavioral sensitivity to ET-1 whereby males show sensitization and females show de-sensitization.
Keywords: endothelin, nociception, neonatal rat
It has long been thought that the immaturity of the nervous system in infants and children precluded the sensory and emotional experience of pain within this patient population. Furthermore, even if they did experience pain, without the cognitive development the painful experience would have little long-term consequence. However, this idea has been replaced by our current understanding that infants and children not only experience pain [2, 30], but in fact, may have greater distress in response to painful stimuli than do older children [13, 30]. In addition, there is good evidence that a history of acute painful events early in life can alter pain intensity and distress upon later pain experiences [26, 29].
The majority of basic science studies in this area have applied an injury to rodents during the first few days after birth which models the third trimester/preterm human infant [1, 20, 27]. Many of these studies utilize exogenous substances such as carrageenan and complete Freud’s adjuvant to produce an injury that endures for days to weeks in a rodent, thus, modeling a prolonged inflammatory pain in a human preterm infant. In contrast, the vast majority of under-treated pain in the hospital setting involves full term infants and young children, that in general, experience acute nociceptive pain, such as that following surgery or during acute procedures such as venipuncture or immunizations [3, 31]. To this end, this study uses endothelin (ET) -1, an endogenous vaso-active peptide normally released at sites of injury and disease, to model an acute nociceptive experience in rats that are developmentally equivalent to a full-term human infant [11].
ET-1 has been selected as the algogen in this model as it is produced endogenously by the body following tissue injury or inflammation and has been established to be an endogenous mediator of nociception [17]. Our lab has previously demonstrated that intraplantar administration of ET-1 induces age specific nociceptive-related paw flinching behaviors in rats in which younger rats exhibit greater and a longer duration of nociceptive-associated behaviors than similarly exposed adult rats [21]. In addition, age-specific sex differences were noted in that younger males had greater behaviors than younger females. The present study tests the postulate that exposure to ET-1 early in development alters subsequent ET-1 induced nociception. The goal of this design is to provide a model in which to study the mechanisms of pain sensitization in the neonatal period. Pain sensitization has been reported in human infants that have undergone circumcision at birth [29], or a surgical procedure in the first 3 months of life [24]. In this study, the first exposure to ET-1 is on postnatal (P) day 7, a time point that is approximately equivalent to the nervous system development of a full-term human infant [11]. The second exposure to ET-1 is on P11, a time point that is approximately equivalent to a human toddler [11].
All methods were approved by the Institutional Animal Care and Use Committee at the University of South Carolina. Efforts were made to limit distress and to use the minimum number of animals for statistical analysis. Male and female Sprague-Dawley rats (Charles River, MA) were housed with dams in a 12/12h light/dark cycle with free access to food and water. Litters were culled at 12 pups/dam with approximate 50:50 male:female ratios. All treatment groups were equally represented within each litter to control for differences in maternal care. No more than two males and two females from each treatment group were used from any one litter.
ET-1 was purchased from American Peptide (Sunnyvale, CA). A stock ET-1 solution was made fresh by dissolving in sterile endotoxin free saline at a concentration of 1 mg/mL. ET-1 (3.3 nmol in 10 μl) was administered intraplantar in the left hindpaw. Four treatment paradigms were employed across eight litters of rats. Group one (n=8-10/sex) was exposed to 3.3 nmoles of ET-1 on both P7 and P11 (ET1(P7) + ET1(P11)). Group two (n=8-10/sex) was exposed to saline on P7 and 3.3 nmoles of ET-1 on P11 (S(P7) + ET1(P11)). Group three (n=8-10/sex) was exposed to saline on both P7 and P11 (S(P7) + S(P11)). Group four (n=8-10/sex) was exposed to 3.3 nmoles of ET-1 on P7 and saline on P11 (ET1(P7) + S(P11)). After intraplantar administration of saline or ET-1 on P7, rats were placed in plexiglas boxes and videotaped for 75 minutes. Animals were maintained at nest temperature by overhead heat lamps. After video recording on P7, animals were returned to the dam in the home cage until P11 when the same procedure was repeated.
The videotapes were analyzed by an experimenter blinded to animal treatments. Akin to the formalin model, intraplantar administration of ET-1 induced spontaneous paw flinching. Paw flinching was counted in fifteen 5 minute intervals for 75 minutes. Significance was determined using a repeated measures ANOVA across time course or one-way ANOVA for the summed behavior over the entire time course followed by Bon Ferroni pairwise comparisons. For animals that were exposed to ET-1 on P7 and P11, a Pearson’s Correlation was used to assess the relationship between paw flinching behaviors on P7 and P11. A value of P <.05 was considered significant. All statistical analysis was done with GraphPad Prism 4.0 (GraphPad Software, Inc., San Diego, CA).
An additional three litters of rats were used to generate tissue for western analysis of ETB receptor expression in the plantar hindpaw skin on P11 following intraplantar administration of 10 μl of 3.3 nmol ET-1, saline, or nothing in the left hindpaw on P7 (n=3-6 rats/sex/treatment). On P11 rats were deeply asphyxiated with CO2 followed by rapid decapitation. Plantar skin from the left hindpaw was quickly removed and frozen on dry ice and stored at −80 °C until homogenization. Skin was glued with the dermal side up to the bottom of a 96 well plate. Skin was then homogenized using a Dremel tool (with tip #191) in 500 μl of lysis buffer (50 mM Tris, 150 nM NaCl, 1% NP-40, 1 mM EDTA, Sigma Protease Inhibitor Cocktail P8340 at 1:100). Following homogenization the sample was incubated for 1 hour on ice with vortexing and trituration of samples every 10 minutes. Samples were spun at 15,700 g for 30 min at 4 °C. The detergent NP-40 is used in the lysis buffer so that membrane bound proteins can be collected in the supernatant. Supernatent was removed and protein quantification was done by BCA protein assay (Pierce, Thermo Scientific). Thirty five μg of total protein was used for polyacrylamide gel electrophoresis followed by protein transfer to nitrocellulose membrane for western analysis. Membranes were blocked in 5% powdered milk in phosphate buffered saline with 1% Tween for 1 hour at room temperature. Membranes were incubated overnight at 4 °C with the ETB receptor antibody (1:400, Abcam, Cambridge, MA). Membranes were washed several times and then placed in HRP labeled secondary donkey anti-rabbit antibody (Jackson Immunoresearch) for 1 hour at room temperature. Membranes were washed several times and bands were visualized using ECL. The same protocol was followed a second time using a primary antibody against β-actin (1:20,000 Sigma) as a loading control. Films were scanned and ETB bands were normalized to β-actin. Significance was determined with one-way ANOVA followed by a Bon Ferroni pairwise comparison.
Administration of ET-1 to P7 rats produced spontaneous paw flinching in male and female rats as previously published (data not shown) [21]. On P11, hindpaw flinching was scored for two 5 minute observation periods for a total of 10 minutes prior to a second exposure to saline or ET-1. In male rats, no statistical difference was observed in the number of hindpaw flinches in 5 minute intervals between naïve P11 rats (3.0 ± 0.37, avg ± SEM, n=8), P11 rats exposed to saline on P7 (1.9 ± 0.62, n=8), and P11 rats exposed to ET-1 on P7 (4.0 ± 0.50, n=8). Similarly, in female rats no statistical difference was observed in the number of hindpaw flinches in 5 minute intervals between naïve P11 rats (2.6 ± 0.06, n=8), P11 rats exposed to saline on P7 (3.8 ± 0.38, n=8), and P11 rats exposed to ET-1 on P7 (2.5 ± 0.21, n=7). Thus, on P11 spontaneous paw flinching behaviors were identical in all groups prior to a second exposure to saline or ET-1 or naïve handling control animals.
Male rats exposed to ET-1 for the second time on P11 displayed an increase in the number of paw flinches across the 75 minute observation as compared to males that were exposed to ET-1 for the first time on P11 (Fig. 1A,B). Intraplantar saline on P11 elicited little paw flinching with no significant difference in behavior between male rats exposed to ET-1 versus saline on P7 (Fig. 1A, B). A positive correlation was observed between ET-1 induced paw flinching on P7 and P11 (Fig. 1C). The greater the behaviors on P7 the larger the behavioral sensitization to ET-1 observed on P11 (R2=0.54, P=0.0149) suggesting that the degree of sensitization is proportional to the magnitude of early nociceptive experience.
Figure 1. ET-1 induced sensitization in males.
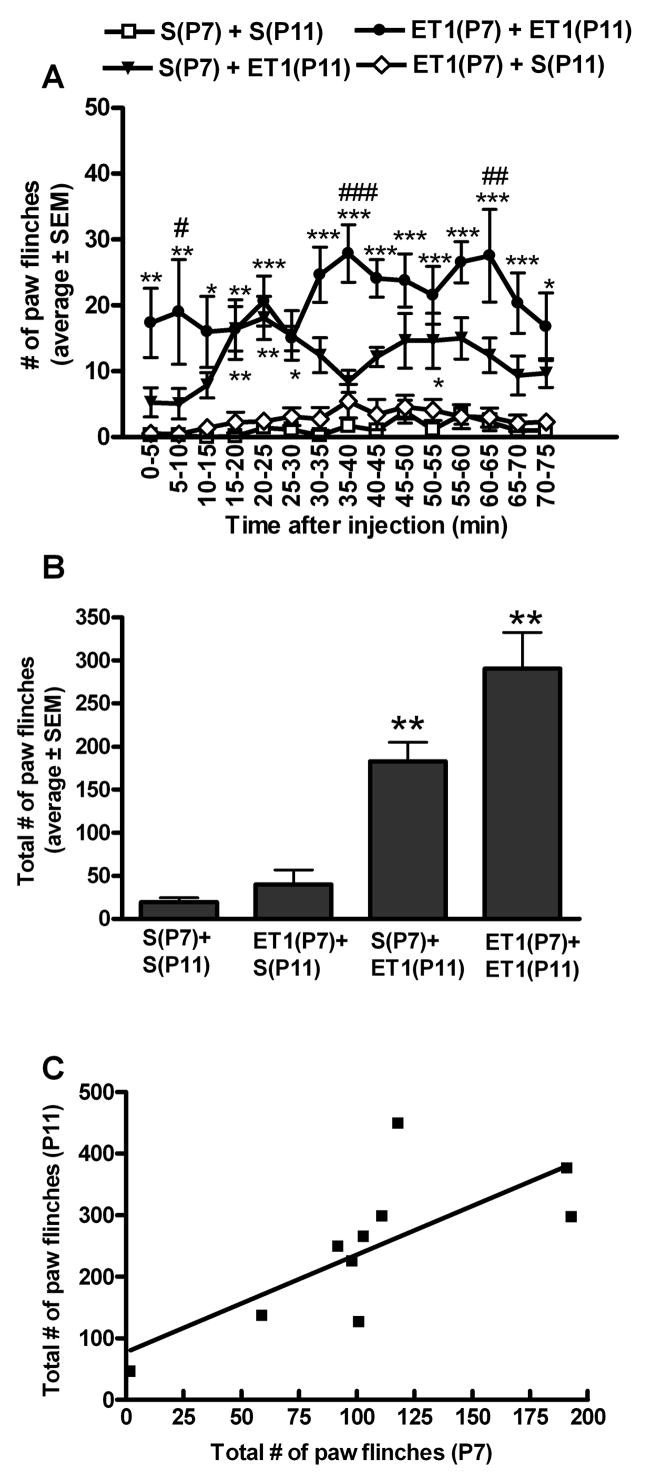
A. In P11 male rats, a second intraplantar exposure to ET-1 induced more spontaneous nociceptive-associated paw flinching compared to P11 male rats exposed to ET-1 for the first time. (*, **, ***p<.05, .01, .001 compared with 3.3(P7) + 3.3(P11) or S(P7) + 3.3(P11) vs. S(P7) + S(P11) or 3.3(P7) + S(P11). #, ##, ####p<.05, .01, .001 compared with 3.3(P7) + 3.3(P11) vs S(P7) + 3.3(P11) ) B. Across the entire 75 minute observation period male rats exposed to ET-1 had significantly more paw flinching compared to saline exposed rats (**P<0.01 compared to animals exposed to saline on P11 independent of the exposure on P7). Rats exposed to ET-1 for the second time on P11 had significantly more paw flinching as compared to those exposed to ET-1 for the first time on P11 (#P<0.05). C. In male rats, a positive correlation between total ET-1 induced paw flinching on P7 and subsequent ET-1 induced paw flinching on P11 was observed (P=0.0149). (n=9-10 males/group).
In contrast, female rats exposed to ET-1 for the second time on P11 had a decrease in the number of paw flinches across the 75 minute observation as compared to females that were exposed to saline on P7 followed by ET-1 on P11 (Fig. 2A,B). Intraplantar saline on P11 elicited little paw flinching with no significant difference in behavior between female rats exposed to ET-1 versus saline on P7 (Fig. 2A, B). A negative correlation was observed between ET-1 induced paw flinching on P7 and P11 (Fig. 2C). The greater the behaviors on P7 the larger the behavioral de-sensitization to ET-1 observed on P11 (R2=0.49, P=0.0243) suggesting that the degree of de-sensitization is proportional to the magnitude of early nociceptive experience.
Figure 2. ET-1 induced de-sensitization in females.
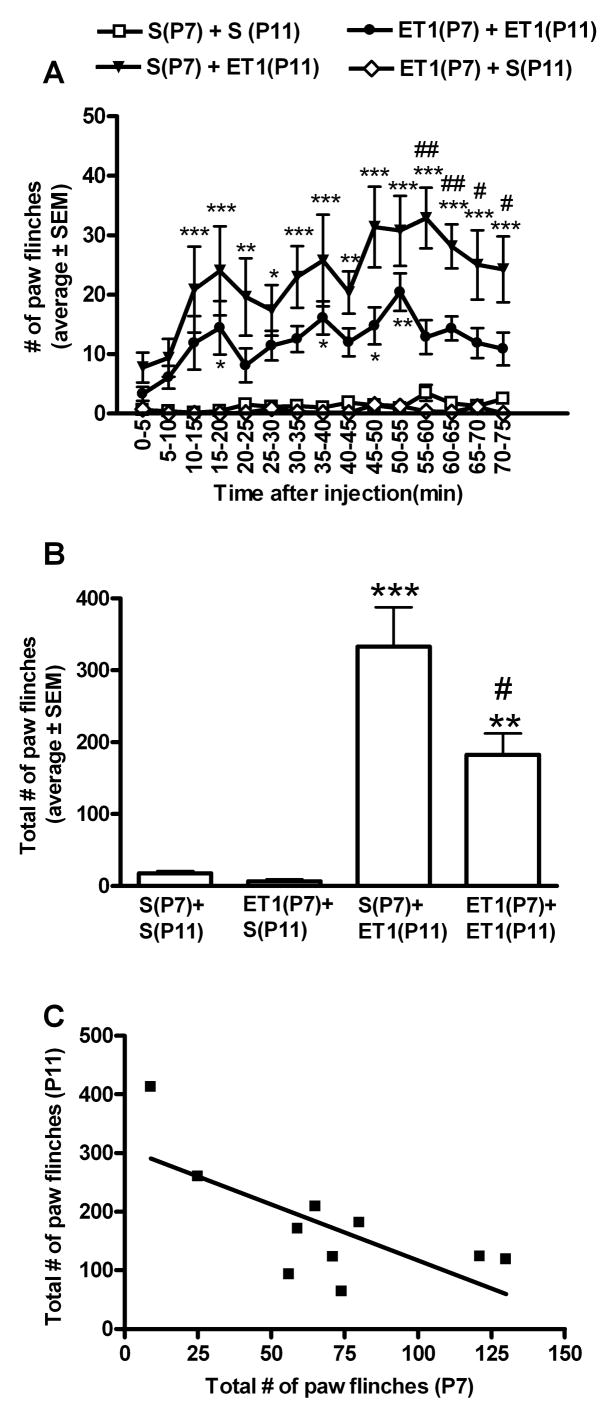
A. In P11 female rats, a second intraplantar exposure to ET-1 induced fewer spontaneous nociceptive-associated paw flinching compared to P11 female rats exposed to ET-1 for the first time. (*, **, ***p<.05, .01, .001 compared with 3.3(P7) + 3.3(P11) or S(P7) + 3.3(P11) vs. S(P7) + S(P11) or 3.3(P7) + S(P11). #, ##, ####p<.05, .01, .001 compared with 3.3(P7) + 3.3(P11) vs S(P7) + 3.3(P11)) B. Across the entire 75 minute observation period female rats exposed to ET-1 had significantly less paw flinching compared to saline exposed rats (**,***P<0.01,0.001 compared to animals exposed to saline on P11 independent of the exposure on P7). Rats exposed to ET-1 for the second time on P11 had significantly less paw flinching as compared to those exposed to ET-1 for the first time on P11 (#P<0.05). C. In female rats, a negative correlation between total ET-1 induced paw flinching on P7 and subsequent ET-1 induced paw flinching on P11 was observed (P=0.0243). (n=8 females/group).
On P11, a two way ANOVA comparison between the sexes showed greater ET-1 induced paw flinching across the time course in females exposed to saline on P7 compared to males exposed to saline on P7 (p<0.01). Furthermore, on P11 there was a statistical difference in the total number of ET-1 induced paw flinches with more flinching in female rats exposed to saline on P7 (333.0 ± 155.2) as compared to male rats exposed to saline on P7 (182.5 ± 84.3; P=0.03). In contrast, a priming exposure to ET-1 on P7 produces an opposite sex-specific sensitivity to ET-1 on P11 whereby ET-1 induced paw flinching across time is significantly higher in male rats exposed to ET-1 on P7 as compared to female rats exposed to ET-1 on P7 (two way ANOVA comparison, p<0.01). Furthermore, on P11 there was a statistical difference in the total number of ET-1 induced paw flinches with more flinching in male rats exposed to ET-1 on P7 (290.4 ± 129.1) as compared to female rats exposed to ET-1 on P7 (182.7 ± 70.6; P=0.05). This suggests that early exposure to ET-1 reverses sex-specific behavioral sensitivity to ET-1 such that sex-specific sensitivity to a repeat exposure to ET-1 (on P11) is opposite to that of a first time exposure to ET-1 on P11 (Figures 1 and 2).
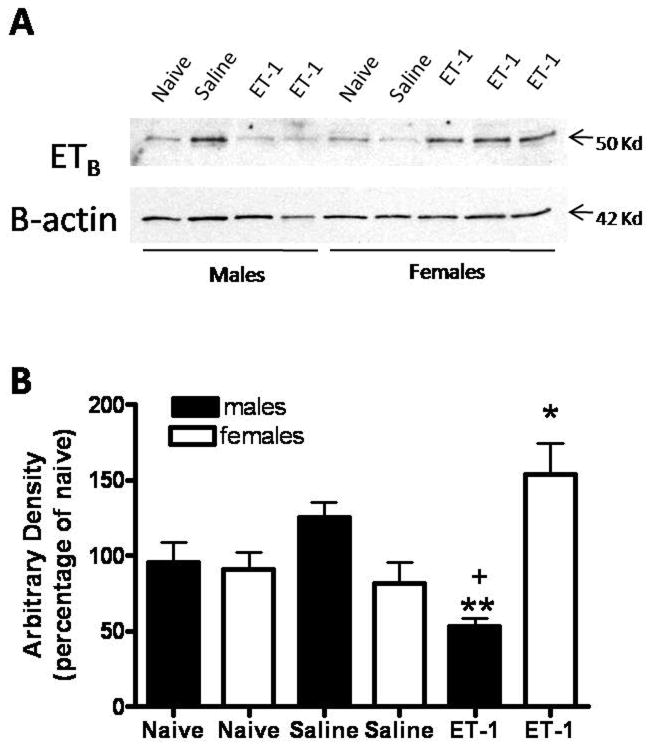
Endothelin-induced nociception in the periphery is mediated by interactions with two receptors, the ETA and the ETB receptors. The ETA receptor has been reported to be pronociceptive and is located in the peripheral epidermal terminals of nociceptors [18]. In contrast, the ETB receptor has been reported to be anti-nociceptive and is located on keratinocytes in the epidermis [18]. In the current study, western analysis was used to examine the expression of ETB receptors in the plantar hindpaw skin on P11 following administration of ET-1 or saline on P7. Expression of ETB in skin on P11 was not statistically different in naïve normal male and female animals (densitometry data not shown). There was an insignificant trend of increased ETB expression in P11 males rats exposed to saline on P7 as compared to female rats exposed to saline on P7 (Fig. 3). Administration of ET-1 on P7 sex-dependently modulates the expression of the ETB receptor in skin on P11 (Fig. 3). In male rats, a statistically significant down-regulation of ETB receptor expression in the left plantar hindpaw skin was observed on P11 in animals exposed to ET-1 on P7 as compared to saline treated (p<0.01) or normal naïve rats (p<0.05) (Fig. 3). In female rats, a statistically significant up-regulation of ETB expression in the left plantar hindpaw skin was observed on P11 in animals exposed to ET-1 on P7 as compared to saline treated rats (p<0.05) (Fig. 3). This suggests that sex-dependent modulation of ET-1 induced paw flinching may be via sex-dependent regulation of ETB receptor expression following exposure to ET-1 on P7.
Figure 3.
Sex-dependent endothelin-1 induced changes in ETB receptor expression in the skin. A. In male rats, hind paw intraplantar administration of ET-1, but not saline, on P7 decreases expression of the ETB receptor in the ipsilateral plantar hind paw skin on P11. In female rats, hind paw intraplantar administration of ET-1, but not saline, on P7 increases expression of the ETB receptor in the ipsilateral plantar hind paw skin on P11. B. Densitometry analysis of ETB receptor expression in the ipsilateral left plantar hindpaw skin. ETB receptor expression is normalized to β actin and expressed at a percentage of expression in skin from same sex normal naïve P11 rats. *,**P<0.05,0.01 compared to saline exposure. +P<0.05 compared to naïve. (n=3-6/sex/treatment).
Conflicting reports on neonatal pain suggests both long-term hyper-and hypo-sensitivity to pain resulting from painful insults early in development [1, 20, 27]. Whether an early painful insult produces hyper- or hypo-sensitivity to pain is postulated to be dependent, at least in part, by the age of the infant as well as the duration of the injury [28]. The sex-differences observed in the present study suggest that sex may also be a factor underlying the discrepancy observed in the clinical literature. In the present study, ET-1 exposure early in development resulted in behavioral sensitization in males (Fig. 1) and de-sensitization in females (Fig. 2). The correlation between ET-1 induced paw flinching on P7 and P11 demonstrates that the magnitude of sensitization and de-sensitization are dependent upon the magnitude of paw flinching during early development. Both male and female rats with the most ET-1-induced paw flinching on P7 had the greatest behavioral sensitization or de-sensitization on P11, respectively. This change in behavioral response to intraplantar ET-1 is accompanied by a sex-dependent change in ETB receptor expression in the plantar hind paw skin (Fig. 3). In male rats, a priming exposure to ET-1 on P7 decreased expression of the ETB receptor in the plantar hindpaw skin and increased behavioral sensitivity to ET-1 on P11. In contrast, when females are exposed to ET-1 priming on P7 there is an increase in expression of the ETB receptor in the plantar hindpaw skin and decreased behavioral sensitivity to ET-1 on P11.
Current literature suggests that the role of ETB in ET-1 induced nociception is highly complex and dependent upon the pathological state of the animal [17]. ETB agonists have been shown to reduce ET-1 induced spontaneous paw flinching in adult male rats at similar doses of ET-1 as used in the current study [18]. In contrast, ETB receptor antagonists have been shown to reduce ET-1 induced mechanical hyperalgesia in naïve animals and in acute and chronic inflammatory models [6]. This apparent contradiction may be related to plasticity within the endothelin system following a priming nociceptive insult. In the ovalbumin model of chronic arthritis, ETB agonists increase licking time in animals exposed to ovalbumin for the first time while antagonists of ETB enhance licking time following a challenge dose of ovalbumin [25]. Similarly, if ET-1 is used as a priming insult by administration prior to an inflammatory insult then administration of an ETB agonist exerts anti-nociceptive effects [10]. Plasticity within ETB appears to be important in nociceptive priming, as illustrated by the evidence that antagonism of ETB enhances carrageenan-induced nociceptive priming while pre-treatment with an ETB agonist prevents nociceptive-induced priming [10]. In line with these reports, our current findings suggest that a priming exposure to ET-1 may sex-dependently modulate the expression of ETB and hence decrease or increase effectiveness of ETB targeted therapies accordingly.
There is a growing literature examining sex differences in pain perception, but few studies discriminate between the activational and organizational role of gonadal hormones. In the cardiovascular system, gonadal steroids have an activational effect modulating endothelin receptor expression and function in the fully developed adult organism [12, 16]. In contrast, organization effects result in sexually dimorphic neuronal connections that underlie sex-dependent physiology and behavior [9]. It has been previously demonstrated that sex-dependent opioid responses in adult animals are a result of an organizational effect of gonadal hormones early in development [8, 19]. Similarly, gonadal hormones have been shown to have an organizational effect on threshold for electrical shock in male rats [7]. Thus, in the present study, ET-1 induced priming may be interacting with these organizational effects resulting in sex-specific behavioral sensitization or de-sensitization to ET-1. This sex-specific alteration in ET-1 induced behavioral sensitivity appears to still be present in young adult rats (unpublished data).
From the current study, the skin may be one anatomical site for these organizational effects. Following a priming exposure to ET-1, ETB expression may be modulated by direct hormonal actions on keratinocytes [23] or indirectly via sex-hormone modulation of immune cells and inflammation. Clinical literature suggests that wound healing is inversely correlated with circulating testosterone levels [4]. While estrogen accelerates wound healing in both human and rodent models [5, 22], these same models suggest that testosterone may prolong wound healing [15]. In addition, androgen receptors are located on immune cells that mediate inflammation [14]. Thus, in neonatal pre-pubescent rodents, a priming exposure to ET-1 may produce a gonadal hormone modulated inflammatory response that is enhanced in male rodents while suppressed in female rodents leading to sex-specific changes in ETB expression and behavioral sensitivity to ET-1.
In summary, these findings show that a nociceptive insult during a developmental window equivalent to a human full-term infant, results in changes in behavioral sensitivity to ET-1 and expression of ETB in the skin on P11. These results provide further evidence for the postulate that pain experiences early in development result in a physiological “memory” of pain which alters pain response later in life [26, 28]. In the current study, changes in behavioral sensitivity to ET-1 appear to be sex-specific with a sensitization in males and a de-sensitization in females that is paralleled by sex-dependent changes in ETB receptor expression in the skin. Understanding the complex interplay of prior ET-1 exposure (as will occur following any tissue injury) and sex-dependent regulation of ETB expression and activity is important for understanding the role of the endothelin system in a number of painful pathological injuries and diseases. This work supports the endothelin system as a rationale therapeutic target for the development of novel analgesics for application in pediatric patients.
Acknowledgments
This work was funded by a University of South Carolina Research and Productive Scholarship Grant, a National Institutes of Health grant R01 DA023593 and the South Carolina Post-Baccalaureate Research Education Programs for Minorities funded by grants R25 GM066526 and R25 GM076277 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anand KJ, Coskun V, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term behavioral effects of repetitive pain in neonatal rat pups. Physiol Behav. 1999;66:627–37. doi: 10.1016/s0031-9384(98)00338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anand KJ, Hickey PR. Halothane-morphine compared with high-dose sufentanil for anesthesia and postoperative analgesia in neonatal cardiac surgery. N Engl J Med. 1992;326:1–9. doi: 10.1056/NEJM199201023260101. [DOI] [PubMed] [Google Scholar]
- 3.Anand KJ, Johnston CC, Oberlander TF, Taddio A, Lehr VT, Walco GA. Analgesia and local anesthesia during invasive procedures in the neonate. Clin Ther. 2005;27:844–76. doi: 10.1016/j.clinthera.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Ashcroft GS, Mills SJ. Androgen receptor-mediated inhibition of cutaneous wound healing. J Clin Invest. 2002;110:615–24. doi: 10.1172/JCI15704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashcroft GS, Mills SJ, Lei K, Gibbons L, Jeong MJ, Taniguchi M, Burow M, Horan MA, Wahl SM, Nakayama T. Estrogen modulates cutaneous wound healing by downregulating macrophage migration inhibitory factor. J Clin Invest. 2003;111:1309–18. doi: 10.1172/JCI16288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baamonde A, Lastra A, Villazon M, Bordallo J, Hidalgo A, Menendez L. Involvement of endogenous endothelins in thermal and mechanical inflammatory hyperalgesia in mice. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:245–51. doi: 10.1007/s00210-003-0841-1. [DOI] [PubMed] [Google Scholar]
- 7.Beatty WW, Fessler RG. Gonadectomy and sensitivity to electric shock in the rat. Physiol Behav. 1977;19:1–6. doi: 10.1016/0031-9384(77)90149-4. [DOI] [PubMed] [Google Scholar]
- 8.Cicero TJ, Nock B, O’Connor L, Meyer ER. Role of steroids in sex differences in morphine-induced analgesia: activational and organizational effects. J Pharmacol Exp Ther. 2002;300:695–701. doi: 10.1124/jpet.300.2.695. [DOI] [PubMed] [Google Scholar]
- 9.Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM. Sexual differentiation of the vertebrate brain: principles and mechanisms. Front Neuroendocrinol. 1998;19:323–62. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- 10.Daher JB, Souza GE, D’Orleans-Juste P, Rae GA. Endothelin ETB receptors inhibit articular nociception and priming induced by carrageenan in the rat knee-joint. Eur J Pharmacol. 2004;496:77–85. doi: 10.1016/j.ejphar.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Dobbing J. Later development of the brain and its vulnerability. In: Davis JA, Dobbing J, editors. Scientific Foundations of Paediatrics. Vol. 2. William Heinemann; London: 1981. pp. 744–59. [Google Scholar]
- 12.Ergul A, Shoemaker K, Puett D, Tackett RL. Gender differences in the expression of endothelin receptors in human saphenous veins in vitro. J Pharmacol Exp Ther. 1998;285:511–7. [PubMed] [Google Scholar]
- 13.Fitzgerald M, McIntosh N. Pain and analgesia in the newborn. Arch Dis Child. 1989;64:441–3. doi: 10.1136/adc.64.4_spec_no.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilliver SC, Ashworth JJ, Ashcroft GS. The hormonal regulation of cutaneous wound healing. Clin Dermatol. 2007;25:56–62. doi: 10.1016/j.clindermatol.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Gilliver SC, Ashworth JJ, Mills SJ, Hardman MJ, Ashcroft GS. Androgens modulate the inflammatory response during acute wound healing. J Cell Sci. 2006;119:722–32. doi: 10.1242/jcs.02786. [DOI] [PubMed] [Google Scholar]
- 16.Kellogg DL, Jr, Liu Y, Pergola PE. Selected contribution: Gender differences in the endothelin-B receptor contribution to basal cutaneous vascular tone in humans. J Appl Physiol. 2001;91:2407–11. doi: 10.1152/jappl.2001.91.5.2407. discussion 2389–90. [DOI] [PubMed] [Google Scholar]
- 17.Khodorova A, Montmayeur J, Strichartz G. Endothelin Receptors and Pain. J of Pain. 2009:1–25. doi: 10.1016/j.jpain.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khodorova A, Navarro B, Jouaville LS, Murphy JE, Rice FL, Mazurkiewicz JE, Long-Woodward D, Stoffel M, Strichartz GR, Yukhananov R, Davar G. Endothelin-B receptor activation triggers an endogenous analgesic cascade at sites of peripheral injury. Nat Med. 2003;9:1055–61. doi: 10.1038/nm885. [DOI] [PubMed] [Google Scholar]
- 19.Krzanowska EK, Ogawa S, Pfaff DW, Bodnar RJ. Reversal of sex differences in morphine analgesia elicited from the ventrolateral periaqueductal gray in rats by neonatal hormone manipulations. Brain Res. 2002;929:1–9. doi: 10.1016/s0006-8993(01)03350-9. [DOI] [PubMed] [Google Scholar]
- 20.Lidow M, Song Z, Ren K. Long-term effects of short-lasting early local inflammatory insult. Neuroreport. 2001;12:399–403. doi: 10.1097/00001756-200102120-00042. [DOI] [PubMed] [Google Scholar]
- 21.McKelvy AD, Mark TR, Sweitzer SM. Age- and sex-specific nociceptive response to endothelin-1. J Pain. 2007;8:657–66. doi: 10.1016/j.jpain.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Mills SJ, Ashworth JJ, Gilliver SC, Hardman MJ, Ashcroft GS. The sex steroid precursor DHEA accelerates cutaneous wound healing via the estrogen receptors. J Invest Dermatol. 2005;125:1053–62. doi: 10.1111/j.0022-202X.2005.23926.x. [DOI] [PubMed] [Google Scholar]
- 23.Pelletier G, Ren L. Localization of sex steroid receptors in human skin. Histol Histopathol. 2004;19:629–36. doi: 10.14670/HH-19.629. [DOI] [PubMed] [Google Scholar]
- 24.Peters JW, Schouw R, Anand KJ, van Dijk M, Duivenvoorden HJ, Tibboel D. Does neonatal surgery lead to increased pain sensitivity in later childhood? Pain. 2005;114:444–54. doi: 10.1016/j.pain.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Piovezan AP, D’Orleans-Juste P, Frighetto M, Souza GE, Henriques MG, Rae GA. Endothelins contribute towards nociception induced by antigen in ovalbumin-sensitised mice. Br J Pharmacol. 2004;141:755–63. doi: 10.1038/sj.bjp.0705663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porter FL, Grunau RE, Anand KJ. Long-term effects of pain in infants. J Dev Behav Pediatr. 1999;20:253–61. doi: 10.1097/00004703-199908000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Ruda M, Qing-Dong L, Hohmann A, Peng Y, Toshiya T. Altered nociceptive neuronal circuits after neonatal peripheral inflammation. Science. 2000;289:628–630. doi: 10.1126/science.289.5479.628. [DOI] [PubMed] [Google Scholar]
- 28.Taddio A, Katz J. The effects of early pain experience in neonates on pain responses in infancy and childhood. Paediatr Drugs. 2005;7:245–57. doi: 10.2165/00148581-200507040-00004. [DOI] [PubMed] [Google Scholar]
- 29.Taddio A, Katz J, Ilersich AL, Koren G. Effect of neonatal circumcision on pain response during subsequent routine vaccination. Lancet. 1997;349:599–603. doi: 10.1016/S0140-6736(96)10316-0. [DOI] [PubMed] [Google Scholar]
- 30.Taddio A, Nulman I, Koren BS, Stevens B, Koren G. A revised measure of acute pain in infants. J Pain Symptom Manage. 1995;10:456–63. doi: 10.1016/0885-3924(95)00058-7. [DOI] [PubMed] [Google Scholar]
- 31.Taddio A, Ohlsson A, Einarson TR, Stevens B, Koren G. A systematic review of lidocaine-prilocaine cream (EMLA) in the treatment of acute pain in neonates. Pediatrics. 1998;101:E1. doi: 10.1542/peds.101.2.e1. [DOI] [PubMed] [Google Scholar]