Abstract
Objective
Arginase stimulates the proliferation of cultured vascular smooth muscle cells (VSMCs); however, the influence of arginase on VSMC growth in vivo is not known. This study investigated the impact of arginase on cell cycle progression and neointima formation following experimental arterial injury.
Methods and Results
Balloon injury of rat carotid arteries resulted in a sustained increase in arginase activity in the vessel wall and the induction of arginase I protein in both the media and neointima of injured vessels. Furthermore, local perivascular application of the potent and selective arginase inhibitors S-(2-boronoethyl)-l-cysteine (BEC) or NG-hydroxy-nor-L-arginine (L-OHNA) immediately after injury markedly attenuated medial and neointimal DNA synthesis and neointima formation. Substantial arginase I protein and arginase activity was also detected in rat cultured aortic VSMCs. Moreover, treatment of VSMCs with BEC or L-OHNA, or knockdown of arginase I protein, arrested cells in the G0/G1 phase of the cell cycle and induced the expression of the cyclin-dependent protein kinase inhibitor, p21.
Conclusion
This study demonstrates that arginase is essential for VSMCs to enter the cell cycle and that arginase I contributes to the remodeling response following arterial injury. Arginase I represents a potentially new therapeutic target for the treatment of vasculoproliferative disorders.
Introduction
The proliferation of vascular smooth muscle cells (VSMCs) plays a critical role in the formation of vascular lesions, and is the major pathophysiologic mechanism responsible for the failure of interventional therapeutic approaches to treat occlusive vascular disorders, including vein bypass failure, transplant arteriosclerosis, and restenosis after balloon angioplasty (1–3). With the recognition of the essential involvement of VSMC proliferation in occlusive vascular disorders, considerable effort has been directed at characterizing biochemical and molecular pathways that regulate the proliferative response of VSMC following arterial injury. However, the exact mechanism and mediators responsible for VSMC activation and neointima formation following injury of the vessel wall remain elusive.
Arginase is a binuclear manganese metalloenzyme that catalyzes the hydrolysis of L-arginine to L-ornithine and urea (see 4,5). Two distinct isoforms of arginase exist, arginase I and II, that are encoded by distinct genes and exhibit different tissue distribution and subcellular localization but possess similar enzymatic properties. Arginase I is a cytosolic enzyme that is abundantly expressed in the liver and plays a central role in the urea cycle while arginase II is a mitochondrial protein that is concentrated in the kidney. Recent studies have documented the presence of arginase I and II in blood vessels from numerous animal species, including humans (6–9). Interestingly, VSMCs and endothelial cells possess arginase activity yet preferentially express different arginase isoforms: VSMCs exclusively express arginase I while endothelial cells predominantly express arginase II (10–13).
Emerging studies indicate that arginase plays an important role in regulating vascular cell function. Arginase impairs endothelial function by competing with endothelial nitric oxide synthase for substrate L-arginine (6–8,12–15). In this respect, we recently reported that arginase contributes to arteriolar nitric oxide dysfunction in salt-sensitive hypertensive rats by depleting endothelial cells of L-arginine (16). Arginase-mediated endothelial dysfunction has also been documented in other models of hypertension as well as in aging, diabetes, and following ischemia-reperfusion (see 4,5). Besides limiting nitric oxide synthesis, arginase generates biologically significant molecules. In particular, the arginase product L-ornithine is further metabolized by ornithine decarboxylase (ODC) to the polyamine putrescine which forms the successive polyamines, spermine and spermidine (17). Polyamines play an integral role in the mitogenic response of cells. VSMC proliferation is preceded by increases in polyamine synthesis while inhibition of polyamine formation blocks cell growth (18–20). Interestingly, we previously reported that VSMCs possess arginase activity and postulated a physiologic role for arginase in augmenting cell growth by optimizing polyamine synthesis in these cells (21). Indeed, subsequent studies found that overexpression of arginase I enhances polyamine generation and VSMC proliferation in culture while arginase inhibition suppresses VSMC growth (20,22). However, the underlying mechanisms by which arginase promotes VSMC proliferation have not been fully characterized. Moreover, the involvement of arginase in governing vascular growth in vivo is not known. Accordingly, the present study investigated the role of arginase in regulating cell cycle progression and neointima formation in a rat carotid artery injury model.
Materials and Methods
Rat Carotid Artery Balloon Injury
Male Sprague Dawley rats (400–450g; Charles River Laboratories, Wilmington, MA) were anesthetized (ketamine, xylazine, and acepromazine; 0.7ml/kg, intramuscularly; VetMed Drugs, Houston, TX) and the left carotid artery injured with a Fogarty 2F embolectomy catheter (Baxter Healthcare Corporation, Houston, TX), as we previously described (23,24). Immediately after injury, a local polymer-based delivery system was used to administer arginase inhibitors to the injured vessel wall. The delivery system consisted of 200µl of a 25% copolymer gel (PLF127; BASF Corporation, Florham Park, NJ) containing BEC (1mg) or L-NOHA (1mg) that was applied in a circumferential manner to the exposed adventitia of the carotid artery. A separate group of animals received empty gel, which has previously been shown to have no effect on vascular remodeling (25). PLF127 gels act as a rate-controlling barrier and serves as a vehicle for the sustained release of drug releasing an accumulative 5.5% original dose of a drug over 3 hours when using a 25% PLF gel (26). Based on this finding, we estimated a delivery dose of 55µg after 3 hours.
Histology
Animals were euthanized and the vasculature perfusion-fixed with 10% buffered formalin. The common carotid artery was excised, paraffin-embedded, and sections (5µm) stained with Verhoff’s-Von Gieson for measurement of vessel dimensions. Microscopic quantification of vessel dimensions was performed using Image-Pro Plus (Media Cybernetics) and Adobe Photoshop software linked through a digital camera (Leaf Microlumina; Leaf Systems, USA) to a Zeiss Axioskop 50 light microscope (Carl Zeiss, Germany) (23–25).
Arginase I Immunohistochemistry
Carotid arteries were perfusion-fixed and embedded in paraffin. Sections (5µm) were dried at room temperature for 20 minutes, fixed in acetone, and treated with 0.3% hydrogen peroxide in methanol to block endogenous peroxidase activity. After washing with PBS, tissues were incubated with BSA (5%) at room temperature for 30 minutes, and then incubated with anti-rabbit arginase I antibody (1:50) at 4°C overnight. Slides were rinsed in PBS, incubated in biotinylated anti-rabbit IgG (1:100) in BSA (1%) for 1 hour, developed with peroxidase-labeled streptavidin and NovaRed (Burlingham, CA), and counterstained with hematoxylin. Slides were viewed and analyzed using a Zeiss Axioskop 50 light microscope (Carl Zeiss, Germany).
DNA Synthesis and Apoptosis
DNA synthesis was determined by measuring the expression of proliferating cell nuclear antigen (PCNA) in the vessel wall by immunostaining (24). Paraffin-embedded tissues were incubated with an anti-PCNA monoclonal antibody (1:25) followed by a biotinylated anti-mouse secondary antibody (1:100). Slides were treated with avidin-biotin block and exposed to DAB black with nuclear fast red counterstain and analyzed by light microscopy. Data are represented as a PCNA-labeling index (LI), defined as the percentage of total cells within a given area positive for PCNA staining. Apoptotic cells were detected by the terminal deoxynucleotidyl transferase (TdT)-measured dUTP nick end-labeling (TUNEL) method and TUNEL-positive cells confirmed microscopically, as we previously described (24).
Endothelial Regrowth
Endothelial regrowth following arterial injury was examined using Evans blue dye, as we previously described (27). Animals were injected intravenously with Evans blue dye (5%) 10 minutes prior to sacrifice. Injured carotid arteries were perfusion-fixed with 10% neutral buffered formalin, removed, cut longitudinally, pinned on a silicon dissecting dish and photographed under a dissecting microscope. Deendothelialized areas were defined as those that stained blue and were determined by planimetry using the Image-Pro Plus program (Media Cybernetics).
Cultured VSMCs
VSMCs were isolated from rat thoracic aorta and arginase activity and polyamine synthesis monitored by measuring the metabolism of radiolabeled L-arginine (10,21). Cell cycle progression was determined by flow cytometry and the expression of arginase I, cyclin D1, cyclin A, cyclin E, p27, p21, and p53 monitored by western and/or northern blotting (23). Complete details of these experiments are available online at http://atvb.ahajournals.org/.
Results
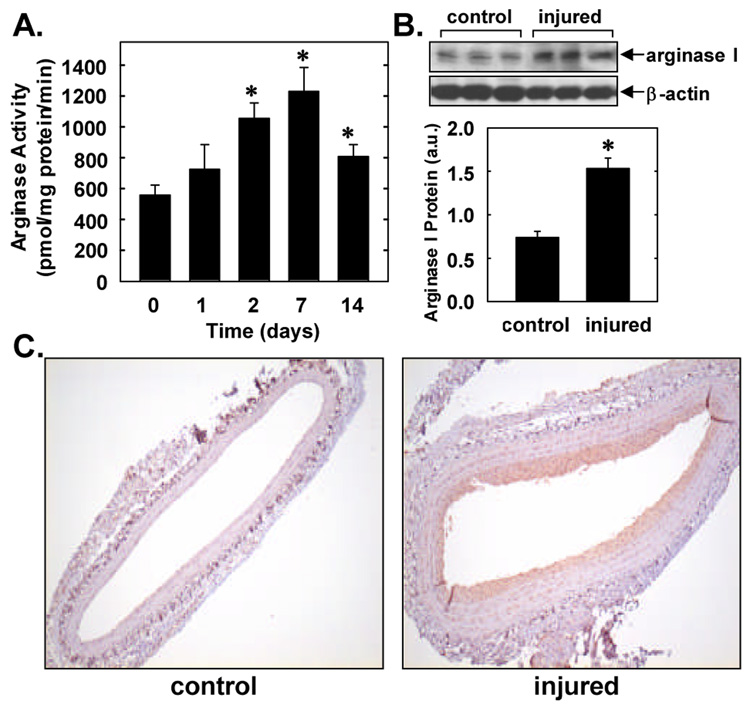
Balloon injury of rat carotid arteries resulted in a time-dependent increase in arginase activity (Figure 1A). A significant increase in arterial arginase activity was detected two days after injury, peaked after one week, and remained elevated two weeks following balloon injury. The peak increase in arginase activity observed one week after arterial injury was associated with an approximate 2-fold increase in arginase I protein expression in the vessel wall (Figure 1B). Immunohistochemistry detected minimal arginase I expression (shown in brown) in control, uninjured carotid arteries (Figure 1C). However, one week after arterial injury there was arginase I staining throughout the vessel wall but intense arginase I expression was observed in the neointima of injured vessels. Omission of the primary antibody or replacement of the primary antibody with non-immune IgG abolished arginase I staining in all sections (data not shown).
Figure 1. Effects of arterial injury on arginase activity and expression.
A. Arginase activity in rat control or balloon injured carotid arteries. Results are mean ± SEM of 5 experiments. *Statistically significant effect of arterial injury. B. Western blot of arginase I protein in control carotid arteries or in vessels 7 days post-injury. Arginase I protein levels were quantified by scanning densitometry, normalized with respect to β-actin, and expressed in arbitrary units (a.u.). Results are mean ± SEM of 3. *Statistically significant effect of arterial injury. C. Immunohistochemistry demonstrating arginase I expression (shown in brown) in control carotid arteries or in vessels 7 days post-injury (magnification 100x). Data are representative of 5 separate experiments.
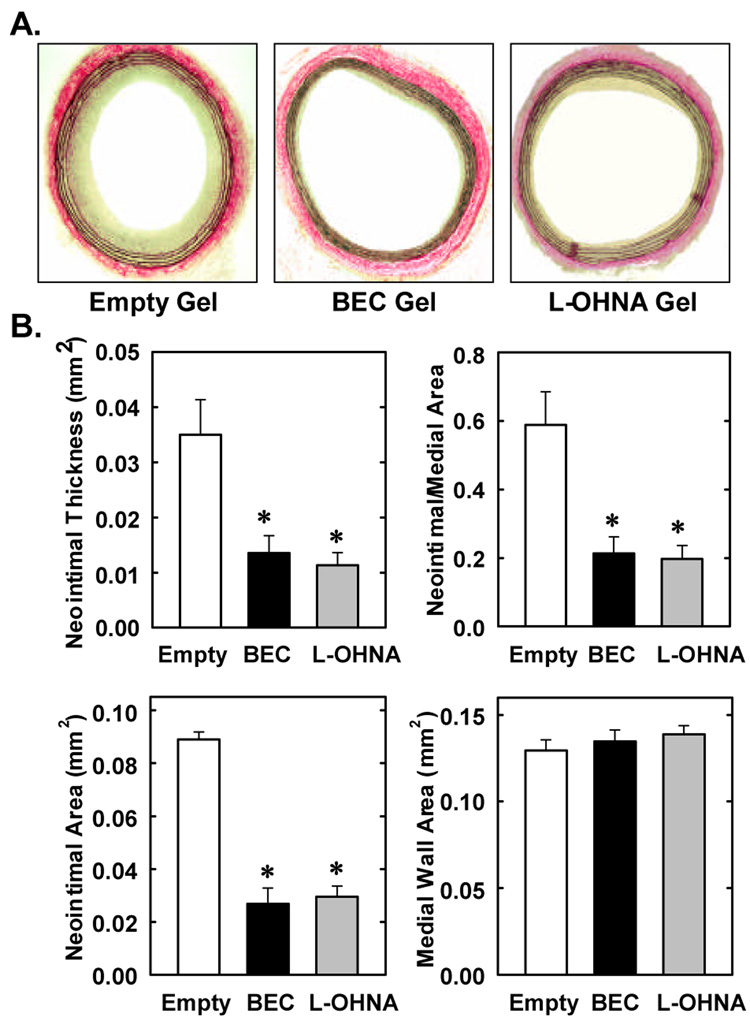
Figure 2A shows representative cross-sections of perfusion-fixed, Verhoff-Van-Giesson-stained tissues obtained from injured animals two weeks after balloon injury. Animals treated with empty gel exhibited a significant and concentric neointima. In contrast, a markedly diminished neointima was observed in vessels exposed to gel containing the arginase inhibitors, BEC or L-OHNA. Morphometric measurements indicate that neointimal area, neointimal thickness, and neointimal/medial wall area ratio were all significantly reduced by BEC- or L-OHNA (Figure 2B). Despite the fact that the neointimal/medial wall ratio was reduced by approximately 65%, medial wall area was not significantly changed by either BEC- or L-OHNA. No significant differences were observed between the three groups of animals for circumference of the left carotid internal elastic laminae (gel control, 2.555±0.041mm; BEC-treated, 2.531±0.083mm; L-OHNA-treated, 2.541±0.066mm) or external elastic lamina (gel control, 2.883±0.048mm; BEC-treated, 2.757±0.0886mm; L-OHNA-treated, 2.852±0.0582mm).
Figure 2. Effects of arginase inhibition on neointima formation after rat carotid artery balloon injury.
A. Injured arteries were treated topically immediately after injury with empty gel or with gel containing BEC (1mg) or L-OHNA (1mg) and harvested two weeks after injury (magnification 100x). B. Morphometric analysis of neointima formation two weeks post-injury. Results are mean ± SEM of 8–10 experiments. *Statistically significant effect of BEC or L-OHNA.
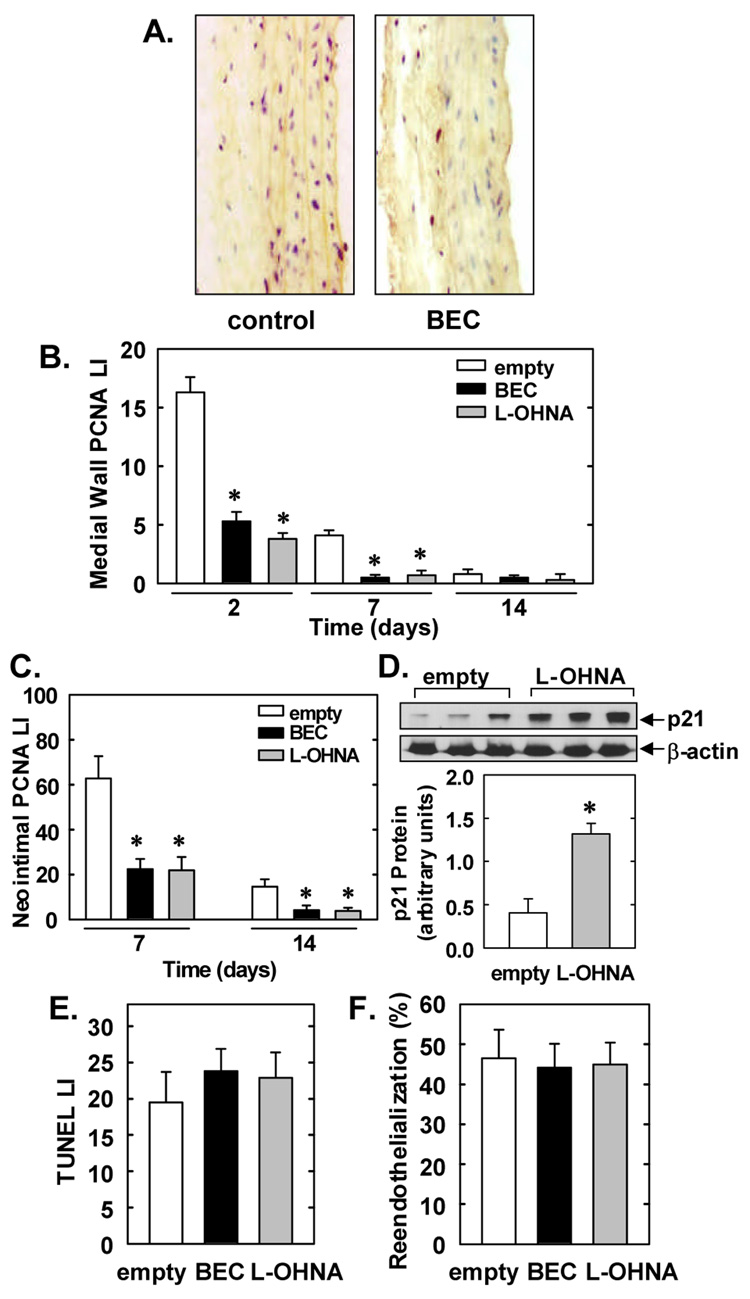
Representative cross-sections of vessels treated with an empty gel demonstrate substantial PCNA staining 2 days following arterial injury whereas BEC-treated arteries are nearly devoid of staining (Figure 3A). Temporal analysis of medial wall DNA replication in empty gel-treated vessels revealed that arterial injury induced substantial PCNA-labeling 2 and 7 days following balloon injury (Figure 3B). However, this increase in medial wall DNA synthesis was significantly attenuated in vessels treated with BEC- or L-OHNA-containing gels. Similarly, the high rate of PCNA-labeling observed in the neointima 7 and 14 days after injury was blocked by BEC or L-OHNA (Figure 3C). Interestingly, treatment of injured arteries with L-OHNA resulted in a significant 2-fold increase in the expression of the cyclin-dependent kinase inhibitor, p21, compared to gel-treated vessels (Figure 3D). Control, uninjured carotid arteries exhibit minimal VSMC apoptosis (1.5±0.3%); however, balloon injury resulted in significant VSMC apoptosis two days after arterial injury (Figure 3E). Application of either BEC or L-OHNA failed to noticeably alter the rate of apoptosis. Finally, BEC or L-OHNA treatment had no effect on endothelial regrowth 14 days following arterial injury (Figure 3F).
Figure 3. Effects of arginase inhibition on DNA synthesis, p21 protein expression, apoptosis and endothelial regrowth after rat carotid artery balloon injury.
A. Representative immunostaining for PCNA (shown in red) in injured carotid arteries 2 days post-injury. B. PCNA labeling index (LI) in the media at 2, 7, and 14 days post-injury. C. PCNA LI in the neointima at 7 and 14 days post-injury. D. Western blot of p21 protein in injured carotid arteries 2 days post-injury. E. TUNEL LI two days post-injury. F. Reendothelialization of carotid arteries 14 days post-injury. Arteries were treated topically with an empty gel, or with gel containing BEC (1mg) or L-OHNA (1mg) immediately after injury. Results are mean ± SEM of 4–6 experiments. *Statistically significant effect of BEC or L-OHNA.
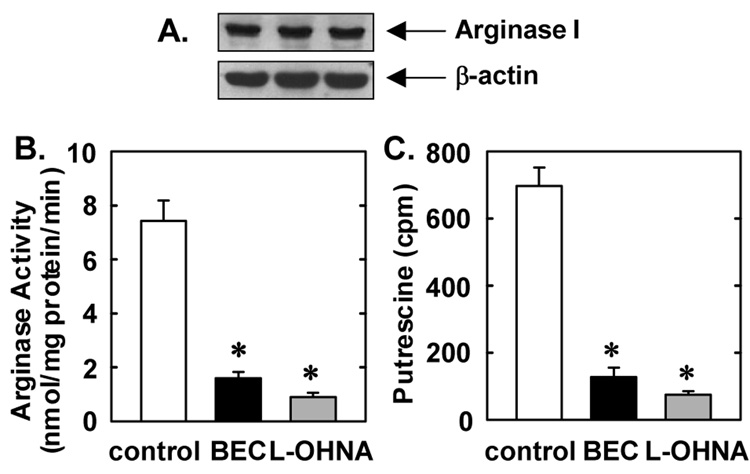
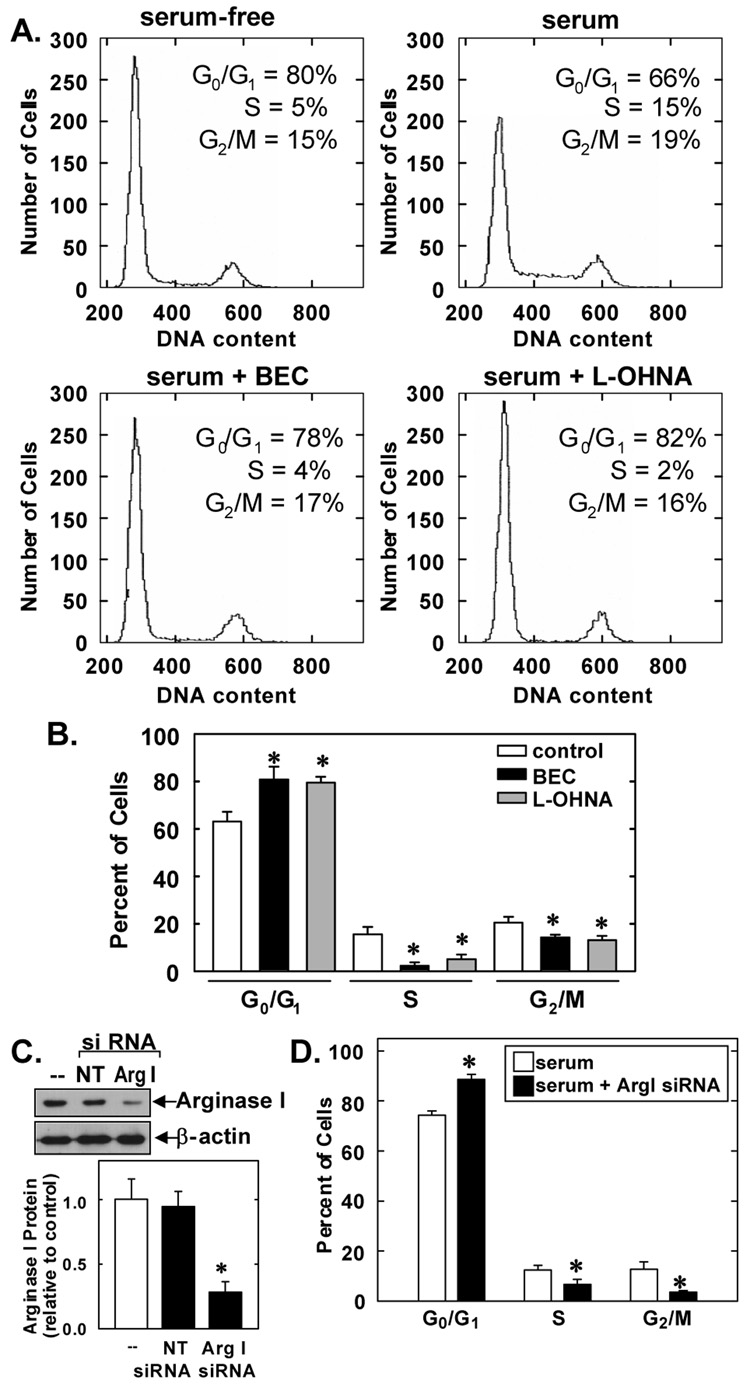
Cultured VSMCs expressed high levels of arginase I protein and substantial arginase activity (Figure 4A and B). Treatment of VSMCs with the arginase inhibitors BEC and L-OHNA markedly reduced arginase activity, confirming the efficacy of these pharmacological inhibitors (Figure 4B). In addition, treatment of VSMCs with BEC or L-OHNA blocked the ability of VSMCs to generate the polyamine putrescine from L-arginine (Figure 4C). Since polyamines play a fundamental role in cell cycle entry (17), succeeding experiments examined whether arginase influenced cell cycle progression in cultured VSMCs. Quiescent VSMCs accumulated in the G0/G1 phase of the cell cycle but the administration of serum for 24 hours induced cell cycle progression and the population of cells in G0/G1 decreased while the number of cells in S and G2/M increased (Figure 5A). However, administration of the arginase inhibitors, BEC or L-OHNA, for 24 hours increased the fraction of cells in the G0/G1 phase of the cell cycle and this was accompanied by a decrease in the percentage of cells in S and G2/M (Figure 5A). BEC or L-OHNA decreased the number of cells in S phase by nearly 80% (Figure 5B). No apparent toxicity was noted with either inhibitor, as reflected by the lack of a sub-G0/G1 fraction (Figure 5A). In addition, trypan blue staining confirmed the lack of toxicity by either arginase inhibitor (data not shown). Treatment of VSMCs with arginase I siRNA also arrested cells in G0/G1 and significantly decreased the percentage of cells in S and G2/M (Figure 5C). In contrast, non-targeting siRNA had no effect cell cycle progression (data not shown). In addition, transfection of VSMCs with arginase I siRNA suppressed arginase I protein expression by nearly 80% whereas the non-targeting siRNA failed to modify arginase I expression, confirming the efficacy and selectivity of the arginase I knockdown approach (Figure 5D).
Figure 4. Arginase expression and activity and in rat cultured VSMCs.
A. Expression of arginase I protein in VSMCs. Data are representative of 3 separate experiments. B. Inhibition of VSMC arginase activity by BEC (2mM) or L-OHNA (2mM). C. Inhibition of VSMC putrescine formation by BEC (2mM) or L-OHNA (2mM). Results are mean ± SEM of 3–5 experiments. *Statistically significant effect of BEC or L-OHNA.
Figure 5. Role of arginase in cell cycle progression in rat cultured VSMCs.
A. Representative histograms of VMSC DNA content in cells treated with serum (5%) for 24 hours in the presence or absence of BEC (2mM) or L-OHNA (2mM). Data are representative of 3 experiments. B. Effect of arginase inhibitors on the distribution of VSMCs in the cell cycle. VSMCs were treated with serum (5%) for 24 hours in the presence or absence BEC (2mM) or L-OHNA (2mM). Results are mean ± SEM of 5 experiments. C. Effect of arginase I siRNA (ArgI siRNA) on the distribution of VSMCs in the cell cycle. VSMCs were pretreated with ArgI siRNA (100nM) for 2 days and then exposed to serum (5%) for 24 hours. Results are mean ± SEM of 4 experiments. *Statistically significant effect of BEC, L-OHNA, or ArgI siRNA.
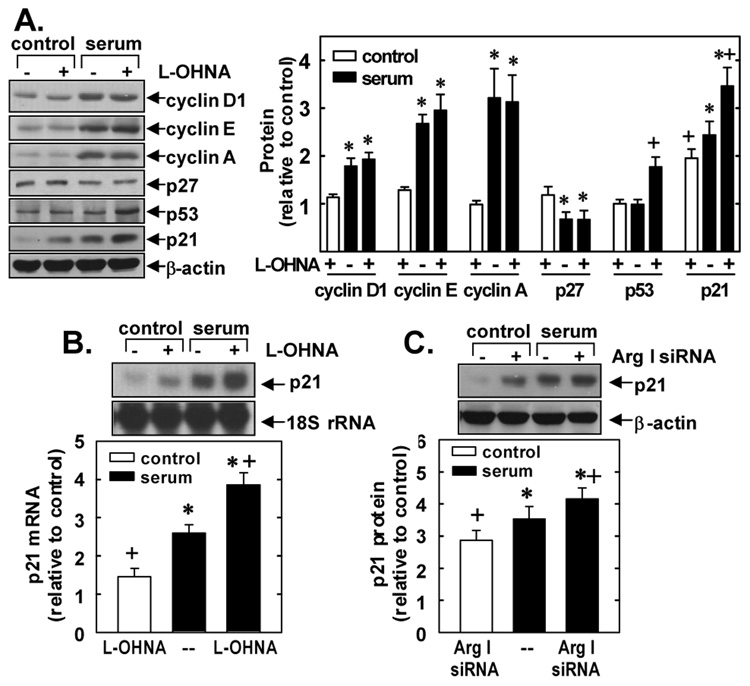
To elucidate the mechanisms by which arginase inhibition disrupts cell cycle progression, we examined the effects of L-OHNA or BEC on cell cycle regulatory proteins. L-OHNA failed to inhibit basal or serum-stimulated protein expression of the G1 cyclins D1, E or A, or the serum-mediated downregulation of the cyclin-dependent kinase inhibitor, p27 (Figure 6A). In contrast, L-OHNA increased the protein expression of the cyclin-dependent kinase inhibitor, p21, and this was associated with an elevation in p53 protein expression in serum-treated cells (Figure 6A). L-OHNA also stimulated p21 mRNA expression in serum-deprived VSMCs and enhanced the serum-mediated induction of p21 mRNA (Figure 6B). Similar findings were also observed with BEC (data not shown). Finally, transfection of VSMCs with arginase I siRNA likewise stimulated the expression of p21 protein (Figure 6C) while non-targeting siRNA failed to modulate p21 expression (data not shown).
Figure 6. Effect of arginase inhibition on VSMC expression of cell cycle regulatory proteins.
A. Effect of L-OHNA on the expression of cyclin D1, E, and A, p21, p27 and p53 protein. B. Effect of L-OHNA and BEC on p21 mRNA expression. C. Effect of arginase I siRNA (ArgI siRNA) on p21 protein expression. VSMCs were treated with serum (5%) in the presence or absence of L-OHNA (2mM) or BEC (2mM) for 24 hours. In some cases, VSMC were pretreated with ArgI siRNA (100nM) for 2 days and then exposed to serum (5%) for 24 hours. Quantification of protein or mRNA expression by scanning densitometry relative to serum-free controls. Results are mean ± of 3–4 experiments. *Statistically significant effect of serum. †Statistically significant effect of BEC, L-OHNA, or ArgI siRNA.
Discussion
The present study demonstrates that arginase plays a fundamental role in vascular growth following injury. Arterial injury stimulates arginase activity and arginase I protein expression in the vessel wall, and local arginase inhibition leads to a significant decline in VSMC DNA synthesis and reduced intimal thickening. In addition, this study shows that arginase promotes the entry of VSMCs into the cell cycle. Arginase inhibition or arginase I knockdown arrests VSMCs in G0/G1 and this is associated with the induction of the cyclin-dependent protein kinase inhibitor, p21. These findings illustrate a critical role for arginase in cell cycle progression and identify arginase I as a novel therapeutic target for the treatment of occlusive vascular disorders.
In the present study, we are the first to demonstrate that arginase plays an integral role in the remodeling response following arterial injury. Balloon injury of rat carotid arteries resulted in a sustained increase in arginase activity in the vessel wall. A significant increase in arginase activity was observed 2 days after injury, peaked at 7 days, and remained elevated after 14 days. The induction of arterial arginase activity following injury was associated with an approximate 2-fold increase in arginase I protein. While arginase I protein expression was minimally expressed in control, uninjured arteries, substantial arginase I protein was detected in the neointima of injured vessels. Since these cells exhibit the highest mitotic activity (28), arginase I expression may parallel the proliferative status of cells. This may explain the absence of arginase I in the quiescent VSMCs resident in the media of control arteries and the strong expression of arginase I noted in our proliferating cultured VSMCs. Our finding that arginase is upregulated in proliferative vascular lesions is consistent with a recent clinical report demonstrating increased arginase activity in hyperplastic human uterine arteries (29). Mechanisms responsible for the induction of arginase activity following arterial injury are not known; however, the generation of growth factors and inflammatory mediators following vascular injury may be involved since a number of mitogens and inflammatory cytokines have been demonstrated to stimulate arginase I gene expression (10,11,21).
To determine the role of arginase in neointima formation, we applied the potent and selective arginase inhibitors BEC or L-OHNA (30) topically to the adventitia of the blood vessel using a specific local delivery copolymer. This drug-targeting approach has been used successfully by our laboratory and others, and elicits a sustained release of agent over the course of several days while avoiding possible non-specific effects associated with the systemic administration of drugs (23–25,31). We found that local perivascular application of BEC or L-OHNA markedly diminished neointima formation after injury. Furthermore, vessel caliber was unaffected by arginase inhibition, indicating that positive remodeling did not occur. Moreover, the inhibition of neointima development by BEC or L-OHNA was independent of overt signs of toxicity or with enhanced apoptosis, but was associated with a significant decline in medial and neointimal VSMC DNA replication. Interestingly, the perivascular administration of L-OHNA stimulated the expression of p21 protein, an established inhibitor of arterial injury-induced intimal thickening (32). Since arginase may also contribute to endothelial cell growth (33), the effect of arginase inhibition on the reendothelialization of injured vessels was examined. Neither BEC nor L-OHNA affected endothelial regrowth following balloon injury. Thus, arginase inhibitors appear to specifically reduce VSMC growth following arterial injury. The ability of arginase inhibitors to selectively target VSMCs may confer a significant therapeutic advantage over currently used antiproliferative drugs that also repress endothelial cell growth (34), an undesired side-effect that enhances neointimal development.
Although arginase has been shown to stimulate VSMC growth, the biological basis for this remains largely unknown. Herein, we demonstrate that arginase promotes the entry of VSMCs into the cell cycle. We found that cultured VSMCs express substantial arginase activity. This result is in agreement with previous reports in primary VSMCs (10,11) but contrasts with a recent study that failed to detect arginase activity in a fetal VSMC line (35), suggesting that arginase activity may be differentially regulated in primary and transformed VSMCs. Variable expression of arginase has also been observed in different breast cancer cells, further emphasizing the cell-specific nature of arginase expression (36). We also found that treatment of VSMCs with arginase inhibitors or knockdown of arginase I protein arrests cells in the G0/G1 phase of the cell cycle. Noteably, arginase inhibition blocks serum-mediated cell cycle progression in the absence of cell death indicating that arginase inhibition acts via cytostatic rather than cytotoxic mechanisms. We further determined that cell cycle arrest following arginase inhibition was not associated with changes in the expression of the G1 cyclins, cyclin D1, E or A, but was linked with an increase in the expression of p21, a known mediator of G1 arrest (37). Similarly, knockdown of arginase I protein induced p21 expression in VSMC. Arginase inhibition also stimulated the expression of the transcription factor, p53, a well established inducer of p21 (38), raising the possibility that the induction of p21 and p53 are causally related in serum-treated VSMCs. Interestingly, our finding that arginase blockade inhibits polyamine biosynthesis may also contribute to the upregulation of p21 expression, since polyamine depletion secondary to ODC inhibition also leads to the induction of p21 and G1 arrest (39,40). Moreover, polyamines have been demonstrated to repress p21 gene transcription (41). Collectively, these findings provide novel insight into the mechanisms behind the antiproliferative actions of arginase inhibitors and highlight a critical role for arginase in cell cycle progression.
In conclusion, the present study demonstrates that arginase plays a fundamental role in cell cycle progression and neointima formation following arterial injury. In particular, we found that balloon injury of rat carotid arteries induces arginase I expression and activity, and that the local application of arginase inhibitors blocks VSMC proliferation and intimal thickening. In addition, we show that arginase inhibition or arginase I knockdown arrests VSMCs in the G0/G1 phase of the cell cycle and this is associated with an increase in p21 expression. These results provide important new insights into the mechanism by which arginase promotes intimal thickening and identify arginase as an attractive therapeutic target in treating occlusive vascular disorders.
Supplementary Material
Acknowledgements
This work was supported by National Institutes of Health Grants HL59976, HL74966, HL82774, and HL81720.
Footnotes
Publisher's Disclaimer: This is an un-copyedited author manuscript that was accepted for publication in Arteriosclerosis, Thrombosis, and Vascular Biology, copyright The American Heart Association. This may not be duplicated or reproduced, other than for personal use or within the “Fair Use of Copyrighted Materials” (section 107, title 17, U.S. Code) without prior permission of the copyright owner, The American Heart Association. The final copyedited article, which is the version of record, can be found at Arteriosclerosis, Thrombosis, and Vascular Biology. The American Heart Association disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.
References
- 1.Orford JL, Selwyn AP, Ganz P, Popma JJ, Rogers C. The comparative pathobiology of atherosclerosis and restenosis. Am. J. Cardiol. 2000;86:6H–11H. doi: 10.1016/s0002-9149(00)01094-8. [DOI] [PubMed] [Google Scholar]
- 2.Gallo R, Padurean A, Jayaraman T, Marx S, Roque M, Adelman S, Chesebro J, Fallon J, Fuster V, Marks A, Badimon JJ. Inhibition of intimal thickening after balloon angioplasty in porcine coronary arteries by targeting regulators of the cell cycle. Circulation. 1999;99:2164–2170. doi: 10.1161/01.cir.99.16.2164. [DOI] [PubMed] [Google Scholar]
- 3.Dzau VJ, Braun-Dellaeus RC, Shedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat. Med. 2002;8:1249–1256. doi: 10.1038/nm1102-1249. [DOI] [PubMed] [Google Scholar]
- 4.Durante W, Johnson FK, Johnson RA. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin. Exp. Pharmacol. Physiol. 2007;34:906–911. doi: 10.1111/j.1440-1681.2007.04638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morris SM., Jr Arginine metabolism: boundaries of our knowledge. J. Nutr. 2007;137:1602S–1609S. doi: 10.1093/jn/137.6.1602S. [DOI] [PubMed] [Google Scholar]
- 6.Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, Burke S, Shoukas AA, Nyhan D, Champion HC, Hare J. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circ. Res. 2003;108:2000–2006. doi: 10.1161/01.CIR.0000092948.04444.C7. [DOI] [PubMed] [Google Scholar]
- 7.Zhang C, Hein TW, Wang W, Chang C-I, Kuo L. Constitutive expression of arginase in microvascular endothelial cells counteracts nitric oxide-mediated vasodilatory function. FASEB J. 2001;15:1264–1266. doi: 10.1096/fj.00-0681fje. [DOI] [PubMed] [Google Scholar]
- 8.Ryoo S, Gupta G, Benjo A, Lim HK, Camara A, Sikka G, Lim HK, Sohi J, Santhanam L, Soucy K, Tuday E, Baraban E, Ilies M, Gerstenblith G, Nyhan D, Shoukas A, Christianson DW, Alp NJ, Champion HC, Huso D, Berkowitz DE. Circ. Res. doi: 10.1161/CIRCRESAHA.107.169573. In press. [DOI] [PubMed] [Google Scholar]
- 9.Marinova GV, Loyaga-Rendon RY, Obayashi S, Ishibashi T, Kubota T, Imamura M, Azuma H. Possible involvement of altered arginase activity, arginase type I and type II expressions, and nitric oxide production in occurrence of intimal hyperplasia in premenopausal human uterine arteries. J. Pharmacol. Sci. 2008;106:385–393. doi: 10.1254/jphs.fp0072275. [DOI] [PubMed] [Google Scholar]
- 10.Durante W, Liao L, Reyna SV, Peyton KJ, Schafer AI. Transforming growth factor-β1 stimulates L-arginine transport and metabolism in vascular smooth muscle cells: role in polyamine and collagen synthesis. Circulation. 2001;103:1121–1127. doi: 10.1161/01.cir.103.8.1121. [DOI] [PubMed] [Google Scholar]
- 11.Wei LH, Jacobs AT, Morris SM, Jr, Ignarro LJ. IL-4 and IL-13 upregulate arginase I expression by cAMP and JAK/STAT6 pathways in vascular smooth muscle cells. Am. J. Physiol. 2000;279:C248–C256. doi: 10.1152/ajpcell.2000.279.1.C248. [DOI] [PubMed] [Google Scholar]
- 12.Ryoo S, Lemmon CA, Soucy KG, Gupta G, White AT, Nyhan D, Shoukas A, Romer LH, Berkowitz DE. Oxidized low-density lipoprotein-dependent endothelial arginase II activation contributes to impaired nitric oxide signaling. Circ. Res. 2006;99:951–960. doi: 10.1161/01.RES.0000247034.24662.b4. [DOI] [PubMed] [Google Scholar]
- 13.Topal G, Brunet A, Walch L, Boucher J-L, David-Dufilho M. Mitochondrial arginase II modulates nitric oxide synthesis through nonfreely exchangeable L-arginine pools in human endothelial cells. J. Pharmacol. Exp. Ther. 2006;318:1368–1374. doi: 10.1124/jpet.106.103747. [DOI] [PubMed] [Google Scholar]
- 14.Lim HK, Lim HK, Ryoo S, Benjo A, Shuleri K, Miriel V, Baraban E, Camara A, Soucy K, Nyhan D, Shoukas A, Berkowitz DE. Mitochondrial arginase II contrains endothelial NOS-3 activity. Am. J. Physiol. Heart. Circ. Physiol. 2007;293:H3317–H3324. doi: 10.1152/ajpheart.00700.2007. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Meininger CJ, Hawker JR, Haynes TE, Kepka-Lenhart D, Mistry SK, Morris SM, Jr, Wu G. Regulatory role of arginase I and II in nitric oxide polyamine, and praline syntheses in endothelial cells. Am. J. Physiol. Endocrinol. Metab. 280:E75–E82. doi: 10.1152/ajpendo.2001.280.1.E75. [DOI] [PubMed] [Google Scholar]
- 16.Johnson FK, Johnson RA, Peyton KJ, Durante W. Arginase inhibition restores arteriolar endothelial function in Dahl rats with salt-induced hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288:R1057–R1062. doi: 10.1152/ajpregu.00758.2004. [DOI] [PubMed] [Google Scholar]
- 17.Tabor CW, Tabor H. Polyamines. Ann. Rev. Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- 18.Durante W, Liao L, Peyton KJ, Schafer AI. Thrombin stimulates vascular smooth muscle cell polyamine synthesis by inducing cationic amino acid transporter and ornithine decarboxylase expression. Circ. Res. 1998;83:217–223. doi: 10.1161/01.res.83.2.217. [DOI] [PubMed] [Google Scholar]
- 19.Durante W, Liao L, Iftikhar I, Cheng K, Schafer AI. Platelet-derived growth factor regulates vascular smooth muscle cell proliferation by inducing cationic amino acid transporter gene expression. J. Biol. Chem. 1996;271:11838–11843. doi: 10.1074/jbc.271.20.11838. [DOI] [PubMed] [Google Scholar]
- 20.Ignarro L, Buga GM, Wei LH, Bauer PM, Wu G, Soldato PD. Role of the arginine-nitric oxide pathway in the regulation of vascular smooth muscle cell proliferation. Proc. Natl. Acad. Sci. USA. 2001;98:4202–4208. doi: 10.1073/pnas.071054698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durante W, Liao L, Peyton KJ, Schafer AI. Lysophosphatidylcholine regulates cationic amino acid transport and metabolism in vascular smooth muscle cells: role in polyamine biosynthesis. J. Biol. Chem. 1997;272:30154–30159. doi: 10.1074/jbc.272.48.30154. [DOI] [PubMed] [Google Scholar]
- 22.Wei LH, Wu G, Morris SM, Jr, Ignarro LJ. Elevated arginase I expression in rat aortic smooth muscle cells increases cell proliferation. Proc. Natl. Acad. Sci. USA. 2001;98:9260–9264. doi: 10.1073/pnas.161294898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Granada JF, Ensenat D, Kaluza GL, Raizner AE, Liu XM, Peyton KJ, Azam MA, Wang H, Durante W. Single perivascular delivery of mitomycin C stimulates p21 expression and inhibits neointima formation in rat arteries. Arterioscler. Thromb. Vasc. Biol. 2005;25:2343–2348. doi: 10.1161/01.ATV.0000184779.01822.9d. [DOI] [PubMed] [Google Scholar]
- 24.Tulis DA, Durante W, Liu XM, Evans AJ, Peyton KJ, Schafer AI. Adenovirus-mediated heme oxygenase-1 gene delivery inhibits injury-induced vascular neointima formation. Circulation. 2001;104:2710–2715. doi: 10.1161/hc4701.099585. [DOI] [PubMed] [Google Scholar]
- 25.Tulis DA, Durante W, Peyton KJ, Champman GB, Evans AJ, Schafer AI. YC-1, a benzyl indazole derivative, stimulates vascular cGMP and inhibits neointima formation. Biochem. Biophys. Res. Commun. 2000;279:646–652. doi: 10.1006/bbrc.2000.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abe T, Sasaki M, Nakajima H, Ogita M, Naitou H, Nagase A, Taguchi K, Miyazaki S. Evaluation of pluronic F127 as a gradual release of anticancer drug. Gan To Kagaku Ryoho. 1990;17:1546–1550. [PubMed] [Google Scholar]
- 27.Tulis DA, Durante W, Peyton KJ, Evans AJ, Schafer AI. Heme oxygenase-1 attenuates vascular remodeling following balloon injury in rat carotid arteries. Atherosclerosis. 2001;155:113–122. doi: 10.1016/s0021-9150(00)00552-9. [DOI] [PubMed] [Google Scholar]
- 28.Clowes AW, Reidy MA, Clowes MM. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab. Invest. 1983;49:327–333. [PubMed] [Google Scholar]
- 29.Layaga-Rendon RY, Sakamoto S, Beppu M, Aso T, Ishizaka M, Takahashi R, Azuma H. Accumulated endogenous nitric oxide synthase inhibitors, enhanced arginase activity, attenuated dimethylarginine dimethylaminohydrolase activity and intimal hyperplasia is premenopausal human uterine arteries. Atherosclerosis. 2005;178:231–239. doi: 10.1016/j.atherosclerosis.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Christianson DW. Arginase: structure, mechanism, and physiological role in male and female sexual arousal. Acc. Chem. Res. 2005;38:191–201. doi: 10.1021/ar040183k. [DOI] [PubMed] [Google Scholar]
- 31.Schachner T, Zou Y, Oberhuber A, Tzankov A, Mairinger T, Laufer G, Bonatti JO. Local application of rapamycin inhibits neointimal hyperplasia in experimental vein grafts. Ann. Thorac. Surg. 2004;77:1580–1585. doi: 10.1016/j.athoracsur.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Yang ZY, Simari RD, Perkins ND, San H, Gordon D, Nabel GJ, Nabel EG. Role of the p21 cyclin-dependent kinase inhibitor in limiting intimal cell proliferation in response to arterial injury. Proc. Natl. Acad. Sci. USA. 1996;93:7905–7910. doi: 10.1073/pnas.93.15.7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H, Meininger CJ, Kelly KA, Hawker JR, Jr, Morris SM, Jr, Wu G. Activities of arginase I and II are limiting for endothelial cell proliferation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282:R64–R69. doi: 10.1152/ajpregu.2002.282.1.R64. [DOI] [PubMed] [Google Scholar]
- 34.Steffel J, Tanner FC. Biological effects of drug-eluting stents in the coronary circulation. Herz. 2007;32:268–273. doi: 10.1007/s00059-007-3000-5. [DOI] [PubMed] [Google Scholar]
- 35.Odenlund M, Holmqvist B, Baldetorp B, Hellstrand P, Nilsson BO. Polyamine synthesis inhibition induces S phase cell cycle arrest in vascular smooth muscle cells. Amino Acids. 2008 doi: 10.1007/s00726-008-0060-7. [DOI] [PubMed] [Google Scholar]
- 36.Singh R, Pervin S, Karimi A, Cederbaum S, Chaudhuri G. Arginase activity in human breast cancer cell lines: Nω-hydroxy-L-arginine selectively inhibits cell proliferation and induces apoptosis in MDA-MB-468 cells. Cancer Res. 60:3305–3312. [PubMed] [Google Scholar]
- 37.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 38.Gartel AL, Tyner AL. Transcriptional regulation of the p21 (WAF1/CIP1) gene. Exp. Cell Res. 1999;246:280–289. doi: 10.1006/excr.1998.4319. [DOI] [PubMed] [Google Scholar]
- 39.Ray RM, Zimmerman BJ, McCormack SA, Patel TB, Johnson LR. Polyamine depletion arrests cell cycle and induces p21Waf1/Cip1, p27Kip1, and p53 in IEC-6 cells. Am. J. Physiol. Cell Physiol. 1999;276:C684–C691. doi: 10.1152/ajpcell.1999.276.3.C684. [DOI] [PubMed] [Google Scholar]
- 40.Kramer DL, Chang BD, Chen Y, Diegelman P, Alm K, Black AR, Roninson IB, Porter CW. Polyamine depletion in human melanoma cells leads to G1 arrest associated with induction of p21WAF1/CIP1/SDI1, changes in expression of p21-regulated genes, and a senescence-like phenotype. Cancer Res. 2001;61:7754–7762. [PubMed] [Google Scholar]
- 41.Liu L, Guo X, Rao JN, Zou T, Marasa BS, Chen J, Greenspon J, Casero RA, Jr, Wang JY. Polyamine-modulated c-Myc expression in normal intestinal epithelial cells regulates p21Cip1 transcription through a proximal promoter region. Biochem. J. 2006;398:257–267. doi: 10.1042/BJ20060217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.