Abstract
In rats, acute exposure to hypoxia causes a decrease in mean arterial pressure (MAP) caused by a predominance of hypoxic vasodilation over chemoreflex-induced vasoconstriction. We previously demonstrated that exposure to chronic intermittent hypoxia (CIH) impairs hypoxic vasodilation in isolated resistance arteries; therefore, we hypothesized that the acute systemic hemodynamic responses to hypoxia would be altered by exposure to CIH. To test this hypothesis, rats were exposed to CIH for 14 days. Heart rate (HR) and MAP were monitored by telemetry. On the first day of CIH exposure, acute episodes of hypoxia caused a decrease in MAP (-9±5 mmHg) and an increase in HR (+45±4 beats/minute). On the 14th day of CIH exposure the depressor response was attenuated (-4±1 mmHg; 44% of the day 1 response) and the tachycardia enhanced (+68±2 beats/minute; 151% of the day 1 response). The observed time-dependent modulation of the acute hemodynamic responses to hypoxia may reflect important changes in neurocirculatory regulation that contribute to CIH-induced hypertension.
Keywords: blood pressure, heart rate, intermittent hypoxia
1. Introduction
Exposure to hypoxia produces a generalized, dose-dependent increase in sympathetic vasoconstrictor outflow caused primarily by engagement of the carotid chemoreceptor reflex (Biesold, D. et al. 1989). This constrictor influence is opposed in most vascular beds by endothelium-dependent and endothelium-independent vasodilation (Fredricks, K. T. et al. 1994;Marshall, J. M. 2000;Weisbrod, C. J. et al. 2001). Consequently, systemic arterial pressure rises very little, if at all, during acute exposures to hypoxia in healthy humans (Xie, A. et al. 2001;Leuenberger, U. A. et al. 2005;Iwasaki, K. et al. 2006;Lusina, S. J. et al. 2006). In rats, arterial pressure actually falls during acute hypoxic exposure (Walker, B. R. 1986;Marshall, J. M. et al. 1988;Marshall, J. M. et al. 1989;Hirakawa, H. et al. 1997;Murasato, Y. et al. 1998).
In contrast to these acute responses, repetitive brief exposures to hypoxia [i.e. chronic intermittent hypoxia (CIH)] causes a sustained increase in blood pressure in rats that persists during subsequent periods of normoxia (Fletcher, E. C. et al. 1992). The mechanisms underlying this carry-over effect are incompletely understood; however, CIH has been shown to increase basal sympathetic outflow and enhance sympathetic activation during subsequent acute hypoxic exposures in rats and humans (Greenberg, H. E. et al. 1999;Xie, A. et al. 2001;Cutler, M. J. et al. 2004;Dick, T. E. et al. 2007;Leuenberger, U. A. et al. 2007). An important contributor to this sympathoexcitation is sensitization of the carotid chemoreceptor, as evidenced by increased rates of carotid sinus nerve discharge during post-exposure normoxia and upon re-exposure to hypoxia (Peng, Y. J. et al. 2003;Peng, Y. J. et al. 2004;Weiss, J. W. et al. 2007). Augmented carotid chemoreflex function has also been observed in humans with obstructive sleep apnea (Narkiewicz, K. et al. 1998), individuals who experience intermittent hypoxia on a nightly basis.
In addition to increases in chemoreflex sensitivity and sympathetic nerve activity, alterations in vascular function may contribute to CIH-induced increases in arterial pressure. In a rat model of sleep apnea, we recently demonstrated that CIH impairs acetylcholine- and hypoxia-induced relaxation in isolated skeletal muscle resistance arteries (Phillips, S. A. et al. 2004). Patients with obstructive sleep apnea exhibit impaired acetylcholine- and flow-mediated vasodilation (Kato, M. et al. 2000;Ip, M. S. et al. 2004;Lattimore, J. L. et al. 2006) and markedly attenuated hypoxic vasodilation (Remsburg, S. et al. 1999) in the forearm compared with control subjects.
The acute hemodynamic effects of hypoxia have been described by many previous investigators (Richardson, D. W. et al. 1966;Kontos, H. A. et al. 1967;Walker, B. R. 1986;Marshall, J. M. et al. 1988;Marshall, J. M. et al. 1990;Hirakawa, H. et al. 1997;Barros, R. C. et al. 2002;Campen, M. J. et al. 2005;Hinojosa-Laborde, C. et al. 2005;Iwasaki, K. et al. 2006). Whether these effects remain stable over time with continued, repetitive hypoxic exposures has not, to our knowledge, been investigated. Therefore, the purpose of this study was to determine the acute and long-term effects of brief, intermittent exposures to hypoxia on systemic arterial pressure and heart rate in rats. Because CIH concomitantly augments chemoreflex-induced sympathoexcitation and impairs endothelium-dependent vasodilation, we hypothesized that it would alter the systemic hemodynamic responses to acute hypoxic exposure. Accordingly, we used radio telemetry monitoring to characterize the mean arterial pressure (MAP) and heart rate (HR) responses of rats to acute hypoxic exposure and to track changes in these responses during a two-week exposure to CIH.
2. Materials And Methods
2.1. Animals
Adult male Sprague-Dawley rats (Harlan Teklad, Madison, WI) were used for all experiments. The rats were housed in our laboratory, and the experimental protocol was approved by the University of Wisconsin-Madison School of Medicine and Public Health's Institutional Animal Care and Use Committee. Rats were allowed ad libitum access to water and standard rat chow (Purina) during exposure to either intermittent hypoxia (CIH) or normoxia (NORM). Room temperature and relative humidity were maintained at 24±1° C. and 30-70%, respectively. Nine rats were exposed to CIH (see below) and five rats served as normoxic controls (NORM)(Table 1).
Table 1.
Characteristics of animals at the conclusion of the 14-day exposure period. The two groups did not differ in age or body weight. Hematocrit was significantly elevated in CIH rats (*p<0.05 vs. NORM by t-test).
| Age (days) | Weight (g) | Hematocrit (%) | |
|---|---|---|---|
| NORM | 107±10 | 398±15 | 50±2 |
| CIH | 103±3 | 388±7 | 58±1* |
2.2. Transmitter Implantation
Prior to initiation of the experimental protocol, radio telemetry transmitters (DSI, St. Paul, MN) were surgically implanted. Briefly, rats were anesthetized with 5% isoflurane in oxygen. A surgical plane of anesthesia was maintained with 1-3% isofluorane in oxygen, delivered via nose cone. Under aseptic conditions the abdomen was opened using a ventral midline incision. The aorta was carefully dissected from surrounding tissue and cannulated using the stem of the catheter attached to the telemetry transmitter. After the catheter was secured in place with glue and a cellulose patch, the transmitter was placed in the peritoneal cavity and sewn into closure of the abdominal wall with non-absorbable sterile suture. The skin was closed with staples. Post-operative analgesia was provided by subcutaneous injections of buprenorphine (0.03 mg/kg, s.c.), given immediately after discontinuation of isoflurane and again 8-12 hours later. Animals were allowed to recover from surgery for 14 days before beginning exposure to intermittent hypoxia or normoxia.
2.3. Chronic Intermittent Hypoxia (CIH) Exposure
Rats were singly housed in Plexiglas cages within the hypoxia chamber and exposed to CIH for 12 hours a day (during the animal's dark cycle from 1800 hours to 0600 hours) for 14 days as previously described (Phillips, S. A. et al. 2004). Briefly, nitrogen was flushed into the chamber at a rate sufficient to achieve a fraction of inspired oxygen (FIO2) of 0.10 within 45 seconds and to maintain this level of FIO2 for an additional minute. Then, oxygen was introduced at a rate sufficient to achieve an FIO2 of 0.21 within 30 seconds and to maintain this level of FIO2 for the remainder of the 4-minute cycle. Airflow was set at a rate sufficient to keep the CO2 level less than 0.5%. Daily checks of chamber oxygen concentrations during hypoxia and normoxia were made using a TED60T oxygen sensor (Teledyne, City of Industry, CA). The noise levels associated with solenoid valve operation and gas switching in the hypoxia chamber were within the limits recommended by the veterinary staff at our institution. It is unlikely that our CIH rats experienced air jet stress because they were housed in individual cages within the larger chamber and the chamber was equipped with baffles at the air inlet site to deflect the jet of air.
2.4. Normoxic (NORM) Exposure
Control (NORM) rats were maintained under normoxic conditions for 14 days. They were singly housed in cages directly adjacent to the hypoxia chamber, where they were subjected to the same light, noise, and temperature stimuli experienced by the hypoxia-exposed rats; however, the noises experienced by NORM rats during operation of the hypoxia chamber were less intense (+5 vs. +18 decibels) than those experienced by the CIH rats.
2.5. Transmitter “Drift” Correction
The zero offset of each telemetry transmitter was recorded before implantation and again after explantation at the end of the experimental period. Correction for transmitter “drift” was made for each animal based on this change in zero offset. In accordance with the recommendation of the manufacturer, we assumed that the downward drift of the pressure reading, which is caused by hydration of the implanted transmitter body, was linear over time. Therefore, for each animal we calculated and applied a correction factor based on linear regression (average value, -0.15 mmHg per day). At the time of transmitter explantation, we compared telemetered pressure readings with those obtained using a mercury manometer. The coefficients of variation for these comparisons in individual rats averaged 2% (range, 0.4-10%).
2.6. Hematocrit
Blood for hematocrit determination was withdrawn from the femoral vein in anesthetized rats at the end of the experimental period. Three samples of blood were placed in glass capillary tubes and centrifuged at 11,500 rpm in an IEC MB microhematocrit centrifuge (International Equipment Co., Needham Heights, MA) for 8 minutes. All samples were measured using a Spiracrit micro-hematocrit reading device (Lourdes Instrument Corp., Brooklyn, NY) and the results were averaged to yield a single value for each rat.
2.7. Data Compilation
Heart rate and blood pressure data were collected at a sampling rate of 500 Hz using Dataquest A.R.T. software (DSI, St. Paul, MN). Data were collected continuously for 4.5 minutes out of every 10 minutes. All of the recorded blood pressure signals were lowpass filtered at 20 Hz and binned in 10 second intervals.
2.7.1. Acute Responses to Hypoxia
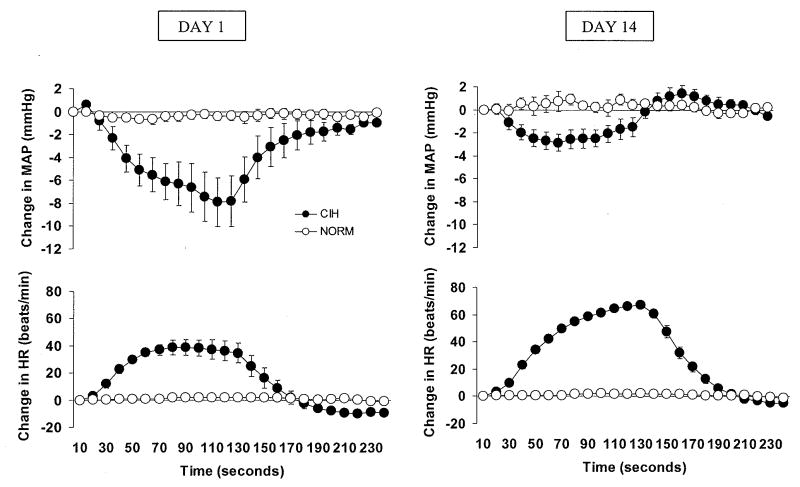
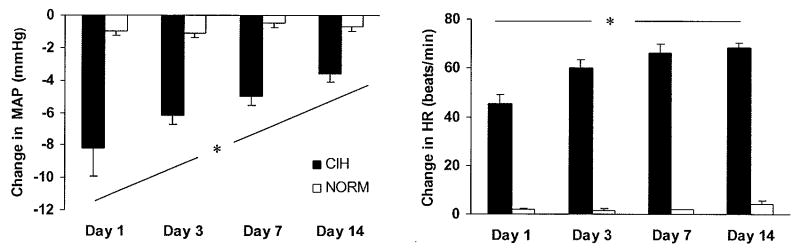
For each rat, we calculated a daily average of the 10-second binned values for HR and MAP during the 180 individual episodes of hypoxia in CIH rats and corresponding normoxic periods in NORM rats. Composite responses were determined by averaging each animal's mean value for each time point across all rats in a given experimental group (Fig. 1). In addition, maximal deviations from baseline in HR and MAP during episodes of hypoxia (or corresponding normoxic periods in NORM rats) were determined for each animal and then averaged across all experimental animals to give a day-by-day assessment of peak hemodynamic responses (Fig. 2).
Figure 1.
On the first day of chronic intermittent hypoxia (CIH) exposure (left panel), hypoxia episodes caused decreases in mean arterial pressure (MAP) and increases in heart rate (HR) (filled circles). During the same sampling intervals, MAP and HR in normoxic control (NORM) rats remained stable (open circles). On the 14th and final day of exposure (right panel), intermittent hypoxia episodes caused decreases in MAP that were smaller and increases in HR that were larger than those observed on the first day of exposure. During the same sampling intervals, MAP and HR in NORM rats remained stable. For each rat, we calculated a daily average of the 10-second binned values for HR and MAP during the 180 individual episodes of hypoxia in CIH rats and corresponding normoxic periods in NORM rats. Composite responses were determined by averaging each animal's mean value for each time point across all rats in a given experimental group.
Figure. 2.
Maximal deviations from baseline in mean arterial pressure (MAP) and heart rate (HR) during episodes of hypoxia or corresponding normoxic periods in normoxic control (NORM) rats were determined for each animal and then averaged across all animals in each group to give a day-by-day assessment of peak hemodynamic responses. Over time, exposure to chronic intermittent hypoxia (CIH) blunted the depressor responses to individual intermittent hypoxia cycles that are typical in the rat (left panel), whereas heart rate responses were augmented (right panel). The filled bars show maximal deviations in MAP and HR in CIH rats, and the open bars show maximal deviations in MAP and HR measured during the same time frames in NORM rats. *P<0.10 for group by time interactions.
2.7.2. Light and Dark Cycle Mean Values
For individual rats, all HR and MAP data collected during the 12-hour light and dark cycles were averaged. Then, the 12-hour mean values for the two experimental groups were computed, with each rat contributing one data point to these averages.
2.8. Statistical Analysis
All data are expressed as mean±SE. Between-group differences in age, weight, and hematocrit were assessed using independent sample t-tests. Time-dependent changes in acute hemodynamic responses to hypoxia were assessed by comparing peak responses on days 1, 3, 7, and 14 using 2-way (group by time) repeated measures ANOVA followed by least squares means tests. Two-way (group by time) repeated measures ANOVA followed by least squares means tests were also used to assess between-group differences in light and dark cycle mean values for MAP and HR. The significance level was set at p<0.10.
3. Results
3.1. General Characteristics of CIH and NORM Rats
Table 1 summarizes the age, weight, and hematocrit data for CIH and NORM rats. At the end of the 14-day exposure period, CIH and NORM did not differ with respect to body weight or age. Hematocrit was significantly elevated in the CIH rats (58±1 vs. 50±2%, p<0.05).
3.2. Acute Hemodynamic Responses to Hypoxia
On the first day of CIH, acute hypoxic exposure caused a decrease in MAP and an increase in HR (Fig. 1, left panel). In contrast, values for MAP and HR recorded at the same time points in the normoxic control rats remained stable. Figure 1 (right panel) shows the acute MAP and HR responses recorded on the 14th (final) day of the CIH exposure.
3.3. Time-Dependent Change in Hemodynamic Responses to Hypoxia during CIH Exposure
The acute effects of hypoxia on systemic hemodynamics did not remain constant over the 14-day CIH exposure; instead, the depressor effect of hypoxia became progressively attenuated and the HR acceleration became progressively augmented. Fig. 2 (left panel) shows group mean values for peak MAP changes caused by acute exposure to hypoxia on days 1, 3, 7, and 14 of the CIH exposure. Two-way, repeated measures ANOVA revealed significant main effects for group (p<0.0001) and time (p=0.0221), and a significant group-by-time interaction (p=0.0787). Post hoc analysis revealed that nadir pressures on day 1 were significantly different from nadir pressures on day 3, 7, and 14 (p=0.0420, p=0.0019, and p<0.0001, respectively). In addition, the nadir pressures on day 3 and day 14 were significantly different from each other (p=0.0102). In the NORM rats, no differences in nadir pressures were noted over time.
Fig. 2 (right panel) shows group mean values for peak HR changes caused by acute exposure to hypoxia on days 1, 3, 7, and 14 of the CIH exposure. Two-way, repeated measures ANOVA revealed significant main effects for group (p<0.0001) and time (p=0.0004), and a significant group-by-time interaction (p=0.0018). Post hoc analysis revealed that peak HR on day 1 was significantly different from peak HR on day 3, 7, and 14 (p<0.0001 for all comparisons). In addition, the peak HR on day 3 and 14 were significantly different from each other (p=0.0209). In the NORM rats, no differences in peak HR were noted over time.
3.4. Effect of CIH on Diurnal MAP and HR
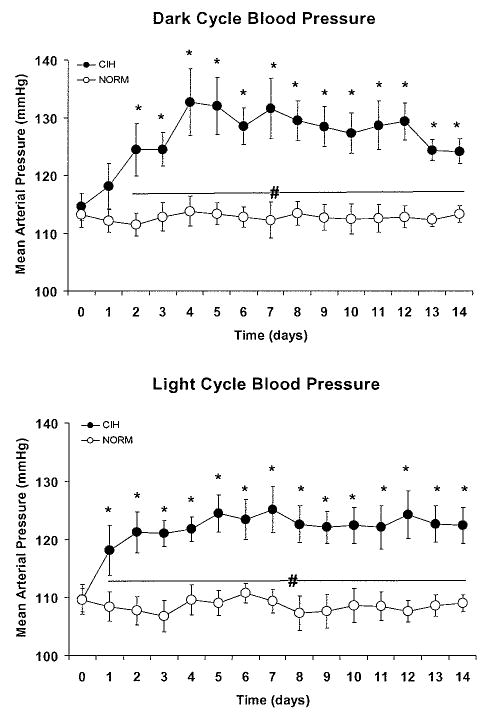
Exposure to CIH for 14 days caused elevations in the 12-hour averages of MAP that were evident during the dark cycle, when the intermittent hypoxia cycles occurred, and also during the light cycle, when the animals were normoxic and unperturbed (Fig. 3). Dark cycle MAP was significantly increased above baseline on day 2 of CIH and remained elevated for the duration of the protocol. Light cycle MAP was significantly increased above baseline after the first 12-hours exposure to CIH on day 1 and remained elevated for the duration of the protocol.
Figure 3.
For each day of the experimental protocol, we computed an average mean arterial pressure (MAP) during the 12-hour light cycle and the 12-hour dark cycle for each rat in the chronic intermittent hypoxia (CIH) and normoxic control (NORM) group. Then, daily group mean values for each cycle were calculated. Day 0 values are the means obtained during a 5-day normoxic baseline period. Exposure to CIH produced increases in MAP above baseline that were evident during the dark cycle (top panel), when intermittent hypoxic cycles occurred, and also during the light cycle (bottom panel), when the animals were unperturbed. *P<0.05 vs. baseline value, # P<0.05 for CIH vs. NORM.
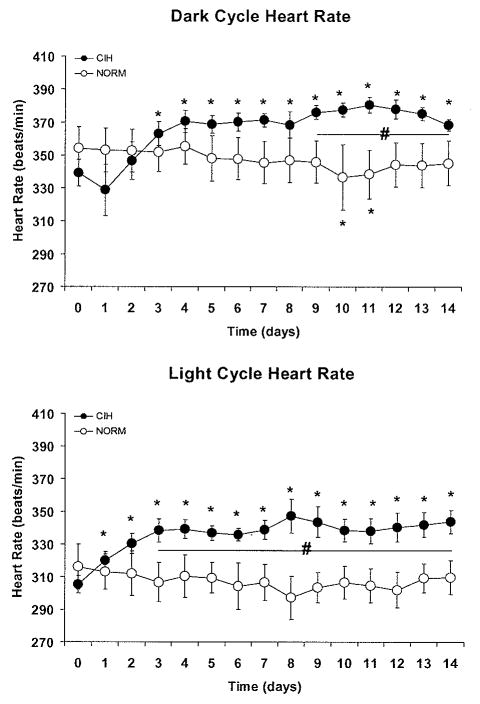
Dark cycle HR was significantly increased above baseline on day 3 of CIH and remained elevated during the dark cycle for the duration of the protocol (Fig. 4). Light cycle heart rate was elevated above baseline beginning on day 1 of CIH and remained elevated during the light cycle for the duration of the protocol.
Figure 4.
Exposure to chronic intermittent hypoxia (CIH) produced increases in heart rate above baseline that were evident during the dark cycle (top panel), when intermittent hypoxic cycles occurred, and also during the light cycle (bottom panel), when the animals were unperturbed. *P<0.05 vs. baseline value, # P<0.05 for CIH vs. NORM. Data points were computed as described in Figure 3.
There were no between-group differences in baseline HR or MAP (Figs. 3 and 4). During both dark and light cycles, MAP was significantly higher in CIH vs. NORM from day 1 to the end of the experimental protocol. During the dark cycle, HR was significantly higher in CIH vs. NORM from day 9 to the end of the experimental protocol. During the light cycle, HR was significantly higher in CIH vs. NORM from day 3 to the end of the experimental protocol.
4. Discussion
The purpose of this study was to test the hypothesis that repetitive, brief exposures to hypoxia, administered 12 hours per day for 14 days, alter the acute systemic hemodynamic responses to these exposures. The major findings were that CIH caused time-dependent: 1) attenuation of the depressor response to hypoxia and 2) enhancement of the HR acceleration caused by hypoxia. In addition, we observed increases in 12-hour mean values of MAP and HR that were apparent during the dark cycle, when the intermittent hypoxia cycles occurred, and also during the light cycles, when the animals were normoxic and unperturbed. The observed time-dependent modulation of the acute hemodynamic responses to hypoxia may be an indirect indicator of the neurocirculatory dysregulation that contributes to CIH-induced hypertension.
4.1. MAP and HR Responses to Acute Episodes of Hypoxia
Previous investigators have reported systemic hypotension and tachycardia in response to acute reductions in PO2 in conscious and anesthetized rats and mice (Walker, B. R. 1986;Marshall, J. M. et al. 1988;Marshall, J. M. et al. 1989;Hirakawa, H. et al. 1997;Campen, M. J. et al. 2005). Our results confirm these previous findings and extend them by demonstrating that the systemic hemodynamic responses to hypoxia do not remain stable over time. Instead, the acute depressor response to hypoxia is progressively attenuated and the HR acceleration is progressively enhanced, in a time-dependent manner, when brief hypoxic exposures are chronically applied. To our knowledge, the present study is the first to report alterations in acute systemic hemodynamic responses to hypoxia during CIH exposure.
4.2. Mechanisms of CIH-Induced Alterations in the Acute Hemodynamic Responses to Hypoxia
In rats, the depressor effect of systemic hypoxia is thought to be caused by a predominance of locally mediated hypoxic vasodilation over carotid chemoreceptor-induced sympathetic vasoconstriction in renal, mesenteric, and skeletal muscle vascular beds (Marshall, J. M. et al. 1988;Marshall, J. M. et al. 1990;Behm, R. et al. 1993;Hayashida, Y. et al. 1996;Skinner, M. R. et al. 1996;Marshall, J. M. 1999). An attenuation of the acute depressor response to hypoxia following 14-day CIH exposure could be caused by an increase in the amount of sympathetic vasoconstriction, a blunting of local hypoxic vasodilation, or the combined effects of these mechanisms.
Previous investigators have shown that repetitive exposure to intermittent hypoxia causes an augmentation of carotid chemoreflex sensitivity that is mediated, at least in part, by reactive oxygen species (Peng, Y. J. et al. 2003;Peng, Y. J. et al. 2006). This chemoreflex sensitization, which enhances the amount of sympathetic activation caused by subsequent exposures to hypoxia (Greenberg, H. E. et al. 1999), could contribute to the time-dependent attenuation of the acute depressor response to hypoxia we observed. In addition, we have previously demonstrated that 14-day exposure to CIH impairs the acute vasodilator response to hypoxia in isolated skeletal muscle resistance arteries (Phillips, S. A. et al. 2004). The mechanisms underlying CIH-induced blunting of hypoxic vasodilation have not been elucidated, however oxidant stress may play a role in this impairment because increased superoxide levels have been observed in mesenteric arteries from CIH-exposed rats (Troncoso Brindeiro, C. M. et al. 2007).
It has been demonstrated previously that CIH in rats elicits a significant increase in hematocrit within 7 days (McGuire, M. et al. 1999;McGuire, M. et al. 2001), and that this increase is accompanied by increases in red blood cell count and hemoglobin concentration (McGuire, M. et al. 2001). In addition, other investigators have observed that CIH results in increased baseline ventilation and augmented hypoxic ventilatory response (Reeves, S. R. et al. 2003;Lai, C. J. et al. 2006). We did not measure red cell count, hemoglobin concentration, or ventilation in our study; however, it is conceivable that the progressively attenuated depressor responses to hypoxia we observed could have been caused, at least in part, by time-dependent reductions in the degree of hypoxemia produced by 10% FIO2. Moreover, it is possible that the observed time-dependent attenuation of the acute depressor response to hypoxia occurred because the cardiac output response, over time, was progressively augmented. We did not measure cardiac output in this study; however, we did observe progressive increases in the acute HR response to hypoxia over the 14-day CIH exposure.
The HR response to systemic hypoxia in freely breathing animals consists of tachycardia which is concomitant with an increase in ventilation (DALY, M. D. et al. 1963;Gandevia, S. C. et al. 1978;Kato, H. et al. 1988). When ventilation is held constant, hypoxic chemoreceptor stimulation results in bradycardia (DALY, M. D. et al. 1963). This suggests that the indirect effects of chemoreflex-induced increases in ventilation supercede the direct effects of chemoreceptor stimulation on HR. CIH exposure increases baseline ventilation and augments the hypoxic ventilatory response (Reeves, S. R. et al. 2003;Rey, S. et al. 2004;Lai, C. J. et al. 2006;Peng, Y. J. et al. 2006;Lusina, S. J. et al. 2006). We believe that CIH-induced chemoreflex sensitization and the resultant augmentation of the hypoxic ventilatory response is a plausible explanation for the potentiation of hypoxic tachycardia we observed.
4.3. Effects of CIH on Diurnal MAP and HR
The diurnal increases in MAP caused by CIH in the present study are similar in magnitude to those reported by previous investigators who used CIH paradigms with different inspired O2 fractions, durations, and frequencies, all of whom administered the intermittent hypoxia during the rat's normal sleep period (the light cycle) (Fletcher, E. C. et al. 1992;Fletcher, E. C. et al. 1995;Lai, C. J. et al. 2006;Troncoso Brindeiro, C. M. et al. 2007). Interestingly, addition of CO2 to prevent hypocapnia or to induce hypercapnia did not affect increases in MAP associated with CIH (Fletcher, E. C. et al. 1995). Viewed collectively, the results of the previous and present studies suggest that: 1) the magnitude of the blood pressure elevation elicited by CIH is the same regardless of whether the exposures are administered during the dark or light cycle, and 2) the addition of CO2 does not enhance the hypertensive effect of intermittent hypoxia.
In our study, CIH caused increases in the 12-hour mean value for HR during both the dark and light cycle. These data are somewhat inconsistent with those of previous investigators who observed CIH-induced increases in HR only during the light-cycle CIH exposure period, with no carry-over into the dark-cycle normoxic period (Hinojosa-Laborde, C. et al. 2005). These investigators employed an experimental paradigm with a hypoxic FIO2 identical to that used in our study (10%), and with a cumulative daily “dose” of hypoxia that was similar to that in our animals (160 vs. 180 minutes). However, because of our shorter duty cycle and longer daily exposure period, our rats were exposed to 100 more normoxia-hypoxia transitions each day. We consider it likely that the number of hypoxia-reoxygenation cycles is a more important determinant of the HR adaptations to CIH than is the total hypoxic exposure time.
4.4. Methodological Considerations
Our control animals were housed in cages adjacent to the hypoxia chamber but were not exposed to cyclic changes in airflow. Both groups of rats were exposed to transient noises associated with solenoid valve operation and airflow change; however, the sound levels were somewhat higher inside the chamber. We consider it unlikely that auditory stimuli were responsible for the acute hemodynamic responses to hypoxia or the time-dependent alterations in these responses that we observed in our study for several reasons. First, rats respond to auditory stimuli with increased MAP and decreased HR (directionally opposite to what we observed) and there is habituation in these responses over time (Rettig, R. et al. 1986;Li, S. G. et al. 1998). Second, there was no change in MAP or HR for 20 seconds after the noise stimuli were presented; therefore, the time course of the MAP and HR responses seems too long to be attributable to auditory stimulation. Finally, our control rats, which were exposed to similar although less intense noises as the CIH rats during hypoxia cycling, did not demonstrate cardiovascular responses to these stimuli.
We considered the possibility that habituation to the alerting response associated with hypoxic exposure could explain the time-dependent changes in the acute hemodynamic responses we observed. Alerting is a behavioral response to unfamiliar or potentially threatening stimuli that elicits increases in MAP and HR, vasoconstriction in renal, splanchnic, and cutaneous vascular beds, and vasodilation in skeletal muscle vascular beds (Yardley, C. P. et al. 1987). We think it is unlikely that our observations during a two-week CIH exposure can be attributed to habituation of the alerting response because the time course for this habituation is known to be very short (i.e. it occurs after a single repetition of a hypoxic stimulus (Marshall, J. M. 1999). Our animals experienced 180 intermittent hypoxia cycles during each 12-hour dark cycle; therefore, we expect that any possible habituation would have occurred early in the first night of exposure, and would have no bearing on our subsequent observations. Instead, diminution of the MAP response and augmentation of the HR response occurred progressively over the 2-week exposure period.
We consider it unlikely that our observations are attributable to changes in the thermal environment associated with gas flow through the hypoxia chamber. Mean, and minimum temperatures were comparable in the chamber and adjacent areas of the room where the normoxic control rats were housed (23.9±0.1 vs. 24.1±0.2 and 23.8±0.1 vs. 23.8±0.2° C., p=0.494 and 0.985, respectively).
The between-group difference in diurnal mean values for arterial pressure we observed could potentially be influenced by differences in the degree of transmitter drift in the two groups of rats. We consider this unlikely, however, because the amount of drift was comparable in the CIH and sham animals (-0.13 ±0.01 vs. -0.17±0.04 mmHg/day, p=0.239).
The acute hemodynamic responses to hypoxia we observed in our rats are comparable to those reported by previous investigators (Walker, B. R. 1986;Hirakawa, H. et al. 1997). In addition, the magnitude of the CIH-induced increases in diurnal blood pressure and heart rate we saw are comparable to those reported by previous investigators who concomitantly observed no effect of intermittent air cycling in normoxic control animals (Fletcher, E. C. et al. 1992;Lai, C. J. et al. 2006;Troncoso Brindeiro, C. M. et al. 2007). Thus, we believe that our findings relative to CIH-induced alterations in cardiovascular regulation can be attributed to intermittent hypoxia and not to associated stresses such as auditory, tactile, or thermal stimuli.
5. Summary and Conclusions
We have shown for the first time that the hypotension and tachycardia associated with acute hypocapnic hypoxia are progressively attenuated and potentiated, respectively, over the course of a 14-day exposure to CIH. We have also shown, in concordance with previous investigators, that CIH exposure causes an increase in diurnal blood pressure that is evident during exposure periods and also during subsequent periods of normoxia. The strikingly similar magnitude of MAP elevation observed in our study vs. previous studies (Fletcher, E. C. et al. 1992;Fletcher, E. C. et al. 1996;Troncoso Brindeiro, C. M. et al. 2007) despite divergent CIH paradigms indicates that intermittent hypoxia per se is the most important pro-hypertensive factor. Supplementation of inspired CO2 and/or exposure of the rats to CIH during their normal sleep period did not result in larger increases in MAP.
CIH-induced alterations in the acute hemodynamic responses to brief, intermittent hypoxia may have important implications for blood pressure regulation and cardiovascular risk in patients with obstructive sleep apnea. Episodes of apnea cause marked increases in blood pressure caused primarily by hypoxemia (Leuenberger, U. et al. 1995). We speculate that these pressor responses may become augmented over time with chronic nightly exposure to intermittent hypoxia, thereby contributing to the loss of normal sleep-related decline in blood pressure (i.e. “non-dipping” pattern) that is commonly seen in patients with sleep apnea (Ziegler, M. G. 2003). Thus, our findings may have clinical relevance because of the known association between “non-dipping” and cardiovascular morbidity (Pickering, T. G. et al. 2001;Cuspidi, C. et al. 2001).
Acknowledgments
This study was supported by a grant from the National Heart Lung and Blood Institute (HL-074072).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Barros RC, Bonagamba LG, Okamoto-Canesin R, de OM, Branco LG, Machado BH. Cardiovascular responses to chemoreflex activation with potassium cyanide or hypoxic hypoxia in awake rats. Auton Neurosci. 2002;97:110–115. doi: 10.1016/s1566-0702(02)00050-4. [DOI] [PubMed] [Google Scholar]
- Behm R, Mewes H, Muinck Keizer WH, Unger T, Rettig R. Cardiovascular and renal effects of hypoxia in conscious carotid body-denervated rats. J Appl Physiol. 1993;74:2795–2800. doi: 10.1152/jappl.1993.74.6.2795. [DOI] [PubMed] [Google Scholar]
- Biesold D, Kurosawa M, Sato A, Trzebski A. Hypoxia and hypercapnia increase the sympathoadrenal medullary functions in anesthetized, artificially ventilated rats. Jpn J Physiol. 1989;39:511–522. doi: 10.2170/jjphysiol.39.511. [DOI] [PubMed] [Google Scholar]
- Campen MJ, Shimoda LA, O'Donnell CP. Acute and chronic cardiovascular effects of intermittent hypoxia in C57BL/6J mice. J Appl Physiol. 2005;99:2028–2035. doi: 10.1152/japplphysiol.00411.2005. [DOI] [PubMed] [Google Scholar]
- Cuspidi C, Macca G, Sampieri L, Fusi V, Severgnini B, Michev I, Salerno M, Magrini F, Zanchetti A. Target organ damage and non-dipping pattern defined by two sessions of ambulatory blood pressure monitoring in recently diagnosed essential hypertensive patients. J Hypertens. 2001;19:1539–1545. doi: 10.1097/00004872-200109000-00004. [DOI] [PubMed] [Google Scholar]
- Cutler MJ, Swift NM, Keller DM, Wasmund WL, Smith ML. Hypoxia-mediated prolonged elevation of sympathetic nerve activity after periods of intermittent hypoxic apnea. J Appl Physiol. 2004;96:754–761. doi: 10.1152/japplphysiol.00506.2003. [DOI] [PubMed] [Google Scholar]
- Daly MD, Scott MJ. The cardiovascular responses to stimulation of the carotid body chemoreceptors in the dog. J Physiol. 1963;165:179–197. doi: 10.1113/jphysiol.1963.sp007051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick TE, Hsieh YH, Wang N, Prabhakar N. Acute intermittent hypoxia increases both phrenic and sympathetic nerve activities in the rat. Exp Physiol. 2007;92:87–97. doi: 10.1113/expphysiol.2006.035758. [DOI] [PubMed] [Google Scholar]
- Fletcher EC, Bao G. The rat as a model of chronic recurrent episodic hypoxia and effect upon systemic blood pressure. Sleep. 1996;19:S210–S212. [PubMed] [Google Scholar]
- Fletcher EC, Bao G, Miller CC., III Effect of recurrent episodic hypocapnic, eucapnic, and hypercapnic hypoxia on systemic blood pressure. J Appl Physiol. 1995;78:1516–1521. doi: 10.1152/jappl.1995.78.4.1516. [DOI] [PubMed] [Google Scholar]
- Fletcher EC, Lesske J, Qian W, Miller CC, III, Unger T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension. 1992;19:555–561. doi: 10.1161/01.hyp.19.6.555. [DOI] [PubMed] [Google Scholar]
- Fredricks KT, Liu Y, Lombard JH. Response of extraparenchymal resistance arteries of rat skeletal muscle to reduced PO2. Am J Physiol. 1994;267:H706–H715. doi: 10.1152/ajpheart.1994.267.2.H706. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, McCloskey DI, Potter EK. Inhibition of baroreceptor and chemoreceptor reflexes on heart rate by afferents from the lungs. J Physiol. 1978;276:369–381. doi: 10.1113/jphysiol.1978.sp012240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg HE, Sica A, Batson D, Scharf SM. Chronic intermittent hypoxia increases sympathetic responsiveness to hypoxia and hypercapnia. J Appl Physiol. 1999;86:298–305. doi: 10.1152/jappl.1999.86.1.298. [DOI] [PubMed] [Google Scholar]
- Hayashida Y, Hirakawa H, Nakamura T, Maeda M. Chemoreceptors in autonomic responses to hypoxia in conscious rats. Adv Exp Med Biol. 1996;410:439–442. doi: 10.1007/978-1-4615-5891-0_67. [DOI] [PubMed] [Google Scholar]
- Hinojosa-Laborde C, Mifflin SW. Sex differences in blood pressure response to intermittent hypoxia in rats. Hypertension. 2005;46:1016–1021. doi: 10.1161/01.HYP.0000175477.33816.f3. [DOI] [PubMed] [Google Scholar]
- Hirakawa H, Nakamura T, Hayashida Y. Effect of carbon dioxide on autonomic cardiovascular responses to systemic hypoxia in conscious rats. Am J Physiol. 1997;273:R747–R754. doi: 10.1152/ajpregu.1997.273.2.R747. [DOI] [PubMed] [Google Scholar]
- Ip MS, Tse HF, Lam B, Tsang KW, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med. 2004;169:348–353. doi: 10.1164/rccm.200306-767OC. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Ogawa Y, Aoki K, Saitoh T, Otsubo A, Shibata S. Cardiovascular regulation response to hypoxia during stepwise decreases from 21% to 15% inhaled oxygen. Aviat Space Environ Med. 2006;77:1015–1019. [PubMed] [Google Scholar]
- Kato H, Menon AS, Chen FJ, Slutsky AS. Contribution of pulmonary receptors to the heart rate response to acute hypoxemia in rabbits. Circulation. 1988;78:1260–1266. doi: 10.1161/01.cir.78.5.1260. [DOI] [PubMed] [Google Scholar]
- Kato M, Roberts-Thomson P, Phillips BG, Haynes WG, Winnicki M, Accurso V, Somers VK. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation. 2000;102:2607–2610. doi: 10.1161/01.cir.102.21.2607. [DOI] [PubMed] [Google Scholar]
- Kontos HA, Levasseur JE, Richardson DW, Mauck HP, Jr, Patterson JL., Jr Comparative circulatory responses to systemic hypoxia in man and in unanesthetized dog. J Appl Physiol. 1967;23:381–386. doi: 10.1152/jappl.1967.23.3.381. [DOI] [PubMed] [Google Scholar]
- Lai CJ, Yang CC, Hsu YY, Lin YN, Kuo TB. Enhanced sympathetic outflow and decreased baroreflex sensitivity are associated with intermittent hypoxia-induced systemic hypertension in conscious rats. J Appl Physiol. 2006;100:1974–1982. doi: 10.1152/japplphysiol.01051.2005. [DOI] [PubMed] [Google Scholar]
- Lattimore JL, Wilcox I, Skilton M, Langenfeld M, Celermajer DS. Treatment of obstructive sleep apnoea leads to improved microvascular endothelial function in the systemic circulation. Thorax. 2006;61:491–495. doi: 10.1136/thx.2004.039164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuenberger U, Jacob E, Sweer L, Waravdekar N, Zwillich C, Sinoway L. Surges of muscle sympathetic nerve activity during obstructive apnea are linked to hypoxemia. J Appl Physiol. 1995;79:581–588. doi: 10.1152/jappl.1995.79.2.581. [DOI] [PubMed] [Google Scholar]
- Leuenberger UA, Brubaker D, Quraishi S, Hogeman CS, Imadojemu VA, Gray KS. Effects of intermittent hypoxia on sympathetic activity and blood pressure in humans. Auton Neurosci. 2005;121:87–93. doi: 10.1016/j.autneu.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Leuenberger UA, Hogeman CS, Quraishi S, Linton-Frazier L, Gray KS. Short-term intermittent hypoxia enhances sympathetic responses to continuous hypoxia in humans. J Appl Physiol. 2007;103:835–842. doi: 10.1152/japplphysiol.00036.2007. [DOI] [PubMed] [Google Scholar]
- Li SG, Randall DC, Brown DR. Roles of cardiac output and peripheral resistance in mediating blood pressure response to stress in rats. Am J Physiol. 1998;274:R1065–R1069. doi: 10.1152/ajpregu.1998.274.4.R1065. [DOI] [PubMed] [Google Scholar]
- Lusina SJ, Kennedy PM, Inglis JT, McKenzie DC, Ayas NT, Sheel AW. Long-term intermittent hypoxia increases sympathetic activity and chemosensitivity during acute hypoxia in humans. J Physiol. 2006;575:961–970. doi: 10.1113/jphysiol.2006.114660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JM. The Joan Mott Prize Lecture. The integrated response to hypoxia: from circulation to cells. Exp Physiol. 1999;84:449–470. [PubMed] [Google Scholar]
- Marshall JM. Adenosine and muscle vasodilatation in acute systemic hypoxia. Acta Physiol Scand. 2000;168:561–573. doi: 10.1046/j.1365-201x.2000.00709.x. [DOI] [PubMed] [Google Scholar]
- Marshall JM, Metcalfe JD. Analysis of the cardiovascular changes induced in the rat by graded levels of systemic hypoxia. J Physiol. 1988;407:385–403. doi: 10.1113/jphysiol.1988.sp017422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JM, Metcalfe JD. Analysis of factors that contribute to cardiovascular changes induced in the cat by graded levels of systemic hypoxia. J Physiol. 1989;412:429–448. doi: 10.1113/jphysiol.1989.sp017625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JM, Metcalfe JD. Effects of systemic hypoxia on the distribution of cardiac output in the rat. J Physiol. 1990;426:335–353. doi: 10.1113/jphysiol.1990.sp018141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire M, Bradford A. Chronic intermittent hypoxia increases haematocrit and causes right ventricular hypertrophy in the rat. Respir Physiol. 1999;117:53–58. doi: 10.1016/s0034-5687(99)00047-x. [DOI] [PubMed] [Google Scholar]
- McGuire M, Bradford A. Chronic intermittent hypercapnic hypoxia increases pulmonary arterial pressure and haematocrit in rats. Eur Respir J. 2001;18:279–285. doi: 10.1183/09031936.01.00078801. [DOI] [PubMed] [Google Scholar]
- Murasato Y, Hirakawa H, Harada Y, Nakamura T, Hayashida Y. Effects of systemic hypoxia on R-R interval and blood pressure variabilities in conscious rats. Am J Physiol. 1998;275:H797–H804. doi: 10.1152/ajpheart.1998.275.3.H797. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, van de Borne PJ, Montano N, Dyken ME, Phillips BG, Somers VK. Contribution of tonic chemoreflex activation to sympathetic activity and blood pressure in patients with obstructive sleep apnea. Circulation. 1998;97:943–945. doi: 10.1161/01.cir.97.10.943. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci U S A. 2003;100:10073–10078. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Rennison J, Prabhakar NR. Intermittent hypoxia augments carotid body and ventilatory response to hypoxia in neonatal rat pups. J Appl Physiol. 2004;97:2020–2025. doi: 10.1152/japplphysiol.00876.2003. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Yuan G, Jacono FJ, Kumar GK, Prabhakar NR. 5-HT evokes sensory long-term facilitation of rodent carotid body via activation of NADPH oxidase. J Physiol. 2006;576:289–295. doi: 10.1113/jphysiol.2006.116020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SA, Olson EB, Morgan BJ, Lombard JH. Chronic intermittent hypoxia impairs endothelium-dependent dilation in rat cerebral and skeletal muscle resistance arteries. Am J Physiol Heart Circ Physiol. 2004;286:H388–H393. doi: 10.1152/ajpheart.00683.2003. [DOI] [PubMed] [Google Scholar]
- Pickering TG, Kario K. Nocturnal non-dipping: what does it augur? Curr Opin Nephrol Hypertens. 2001;10:611–616. doi: 10.1097/00041552-200109000-00010. [DOI] [PubMed] [Google Scholar]
- Reeves SR, Gozal E, Guo SZ, Sachleben LR, Jr, Brittian KR, Lipton AJ, Gozal D. Effect of long-term intermittent and sustained hypoxia on hypoxic ventilatory and metabolic responses in the adult rat. J Appl Physiol. 2003;95:1767–1774. doi: 10.1152/japplphysiol.00759.2002. [DOI] [PubMed] [Google Scholar]
- Remsburg S, Launois SH, Weiss JW. Patients with obstructive sleep apnea have an abnormal peripheral vascular response to hypoxia. J Appl Physiol. 1999;87:1148–1153. doi: 10.1152/jappl.1999.87.3.1148. [DOI] [PubMed] [Google Scholar]
- Rettig R, Geyer MA, Printz MP. Cardiovascular concomitants of tactile and acoustic startle responses in spontaneously hypertensive and normotensive rats. Physiol Behav. 1986;36:1123–1128. doi: 10.1016/0031-9384(86)90489-0. [DOI] [PubMed] [Google Scholar]
- Rey S, Del RR, Alcayaga J, Iturriaga R. Chronic intermittent hypoxia enhances cat chemosensory and ventilatory responses to hypoxia. J Physiol. 2004;560:577–586. doi: 10.1113/jphysiol.2004.072033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson DW, Kontos HA, Shapiro W, Patterson JL., Jr Role of hypocapnia in the circulatory responses to acute hypoxia in man. J Appl Physiol. 1966;21:22–26. doi: 10.1152/jappl.1966.21.1.22. [DOI] [PubMed] [Google Scholar]
- Skinner MR, Marshall JM. Studies on the roles of ATP, adenosine and nitric oxide in mediating muscle vasodilatation induced in the rat by acute systemic hypoxia. J Physiol. 1996;495(Pt 2):553–560. doi: 10.1113/jphysiol.1996.sp021615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troncoso Brindeiro CM, da Silva AQ, Allahdadi KJ, Youngblood V, Kanagy NL. Reactive oxygen species contribute to sleep apnea-induced hypertension in rats. Am J Physiol Heart Circ Physiol. 2007;293:H2971–H2976. doi: 10.1152/ajpheart.00219.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BR. Role of vasopressin in the cardiovascular response to hypoxia in the conscious rat. Am J Physiol. 1986;251:H1316–H1323. doi: 10.1152/ajpheart.1986.251.6.H1316. [DOI] [PubMed] [Google Scholar]
- Weisbrod CJ, Minson CT, Joyner MJ, Halliwill JR. Effects of regional phentolamine on hypoxic vasodilatation in healthy humans. J Physiol. 2001;537:613–621. doi: 10.1111/j.1469-7793.2001.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JW, Liu MD, Huang J. Sleep Apnoea & Hypertension: Physiological bases for a causal relation: Physiological basis for a causal relationship of obstructive sleep apnoea to hypertension. Exp Physiol. 2007;92:21–26. doi: 10.1113/expphysiol.2006.035733. [DOI] [PubMed] [Google Scholar]
- Xie A, Skatrud JB, Puleo DS, Morgan BJ. Exposure to hypoxia produces long-lasting sympathetic activation in humans. J Appl Physiol. 2001;91:1555–1562. doi: 10.1152/jappl.2001.91.4.1555. [DOI] [PubMed] [Google Scholar]
- Yardley CP, Hilton SM. Vasodilatation in hind-limb skeletal muscle evoked as part of the defence reaction in the rat. J Auton Nerv Syst. 1987;19:127–136. doi: 10.1016/0165-1838(87)90006-3. [DOI] [PubMed] [Google Scholar]
- Ziegler MG. Sleep disorders and the failure to lower nocturnal blood pressure. Curr Opin Nephrol Hypertens. 2003;12:97–102. doi: 10.1097/00041552-200301000-00016. [DOI] [PubMed] [Google Scholar]