Abstract
How active membrane conductance dynamics tunes neurons for specific time-varying stimuli remains poorly understood. We studied the biophysical mechanisms by which spike frequency adaptation shapes visual stimulus selectivity in an identified visual interneuron of the locust. The lobula giant movement detector (LGMD) responds preferentially to objects approaching on a collision course with the locust. Using calcium imaging, pharmacology and modeling, we show that spike frequency adaptation in the LGMD is mediated by a Ca2+-dependent potassium conductance closely resembling those associated with ‘small-conductance’ (SK) channels. Intracellular block of this conductance minimally affected the LGMD’s response to approaching stimuli, but substantially increased its response to translating ones. Thus, spike frequency adaptation contributes to the neuron’s tuning by selectively decreasing its responses to nonpreferred stimuli. Our results identify a new mechanism by which spike frequency adaptation may tune visual neurons to behaviorally relevant stimuli.
Collision avoidance is crucial to an animal’s survival, in the contexts of both locomotion and escape. Neural circuitry mediating collision avoidance must be tuned to approaching and not translating visual stimuli, as only the former should reliably elicit collision-avoidance behaviors. In the visual system of orthopteran insects such as locusts, two identified neurons are thought to be important in mediating such behaviors. The LGMD1 is a higher-order visual interneuron located in the third neuropil of the optic lobe that receives input from an entire visual hemifield2. The LGMD outputs, with one-to-one spike correspondence, onto the descending contralateral movement detector (DCMD), a neuron having the largest axon in the locust nerve cord and projecting to motor centers involved in flight steering and jump escape behaviors3.
The preference of the LGMD/DCMD system for objects approaching on a collision course with the insect—or their two-dimensional representations, looming stimuli4–7—combined with the DCMD’s morphology and connectivity, strongly suggest that this system is involved in visually guided escape behavior and locomotion. Indeed, the LGMD and DCMD are exquisitely tuned to a key property of looming stimuli: their firing rate peaks reliably a fixed delay after the stimulus exceeds a threshold angular size on the eye8,9. Behaviorally, it has been demonstrated that the timing of the phases of the locust jump also occur after the crossing of fixed angular threshold sizes10. In other animals, similar looming responses have been observed both behaviorally11 and electrophysiologically12,13. Despite the pervasiveness of looming-selective neurons and behaviors across species, the biophysical mechanisms underlying this selectivity remain poorly understood. Because the LGMD/DCMD system is amenable to a variety of electrophysiological techniques in vivo, it represents an excellent model system for studying the underlying biophysics of neural computations mediating collision-avoidance behaviors.
Two known processes that contribute to declining firing rates in the LGMD during sustained stimulation are feed-forward inhibition14,15 and spike-frequency adaptation16. Feed-forward inhibition is transiently activated in response to large and rapid luminance changes14 and is therefore unlikely to contribute to the sustained suppression of the responses to translating objects. Though the role of spike-frequency adaptation in the response of the LGMD to visual stimuli has not yet been investigated, it may give rise to selectivity for looming versus translating stimuli: a translating stimulus activates a constant number of photoreceptors per unit time as it crosses the visual field, making it potentially susceptible to adaptation; in contrast, a looming stimulus activates a rapidly increasing number of photoreceptors as it expands, which in principle could allow it to overcome adaptation. To be effective, such adaptation would have to occur on a timescale fast enough to shut off the translating response but slow enough to allow a looming response to build up. Phenomenologically, spike-frequency adaptation in the LGMD is similar to the intermediate (medium)-duration adaptation observed in many neurons16. As it takes about a hundred milliseconds to activate fully, the observed adaptation could potentially satisfy the aforementioned timescale constraints. Therefore, we first investigated the biophysical mechanisms underlying spike-frequency adaptation in the LGMD. Next, we directly examined the effect of adaptation on the LGMD’s response to various classes of visual stimuli in vivo. Finally, we were able to reproduce the visual responses using a model implementing spike-frequency adaptation. Our results suggest that adaptation is indeed involved in shaping the LGMD’s selectivity for looming relative to translating stimuli, revealing a new mechanism by which spike-frequency adaptation may affect the processing of sensory stimuli.
RESULTS
LGMD responds better to looming than translating stimuli
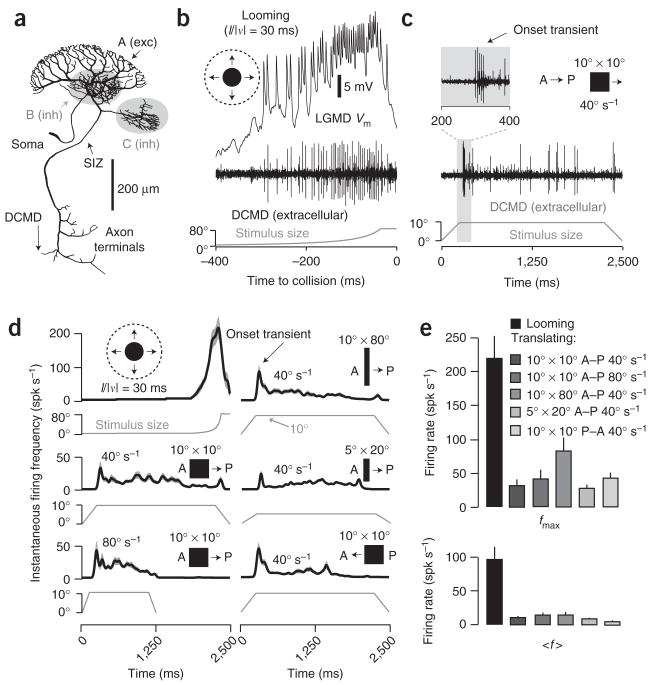
The LGMD consists of a large dendritic field (A) thought to receive ~15,000 retinotopic, motion-sensitive excitatory inputs, and two smaller fields (B, C), each receiving ~500 feed-forward inhibitory inputs best activated by large luminance transients (Fig. 1a). The spike initiation zone is located ~260 μm from the base of field A (ref. 17). The LGMD and its postsynaptic partner the DCMD respond vigorously and reproducibly to looming stimuli (Fig. 1b). Such stimuli elicit a rapid increase in firing, with peak rates often in excess of 300 spikes s−1 and spike counts ~50. In contrast, translating stimuli usually elicit brief onset transients (~50 spikes s−1) followed by much lower sustained firing rates (Fig. 1c), independent of velocity, size, aspect ratio and direction of motion (Fig. 1d,e). For example, the ~20 spikes observed during the translating motion depicted in Figure 1c constitute less than half of those obtained over 400 ms for a looming stimulus (Fig. 1b), despite a much longer presentation time. Thus, the mean firing rate for a looming stimulus over the last 400 ms of approach amounts to ~100 spikes s−1, compared to ~10 spikes s−1 for a translating stimulus.
Figure 1.
LGMD morphology and response to looming versus translating stimuli. (a) LGMD reconstruction17 showing excitatory (A; exc) and inhibitory (B, C; inh) dendrites. (b) Response to looming (l/|v| = 30 ms). Top, LGMD membrane potential (Vm). Middle, corresponding nerve cord recording showing DCMD spikes. Bottom, the approaching disk’s retinal angle as a function of time to collision (t = 0). (c) Response to a 10° × 10° square, translating at 40° s−1 in the A–P direction. The onset transient is magnified in the inset. Stimulus size is the retinal angle subtended in the azimuth direction. The initial size increase is due to the object’s moving onto the screen. (d) Responses to assorted stimuli. Dark lines, gaussian-convolved instantaneous firing frequency (spikes s−1); gray envelopes, s.e.m. (N = 25 trials, 5 per locust). Left column: a looming disk (l/|v| = 30 ms; top) and 10° × 10° squares translating at 40° s−1 (middle) or 80° s−1 (bottom) in the A–P direction. Right column: 10° × 80° (top) and 5° × 20° (middle) rectangles translating at 40° s−1 in the A–P direction and a 10° × 10° square translating at 40° s−1 in the P–A direction (bottom). (e) Peak (fmax) and mean (〈f〉) firing rate in response to visual stimuli. 〈f〉 for looming was for the last 500 ms of approach; 〈f〉 for translation was for the steady state period (Methods). Error bars, s.e.m. (N = 25 trials, as in d); spk, spikes.
Rotating discs2, translating gratings14 and expanding edges6 also elicit weaker responses than looming stimuli. Furthermore, looming stimuli elicit vigorous motor responses, well correlated with LGMD and DCMD activity10,18, whereas translating stimuli do not19. These results therefore suggest that the LGMD is tuned to convey behaviorally relevant, looming-related information to downstream motor centers responsible for escape behaviors.
SK-like K+ conductance mediates spike-frequency adaptation
Because spike-frequency adaptation (SFA) has been observed in the LGMD16, we hypothesized that it could suppress responses to translating stimuli. To test this hypothesis, we first sought to characterize the biophysical mechanisms underlying SFA.
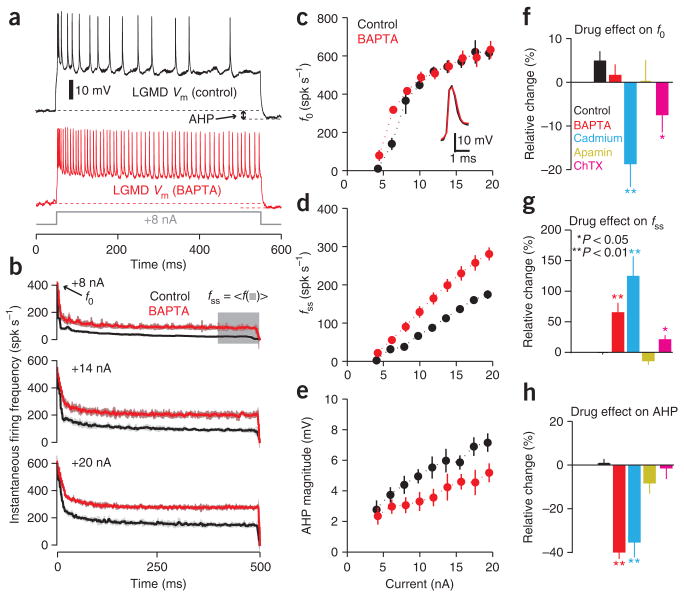
During depolarizing current pulses, the LGMD’s firing rate adapts and an after-hyperpolarization (AHP) is observed upon current injection termination (Fig. 2a, top). Intracellular iontophoresis of the calcium chelator BAPTA20 caused a decrease in SFA and AHP magnitude, implying a calcium dependent process (Fig. 2a, bottom). In the LGMD, SFA is characterized as a function of current amplitude by the initial firing rate (f0), the steady-state firing rate (fss) and the adaptation time constant (τadapt), obtained by fitting an exponential to the instantaneous firing rate’s decay16 (Fig. 2b). After BAPTA iontophoresis, f0 remained largely unchanged (Fig. 2c), whereas fss increased (Fig. 2b,d). The time constant of adaptation, τadapt, also increased: in the case of the +8 nA current pulse illustrated in Figure 2b, it had values of 7.9 ± 2.4 ms and 13.9 ± 4.4 ms before and after BAPTA iontophoresis, respectively (mean ± s.e.m., N = 10 trials, P = 1.83 × 10−4; Wilcoxon rank-sum test). Concurrently, the AHP declined considerably (Fig. 2e). When averaged across current levels and individual locusts, BAPTA had no statistically significant effect on f0 relative to control (Fig. 2f), but produced an increase in fss (Fig. 2g) and a reduction in AHP (Fig. 2h; Supplementary Fig. 1 online shows data for individual locusts). We corroborated the calcium dependence of SFA by perfusing the brain with saline containing the voltage-gated calcium channel blocker cadmium (Cd2+, Methods). Like BAPTA, it caused an increase in fss and a reduction in AHP (Fig. 2g,h; see Supplementary Fig. 2 online for single-locust data). In contrast to BAPTA, Cd2+ produced a decrease in f0 and broadened the action potentials (compare insets in Fig. 2c and Supplementary Fig. 2a), suggesting it had effects on another, BAPTA-insensitive mechanism. On average, the relative adaptation16, Fadapt = (f0 − fss)/f0, decreased from 0.82 ± 0.02 to 0.69 ± 0.02 in the BAPTA and Cd2+ experiments (mean ± s.e.m., N = 10 locusts; P = 0.001).
Figure 2.
Spike frequency adaptation in the LGMD results from an SK-like KCa conductance. (a) LGMD membrane potential (Vm) during current injection (+8 nA, 500 ms), before (black) and after (red) BAPTA iontophoresis. Arrow, example after-hyperpolarization. (b) Instantaneous firing rate during depolarization before (black) and after (red) BAPTA iontophoresis (N = 10 trials, 1 locust; s.d. envelopes). Time interval used for steady-state frequency (fss) is highlighted in gray. (c–e) Initial firing frequency (f0; c), steady-state frequency (fss; d), and AHP magnitude (e) as a function of current before (black) and after (red) BAPTA (10 repetitions per current level; error bars, s.d.); inset, effect of BAPTA on action potential shape. (f–h) Summary of drug effects, obtained by measuring the mean relative change pooled across current levels triggering spiking and averaging across N = 5 locusts for control (rundown; see Methods), BAPTA, cadmium (Cd2+) and charybdotoxin (ChTX); N = 3 for apamin (error bars, s.e.m.). (f) Cd2+ and ChTX reduced f0 relative to control (P = 0.008 and P = 0.032, respectively; Wilcoxon rank-sum test). BAPTA and apamin yielded no change (P = 0.421 and P = 0.393, respectively). (g) BAPTA and Cd2+ gave rise to fss increases (P = 0.008 for both), as did ChTX (P = 0.032). Apamin had no effect (P = 0.071). (h) BAPTA and Cd2+ gave rise to decreases in AHP (P = 0.008 for both); apamin and ChTX had no effect (P = 0.143 and P = 0.841, respectively). Spk, spikes.
These experiments demonstrated that a calcium-dependent hyperpolarizing conductance contributed to SFA. Such conductances often produce a prominent AHP21, and the effects of BAPTA and Cd2+ suggested that the LGMD’s AHP was similarly mediated. Several more arguments support this hypothesis. First, the relative level of adaptation, Fadapt, after intracellular current injection in the LGMD16 was well correlated with AHP magnitude (ρ = −0.60, N = 23 locusts). Second, the change in AHP magnitude after treatment with BAPTA or Cd2+ was highly correlated with the change in Fadapt (ρ = 0.76; N = 10 locusts). Finally, if the AHP is governed by the same mechanism as adaptation, the time constant of AHP recovery, τAHP, will be determined by the time constant governing the decline in free calcium, τCa (ref. 22). In the LGMD, SFA in response to intracellular current injection has been shown to conform to a model allowing a direct estimation of τCa (refs. 16,23). As observed previously16, τAHP (126.3 ± 8.9 ms) and τCa (132.3 ± 7.4 ms) proved to have indistinguishable values (mean ± s.e.m., N = 23 locusts, P = 0.843; Wilcoxon rank-sum test). These results allowed us to use the AHP to distinguish between two classes of calcium-dependent hyperpolarizing conductances that could mediate both SFA and the AHP in the LGMD: calcium-dependent potassium (KCa)24 and chloride (ClCa)25 conductances. We depolarized the neuron from a slightly hyperpolarized holding potential before and after perfusion with high-potassium saline. After perfusion, the AHP was abolished, indicating that SFA was mediated by KCa (N = 3 locusts; Supplementary Fig. 3 online).
We next investigated the type of KCa channels involved. The kinetics of adaptation and AHP recovery were consistent with those of systems wherein intracellular calcium interacting with KCa channels of small conductance (SK) drives adaptation and AHP recovery (refs. 21,26). Though SK channels are known to be present in insects, the most common antagonist, apamin, has so far proven ineffective27. Consistent with this, no effect on SFA was produced by apamin (Fig. 2f–h). Additionally, several SK antagonists and agonists failed to produce an effect (Supplementary Pharmacology and Supplementary Fig. 4 online). To rule out the involvement of the other types of KCa channel (BK and IK), we used the BK and IK blocker charybdotoxin (ChTX), known to be effective in locust neurons28. Like Cd2+, ChTX reduced f0 (Fig. 2f) and broadened the action potential (Supplementary Fig. 2), suggesting the presence of a BK conductance, which is known to act on the submillisecond timescale and shape the action potential and f0 (ref. 29). This also makes it unlikely that diffusion failure explains the apamin insensitivity, as ChTX has a similar molecular weight. The slight increase in fss observed with ChTX is probably due to its effect on f0, as reducing f0 will result in reduced calcium influx through voltage-gated calcium channels.
The lack of an effect of ChTX on the AHP and its minimal impact on fss suggest that neither BK nor IK conductances directly contribute to SFA. Thus, although we could not definitively isolate the SFA-mediating KCa conductance pharmacologically, the kinetics of SFA and the AHP, the BAPTA and Cd2+ sensitivity, the dependence of the AHP on extracellular K+ concentration, and the exclusion of BK and IK channels suggest that SFA is mainly due to an SK-like conductance with nontraditional pharmacological properties.
Adaptation suppresses in vivo response to translation
Because BAPTA effectively suppressed SFA after intracellular iontophoresis, we used it to test the hypothesis that SFA mediates the selectivity for looming over translating stimuli in vivo.
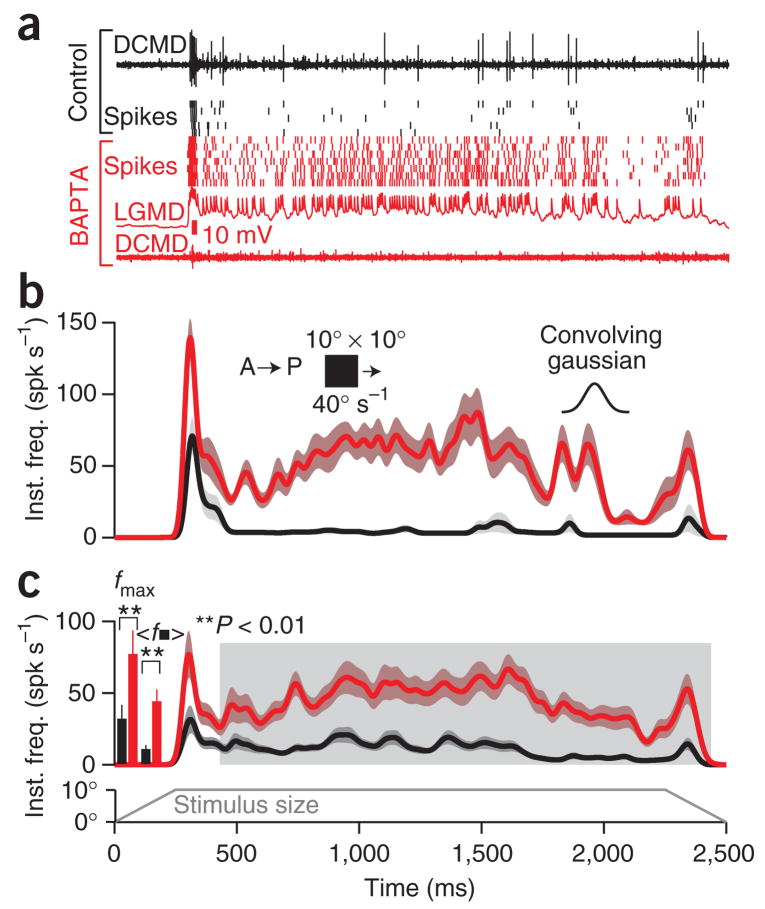
Figure 3a shows an extracellular nerve cord recording and DCMD spike rasters obtained in response to translating squares before BAPTA iontophoresis. For trials obtained after intracellular BAPTA iontophoresis in the LGMD, spikes were obtained from the intracellular LGMD recording. BAPTA abolished the 1:1 correspondence between LGMD and DCMD spikes, presumably by means of action at the LGMD axon terminal (Fig. 3a, bottom). This demonstrates that molecules like BAPTA diffuse effectively throughout the LGMD. Furthermore, it suggests that chemical transmission predominates at this mixed electrical–chemical synapse30, as calcium is crucial for chemical transmission31 but reduces gap junction conductance32. The LGMD’s firing rate increased throughout the stimulus presentation after BAPTA iontophoresis, though the relative increase for the period after the onset transient was greater (Fig. 3b). This held true across individual locusts (Fig. 3c), with a larger relative change in the mean steady-state frequency, 〈f■〉 (Fig. 3c), than in the maximal frequency, fmax (Table 1), because basal firing was much lower at steady-state. The period of sustained firing >50 spikes s−1 increased several-fold after BAPTA iontophoresis (Table 1).
Figure 3.
Intracellular block of spike frequency adaptation (SFA) in vivo enhances the responses to translating motion. Stimulus consisted of a 10° × 10° square translated in the A–P direction at 40° s−1. The size of the stimulus in the azimuth direction as a function of time is indicated at the bottom of c. (a) Top trace, extracellular nerve cord recording with DCMD spikes for a single trial. The black rasters indicate DCMD spike times for different presentations of the same stimulus. The red rasters show LGMD spike times after BAPTA iontophoresis (N = 7). The red nerve cord recording (bottom) was acquired simultaneously with an LGMD membrane potential trace (second from bottom); there was complete breakdown of LGMD:DCMD spike correspondence. (b) Gaussian-convolved (inset; σ = 20 ms) average instantaneous firing rate (inst. freq.) for the locust in a before (black) and after (red) BAPTA iontophoresis. Envelopes indicate s.e.m. (c) Instantaneous firing rate across five locusts before and after BAPTA iontophoresis (conventions as in b; five trials per condition per locust, N = 25 total). Shaded region was used to compute steady state firing frequency, 〈f■〉 (Methods). Left bar plots: increase in fmax and 〈f■〉 after BAPTA iontophoresis (mean; error bars, s.e.m.; P = 7.39 × 10−4 and P = 5.85 × 10−9, respectively; Wilcoxon rank-sum test; see Table 1).
Table 1.
BAPTA produces a stronger increase in firing rate and longer sustained responses for translating stimuli than for looming stimuli
|
fmax (spikes s−1) |
〈f■〉, 〈f〉 (spikes s−1) |
Longest contiguous time f > 50 spikes s−1 (ms) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stimulus class | Control | BAPTA | Δ (%) | P-value | Control | BAPTA | Δ (%) | P-value | Control | BAPTA | P-value |
| Translating rectangles | |||||||||||
| 10°× 10°, 40° s−1, A–P | 32 ± 5 | 77 ± 10 | 144 | 7 ± 10−4 | 11 ± 1 | 44 ± 3 | 308 | 6 × 10−9 | 30 ± 6 | 397 ± 66 | 2 × 10−9 |
| 10° × 10°, 80° s−1, A–P | 41 ± 7 | 81 ± 10 | 100 | 0.001 | 15 ± 2 | 52 ± 6 | 237 | 6 × 10−7 | 40 ± 7 | 353 ± 63 | 4 × 10−8 |
| 10° × 10°, 160° s−1, A–P | 59 ± 6 | 93 ± 6 | 58 | 9 × 10−4 | 18 ± 2 | 58 ± 7 | 231 | 1 × 10−6 | 52 ± 8 | 260 ± 38 | 9 × 10−7 |
| 10° × 10°, 40° s−1, P–A | 42 ± 5 | 92 ± 6 | 118 | 2 × 10−6 | 8 ± 1 | 31 ± 4 | 276 | 2 × 10−6 | 26 ± 6 | 213 ± 37 | 1 × 10−7 |
| 10° × 10°, 80° s−1, P–A | 51 ± 4 | 100 ± 7 | 95 | 4 × 10−5 | 9 ± 2 | 42 ± 6 | 353 | 2 × 10−6 | 35 ± 6 | 217 ± 38 | 4 × 10−7 |
| 10° × 10°, 160° s−1, P–A | 58 ± 5 | 102 ± 10 | 77 | 4 × 10−4 | 12 ± 2 | 46 ± 7 | 278 | 8 × 10−5 | 40 ± 6 | 128 ± 20 | 7 × 10−5 |
| 10° × 80°, 40° s−1, A–P | 82 ± 9 | 137 ± 9 | 66 | 1 × 10−4 | 16 ± 2 | 52 ± 7 | 232 | 6 × 10−5 | 110 ± 16 | 651 ± 106 | 2 × 10−6 |
| 10° × 80°, 80° s−1, A–P | 81 ± 10 | 122 ± 9 | 50 | 0.005 | 11 ± 2 | 33 ± 6 | 191 | 0.035 | 83 ± 14 | 282 ± 57 | 0.034 |
| 10° × 80°, 160° s−1, A–P | 95 ± 10 | 130 ± 11 | 37 | 0.044 | 11 ± 2 | 18 ± 4 | 58 | 0.461 | 74 ± 7 | 140 ± 21 | 0.200 |
| Average | 83 | 242 | |||||||||
| Looming disks | |||||||||||
| l/|v| = 10 ms | 366 ± 50 | 541 ± 152 | 48 | 0.430 | 81 ± 7 | 116 ± 26 | 42 | 0.021 | 229 ± 14 | 245 ± 16 | 0.573 |
| l/|v| = 30 ms | 220 ± 32 | 236 ± 24 | 7 | 0.569 | 97 ± 9 | 114 ± 11 | 18 | 0.033 | 340 ± 26 | 375 ± 26 | 0.303 |
| l/|v| = 50 ms | 138 ± 16 | 211 ± 25 | 53 | 0.005 | 75 ± 8 | 112 ± 13 | 49 | 0.960 | 309 ± 33 | 306 ± 28 | 0.960 |
| Average | 36 | 36 | |||||||||
With the exception of the fastest-moving 10° × 80° rectangle, BAPTA produced significant increases for both maximal response frequency, fmax, and the mean response during the steady state period, 〈f■〉, for all translating stimuli. The relative increase in looming response was much smaller and significant only for the weakest stimulus (l/|v| = 50 ms). The longest contiguous period of sustained firing above 50 spikes s−1 was greatly increased for translating but not looming stimuli. All values are mean ± s.e.m. (N = 25 trials, pooled from 5 locusts with 5 trials each; Wilcoxon rank-sum test comparing the control (before BAPTA) with the response after BAPTA). Δ, percentage change of the mean after BAPTA treatment. The two average rows give mean relative changes across all translating and looming conditions, respectively. The raw data for looming and translating stimuli are shown in Figure 4 and Supplementary Figure 5, respectively.
To determine whether this phenomenon was dependent on the type of translating stimulus, we tested a range of speeds, motion directions, and shapes. We used 10° × 10° squares and 10° × 80° rectangles moving at 40°, 80°, and 160° s−1 in both anterior–posterior (A–P) and posterior–anterior (P–A) directions (Table 1; individual responses in Supplementary Fig. 5 online). We observed an increased response after BAPTA iontophoresis for every tested stimulus. The relative increase in firing for the steady-state period was always larger than for the peak rate. The steady-state firing rate change after BAPTA iontophoresis was weakest for rectangles, failing to reach statistical significance (P < 0.05) for 160° s−1 A–P motion (Table 1). This was presumably because these larger and faster stimuli activate feed-forward inhibition onto the LGMD15, reducing the importance of SFA in mediating stimulus selectivity. In summary, we observed a consistent increase in response after BAPTA iontophoresis, resulting in a prolonged period of sustained firing, and supporting the hypothesis that SFA prevents the LGMD from responding to translating stimuli.
Adaptation minimally affects looming response in vivo
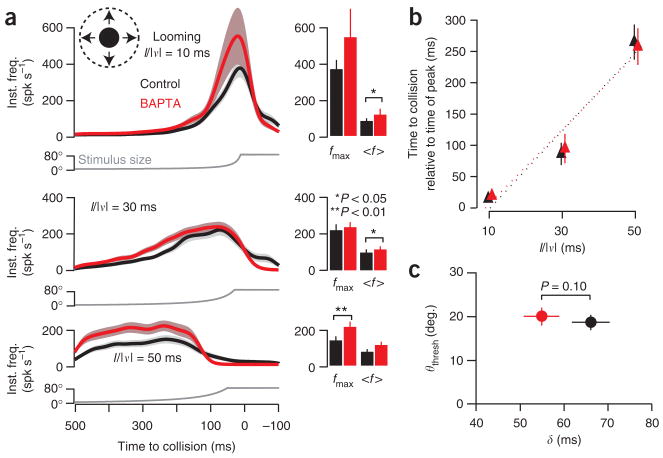
To determine the contribution of SFA to the looming response, we presented, before and after BAPTA iontophoresis, stimuli simulating approaching discs with three values of the ratio of the radius (l) to constant approach speed (|v|): l/|v| = 10, 30 and 50 ms. Although BAPTA increased the firing rate, the overall time course of the response was nearly unaffected (Fig. 4a). A statistically significant increase in fmax was observed only for the slowest approach (l/|v| = 50 ms); the two faster speeds (l/|v| = 10 and 30 ms) produced a significant effect on 〈f〉. In all cases, however, the relative change was much smaller than for translating stimuli and the period of sustained firing was minimally affected (Table 1). Furthermore, the overlap between the responses before and after BAPTA increased with faster approaches, a result consistent with the greater effectiveness of adaptation on slower and weaker stimuli.
Figure 4.
Block of spike frequency adaptation has little effect on the time-course of looming response. (a) Responses to looming disks approaching with l/|v| = 10, 30 and 50 ms. Gaussian-convolved average instantaneous firing rate (inst. freq.) before (black) and after BAPTA iontophoresis (red). Envelopes indicate s.e.m. (5 locusts, 5 trials per locust per condition; N = 25). The bar plots depict the corresponding mean (〈f〉) and peak (fmax) firing frequencies (lines indicate s.e.m.; see Table 1). The BAPTA-induced change in fmax was significant only for l/|v| = 50 ms (P = 0.035), whereas the change in 〈f〉 was significant for l/|v| = 10 and 30 ms (P = 0.021 and 0.033, respectively). (b) Firing rate peak relative to collision time for the same three l/|v| values, before (black) and after (red) BAPTA. Solid lines indicate s.e.m. Dotted lines represent linear fits. Peak times after BAPTA shifted from 14.4 ± 3.1 ms (mean ± s.e.m.) to 19.3 ± 2.4 ms, 86.2 ± 17.9 ms to 94.8 ± 23.7 ms, and 265.1 ± 28.1 ms to 257.7 ± 29.3 ms for l/|v| = 10, 30 and 50 ms, respectively (P = 0.15, 1 and 1, respectively; N = 25). (c) Threshold angle (θthresh) as a function of delay to firing rate peak (δ). After BAPTA iontophoresis, θthresh shifted from 18.8° ± 1.7° to 19.9° ± 2.1° (P = 1, N = 25) and δ shifted from 66.1 ± 4.3 ms to 54.9 ± 4.1 ms (P = 0.1, N = 25). Same conventions as in b.
The LGMD’s firing rate in response to looming stimuli peaks at a fixed delay, δ, after the stimulus exceeds a threshold angle, θthresh (ref. 33). The time of peak, the preceding increase, and the subsequent decrease in firing rate are each related to the timing of distinct phases of jump escape in behaving locusts10. We therefore examined the influence of BAPTA on peak timing. We observed no significant change (Fig. 4b). Moreover, θthresh and δ showed no significant change after BAPTA iontophoresis (Fig. 4c). We therefore conclude that the SK-like KCa conductance does not play a substantive role in shaping the time course of the response to looming stimuli.
Ca2+ enters the LGMD proximal to the spike initiation zone
Having established the involvement of an SK-like KCa current in SFA, and having demonstrated its role in visual processing, we wanted to localize the underlying conductance. Theoretical arguments indicate that if SFA is acting as an inhibitory conductance, it will be most effective if the underlying conductance is localized between the excitatory inputs and the spike initiation zone34,35 (SIZ). This is because a proximal conductance has more influence than a distal one and because on-path inhibition increases the electrotonic distance between the site of excitatory input and the SIZ.
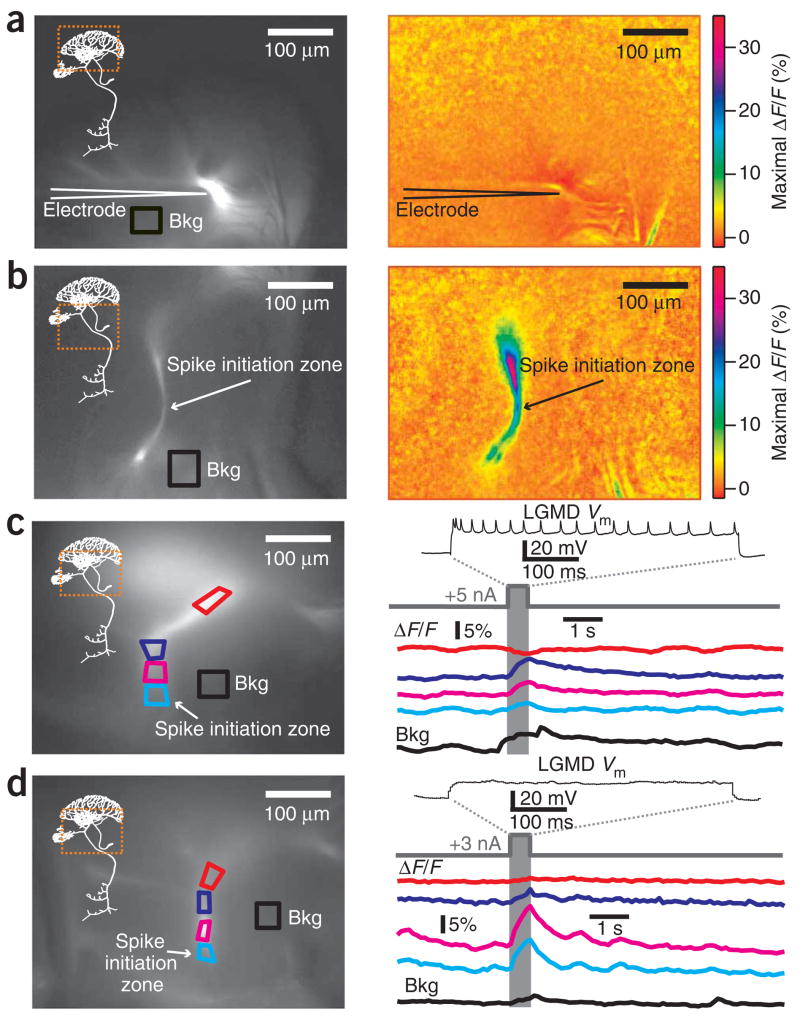
KCa conductances require calcium for activation, usually within localized microdomains21. Therefore, we used imaging to localize the site of Ca2+ influx in the LGMD during depolarizing current injections and, by extension, the presumed location of functional KCa channels. Fig. 5a (left) shows an LGMD filled with the calcium indicator Oregon Green BAPTA-I using an electrode positioned at the base of the excitatory dendritic field. We measured background-subtracted maximal changes in fluorescence (ΔF/F) during depolarizing current pulses. We observed no calcium influx in the excitatory dendrites, despite the electrode’s proximity (Fig. 5a, right). For the same neuron, we also imaged the region near the SIZ (Fig. 5b, left). A large influx of calcium was observed along the main process between the SIZ and the excitatory dendritic field (Fig. 5b, right). This result was reproduced in four other neurons (for example, Fig. 5c,d, each of which shows a different neuron), revealing that only about 100–200 μm of the main process immediately adjacent to the SIZ experienced calcium influx. Moreover, calcium influx occurred both with supra- and subthreshold depolarization (Fig. 5c and 5d, respectively), suggesting that low-voltage activated calcium channels contribute to the observed influx (on the basis of the LGMD’s electrotonic structure17, the dendritic depolarization illustrated in Fig. 5d would have produced a ~5 mV depolarization at the SIZ). The lack of any ΔF/F signal in the excitatory dendrites, despite the electrode’s proximity and large back-propagating spikes, suggests that voltage-gated calcium channels are largely absent from the excitatory dendrites.
Figure 5.
Calcium entry is confined to a region of the LGMD close to the spike initiation zone during depolarizing current injection. (a) Basal fluorescence image of the excitatory dendritic field (left) and maximal ΔF/F (right) in response to depolarizing current (500 ms, +9 nA). Bkg, region used for background subtraction. Dashed square (left panel) shows the LGMD region imaged in right panel. (b) Raw fluorescence image of the SIZ (left) and maximal ΔF/F (right) for the same neuron, penetration site and current shown in a. (c) Basal resting fluorescence image of a second LGMD neuron (left), with four regions of interest (ROIs) demarcated in different colors. Top trace (right), membrane potential (Vm) during a dendritic depolarizing current pulse (500 ms, +5 nA). Colored traces, time course of ΔF/F in the ROIs. Bottom trace, ΔF/F used for background subtraction. (d) Basal resting fluorescence image (left) and Vm response (right, top) in a third LGMD neuron during subthreshold dendritic current pulse (500 ms, +3 nA). The subsequent traces show ΔF/F in the ROIs indicated on the left. Calcium influx does not depend on action potential firing. Electrode is outside field of view in b–d.
SK-like conductance model reproduces physiological response
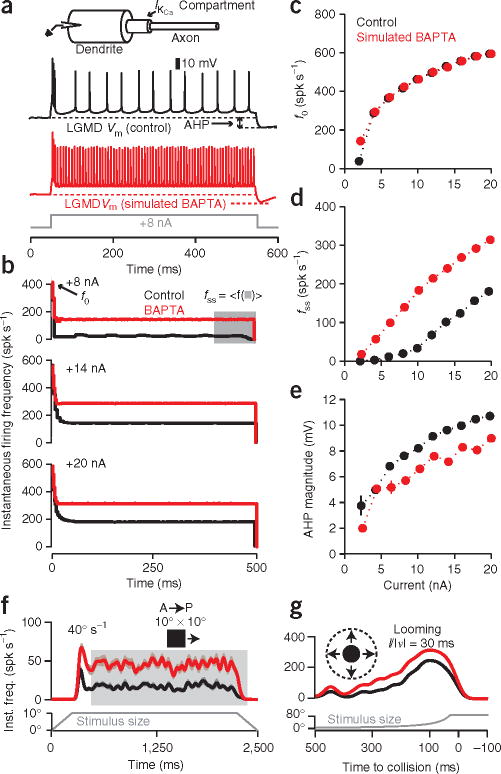
To confirm our experimental results, we next constructed a reduced compartmental model of the LGMD (see Methods) and attempted to reproduce the in vivo results. SFA in the LGMD conforms16 to the predictions of a pyramidal neuron model23 based on an SK-like KCa conductance. We modified it to take into account the morphology of the LGMD and the Ca2+ imaging data in Figure 5. In addition to a dendritic and axonal compartment containing conductances responsible for spike generation, we included an intermediate compartment containing the KCa conductance and a first-order mechanism governing the decline in free calcium with time constant τCa = 132 ms (Fig. 6a, inset; see Methods). The model was able to qualitatively replicate SFA and the AHP observed in the LGMD in response to current pulses, although it showed some differences from the experimental data (Fig. 6a,b). Nonetheless, the relationships between current magnitude and f0, fss, or AHP magnitude were well reproduced overall (Fig. 6c–e), confirming that a simple model based on an SK-like KCa conductance can largely, if not perfectly, account for the experimental responses.
Figure 6.
LGMD compartmental model reproduces in vivo current injection and visual stimulation results, both before and after block of spike frequency adaptation by simulated BAPTA iontophoresis. (a) The model consisted of dendritic and axonal compartments connected by a KCa compartment (inset, top; see Methods). The electrode was positioned in the dendritic compartment. Membrane potential (Vm) traces show model response to a depolarizing current (500 ms, +8 nA) without (black) and with (red) simulated BAPTA iontophoresis. (b) The model gave rise to adaptation with a time course similar to that observed in vivo (Fig. 2b). (c–e) Sensitivity of SFA parameters to simulated BAPTA: f0 did not change (c), whereas fss increased (d) and AHP declined (e). Error bars, s.d. (N = 10 repetitions per current level; see Methods). (f) Gaussian-convolved average response of the model to simulated translation of a 10° × 10° square in the A–P direction at 40° s−1 before (black) and after (red) simulated BAPTA iontophoresis. Inst. freq., instantaneous firing rate. The fmax increased 103%, from 51 ± 5 to 104 ± 5 Hz, and 〈f■〉 increased 152%, from 18 ± 0.3 to 45 ± 1 Hz, after simulated BAPTA iontophoresis (mean ± s.e.m.; N = 25 trials, variability described in Methods). (g) Gaussian-convolved average response of the model to simulated looming stimulus (l/|v| = 30 ms) before (black) and after (red) simulated BAPTA iontophoresis: fmax increased 26%, from 248 ± 2 to 314 ± 1 Hz, and 〈f〉 increased 51%, from 102 ± 1 to 153 ± 1 Hz (mean ± s.e.m.; N = 25 trials).
To test the effectiveness of the model in reproducing visual responses, we had to simulate the effect of intracellular BAPTA iontophoresis. On the basis of the formula derived previously23 for computing τCa and the LGMD data shown in Figure 2, we observed that τCa declined from 98.4 ± 11.5 ms to 70.0 ± 2.9 ms after BAPTA iontophoresis (mean ± s.e.m.; N = 7 current levels, P = 0.002; Wilcoxon rank-sum test). This suggested modeling BAPTA iontophoresis as a decrease in τCa, resulting in a faster decline of free calcium. We found that decreasing τCa—albeit by a larger value than observed experimentally, from 132 to 20 ms—reproduced qualitatively the responses to current injection after BAPTA iontophoresis in vivo (Fig. 6a–e).
After confirming our ability to approximate the current injection results, we turned our attention to the contribution the simulated KCa conductance could make to visual processing. We used parameters obtained from simulations of visual stimuli in a detailed passive compartmental model of the LGMD17 to mimic the LGMD’s response to translating stimuli before and after simulated BAPTA iontophoresis (Fig. 6f). As with the in vivo response, the model produced an onset transient followed by reduced firing. Elimination of feed-forward inhibition reduced this transient and completely abolished it after simulated BAPTA iontophoresis (Supplementary Fig. 6 online). Also, simulated BAPTA iontophoresis resulted in an increase in response, with a larger relative increase for the steady state component, as observed experimentally. The agreement with experimental results was obtained even though the parameters of adaptation were fit independently from those of visually stimulated excitatory synapses17. Finally, we simulated the response of the LGMD to looming stimuli before and after BAPTA iontophoresis (Fig. 6g). As in vivo, the relative increase in response was much smaller for looming stimuli. Moreover, our model reproduced the observed insensitivity to BAPTA iontophoresis of the peak time, as well as of θthresh and δ (Supplementary Fig. 7 online). Thus, the model supported our central claim: that SFA in the LGMD mediated by the SK-like KCa conductance suppresses the response to translation while having little effect on looming responses.
DISCUSSION
Our results suggest that spike-frequency adaptation (SFA) in the LGMD is mostly mediated by an SK-like calcium dependent potassium (KCa) conductance. Furthermore, we showed that this conductance suppressed the response to translating stimuli but had little effect on looming responses. Thus, SFA contributes to the selectivity of the LGMD for looming over translating stimuli.
Mechanisms contributing to SFA in neurons include calcium-activated chloride25, calcium-activated potassium21,24, and sodium-activated potassium36 conductances. Our BAPTA, Cd2+ and high-potassium saline experiments showed the presence of a KCa conductance contributing to SFA in the LGMD. The failure of the BK and IK blocker charybdotoxin28 to influence SFA, combined with the characteristics of adaptation in the LGMD, suggests that SFA is mediated by SK channels16,21. The lack of an effect of apamin and other drugs targeting SK channels (Fig. 2f–h; Supplementary Fig. 4) precludes a definitive conclusion. However, the ineffectiveness of apamin in insects is well documented27, and the lack of effect of other SK drugs occurs under some circumstances in vertebrates as well37. The hypothesis of an SK-like conductance mediating SFA is further supported by results from our three-compartment model. In the model, the KCa conductance is voltage independent, with activation and deactivation kinetics controlled by calcium accumulation and clearance, respectively, as is often—though not always38—thought to be the case for SK channels39,40. The model reproduced qualitatively the main properties of SFA in the LGMD and confirmed its effect on translating stimuli. The model did not reproduce exactly all our experimental results, though this was to be expected given the reduced electrotonic structure of the simulated LGMD, its simplified calcium dynamics, and the possibility that it may lack as-yet-uncharacterized conductances.
It is likely that further mechanisms contribute to SFA because it was not entirely abolished by BAPTA or Cd2+. However, the high correlation between the change in AHP and Fadapt after BAPTA treatment suggests that the mechanism underlying the AHP is the main contributor to SFA. Imaging revealed calcium influx to occur between the excitatory dendritic field and the spike initiation zone (Fig. 5), suggesting that the localization of the KCa conductance is optimized for maximal impact of its inhibitory effect35. KCa can therefore suppress spiking even with relatively strong excitation, as illustrated by our in vivo translating stimulus experiments. Thus, even if other mechanisms contribute to SFA, the KCa conductance is likely to be a dominant factor in shaping LGMD responses to these stimuli. Similar compartmentalized voltage-dependent calcium influx has been observed in motion-sensitive blowfly neurons41,42, and, in some of them, a calcium-dependent adaptation mechanism is also likely to be present43.
We demonstrated that SFA mediated by the SK-like KCa conductance is important in decreasing the response to translation. It is likely that lateral inhibition and, especially with larger stimuli, feed-forward inhibition also reduce these responses14,15. For instance, feed-forward inhibition is likely to contribute to the observed onset transients (Figs. 1d and 3). Indeed, the onset transients were largely abolished in our model without feed-forward inhibition (Supplementary Fig. 6). The relatively large firing rate decrease caused by SFA in response to translating stimuli was also observed in simulations without feed-forward inhibition. This, combined with the fact that smaller stimuli elicit little feed-forward inhibition15, suggests that the SK-like KCa conductance is the main driver of selectivity against translating stimuli.
The link between calcium-dependent spike-frequency adaptation and its effect on stimulus selectivity in sensory neurons has been studied in several systems. Calcium-dependent SFA has been implicated in auditory forward masking in the cricket22. In flies, visual motion adaptation in tangential cells is often accompanied by a prominent AHP44, which correlates with calcium influx43. In weakly electric fish, SK channels contribute to the frequency tuning of pyramidal neurons in the electrosensory lateral line lobe45. Finally, experimental evidence and modeling suggest that calcium-dependent SFA may be partially responsible for contrast adaptation and temporal decorrelation in primary visual cortex46,47. In these contexts, the computational role of SFA is to act as a high-pass filter on largely stationary inputs. Our study suggests a novel role for SFA in the context of dynamically changing inputs: SFA will select for stimuli with temporal profiles of increasing strength. In the particular case of looming stimuli, the angular size subtended by the approaching object at the retina increases supralinearly (that is, with positive acceleration). Thus, as the approach progresses, excitation increases rapidly as an ever greater number of photoreceptors are stimulated per unit time. In the LGMD, such stimuli are able to overwhelm SFA, leading to minimal changes in response whether SFA is present or not. In contrast, visual stimuli translating at constant speed activate an approximately constant number of photoreceptors per unit time, leading to rapid adaptation. Thus, only an accelerating stimulus, such as a looming stimulus, is able to overcome adaptation. More broadly, selectivity for acceleration could also prove useful in the neural processing of a range of dynamic stimuli in visual, auditory, proprioceptive and electrosensory systems.
Electrophysiological studies have revealed a great diversity of ionic conductances and distinct neuron types showing very specific conductance distributions48. Studies in model systems such as the LGMD, for which relatively detailed knowledge of a behavioral role exists, reveal that the functions of individual conductances are often highly specific in their contribution to the overall neural computations carried out by their respective neurons.
METHODS
Electrophysiology
Mature female locusts (Schistocerca americana) were dissected as described previously17. Thin-walled electrodes (WPI) filled with a 2 M potassium acetate, 0.5 M KCl solution were used for LGMD recordings. In BAPTA experiments, electrodes were filled with 2 M potassium acetate, 200 mM BAPTA. BAPTA was iontophoresed intracellularly into the LGMD during ~30 min of 1 s ON, 1 s OFF, −10 nA current pulses. All recordings were carried out in discontinuous current clamp mode, with a −1 nA holding current. Extracellular DCMD recordings were obtained as described previously8.
The LGMD was identified as the neuron whose spiking matched that of the DCMD. Current pulses lasted 500 ms, with amplitudes ranging from +2 to +20 nA in 2-nA increments. In a block, each current level was repeated 5 times, with 10-s intervals between pulses. For analysis of drug effects, the last two control and drug blocks were used to compute changes in SFA parameters. The changes observed in control rundown experiments were used as reference for statistical comparison (Fig. 2f–h and Supplementary Figs. 1 and 4).
Data were analyzed using custom MATLAB programs (MathWorks). Instantaneous firing frequency curves were calculated as described previously16. Averages across locusts were computed using mean relative changes across all current levels that elicited spiking before and after drug treatment. The AHP time constant was fitted as described previously16. To examine the relationship between Fadapt and AHP magnitude, as well as the change thereof, correlation coefficients were computed individually for each locust across multiple current levels; the mean of these is given. We used a threshold of 50 spikes s−1 to quantify the change in duration of sustained firing (Table 1) because this number corresponds approximately to the firing rate observed around the earliest behavioral stage of jump escape behaviors10. All statistical comparisons were carried out using a nonparametric Wilcoxon rank-sum test.
Pharmacology
Unless otherwise noted, all drugs were obtained from Sigma-Aldrich. During dissections and recordings, the brain was bathed in locust saline (165 mM Cl−, 144 mM Na+, 5 mM K+, 5 mM Ca2+, 5 mM Mg2+, 4 mM HCO3−, 6.3 mM HEPES buffer, 146 mM sucrose). Cadmium saline (as before, but with 1 mM Cd2+, 0 mM Ca2+, 10 mM Mg2+) was added using a gravity perfusion system, as was high-K+ saline (95 mM, versus 5 mM in control). To increase [K+] without changing [Cl−], [Na+] was reduced to 54 mM, which did not abolish spiking. Apamin (Calbiochem), scyllatoxin, UCL1684, NS309 (Tocris Biosciences) and ChTX aliquots were prepared in advance and stored at −20 °C. Apamin, scyllatoxin and ChTX were added directly to the bath in concentrated form, reaching final concentrations of 600 nM, 1 μM and 2 μM, respectively, by passive diffusion (bath volume ~5 ml). All agents were dissolved in H2O, with the following exceptions: scyllatoxin (25 mM HEPES), UCL1684 (DMSO) and NS309 (DMSO).
Imaging
Calcium imaging was performed using a BX51WI microscope (Olympus) with a 20×, 0.95 numerical aperture objective, fluorescein isothiocyanate filter set, and Rolera XR CCD camera (QImaging) operating at 10 Hz. Cells were filled following the same iontophoresis protocol as was used for BAPTA filling in electrophysiology experiments, using electrodes containing 10 μl of a 5 mM aqueous solution of Oregon green BAPTA-I (Molecular Probes) at their tip. Electrodes were backfilled with 2 M potassium acetate. The ability of BAPTA to diffuse to the synaptic terminal, as well as the effectiveness of Lucifer yellow fills in previous studies17, suggests that there are no problems with dye diffusion. For stimulation, we used two or three 500-ms depolarizing current amplitudes (typically, +1, +5, +9 nA), with 15-s interstimulus intervals and 3–5 repetitions per amplitude. Each pixel’s ΔF/F time series was obtained by first carrying out background subtraction and then averaging over a 5 × 5 pixel area centered at the given pixel. Trials containing motion artifacts were excluded.
Visual stimulation
The following stimuli were presented on a cathode ray tube monitor as described previously17: (i) Disks approaching on a collision course with the eye and characterized by l/|v| ratios of 10, 30 and 50 ms (looming stimuli). (ii) Squares and rectangles translating in AP and PA directions across 80° of visual space from an azimuth of 50 to 130°, centered at an elevation of 0° (that is, at the eye equator; coordinate system as described previously2). Both translating stimuli were presented at speeds of 40° s−1, 80° s−1, and 160° s−1 and subtended 10° × 10° and 10° × 80°, respectively (azimuth × elevation; measured when the stimulus was aligned with the center of the eye, perpendicular to the longitudinal body axis). (iii) A series of 5° × 20° rectangles moving in an AP direction at 40° s−1 (‘aspect ratio’ stimuli). All stimuli were dark (0.57 cd m−2) on a bright background (79 cd m−2), with a 45 s interstimulus interval. A block constituted of a single presentation of all 15 stimuli, and 5 blocks were presented before and after BAPTA iontophoresis (except for aspect ratio stimuli, which were presented to different locusts without BAPTA iontophoresis).
Eyes were waxed into position, with eye striations serving as reference. Eye health was regularly monitored using rotating disks17 positioned across the visual field, and locusts whose receptive fields deteriorated were rejected. We obtained mean instantaneous firing frequency curves by averaging the individual instantaneous frequency curves of the five trials presented to the locust for a given condition, then convolving with a gaussian window (σ = 20 ms). All 25 trials (N = 5 locusts; N = 5 presentations per condition per locust) were pooled in the BAPTA versus control analysis. For translating stimuli, the steady state period refers to the time from the first local minimum after fmax to the time of the last frequency above 3 spikes s−1.
Simulations
A detailed description of the model is provided in Supplementary Methods online. Briefly, the three simulated compartments corresponded to the dendrites, the region where the KCa conductance was localized, and the axon (Fig. 6a, inset). We used the Hodgkin-Huxley formalism to simulate Ih (inward rectifier, dendritic), ICa (allowing calcium entry in the KCa compartment), and the two action potential–generating conductances, IKDR (delayed-rectifier K+, axonal) and INa (fast sodium, axonal). A calcium-sensitive potassium current, IAHP, was also included in the KCa compartment, and the decline in free calcium was simulated using a first-order process with a time constant, τCa, of 132 ms. The effect of intracellular BAPTA iontophoresis was simulated by reducing this value to 20 ms. The properties of these conductances were fitted to the observed physiological data (Fig. 2).
Visual stimulation was simulated using synapses whose properties were based on previously obtained estimates for excitatory input strength17 and the relative influence of feed-forward inhibition15. Excitatory synaptic input arrived at the dendritic compartment, whereas inhibitory input arrived at the KCa compartment. Spontaneous excitatory and inhibitory input was added, as was a visual-input timing jitter. Model output was analyzed like experimental data, with N = 25 runs for all visual simulations.
Supplementary Material
Note: Supplementary information is available on the Nature Neuroscience website.
Acknowledgments
We would like to thank K. Josic and H. Krapp for comments. This work was supported by grants from the US National Institute of Mental Health. The use of the QNX 6 OS was made possible by QNX Software Systems’ Educational Program.
References
- 1.O’Shea M, Williams JLD. The anatomy and output connection of a locust visual interneurone: the lobular giant movement detector (LGMD) neurone. J Comp Physiol. 1974;91:257–266. [Google Scholar]
- 2.Krapp HG, Gabbiani F. Spatial distribution of inputs and local receptive field properties of a wide-field, looming sensitive neuron. J Neurophysiol. 2005;93:2240–2253. doi: 10.1152/jn.00965.2004. [DOI] [PubMed] [Google Scholar]
- 3.Burrows M. The neurobiology of an insect brain. Oxford University Press; Oxford, UK: 1996. [Google Scholar]
- 4.Schlotterer GR. Response of the locust descending movement detector neuron to rapidly approaching and withdrawing visual stimuli. Can J Zool. 1977;55:1372–1376. [Google Scholar]
- 5.Rind FC, Simmons PJ. Orthopteran DCMD neuron: a reevaluation of responses to moving objects. I Selective responses to approaching objects. J Neurophysiol. 1992;68:1654–1666. doi: 10.1152/jn.1992.68.5.1654. [DOI] [PubMed] [Google Scholar]
- 6.Simmons PJ, Rind FC. Orthopteran DCMD neuron: a reevaluation of responses to moving objects. II Critical cues for detecting approaching objects. J Neurophysiol. 1992;68:1667–1682. doi: 10.1152/jn.1992.68.5.1667. [DOI] [PubMed] [Google Scholar]
- 7.Judge S, Rind F. The locust DCMD, a movement-detecting neurone tightly tuned to collision trajectories. J Exp Biol. 1997;200:2209–2216. doi: 10.1242/jeb.200.16.2209. [DOI] [PubMed] [Google Scholar]
- 8.Gabbiani F, Krapp HG, Laurent G. Computation of object approach by a wide-field, motion-sensitive neuron. J Neurosci. 1999;19:1122–1141. doi: 10.1523/JNEUROSCI.19-03-01122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matheson T, Rogers SM, Krapp HG. Plasticity in the visual system is correlated with a change in lifestyle of solitarious and gregarious locusts. J Neurophysiol. 2004;91:1–12. doi: 10.1152/jn.00795.2003. [DOI] [PubMed] [Google Scholar]
- 10.Fotowat H, Gabbiani F. Relationship between the phases of sensory and motor activity during a looming-evoked multistage escape behavior. J Neurosci. 2007;27:10047–10059. doi: 10.1523/JNEUROSCI.1515-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto K, Nakata M, Nakagawa H. Input and output characteristics of collision avoidance behavior in the frog Rana catesbeiana. Brain Behav Evol. 2003;62:201–211. doi: 10.1159/000073272. [DOI] [PubMed] [Google Scholar]
- 12.Sun H, Frost BJ. Computation of different optical variables of looming objects in pigeon nucleus rotundus neurons. Nat Neurosci. 1998;1:296–303. doi: 10.1038/1110. [DOI] [PubMed] [Google Scholar]
- 13.Preuss T, Osei-Bonsu PE, Weiss SA, Wang C, Faber DS. Neural representation of object approach in a decision-making motor circuit. J Neurosci. 2006;26:3454–3464. doi: 10.1523/JNEUROSCI.5259-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowell CHF, O’Shea M. The neuronal basis of a sensory analyser, the acridid movement detector system. IV The preference for small field stimuli. J Exp Biol. 1977;68:157–185. doi: 10.1242/jeb.68.1.157. [DOI] [PubMed] [Google Scholar]
- 15.Gabbiani F, Cohen I, Laurent G. Time-dependent activation of feed-forward inhibition in a looming-sensitive neuron. J Neurophysiol. 2005;94:2150–2161. doi: 10.1152/jn.00411.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gabbiani F, Krapp HG. Spike-frequency adaptation and intrinsic properties of an identified, looming-sensitive neuron. J Neurophysiol. 2006;96:2951–2962. doi: 10.1152/jn.00075.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peron SP, Krapp HG, Gabbiani F. Influence of electrotonic structure and synaptic mapping on the receptive field properties of a collision-detecting neuron. J Neurophysiol. 2007;97:159–177. doi: 10.1152/jn.00660.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santer RD, Yamawaki Y, Rind FC, Simmons PJ. Preparing for escape: an examination of the role of the DCMD neuron in locust escape jumps. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2008;194:69–77. doi: 10.1007/s00359-007-0289-8. [DOI] [PubMed] [Google Scholar]
- 19.Rowell CH. The orthopteran descending movement detector (DMD) neurones: a characterisation and review. Z vergl Physiol. 1971;73:167–194. [Google Scholar]
- 20.Tsien RY. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980;19:2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- 21.Stocker M. Ca2+-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci. 2004;5:758–770. doi: 10.1038/nrn1516. [DOI] [PubMed] [Google Scholar]
- 22.Sobel EC, Tank DW. In Vivo Ca2+ Dynamics in a Cricket Auditory Neuron: An Example of Chemical Computation. Science. 1994;263:823–826. doi: 10.1126/science.263.5148.823. [DOI] [PubMed] [Google Scholar]
- 23.Wang XJ. Calcium coding and adaptive temporal computation in cortical pyramidal neurons. J Neurophysiol. 1998;79:1549–1566. doi: 10.1152/jn.1998.79.3.1549. [DOI] [PubMed] [Google Scholar]
- 24.Faber ES, Sah P. Calcium-activated potassium channels: multiple contributions to neuronal function. Neuroscientist. 2003;9:181–194. doi: 10.1177/1073858403009003011. [DOI] [PubMed] [Google Scholar]
- 25.Scott RH, Sutton KG, Griffin A, Stapleton SR, Currie KP. Aspects of calcium-activated chloride currents: a neuronal perspective. Pharmacol Ther. 1995;66:535–565. doi: 10.1016/0163-7258(95)00018-c. [DOI] [PubMed] [Google Scholar]
- 26.Bond CT, Maylie J, Adelman JP. SK channels in excitability, pacemaking and synaptic integration. Curr Opin Neurobiol. 2005;15:305–311. doi: 10.1016/j.conb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Wicher D, Walther C, Wicher C. Non-synaptic ion channels in insects–basic properties of currents and their modulation in neurons and skeletal muscles. Prog Neurobiol. 2001;64:431–525. doi: 10.1016/s0301-0082(00)00066-6. [DOI] [PubMed] [Google Scholar]
- 28.Heidel E, Pflüger HJ. Ion currents and spiking properties of identified subtypes of locust octopaminergic dorsal unpaired median neurons. Eur J Neurosci. 2006;23:1189–1206. doi: 10.1111/j.1460-9568.2006.04655.x. [DOI] [PubMed] [Google Scholar]
- 29.Gu N, Vervaeke K, Storm JF. BK potassium channels facilitate high-frequency firing and cause early spike frequency adaptation in rat CA1 hippocampal pyramidal cells. J Physiol (Lond) 2007;580:859–882. doi: 10.1113/jphysiol.2006.126367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Killmann F, Schürmann FW. Both electrical and chemical transmission between the lobula giant movement detector and the descending contralateral movement detector neurons of locusts are supported by electron microscopy. J Neurocytol. 1985;14:637–652. doi: 10.1007/BF01200802. [DOI] [PubMed] [Google Scholar]
- 31.Neher E, Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59:861–872. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 32.Peracchia C. Chemical gating of gap junction channels; roles of calcium, pH and calmodulin. Biochim Biophys Acta. 2004;1662:61–80. doi: 10.1016/j.bbamem.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 33.Gabbiani F, Krapp HG, Koch C, Laurent G. Multiplicative computation in a visual neuron sensitive to looming. Nature. 2002;420:320–324. doi: 10.1038/nature01190. [DOI] [PubMed] [Google Scholar]
- 34.Rall W. Theoretical significance of dendritic trees for neuronal input-output relations. In: Reiss RF, editor. Neural Theory and Modeling. Stanford University Press; Palo Alto, CA: 1964. [Google Scholar]
- 35.Koch C, Poggio T, Torre V. Retinal ganglion cells: a functional interpretation of dendritic morphology. Phil Trans R Soc Lond B. 1982;298:227–263. doi: 10.1098/rstb.1982.0084. [DOI] [PubMed] [Google Scholar]
- 36.Bhattacharjee A, Kaczmarek LK. For K+ channels, Na+ is the new Ca2+ Trends Neurosci. 2005;28:422–428. doi: 10.1016/j.tins.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Wittekindt OH, et al. An Apamin and Scyllatoxin-Insensitive Isoform of the Human SK3 Channel. Mol Pharmacol. 2004;65:788–801. doi: 10.1124/mol.65.3.788. [DOI] [PubMed] [Google Scholar]
- 38.Teagarden M, Atherton JF, Bevan MD, Wilson CJ. Accumulation of cytoplasmic calcium, but not apamin-sensitive afterhyperpolarization current, during high frequency firing in rat subthalamic nucleus cells. J Physiol (Lond) 2008;586:817–833. doi: 10.1113/jphysiol.2007.141929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sah P. Ca2+-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci. 1996;19:150–154. doi: 10.1016/s0166-2236(96)80026-9. [DOI] [PubMed] [Google Scholar]
- 40.Hirschberg B, Maylie J, Adelman JP, Marrion NV. Gating of recombinant small-conductance Ca-activated K+ channels by calcium. J Gen Physiol. 1998;111:565–581. doi: 10.1085/jgp.111.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haag J, Borst A. Spatial distribution and characteristics of voltage-gated calcium signals within visual interneurons. J Neurophysiol. 2000;83:1039–1051. doi: 10.1152/jn.2000.83.2.1039. [DOI] [PubMed] [Google Scholar]
- 42.Single S, Borst A. Different mechanisms of calcium entry within different dendritic compartments. J Neurophysiol. 2002;87:1616–1624. doi: 10.1152/jn.00215.2001. [DOI] [PubMed] [Google Scholar]
- 43.Kurtz R, Dürr V, Egelhaaf M. Dendritic calcium accumulation associated with direction-selective adaptation in visual motion-sensitive neurons in vivo. J Neurophysiol. 2000;84:1914–1923. doi: 10.1152/jn.2000.84.4.1914. [DOI] [PubMed] [Google Scholar]
- 44.Harris RA, O’Carroll DC, Laughlin SB. Contrast gain reduction in fly motion adaptation. Neuron. 2000;28:595–606. doi: 10.1016/s0896-6273(00)00136-7. [DOI] [PubMed] [Google Scholar]
- 45.Ellis LD, et al. SK channels provide a novel mechanism for the control of frequency tuning in electrosensory neurons. J Neurosci. 2007;27:9491–9502. doi: 10.1523/JNEUROSCI.1106-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanchez-Vives MV, Nowak LG, McCormick DA. Cellular mechanisms of long-lasting adaptation in visual cortical neurons in vitro. J Neurosci. 2000;20:4286–4299. doi: 10.1523/JNEUROSCI.20-11-04286.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang XJ, Liu Y, Sanchez-Vives MV, McCormick DA. Adaptation and temporal decorrelation by single neurons in the primary visual cortex. J Neurophysiol. 2003;89:3279–3293. doi: 10.1152/jn.00242.2003. [DOI] [PubMed] [Google Scholar]
- 48.Migliore M, Shepherd GM. Emerging rules for the distributions of active dendritic conductances. Nat Rev Neurosci. 2002;3:362–370. doi: 10.1038/nrn810. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Note: Supplementary information is available on the Nature Neuroscience website.