Abstract
Building upon our initial studies in young adult surgically menopausal monkeys, this study examined the effects of a novel schedule of administration of estradiol therapy alone, or in combination with progesterone, on visual and spatial recognition memory in older monkeys. Monkeys were preoperatively trained on a delayed matching-to-sample task and a delayed response task. At the time of ovariectomy, monkeys began their hormonal treatments and were cognitively assessed at 2, 12 and 24 weeks following treatment initiation. A schedule of hormone administration was used that closely modeled the normal fluctuations of hormones during the course of a normal primate menstrual cycle. Monkeys receiving placebo had lower levels of accuracy than monkeys receiving estrogen therapies on the delayed matching-to-sample task that were not apparent until 12 weeks following initiation of therapy and were no longer detected at the 24-week assessment. There was no effect of hormone therapy on accuracy in the delayed response task at any of the postoperative assessments. In both tasks, monkeys treated with estrogen plus progesterone had longer choice response latencies, especially on trials in which they made errors; however these effects did not influence accuracy measures in these animals. Our findings indicate that visual recognition ability may be more sensitive than spatial recognition memory to this novel hormone therapy regimen, that treatment with estradiol plus progesterone was equivalent to that of estradiol alone, and that neither therapy had significant negative impact on memory profiles.
Keywords: nonhuman primates, ovariectomy, postmenopausal, delayed matching-to-sample, delayed response
For postmenopausal women, the question of whether to start or to continue with hormone therapy to alleviate menopausal symptoms and decrease risks for dementia and cognitive decline has become more difficult to answer given the recent results from the Women's Health Initiative (WHI) study (Writing Group for Women's Health Initiative Investigators, 2002; Women's Health Initiative Steering Committee, 2004) and its ancillary study, the Women's Health Initiative Memory Study (WHIMS) (Rapp et al., 2003b; Shumaker et al., 2003; Espeland et al., 2004; Shumaker et al., 2004). In the wake of the surprising results of these studies, many have suggested that several critical factors present in these studies contributed to the results, including the age and health of the subjects, delayed timing of hormone therapy initiation, the particular formulations and doses of hormones used, and the continuous nature of the hormone regimen (Mikkola et al., 2004; Ancelin and Ritchie, 2005; Sherwin, 2005; Maki, 2006; Manson et al., 2006).
In an effort to answer some of the questions about estrogen and cognition in ways that are not entirely possible by studying postmenopausal women, our laboratory and others have used animal models of menopause in which we have control over many variables that may contribute to cognitive function. In one of the first monkey studies to examine the effects of estrogen therapy (ET) on memory, we demonstrated that aspects of visual and spatial memory were unaltered following surgical menopause or ET in young adult monkeys (Voytko, 2000). Subsequent studies in young monkeys corroborated these initial findings (Lacreuse and Herndon, 2003; Hao et al., 2007). In contrast, studies in older monkeys indicate that both spatial and visual memory are sensitive to the loss of estrogen and ET (Roberts et al., 1997; Lacreuse et al., 2000; Lacreuse et al., 2002; Rapp et al., 2003a; Tinkler and Voytko, 2005). Timing of hormone therapy is important with greater benefits associated with therapy that is initiated soon after ovariectomy in rats (Gibbs, 2000; Daniel et al., 2006). In monkeys, ET given within six months of ovariectomy improved memory (Rapp et al., 2003a; Tinkler and Voytko, 2005), but did not if therapy was delayed for more than 10 years (Lacreuse et al., 2002).
With an interest in designing therapies that are more in line with the natural menstrual cycle, cyclic schedules of hormone administration recently have been investigated. Intermittent schedules of hormones improved acquisition and memory in a variety of tasks in rodents (Gibbs, 2000; Sandstrom and Williams, 2001; Korol and Kolo, 2002; Markowska and Savonenko, 2002; Bimonte-Nelson et al., 2006). In an innovative approach in monkeys, Rapp and colleagues employed a long-term cyclic administration of ET (Rapp et al., 2003a). Monkeys receiving this intermittent delivery of ET had better performance on a delayed response task and a delayed nonmatch-to-sample task than placebo-treated monkeys (Rapp et al., 2003a). This same therapy schedule however did not influence memory in the delayed response task in young ovariectomized monkeys (Hao et al., 2007).
Although the majority of animal studies have used ET alone, several studies in rodents found that estradiol combined with natural progesterone (E+P) enhances spatial and nonspatial memory in a manner equivalent to ET (Dohanich et al., 1994; Gibbs, 2000; Sandstrom and Williams, 2001; Markham et al., 2002; Frye et al., 2007). While the effects of E+P on cognitive function of surgically menopausal monkeys have not been reported, E+P positively influences neurotransmitter systems that are known to influence cognition in primates (Kritzer and Kohama, 1998,1999; Kritzer et al., 2003); in contrast to conjugated equine estrogen plus medroxyprogesterone therapy, the formulation used in WHI/WHIMS, that has either no effect or detrimental effects in the primate brain (Gibbs et al., 2002; Gibbs et al., 2006).
The present study was undertaken in an effort to build upon our initial studies of memory in young monkeys (Voytko, 2000) and to further investigate the most appropriate hormone formulations and administration schedules that will benefit cognitive function in primates. In this study, older monkeys were used to investigate the effects of estradiol alone or in combination with natural progesterone on both visual and spatial recognition memory function. Thus, this study is the first to investigate the effects of combined E+P on memory in ovariectomized monkeys and to compare this combined therapy to that of ET alone. Our hypotheses were that recognition memory would be preserved or enhanced in monkeys treated with ET or E+P and that these therapies would be comparable in their effects. These hypotheses were based on the previously mentioned studies in rodents that showed that E+P was equivalent to ET in improving memory and on the studies in primates that demonstrated that E+P has positive effects in several neurochemical systems in the brain that can influence memory. Importantly, as a result of the pressing need to investigate alternate schedules and regimens of hormone therapy to address the concerns raised by the results of WHIMS in postmenopausal women, a novel hormone therapy schedule was used that closely mirrored the hormonal fluctuation patterns that occur over the course of a normal primate menstrual cycle. This hormone therapy was initiated at the time of ovariectomy in the monkeys to address the concern that early therapy may be more beneficial than delayed therapy for postmenopausal women (Henderson, 2005; Maki, 2006; Sherwin, 2006).
Experimental Procedures
Subjects
Twenty-five female rhesus (Macaca mulatta) monkeys (mean ± SEM at time of ovariectomy and treatment initiation: 19.7 ± 0.5 years) were the subjects of these experiments. The animals were individually housed in a climate-controlled room. Water was available ad libitum and rations of monkey chow were provided after the daily behavioral sessions. They received environmental enrichment in the form of novel toys and foods regularly. The monkeys had been examined on a test of executive function prior to being examined on the memory tests of this study. All procedures involving animals were conducted according to the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) and approved by the Wake Forest University School of Medicine Animal Care and Use Committee. All efforts were made to minimize the number of animals used and their suffering.
Experimental Design
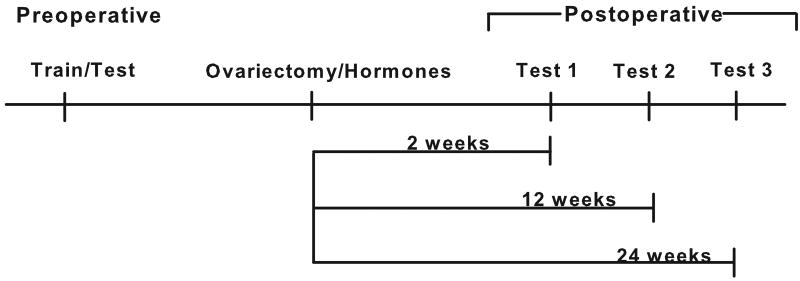
The experimental design is depicted in Figure 1. Monkeys were trained and tested on the visual and spatial recognition memory tasks prior to ovariectomy and initiation of hormone treatment and reexamined again postoperatively at 2, 12, and 24 weeks following surgery. Monkeys were tested five days a week and pre- and postoperative behavioral sessions were conducted without regard to the time of day for all animals. Preoperative training/testing took place without regard to the phase of the menstrual cycle. Postoperative testing of monkeys receiving ET or E+P was conducted throughout their treatment schedule and was not confined to only a particular phase of their schedule to mirror the conditions that hormonally-treated postmenopausal women normally experience in their daily lives. Monkeys were randomized to treatment group based on their preoperative mean performance on the delayed response and delayed matching-to-sample tasks.
Figure 1.
Experimental Design. Monkeys were preoperatively trained and tested on the memory tasks and tested at three time points following ovariectomy and initiation of hormone therapy.
Ovariectomy
A staff veterinarian ovariectomized the monkeys using sterile surgical procedures. To perform the ovariectomies, the monkeys were sedated with ketamine (5-10 mg/kg, i.m.), the trachea intubated, and the animals maintained on 2.5% isoflurane and oxygen during the surgery. A midline incision was made and the ovaries were exteriorized. The vasculature was ligated, the ovaries removed and the incision was then sutured closed. The monkeys were monitored postoperatively until recovered from anesthesia and then on a daily basis until sutures were removed. Butorphanol (0.025 mg/kg, i.m.) was administered to alleviate pain 3-4 hours after surgery in a one time dose. Surgically related postoperative complications were not noted in any of the monkeys.
Hormone Treatments
During surgery to remove the ovaries, 8 monkeys were subcutaneously implanted with a 3.5 cm length of empty Silastic tubing (PL, placebo group) and 17 of the monkeys were implanted subcutaneously with a 3.5 cm length of Silastic tubing (0.335 cm ID and 0.465 cm OD) containing a 3 cm packed column of 17β-estradiol (Steraloids Inc., Wilton, NH). Implants of this size have been used successfully in previous monkey studies and have remained viable for greater than 14 months following implantation (Adams et al., 1990; Voytko, 2000, 2002). Pilot studies in rhesus monkeys indicated that implants of this size would deliver and maintain estradiol levels at 40-80 pg/ml. All monkeys with estradiol implants received an injection of estradiol valerate (0.10-0.20 mg/cc, Pfizer, NY) on Day 12 to simulate the peak in estradiol that normally occurs prior to ovulation in the menstrual cycle. Pilot studies indicated that injections of this size would achieve estradiol peaks ranging from 300-700 pg/ml. Nine of the monkeys receiving estradiol implants comprised the estrogen alone group (ET) while the remaining eight monkeys with implants comprised the estrogen plus progesterone group (E+P) and were given oral doses of progesterone (0.2mg/kg, Prometrium, USP, Solvay Phamaceuticals) for 12 days beginning on day 16. Oral dosing of progesterone was chosen because of the reoccurring cyclical nature of the therapy schedule of this hormone. The progesterone dosing schedule duration and start day was based on the normal menstrual cycle of macaque monkeys (Goodman et al., 1977; Downs and Urbanski, 2006). The progesterone was placed in various foods or treats for dosing and each animal was closely observed during the dosing to ensure that the foods/treats were totally consumed. This schedule of estradiol injections and progesterone dosing was repeated monthly through the course of the study.
Hormone Assays
Awake blood sampling took place frequently to monitor levels of estradiol and progesterone throughout the study. Blood samples were drawn from the saphenous vein while the animal was within a specially constructed transfer cage. For monkeys receiving estradiol, blood samples were taken the day before the estrogen injection to determine the status of estradiol delivery by the implants. Blood was again sampled six hours following the estrogen injection to determine peak levels of estradiol attained by the injection. For E+P monkeys, blood also was drawn on days 1, 6 and 12 of progesterone dosing to assay levels of progesterone. A blood sample also was taken 24 hours after the final dose of progesterone to verify that progesterone levels were no longer elevated. This schedule of blood sampling was repeated monthly through the course of the study. Blood was collected in sterile serum tubes, allowed to clot, centrifuged, and then serum was aliquoted into separate tubes and frozen at −20 °C. Serum levels of estradiol (E2) and progesterone (P4) were measured with a Roche Diagnostics Elecsys 2010 clinical assay platform by the Oregon National Primate Research Center Endocrine Services Core Laboratory. These assays had been previously validated against traditional extraction RIA's (Hess et al., 1981). The sensitivity for E2 was 12 pg/ml and for P4 was 0.05 ng/ml. The coefficient of variation for both hormones was <10%.
Behavioral Apparatus
The monkeys were behaviorally trained and tested in computer-controlled apparatuses that were modified from one previously described (Voytko et al., 1994). In the test chamber, monkeys faced a 17” touchscreen monitor (KDS Pixel Touch Inc.) onto which stimuli were projected. Speakers were mounted in the chamber to deliver different sounds for correct and incorrect responses and to provide continuous white noise during testing. A fan provided nonstop airflow within the testing chamber and a closed-circuit camera allowed visualization of the monkeys during their test sessions. Banana pellets were delivered via a pellet dispenser as reward for correct responses. To provide auditory feedback, the sound of chimes was delivered for correct responses and a low-pitched tone was delivered for incorrect responses for all tasks. To provide visual feedback, the monitor screen turned black after correct responses and turned blue after incorrect responses for all tasks. To signal the end of the test session, the monitor screen turned red.
Behavioral Tasks
Dr. James Herndon at the Yerkes National Primate Research Center graciously provided Touchscreen computer software containing the memory tasks used in this study. Modifications to the memory tasks were made in our laboratory to fit our individual testing requirements. All animals received the tasks in the order in which they are described.
Delayed Match-to-Sample (DMS) Task
This task assesses visual recognition memory and the procedures followed those used previously for modified or identical versions of this task (Voytko et al., 1994; Lacreuse and Herndon, 2003). Each trial of DMS consisted of three phases: cue, delay, and choice. A trial began with the appearance of an object (sample) to the center of the monitor (“cue phase”). After a response to the object, a reward was delivered, the sample removed, and the screen darkened for a period of time (“delay phase”). After the delay, the sample object (match) and a novel object (nonmatch) appeared on each side of the monitor (“choice phase”). The left-right appearance of these stimuli was determined pseudorandomly. The monkey received a reward for responding to the match stimulus. Any response to the nonmatch stimulus resulted in no reward and immediate removal of all stimuli from the screens. Training began with a 0 sec. delay, with the delay increasing by 1 sec. each time 85% correct accuracy was achieved in a block of 20 trials (17/20 correct trials). Training continued until a 10 sec. delay was reached. A 15 sec. intertrial interval (ITI) was used and a daily session consisted of 100 trials. Once the animals learned the task, they were then trained on a variable delay version of the task (VDMS) in which delays of 1, 10, 30, 60 sec. were randomly presented within a session. A daily session consisted of 100 trials (25 trials per delay). Training continued on this version of the task until performance was stable across all delays as evidenced by minimal fluctuations in accuracy at each delay.
During the preoperative assessment and each postoperative assessment of VDMS, the animals were tested using the randomly presented delays of 1, 10, 30, 60 sec. Test data were collected over four days to obtain a total of 100 trials/delay. The dependent measures were percent correct accuracy at each delay and response latency (in sec) during the choice phase. Response latency was measured for all trials (total trials), and for trials in which correct responses were made (correct trials) and for trials in which an error was made (incorrect trials). In addition to the variable delay version of DMS, the monkeys also were tested pre- and postoperatively on two additional DMS versions with only one delay, either a 2 min. (DMS2) or a 10 min. (DMS10) delay. In these single version DMS assessments, the monkeys remained in the behavioral apparatus during the delays. For DMS2, the animals were tested for 25 trials/day for 2 days for a total of 50 trials. For the DMS10, the animals were tested for 5 trials/day for 10 days for a total of 50 trials. Only monkeys that performed at ≥ 60% correct on DMS2 were then tested on DMS10 at any of the assessments.
Delayed Response (DR) Task
This task assesses spatial recognition memory and the procedures followed those used previously (Voytko et al., 1994; Voytko, 2000). Each trial of DR consisted of 3 phases: cue, delay, choice. A trial began with a colored flashing circle appearing on either the right or left side of the monitor (“cue phase”). No response was required to the flashing cue. The flashing cue was removed and the screen was darkened for a period of time (“delay phase”). After the delay, a yellow square appeared on both the right and left sides of the monitor (“choice phase”) and the correct strategy to receive a reward was to respond to the square that appeared in the same location as the flashing cue presented during the cue phase. Any response to the incorrect stimulus resulted in no reward and immediate removal of all stimuli from the screens. Training began with a 0 sec. delay, with the delay increasing by 1 sec. each time 85% correct accuracy was achieved in a block of 20 trials (17/20 correct trials). Training continued until a 10 sec. delay was reached. A 15 sec. ITI was used and a daily session consisted of 100 trials. Once the animals learned the task, they were then trained on a variable delay version of the task in which delays of 1, 10, 30, 60 sec. were randomly presented within a session. A daily session consisted of 100 trials (25 trials per delay). Training continued on this version of the task until performance was stable across all delays as evidenced by minimal fluctuations in accuracy at each delay.
During the preoperative assessment and each postoperative assessment of DR, the animals were tested using the randomly presented delays of 1, 10, 30, 60 sec. Test data were collected over four days to obtain a total of 100 trials/delay. The dependent measures were percent correct accuracy at each delay and response latency (in sec) during the choice phase. Response latency was measured for all trials (total trials), and for trials in which correct responses were made (correct trials) and for trials in which an error was made (incorrect trials).
Statistical Analyses
Data were analyzed using repeated measures analyses of variance stratified by time and by group. Time analyses were performed with and without inclusion of the preoperative data so that comparisons could be made of data collected before and after ovariectomy/treatment, as well as only once treatment was initiated. Post hoc comparisons were performed with Tukey's HSD test. Initial analyses compared all three treatment groups (PL, ET, E+P) to reveal any differences that may exist between each of the estrogen groups and the PL group. Additional analyses were performed to determine if there were differences just between the estrogen treated groups (ET vs E+P) and if no differences existed, the data from these two groups then were combined into a single group of estrogen treated monkeys (E) and compared to the PL monkeys. For analyses of choice response latencies, latencies greater than 15 sec (distracted responses) were eliminated from the analyses. Regression analyses were performed to determine relationships between hormone levels and the dependent measures.
Results
Hormone Levels
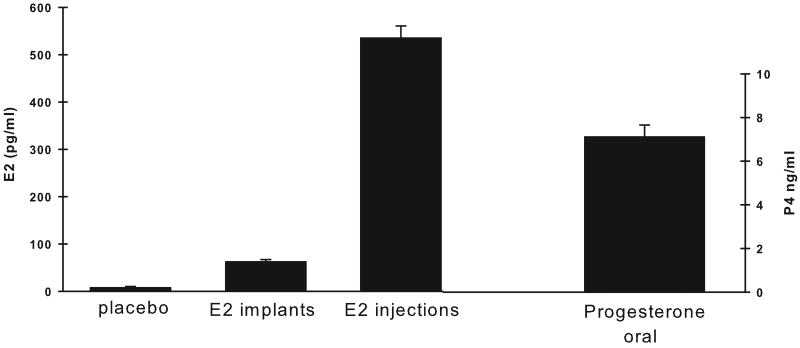
Average levels of E2 and P4 achieved by the hormone regimen used in the present study are illustrated in Figure 2. Basal serum E2 levels were different between the treatment groups (F (2,22) = 198.11, p < 0.001). Post hoc analyses indicated that the monkeys with estradiol implants had significantly greater basal levels of E2 compared to the placebo monkeys (ET vs PL, p < 0.001; E+P vs PL, p < 0.001). Table 1 shows the E2 levels produced by the implants and by the estradiol valerate injections during each postoperative assessment in the ET and E+P monkeys. The two estrogen-treated groups of monkeys did not differ in their serum E2 levels produced either by the implants or by the monthly injections (p's > 0.05) and E2 levels produced by the injections were within the physiological increased levels that occur during the normal primate menstrual cycle just prior to ovulation in young premenopausal monkeys (Downs and Urbanski, 2006). Oral doses of Prometrium in E+P monkeys produced serum progesterone levels that averaged 7 ng/ml. In contrast, PL and ET monkeys had nearly undetectable progesterone levels (i.e., < 0.05 ng/ml).
Figure 2.
Average serum levels of ovarian hormones over the course of the study. Levels of estradiol are shown for placebo monkeys and for monkeys receiving estradiol implants and monthly injections of estradiol. Estradiol levels were greater in monkeys receiving therapy than placebo monkeys. Average levels of progesterone are shown for those monkeys receiving oral doses of prometrium in 12 day blocks each month over the course of the study. E2, estradiol. Bars = SEM.
Table 1.
Levels of Estrogen (pg/ml) produced by implants and injections
| Postop 1 implant | Postop 1 injection | Postop 2 implant | Postop 2 injection | Postop 3 implant | Postop 3 injection | |
|---|---|---|---|---|---|---|
| ET | 67.7 + 2.1 | 625.7 + 40.5 | 67.9 + 2.4 | 630.6 + 61.0 | 74.1 + 5.6 | 456.8 + 51.4 |
| E+P | 66.2 + 5.1 | 704.8 + 66.2 | 66.3 + 3.4 | 459.4 + 59.6 | 65.3 + 3.0 | 471.1 + 73.7 |
DMS
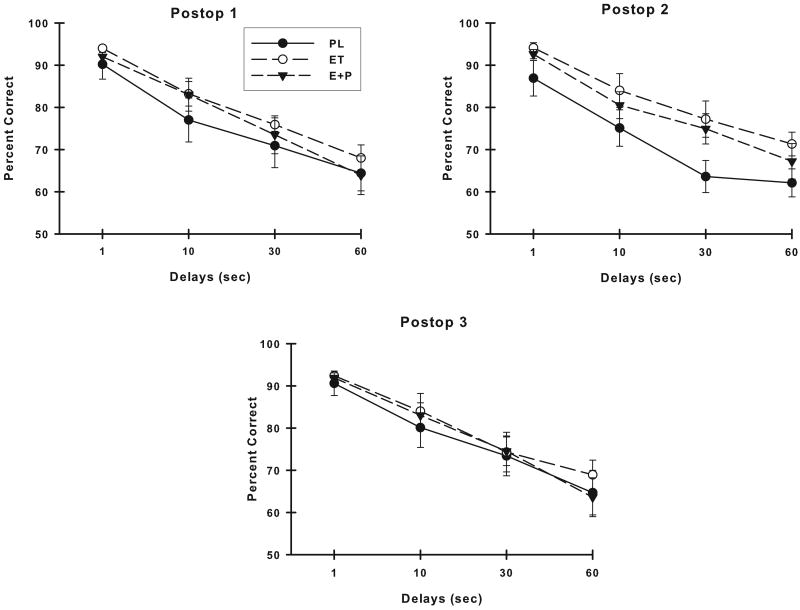
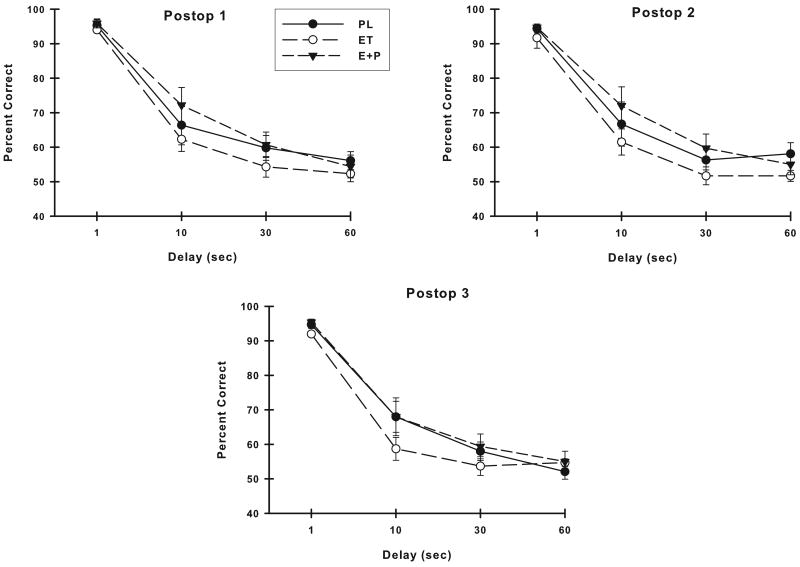
There were no differences between the groups of monkeys in their preoperative VDMS accuracy (group or group × delay, p > 0.05). Figure 3 illustrates the VDMS accuracy of the three treatment groups at each of the three postoperative assessments. Analyses of these VDMS scores suggested a difference on VDMS between the PL, ET and E+P monkeys at the Postop #2 assessment (group, F (2,22) = 2.63, p = 0.09). The groups did not differ on VDMS at either of the other two postoperative assessment periods (group or group × delay, p's > 0.05). At all time points, the monkeys performed worse with increasing delays (Postop #1: F (3,66) = 83.05, p < 0.001; Postop #2: F (3,66) = 91.43, p < 0.001; Postop #3: F (3,66) = 77.93, p < 0.001) but no other effects were significant. Comparisons of VDMS accuracy of the ET and E+P monkeys were conducted to determine if these groups could be combined into an overall group of estrogen-treated monkeys (E group of monkeys) for comparison with PL monkeys. There was no difference between ET and E+P monkeys in their VDMS accuracy at any of the postoperative assessments (group or group × delay, p's > 0.05), thus VDMS scores from these two estrogen-treated groups were combined and compared to the scores of the PL monkeys. Placebo-treated monkeys performed worse than E monkeys on VDMS at all postoperative assessments but this difference was significant only at the Postop #2 assessment (group, F (1,23) = 4.96, p = 0.03). Although the analysis did not indicate that the groups differed at only specific delays (delay × group, p > 0.05), it was clear from examining the VDMS performance of the treatment groups that the PL monkeys had the greatest difference in performance from E monkeys at the 30 sec delay.
Figure 3.
Accuracy on VDMS at each postoperative assessment. Placebo monkeys were significantly worse than monkeys receiving estrogen therapies at the second postoperative evaluation. All monkeys had poorer accuracy with increasing delays at each assessment. PL, placebo; ET, estrogen alone; E+P, estrogen plus progesterone. Bars = SEM.
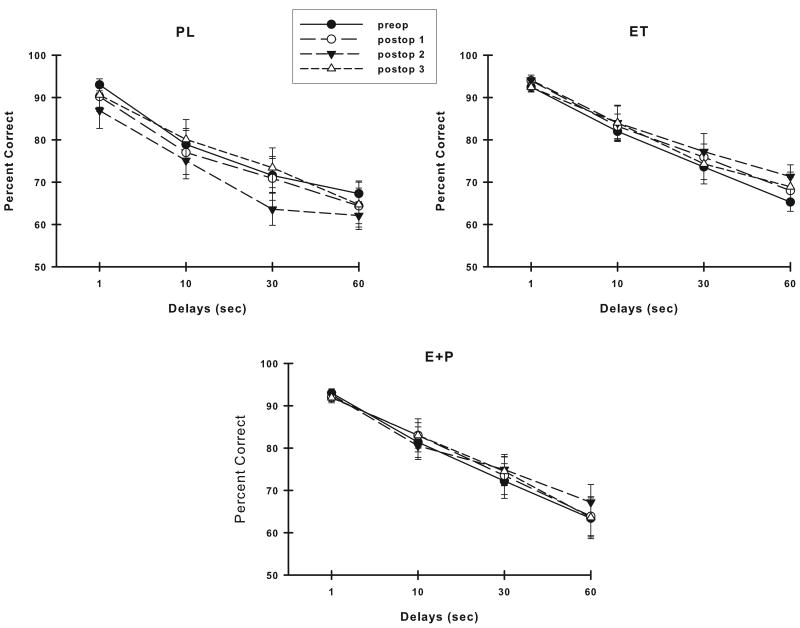
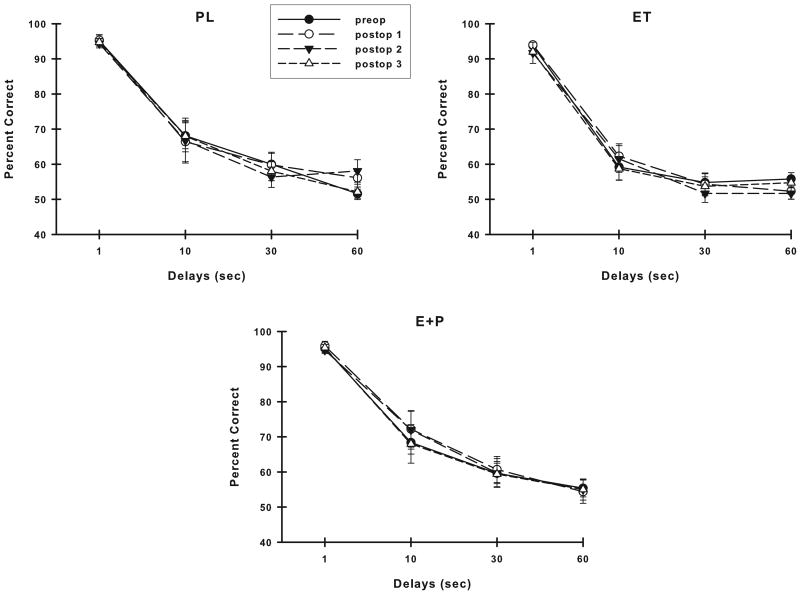
Figure 4 illustrates the VDMS accuracy of each group of monkeys across their assessments. Analysis of these VDMS scores revealed that the accuracy of PL monkeys differed with time (preop through postop: F (3,21) = 2.70, p = 0.07; postop only: F (2,14) = 3.96, p = 0.04) and this effect was likely related to the lower VDMS accuracy at the Postop #2 assessment compared to the other assessments. In contrast, the estrogen-treated monkeys maintained their levels of VDMS accuracy across all assessments (p > 0.05 for ET or E+P, either preop to postop or postop only).
Figure 4.
Accuracy on VDMS across time in each group of monkeys. Placebo monkeys were significantly worse with time, while monkeys receiving estrogen therapies maintained their accuracy across time. Abbreviations as in Fig. 3.
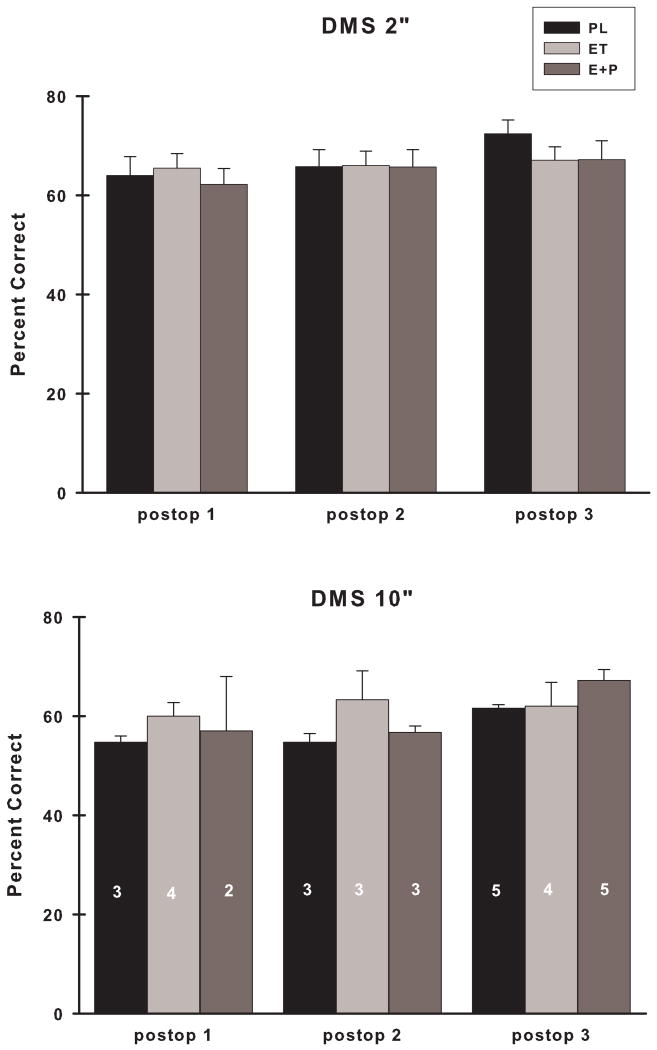
There were no differences between the groups of monkeys in their preoperative performance on DMS at the 2 minute delay (DMS2) (p > 0.05). Postoperatively, there was no difference in DMS2 accuracy between PL, ET and E+P monkeys at any of the postoperative assessments (Fig.5; group, p's > 0.05). Examination of DMS2 scores for each group of monkeys across assessments revealed that the performance of PL monkeys improved with time (preop to postop: time, F (3,18) = 2.35, p = 0.10; postop only: time, F (2,12) = 8.01, p = 0.006), but similar improvements did not occur for either ET or E+P monkeys (time, p's > 0.05). Some monkeys (n = 5: PL = 2, ET = 1, E+P = 2) were not tested on DMS with a 10 minute delay (DMS10) because their scores were < 60% accurate on DMS2. Of those monkeys who were tested on DMS10, there were no differences between the groups of monkeys in their preoperative performance on DMS10 (p > 0.05). Postoperatively, there was no difference between PL, ET and E+P monkeys on their accuracy on DMS10 at any of the postoperative assessments (Fig. 5; group, p > 0.05). Because of inconsistency in the performance of the animals on DMS10 across the assessments, within group analyses could not be performed. Comparison of ET and E+P accuracy on DMS2 and DMS10 revealed no differences between these groups at any of the postoperative assessments (group, p's > 0.05) and when these groups were combined into a collective E group and compared to PL monkeys, no differences between these groups emerged for either DMS2 or DMS10 (group, p's > 0.05).
Figure 5.
Accuracy on DMS using a two minute and ten minute delay at each of the postoperative assessments. Numbers of monkeys in each group at each time point are indicated in the individual bar graphs. There were no differences in accuracy between the groups at either delay. DMS 2”, two minute delay; DMS 10”, ten minute delay. Remainder of abbreviations as in Fig. 3.
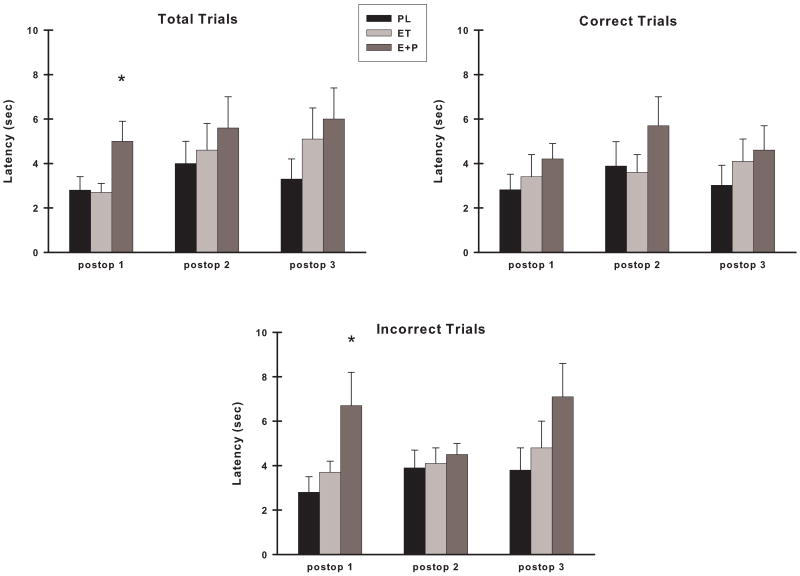
There were no differences in VDMS response latencies between the three groups preoperatively on any of the types of trials (total, correct or incorrect) (group, p's > 0.05). Postoperatively, 12 latencies were greater than 15 sec and thus were omitted from the analyses. This number represented 0.05% of the total VDMS latencies that were measured postoperatively. There was a difference between the groups at the Postop #1 assessment for response latencies for total trials (F (2,20) = 4.01, p = 0.03) and for incorrect trials (F (2,19) = 3.62, p = .04) (Fig. 6). Post hoc analyses indicated that for total trials, the E+P monkeys had longer response latencies than ET monkeys (p = 0.04) and a suggestion of the same for PL monkeys (p = 0.07). For incorrect trials, E+P monkeys had longer response latencies than PL monkeys (p = 0.05) and a trend for the same for ET monkeys (p = 0.10). There were no differences in latencies between ET and PL monkeys for any types of trials at the Postop #1 assessment period. In addition, there were no differences between the groups of monkey in their choice response latencies for any other postoperative assessment. Moreover, in repeated measures analyses, all groups of monkeys maintained their levels of performance across all assessments (preop to postop or postop only) for total, correct and incorrect trials (time, p > 0.05 for each group of monkeys).
Figure 6.
Choice response latency on VDMS at each postoperative assessment across all trials (Total Trials), on trials in which a correct response was made (Correct Trials), and on trials in which an error was made (Incorrect Trials). E+P monkeys had significantly longer latencies for total and incorrect trials during the first postoperative assessment. Abbreviations as in Fig. 3. *, p <0.05 compared to other two groups.
DR
There was no difference between the groups preoperatively on DR accuracy (p > 0.05). There were no differences between the groups on DR accuracy at any of the postoperative assessment periods (Fig. 7; group, p's > 0.05). At all time points, the monkeys performed worse with increasing delays (Postop #1: F (3,66) = 175.95, p < 0.001; Postop #2: F (3,66) = 131.24, p < 0.001; Postop #3 F (3,66) = 148.22, p < 0.001), but no other effects were significant. Comparisons of DR accuracy of the ET and E+P monkeys were conducted to determine if these groups could be combined into an overall group of estrogen-treated monkeys (E group of monkeys) for comparison with PL monkeys. These groups of estrogen monkeys did not differ at the Postop #1 or Postop #3 assessments, but they did differ at the Postop# 2 evaluation (group, F (1,15) = 6.13, p = 0.02) where E+P monkeys had higher accuracies at the middle delays and thus their scores were not combined for additional comparisons with the PL monkeys. Examination of VDR scores for each group of monkeys across assessments (preop to postop or postop only) revealed stable levels of accuracy for each treatment group (Fig. 8; time, p > 0.05 for all groups).
Figure 7.
Accuracy on VDR at each postoperative assessment. There were no differences in accuracy between the groups at any of the assessment periods. All monkeys had poorer accuracy with increasing delays at each assessment. Abbreviations as in Fig. 3
Figure 8.
Accuracy on VDR across time in each group of monkeys. All groups of monkeys maintained their levels of accuracy across time. Abbreviations as in Fig. 3.
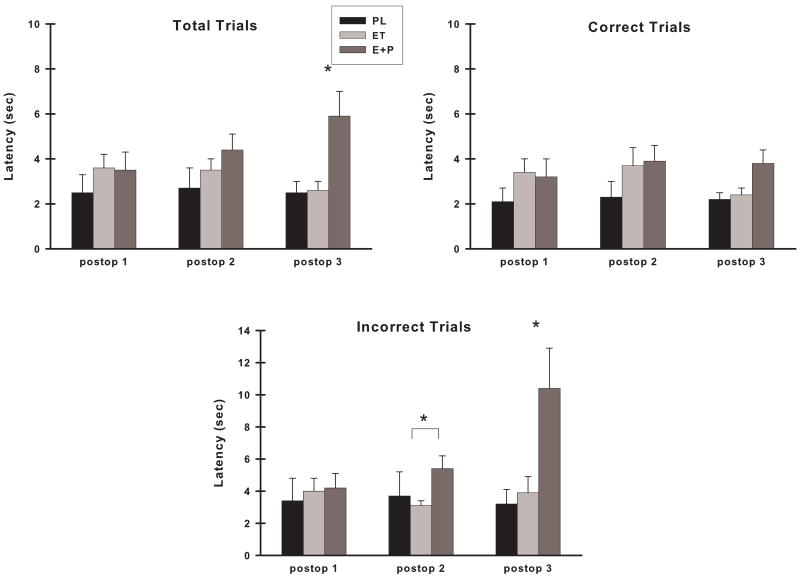
Preoperatively, there were no differences between the groups in their choice response latency on any of the types of trials (group, p's >0.05). Postoperatively, no response latencies were omitted from the analyses. Choice response latency on DR total trials was not different between groups for Postop #1 or #2 assessments, however there were group differences at the Postop #3 assessment (Fig. 9; F (2,21) = 7.06, p = 0.005) and post hoc analyses indicated that E+P monkeys were significantly slower in responding across all trials than either ET (p = 0.009) or PL (p = 0.01) monkeys. Subsequent analyses were conducted for both correct and incorrect trials during Postop #3 to determine whether group differences in choice response latency in total DR trials were related to whether the animals made a correct or incorrect response. There was a difference between the groups in choice response latency on correct trials (Fig. 9; F (2,21) = 3.42, p = 0.05) and further analyses suggested that E+P monkeys were slightly slower in responding to the choice than ET (p = 0.09) or PL (p = 0.07) monkeys. For incorrect trials, the difference between the groups was much larger and probing of a group effect (Fig. 9; F (2,21) = 5.89, p = 0.009) revealed that E+P monkeys were significantly slower in responding on incorrect trials than either ET (p = 0.02) or PL (p = 0.01) monkeys. In additional comparisons of DR latency among only estrogen treated monkeys, ET monkeys also were found to be faster than E+P monkeys on incorrect trials at the Postop #2 assessment (F (1,15) = 8.10, p = 0.01).
Figure 9.
Choice response latency on VDR at each postoperative assessment across all trials (Total Trials), on trials in which a correct response was made (Correct Trials), and on trials in which an error was made (Incorrect Trials). The groups of monkeys differed in their latencies during the third postoperative assessment at which time the E+P monkeys had significantly longer latencies than the other monkeys, particularly on total and incorrect trials. Abbreviations as in Fig. 3. *, p <0.05 compared to other two groups; * with bracket, significant difference between ET and E+P at p < 0.05.
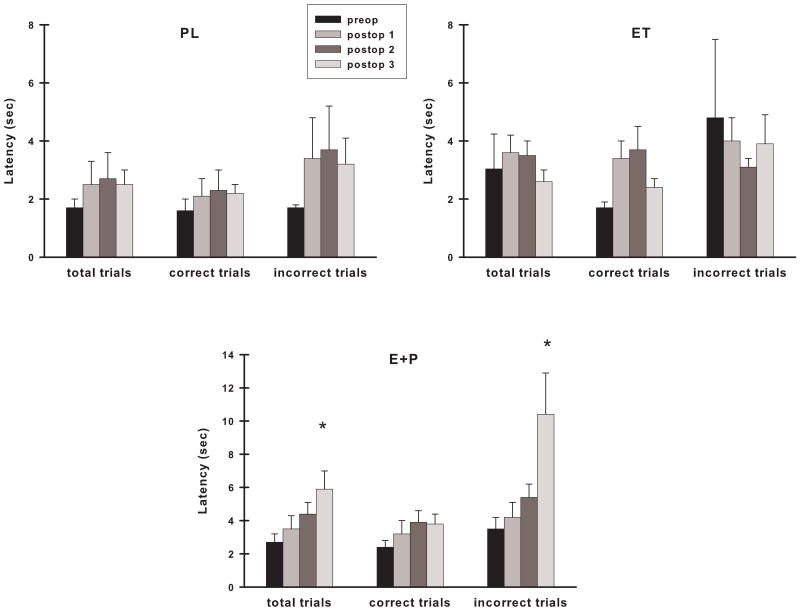
Analyses of DR choice response latencies within each treatment group indicated that E+P monkeys demonstrated increased response latencies with time for total trials (Fig. 10; preop to postop: time, F (3,21) = 4.84, p = 0.01; postop only: time, F (2,14) = 4.00, p = 0.04) and for incorrect trials (preop to postop: time, F(3,21) = 6.41, p = 0.003; postop only: time, F (2,14) = 6.33, p = 0.01), but not for correct trials (time, p's > 0.05). These results were related to the maximal increase in response latency found during the Postop #3 assessment as there was no significant increase in response latency of E+P monkeys across time if this last assessment is not included in the analyses. Choice response latency of ET monkeys on correct trials showed an increase postoperatively compared to their preoperative performance (Fig. 10; time, F (3,24) = 3.75, p = 0.02) but there was no difference across their postoperative assessments (time, p > 0.05). There were no differences in DR choice response latencies for ET monkeys on the other types of trials (time, p's > 0.05). In contrast, PL monkeys did not demonstrate a significant difference in their choice response across all assessments for total, correct, and incorrect trials (time, p's> 0.05).
Figure 10.
Choice response latency on VDR across time in each group of monkeys. Monkeys receiving the combined hormone therapy had significantly longer latencies on both total and incorrect trials over time that was related to their elevated latencies during the third postoperative assessment. Monkeys treated with estrogen alone showed an increase in response latency on correct trials only in analyses that included their preoperative performance. Placebo monkeys maintained their performance across time. Abbreviations as in Fig. 3. *, p <0.05 compared to other two groups.
Hormone Correlations with Behavior
There were no significant correlations between levels of E2 and any of the dependent measures in the monkeys treated with estrogen. Similarly, there were no significant correlations between levels of P4 and any of the dependent measures in the monkeys treated with progesterone.
Discussion
The present study is unique in three major factors that are critical to furthering our understanding of the effects of ovarian hormones on cognitive processes in primates. First, it is the first to examine and compare the effects of estrogen therapy alone and in combination with progesterone on memory function in a monkey model of menopause. Second, it is the first in any animal model of menopause to use a hormone therapy administration schedule that most closely mimics the normal primate menstrual cycle fluctuations of estradiol and progesterone. Finally, because of the nature of our experimental design, we could evaluate performance patterns across time in the different treatment groups. Our results suggest that visual recognition ability may be more sensitive than spatial recognition to hormone therapy in older monkeys, that these effects are delayed in appearance, and that these effects are transient.
Hormone Therapy and Schedule
In a normal menstrual cycle of a rhesus monkey, basal estradiol levels are approximately 50 pg/ml (Downs and Urbanski, 2006). At midcycle, a peak of estradiol occurs just prior to ovulation in which estradiol levels can reach 300-600 pg/ml followed by a sharp decrease back to basal levels. A rise in progesterone occurs approximately 2-3 days following the estradiol peak and remains at ∼ 4-9 ng/ml for 12 days. In the present study, we used a novel hormone therapy regimen to create a hormonal milieu that would be similar to this normal primate menstrual cycle. Natural progesterone was chosen over medroxyprogesterone as the progestin to provide to the monkeys in this study because of evidence that medroxyprogesterone antagonizes the beneficial effects produced by estrogen (Williams et al., 1994; Adams et al., 1997; Nilsen and Brinton, 2002, 2003).
Visual Recognition Memory
Visual recognition in the DMS task was altered following ovariectomy in older female monkeys who were treated with placebo compared to monkeys receiving estrogen. Moreover, monkeys treated with estrogen plus progesterone were equivalent in their visual memory capacity to monkeys treated with estrogen alone. Our observations are in line with those reported recently in older monkeys on a nonmatching version of the DMS task. Older monkeys treated with a single injection of estradiol every three weeks performed at higher levels of accuracy than placebo monkeys on this task, particularly at delays of 30 sec and 2 minutes, but not at delays of 10 minutes (Rapp et al., 2003a). Similarly, performance at a 10 minute delay in this task was equivalent between older monkeys receiving short-term continuous estrogen therapy and those receiving placebo (Lacreuse et al., 2002). While we did not find a difference between placebo treated monkeys and either of the estrogen groups at the 2 or 10 minute delays of the DMS task, we did note that the maximum difference between placebo and estrogen monkeys occurred at the 30 sec delay in our VDMS task, similar to the findings of Rapp (Rapp et al., 2003a).
Our experimental design was unique in that we examined performance patterns of the treatment groups both cross-sectionally and longitudinally across time, something that few other studies of older monkey models of menopause have done. In doing so, we found that VDMS performance was altered with time in placebo monkeys but preserved in monkeys receiving estrogen alone or in combination with progesterone. While the impairments in the placebo monkeys were most pronounced by three months following surgery (Postop #2 evaluation), they were resolved by approximately six months following ovariectomy (Postop #3 evaluation). Thus, while some time is required for the loss of ovarian hormones to have a significant negative impact on visual recognition ability, the effects may not be long lasting. Interestingly, the time at which we conducted our third postoperative assessment matches the approximate time at which Rapp first began to behaviorally evaluate their older ovariectomized monkeys and later found impairments in their placebo monkeys on the nonmatch-to-sample task (Rapp et al., 2003a). That our placebo monkeys were no longer demonstrating a difference from estrogen monkeys on the VDMS task after six months of ovariectomy may be related to practice effects because of the repeated testing that we performed, in contrast to the one time evaluation by Rapp (Rapp et al., 2003a).
Visual recognition memory has been assessed in ovariectomized rodents using an object recognition task that is somewhat analogous to the DMS task. Similar to our observations in monkeys receiving estrogen, estrogen-treated young (Luine et al., 2003; Walf et al., 2006) and older (Fernandez and Frick, 2004; Vaucher et al., 2002) ovariectomized rats and mice displayed better object recognition memory than placebo treated animals as evidenced by the greater amount of time they spent exploring the novel object in this task. Object recognition memory has yet to be examined in ovariectomized rodents given the combined estrogen plus progesterone therapy.
In the present study, monkeys treated with estrogen plus progesterone had choice response latencies that were significantly longer than the other groups of monkeys, but only during the first postoperative assessment period and primarily on trials in which they made an error. These results suggest that a generalized motoric slowing was not the cause of the increased latencies. Instead, poor recall of the correct stimulus may have resulted in an increased amount of time in which to decide to which stimulus a response should be made. Although monkeys receiving estrogen combined with progesterone may have taken more time to process information on those trials in which they committed an error, they did not demonstrate an increased error rate that affected their accuracy. Moreover, this slowing of choice response was not permanent.
Spatial Recognition Memory
Accuracy in the DR task was not sensitive to the loss of ovarian hormones or treatment with two different hormone therapies. While studies in young adult monkeys agree that memory in the DR task is unaffected by ovariectomy or estrogen therapy (Voytko, 2000; Hao et al., 2007), the findings have been more variable in older monkeys. Similar to our observations here, accuracy on a DR task was not affected by daily oral treatments with estradiol in a cross-over study of older female monkeys (Lacreuse et al., 2002). In the study by Rapp and colleagues (Rapp et al., 2003a), there were no differences in acquisition of the DR task between ovariectomized older female monkeys receiving a cyclical schedule of estrogen or those receiving placebo, however when accuracy at longer delays was subsequently examined, the estrogen monkeys displayed significantly better overall performance on the DR task. As the monkeys of that study were tested only at one time point, it is unknown if the benefits of estrogen on DR performance were stable with time. Indeed, in a preliminary small study of our own (Tinkler and Voytko, 2005), we found that DR accuracy was preserved in monkeys receiving continuous estradiol compared to placebo monkeys when they were evaluated at two months following ovariectomy and treatment initiation. It is possible however that the estrogen effects were only transient as suggested by the absence of estrogen effects in the present study when DR was tested at approximately three months following ovariectomy and treatment initiation (Postop #2 evaluation).
In ovariectomized rats, the effects of estrogen on spatial recognition memory has been evaluated using the delayed matching-to-position (DMP) task in a t-maze that is analogous to the DR task used in the present study. While estrogen improved acquisition in the DMP task in both young and old ovariectomized rats (Gibbs, 1999, 2000; Gibbs et al., 2004), it subsequently had no effect on spatial memory when the animals were tested with increasing delays (O'Neal et al., 1996; Gibbs, 1999; Gibbs et al., 2004). The absence of an estrogen effect on memory in the DMP task in rats agrees with the findings on the DR task in the present study in ovariectomized monkeys. Our observations of similar performance in monkeys receiving estrogen therapy alone with those receiving estrogen combined with progesterone mirrors observations made in several studies comparing these hormone therapies in rats (Dohanich et al., 1994; Gibbs, 2000; Sandstrom and Williams, 2001).
Choice response latency of monkeys receiving combined hormone therapy was increased across the repeated testing of DR in the current study and was longer than those of the other groups of monkeys. These effects were solely linked to the longer latencies of monkeys receiving estrogen plus progesterone at the last postoperative assessment and were predominantly related to their increased response latencies on trials in which they made errors. These findings mirror the increased latencies in this same group of monkeys on the VDMS task and again could reflect a hesitancy in responding on those trials because of poor recall of the correct location. However, as in the VDMS task, DR accuracy was not adversely affected. Monkeys treated with estrogen alone had significantly faster response latencies on error trials and poorer accuracy in DR compared to monkeys receiving the combined therapy in the second postoperative evaluation. These findings suggest that the estrogen alone monkeys may not have taken a sufficient amount of time to process information before making a choice response leading to an increased number of errors and reduced accuracy compared to the combined therapy monkeys. Indeed, examination of the data indicate that the monkeys given estrogen alone had shortened response latencies on incorrect trials in the second postoperative assessment compared to their first postoperative evaluation. It is not clear why response times of monkeys receiving only estrogen should have been affected as estrogen does not influence choice reaction times in postmenopausal women (Rauramo et al., 1975; Vanhulle and Demol, 1976; Polo-Kantola et al., 1998).
Conclusions
We found a transient selective preservation of visual recognition, but not spatial recognition, ability in surgically menopausal older monkeys receiving a more natural regimen of hormone therapy. Importantly, we also found that our treatment of estradiol plus progesterone was equivalent to that of estradiol alone and that neither therapy had significant negative impact on memory profiles. Given the selective and transient nature of our observations, the implications for use of these therapies to preserve memory of postmenopausal women are somewhat limited. Additional studies are required to determine whether this regimen of hormone therapy may be effective for treating the physiological and somatic symptoms associated with menopause. Because macaque monkeys share many parallels in reproductive and endocrine profiles with women (Jewitt and Dukelow, 1972; Goodman et al., 1977), including menopause (Walker, 1995; Gilardi et al., 1997; Shideler et al., 2001; Downs and Urbanski, 2006), the macaque monkey will remain a particularly critical animal model in which to continue to investigate alternate hormone therapies that will provide information that may be valuable in developing effective therapies for postmenopausal women.
Acknowledgments
This research was supported by grant AG13204 from the National Institute on Aging.
List of Abbreviations
- DMP
delayed matching-to-position
- DMS
delayed matching-to-sample
- DMS2
delayed matching-to-sample with a 2 minute delay
- DMS10
delayed matching-to-sample with a 10 minute delay
- DR
delayed response
- E
collective group of estrogen and estrogen plus progesterone monkeys
- E2
estradiol
- E+P
estrogen plus progesterone monkeys
- ET
estrogen monkeys
- P4
progesterone
- PL
placebo monkeys
- VDMS
variable delayed matching-to-sample
- WHI
women's health initiative
- WHIMS
women's health initiative memory study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams MR, Register TC, Golden DL, Wagner JD, Williams JK. Medroxyprogesterone acetate antagonizes inhibitory effects of conjugated equine estrogens on coronary artery atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:217–221. doi: 10.1161/01.atv.17.1.217. [DOI] [PubMed] [Google Scholar]
- Adams MR, Kaplan JR, Manuck SB, Koritnik DR, Parks JS, Wolfe MS, Clarkson TB. Inhibition of coronary artery atherosclerosis by 17-beta estradiol in ovariectomized monkeys: Lack of an effect of added progesterone. Arteriosclerosis. 1990;10:1051–1057. doi: 10.1161/01.atv.10.6.1051. [DOI] [PubMed] [Google Scholar]
- Ancelin ML, Ritchie K. Lifelong endocrine fluctuations and related cognitive disorders. Curr Pharm Design. 2005;11:4229–4252. doi: 10.2174/138161205774913228. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Francis KR, Umphlet C, Granholm AC. Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. Eur J Neurosci. 2006;24:229–242. doi: 10.1111/j.1460-9568.2006.04867.x. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147:607–614. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- Dohanich GP, Fader AJ, Javorsky DJ. Estrogen and estrogen-progesterone treatments counteract the effect of scopolamine on reinforced T-maze alternation in female rats. Behav Neurosci. 1994;108:988–992. doi: 10.1037//0735-7044.108.5.988. [DOI] [PubMed] [Google Scholar]
- Downs JL, Urbanski HF. Neuroendocrine changes in the aging reproductive axis of female rhesus macaques (Macaca mulatta) Biol Reprod. 2006;75:539–546. doi: 10.1095/biolreprod.106.051839. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J Women's Health Initiative Memory Study. Conjugated equine estrogens and global cognitive function in postmenopausal women. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Frick KM. Chronic oral estrogen affects memory and neurochemistry in middle-aged female mice. Behav Neurosci. 2004;118:1340–1351. doi: 10.1037/0735-7044.118.6.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88:208–16. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Horm Behav. 1999;36:222–233. doi: 10.1006/hbeh.1999.1541. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging. 2000;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Nelson D, Anthony MS, Clarkson TB. Effects of long-term hormone replacement and of tibolone on choline acetyltransferase and acetylcholinesterase activities in the brains of ovariectomized cynomolgous monkeys. Neuroscience. 2002;113:907–914. doi: 10.1016/s0306-4522(02)00239-7. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Gabor R, Cox T, Johnson DA. Effects of raloxifine and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychneuroendocrinology. 2004;29:741–748. doi: 10.1016/S0306-4530(03)00118-5. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Edwards D, Lazar N, Nelson D, Talameh J. Effects of long-term hormone treatment and of tibolone on monoamines and monoamine metabolites in the brains of ovariectomised cynomolgous monkeys. J Neuroendocrinol. 2006;18:643–654. doi: 10.1111/j.1365-2826.2006.01463.x. [DOI] [PubMed] [Google Scholar]
- Gilardi KVK, Shideler SE, Valverde CR, Roberts JA, Lasley BL. Characterization of the onset of menopause in the rhesus macaque. Biol Reprod. 1997;57:335–340. doi: 10.1095/biolreprod57.2.335. [DOI] [PubMed] [Google Scholar]
- Goodman AL, Descalzi CD, Johnson DK, Hodgen GD. Composite pattern of circulating LH, FSH, estradiol, and progesterone during the menstrual cycle in cynomolgus monkeys. Proc Soc Exp Biol Med. 1977;155:479–481. doi: 10.3181/00379727-155-39834. [DOI] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Janssen WGM, Lou W, Lasley BL, Hof PR, Morrison JH. Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. PNAS. 2007;104:11465–11470. doi: 10.1073/pnas.0704757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW. Only a matter of time? Hormone therapy and cognition. Menopause. 2005;12:1–3. doi: 10.1097/00042192-200512010-00002. [DOI] [PubMed] [Google Scholar]
- Hess DL, Spies HG, Hendrickx AG. Diurnal steroid patterns during gestation in the rhesus macaque: onset, daily variation, and the effects of dexamethasone treatment. Biol Reprod. 1981;24:609–16. doi: 10.1095/biolreprod24.3.609. [DOI] [PubMed] [Google Scholar]
- Jewitt DA, Dukelow WR. Cyclicity and gestation length of Macaca fascicularis. Primates. 1972;13:327–330. [Google Scholar]
- Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci. 2002;116:411–420. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Kohama SG. Ovarian hormones influence the morphology, distribution, and density of tyrosine hydroxylase immunoreactive axons in the dorsolateral prefrontal cortex of adult rhesus monkeys. J Comp Neurol. 1998;396:1–17. [PubMed] [Google Scholar]
- Kritzer MF, Kohama SG. Ovarian hormones differentially influence immunoreactivity for dopamine β–hydroxylase, choline acetyltransferase, and serotonin in the dorsolateral prefrontal cortex of adult rhesus monkeys. J Comp Neurol. 1999;409:438–451. doi: 10.1002/(sici)1096-9861(19990705)409:3<438::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Adler A, Bethea CL. Ovarian hormone influences on the density of immunoreactivity for tyrosine hydroxylase and serotonin in the primate corpus striatum. Neuroscience. 2003;122:757–772. doi: 10.1016/s0306-4522(03)00548-7. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Herndon JG. Estradiol selectively affects processing of conspecifics' faces in female rhesus monkeys. Psychoneuroendocrinology. 2003;28:885–905. doi: 10.1016/s0306-4530(02)00104-x. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Herndon JG, Moss MB. Cognitive function in aged ovariectomized female rhesus monkeys. Behav Neurosci. 2000;114:506–513. doi: 10.1037//0735-7044.114.3.506. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Wilson ME, Herndon JG. Estradiol, but not raloxifine, improves aspects of spatial working memory in aged ovariectomized rhesus monkeys. Neurobiol Aging. 2002;23:589–600. doi: 10.1016/s0197-4580(02)00002-7. [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Maki PM. Hormone therapy and cognitive function: is there a critical period for benefit? Neuroscience. 2006;138:1027–1030. doi: 10.1016/j.neuroscience.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Manson JE, Bassuk SS, Harman SM, Brinton EA, Cedars MI, Lobo R, Merriam GR, Miller VM, Naftolin F, Santoro N. Postmenopausal hormone therapy: new questions and the case for new clinical trials. Menopause. 2006;12:139–147. doi: 10.1097/01.gme.0000177906.94515.ff. [DOI] [PubMed] [Google Scholar]
- Markham JA, Pych JC, Juraska JM. Ovarian hormone replacement to aged ovariectomized female rats benefits acquisition of the morris water maze. Horm Behav. 2002;42:284–293. doi: 10.1006/hbeh.2002.1819. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Savonenko AV. Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. J Neurosci. 2002;22:10985–10995. doi: 10.1523/JNEUROSCI.22-24-10985.2002. 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkola TS, Clarkson TB, Notelovitz M. Postmenopausal hormone therapy before and after the women's health initiative study: what consequences? Ann Med. 2004;36:402–413. doi: 10.1080/07853890410035430. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology. 2002;143:205–212. doi: 10.1210/endo.143.1.8582. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Divergent impact of progesterone and medroxyprogesterone acetate (provera) on nuclear mitogen-activated protein kinase signaling. PNAS. 2003;100:10506–10511. doi: 10.1073/pnas.1334098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal MF, Means LW, Poole MC, Hamm RJ. Estrogen affects performance of ovariectomized rats in a two-choice water-escape working memory task. Psychoneuroendocrinology. 1996;21:51–65. doi: 10.1016/0306-4530(95)00032-1. [DOI] [PubMed] [Google Scholar]
- Polo-Kantola P, Portin R, Polo O, Helenius H, Irjala K, Erkkola R. The effect of short-term estrogen replacement therapy on cognition: a randomized, double-blind, cross-over trial in postmenopausal women. Obstet Gynecol. 1998;91:459–466. doi: 10.1016/s0029-7844(97)00700-x. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003a;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, Gass ML, Stefanick ML, Lane DS, Hays J, Johnson KC, Coker LH, Dailey M, Bowen D WHIMS Investigators. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the women's health initiative memory study: a randomized controlled trial. JAMA. 2003b;289:2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- Rauramo L, Lagerspetz K, Engblom P, Punnonen R. The effect of castration and peroral estrogen therapy on some psychological functions. Front Horm Res. 1975;3:94–104. doi: 10.1159/000398269. [DOI] [PubMed] [Google Scholar]
- Roberts JA, Gilardi KVK, Lasley B, Rapp PR. Reproductive senescence predicts cognitive decline in aged female monkeys. NeuroReport. 1997;8:2047–2051. doi: 10.1097/00001756-199705260-00048. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Memory retention is modulated by acute estrodial and progesterone replacement. Beh Neurosci. 2001;115:384–393. [PubMed] [Google Scholar]
- Sherwin BB. Surgical menopause, estrogen, and cognitive function in women: what do the findings tell us? Ann NY Acad Sci. 2005;1052:3–10. doi: 10.1196/annals.1347.001. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. The critical period hypothesis: can it explain discrepancies in the oestrogen-cognition literature? J Neuroendocrinol. 2006;19:77–81. doi: 10.1111/j.1365-2826.2006.01508.x. [DOI] [PubMed] [Google Scholar]
- Shideler SE, Gee NA, Chen J, Lasley BL. Estrogen and progesterone metabolites and follicle-stimulating hormone in the aged macaque female. Biol Reprod. 2001;65:1718–1725. doi: 10.1095/biolreprod65.6.1718. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the women's health initiative memory study: a randomized controlled trial. JAMA. 2003;289:2652–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, et al. Conjugated equine estrogens and incidence of dementia and mild cognitive impairment in postmenopausal women. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Tinkler GP, Voytko ML. Estrogen modulates cognitive and cholinergic processes in surgically menopausal monkeys. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:423–431. doi: 10.1016/j.pnpbp.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Vanhulle G, Demol R. A double-blind study into the influence of estriol on a number of psychological tests in post-menopausal women. In: van Keep PA, Greenblatt RB, Albeaux-Fernet M, editors. Consensus on menopause research. London: MTP Press; 1976. pp. 94–99. [Google Scholar]
- Vaucher E, Reymond I, Najaffe R, Satyabrata K, Quirion R, Miller MM, Franklin KBJ. Estrogen effects on object memory and cholinergic receptors in young and old female mice. Neurobiol Aging. 2002;23:87–95. doi: 10.1016/s0197-4580(01)00250-0. [DOI] [PubMed] [Google Scholar]
- Voytko ML. The effects of long-term ovariectomy and estrogen replacement therapy on learning and memory in monkeys. Behav Neurosci. 2000;114:1078–1087. [PubMed] [Google Scholar]
- Voytko ML. Estrogen and the cholinergic system modulate visuospatial attention in monkeys. Behav Neurosci. 2002;116:187–197. doi: 10.1037//0735-7044.116.2.187. [DOI] [PubMed] [Google Scholar]
- Voytko ML, Olton DS, Richardson RT, Gorman LK, Tobin JR, Price DL. Basal forebrain lesions in monkeys disrupt attention but not learning and memory. J Neurosci. 1994;14:167–186. doi: 10.1523/JNEUROSCI.14-01-00167.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker ML. Menopause in female rhesus monkeys. Am J Primatol. 1995;35:59–71. doi: 10.1002/ajp.1350350106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JK, Honoré ED, Washburn SA, Clarkson TB. Effects of hormone replacement therapy on reactivity of atherosclerotic coronary arteries in cynomolgus monkeys. J Am Coll Cardiol. 1994;24:1757–1761. doi: 10.1016/0735-1097(94)90184-8. [DOI] [PubMed] [Google Scholar]
- Women's Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]