Abstract
Vernix suspended in amniotic fluid is normally swallowed by the late term fetus. We hypothesized that branched chain fatty acids (BCFA), long known to be major vernix components, would be found in meconium and that the profiles would differ systematically. Vernix and meconium were collected from term newborns and analyzed. BCFA-containing lipids constituted about 12% of vernix dry weight, and were predominantly saturated, and had 11 to 26 carbons per BCFA. In contrast, meconium BCFA had 16 to 26 carbons, and was about 1% of dry weight. Meconium BCFA were mostly in the iso configuration, whereas vernix BCFA contained dimethyl and middle chain branching, and five anteiso BCFA. The mass of BCFA entering the fetal gut as swallowed vernix particles is estimated to be 180 mg in the last month of gestation while the total mass of BCFA found in meconium is estimated to be 16 mg, thus most BCFA disappear from the fetal gut. The BCFA profiles of vernix and meconium show that BCFA are major components of normal healthy term newborn gastrointestinal tract. BCFA are candidates for agents that play a role in gut colonization and should be considered a nutritional component for the fetus/newborn.
Keywords: branched chain fatty acids, vernix, meconium
Branched chain fatty acids (BCFA) are mostly saturated fatty acids (SFA) with one or more methyl branches on the carbon chain. BCFA are synthesized mainly by the skin and have long been known to be a major component of vernix caseosa (10–20% dry weight) (1). Among terrestrial animals, vernix is unique to humans, and is not found in other land mammals, including other primates (2). Vernix is made of sebum and fetal corneocytes (1, 3) and is produced by fetal skin starting at 24 weeks gestational age and continuing until term birth (4). During the third trimester vernix sloughs off as particulates that become suspended in amniotic fluid (3, 5), possibly aided by lung surfactant phospholipids that also enter the amniotic fluid. The fetus normally swallows amniotic fluid in amounts approaching 500 ml at the end of gestation (6, 7) and with it vernix. Thus, the late term fetal gut is normally exposed to vernix and its BCFA, increasingly so as parturition approaches.
Vernix dry matter is composed of approximately equal amounts of protein and lipids (2, 8). Lipid fractions in vernix have been comprehensively characterized (9–11) and shown to be 25–30% sterol esters (SE), 18–36% triglycerides (TAG), 12–16% wax esters (WE), 9% squalene, 5% ceramides, and low levels of non-esterified fatty acid (NEFA) fraction was also detected by some (10, 12) but not by others (13). BCFA are found in all acyl-carrying lipid classes, WE (16– 53%) and SE (27–62%) (9–11, 13), as well as in the TAG (18–21%) and NEFA (21%) fractions (10).
Apart from skin (1, 9, 14), BCFA are at very low levels in internal tissue (14), but are also found in human milk (15–17) at concentrations as high as 1.5%w/w of total fatty acids (FA). This level is comparable to and in some cases greater than that of docosahexaenoic acid (DHA, 22:6n-3) and arachidonic acid (ARA, 20:4n-6) in the same milk. For instance, a 1981 publication reported the concentration of anteiso 17:0 in Australian women’s colostrum to be 0.45%w/w of total FA, exceeding the concentrations of DHA (0.32%w/w) and ARA (0.4%w/w) (17).
Meconium, the newborn’s first fecal pass, first appears in the fetal GI tract at around 12 weeks of gestational age, and is normally passed after birth (18–20). It consists of amniotic fluid residue, skin and gastrointestinal (GI) epithelial cells, GI secretions and enzymes, lipids, sugars, proteins, cholesterol, sterols, bile acid and salts (18, 19, 21, 22). Meconium contains 12% dry weight lipid (21), and there is only one unconfirmed study reporting BCFA in meconium (23). There are no studies linking BCFA composition of vernix and meconium in the same infants.
We hypothesized that vernix BCFA of term newborns would survive the alimentary canal and be found in meconium. Our test of this hypothesis led us to characterize the relative BCFA profiles of vernix and meconium to establish the degree to which the profile is altered by the sterile fetal gut in utero.
Methods
This study was approved by the Cornell University and the Cayuga Medical Center Institutional Review Boards (IRB) on the Use of Human Subjects. The IRB approved an exemption from the requirement to obtain individual informed consent because the human materials sampled, vernix and meconium, are deemed to be medical waste and no individually identifiable information was obtained from participants.
Sample collection
Eighteen samples of vernix and meconium were collected from 18 normal term newborns at Cayuga Medical Center in Ithaca, NY. Vernix was removed from the shoulder regions in the birthing room, placed in clean tubes and stored at −80°C until analysis. Meconium was collected from diapers and similarly transferred into clean tubes and stored at −80°C until analysis.
FA analysis
Total lipids were extracted from the vernix and the meconium samples according to a modified Bligh and Dyer method (24). Fatty acids are overwhelmingly found in mammalian pools, such as vernix and meconium, as acyl moieties, which are constituents of higher molecular weight lipid molecules such as TAG, SE, and WE. For detailed molecular analysis, fatty acyl groups are hydrolyzed and fatty acid methyl esters (FAME) synthesized for analysis. FAME were prepared using 14% BF3 in methanol (Sigma Chemical, St. Louis, MO.). Butylated hydroxytoluene (BHT) was added to methanol as an antioxidant. Heptadecanoic acid (Sigma Chemical, St Louis, MO) in chloroform was used as an internal standard. This routine step obscures heptadecanoic acid, which is normally rare in mammalian tissue but is present in vernix and meconium. Because of the extraordinary diversity of FA in these samples, any internal standard interferes with analysis of one or more FA in some of the samples. A correction was applied to estimate the extent of interference, and the signals were carefully calibrated against external standards.
FAME analyses were performed using a Hewlett Packard 5890 series II gas chromatograph (GC). A BPX-70 column (60m×0.32mm×0.25µm, SGE, Austin, TX) was used for the analysis with H2 as the carrier gas. FAME identities were determined by a chemical ionization (CI) and electron impact (EI) mass spectrometry (MS), using a Varian Star 3400 GC coupled to a Varian Saturn 2000 ion trap MS. BCFA FAME identities were based on GC retention time of each substance and its electron impact mass spectra. FAME mass spectral assignments were confirmed by conversion of the FAME to picolinyl ester derivatives according to the method described by Christie <www.lipidlibrary.co.uk> and Yang (25), followed by GC/MS analysis and comparison to literature spectra (26–31).
An equal weight FAME mixture (68A; Nuchek Prep, Elysian, MN) was used to calculate response factors. The following were also used as standards: n-11:0 up to n-24:0 (Nuchek Prep, Elysian, MN); iso 13:0, anteiso 13:0, iso 15:0, anteiso 15:0; iso 17:0, anteiso 17:0 (Larodan Fine Chemicals AB, Malmo, Sweden) and 10 methyl hexadecanoic acid (Matreya LLC, Pleasant Gap, PA). FA levels were expressed as weight % of total fatty acids for all 11 to 32 carbons FA.
Statistics
Data are expressed as mean±SD for study population characteristics, and as mean±SEM for FA analysis. Statistical analyses were made using JMP 6 (SAS Institute, Cary, NC). Differences in mean of each FA were calculated using one sample t-test for non-zero differences, with p<0.05 declared significant.
Results
Subjects
Characteristics of the study population are presented in Table 1. No complications were present for any of the newborns other than as noted. All but two newborns were by vaginal delivery. Six mothers received antibiotic treatment during pregnancy; five of them gave birth to female infants.
Table 1.
Characteristics of study population (mean±SD (range)).
| Mother’s age (years) | 29±5.8 | 18–42 |
| Gestational age (weeks) | 40± | 38–41 |
| Birthweight (kg) | 3.3±0.5 | 2.3–4.4 |
| Gender | 10 female, 8 males | |
| Delivery | 16 vaginal, 2 CS* | |
| Antibiotics | 5 female, 1 male | |
Cesarian section
Overall FA distribution
A profile of FA classes is shown in Table 2. Comparisons of all classes were significant at the p<0.05 level. BCFA constituted almost a third (29.1±1.5%w/w) of all FA in vernix and were significantly higher compared to the mean levels in meconium (17.5±1.3%w/w; p<0.05). This drop in BCFA was accompanied by a reciprocal increase in normal (n-) saturated FA (n-SFA) specifically, 34±1.9%w/w in vernix and 51.3±3.0%w/w in meconium (p<0.05). Differences in n-monounsaturated fatty acids (MUFA) and polyunsaturated fatty acid (PUFA) were modest by comparison.
Table 2.
Profile of FA classes (%w/w) in vernix and meconium (mean±SEM)
| FA | Vernix | Meconium |
|---|---|---|
| BCFA | 29.1±1.5* | 17.5±1.3 |
| n-SFA | 34±1.9* | 51.3±3.0 |
| n-MUFA | 31.0±1.7* | 22.4±2.1 |
| PUFA | 3.9±0.4* | 7.1±1.1 |
p<0.05
Overall, BCFA hydrolyzed from their native lipid classes constituted 5.8% of dry weight of vernix, corresponding to approximately 12% of dry weight of vernix within the native BCFA-containing lipids. Meconium had 0.55% dry weight of hydrolyzed BCFA and an estimated 1% of BCFA-containing lipids.
BCFA distribution in vernix and meconium
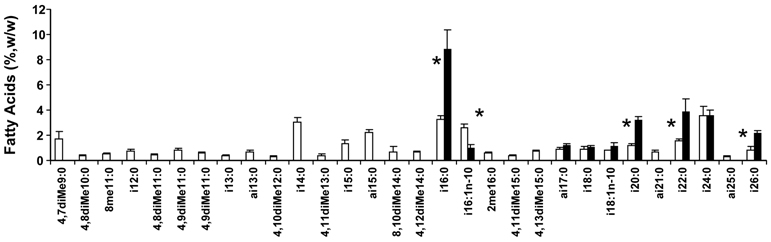
Figure 1 is a graphical summary of the BCFA profiles for vernix and meconium for those BCFA detected in samples from at least 3 newborns, presented left to right in order of carbon number. In total, 30 BCFA were identified in vernix while nine were also detected in meconium. Vernix BCFA ranged from 11 to 26 carbon atoms and were primarily saturated apart from two iso monounsaturates. iso BCFA, anteiso BCFA, middle chain monomethyl BCFA and dimethyl BCFA were all detected among vernix BCFA. In contrast, meconium BCFA had a much more restricted range of carbon numbers, from 16 to 26 carbons. Of the nine meconium BCFA, eight were iso BCFA, of which two were MUFA, and one was anteiso.
Figure 1.
BCFA methyl esters profiles for vernix and meconium (means±SEM, n≤18 newborn) listed left to right in order of molecular weight. Means are for those FA appearing in samples from at least three newborns. iso-BCFA have a dimethyl terminal structure: iso-16:0 is synonymous with 14-methyl-15:0 (14-methyl-pentadecanoic acid). anteiso-BCFA have a methyl branch at the n-2 position: anteiso-17:0 is synonymous with 14-methyl-16:0 (14-methylhexadecanoic acid). i=iso; ai=anteiso; Me=methyl; diMe = two methyl branches. Key: □ vernix; ■ meconium. *p<0.05
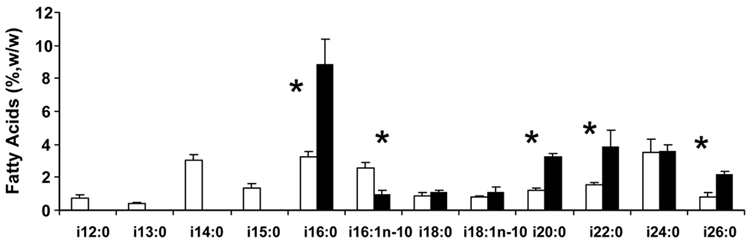
The profile of the iso BCFA in vernix and meconium is shown in Figure 2. The vernix iso BCFA profile had odd and even carbon numbered FA from iso-12:0 to iso-16:0, and only even carbon numbers at greater chain lengths. In contrast, meconium iso BCFA was dominated by the shortest chain BCFA in its profile, iso-16:0, which was more than twice the relative concentration of any other BCFA. Five of the eight iso-BCFA appearing in both vernix and meconium were a significantly different proportion of BCFA in the respective profiles; the preponderance of longer chains in meconium lead to significant differences in three of the four iso-BCFA of chain numbers from 20 to 26 carbons.
Figure 2.
Iso BCFA methyl esters in vernix and meconium (means±SEM, n≤18 newborn) listed left to right in order of molecular weight. i=iso. Key: □ vernix; ■ meconium. *p<0.05
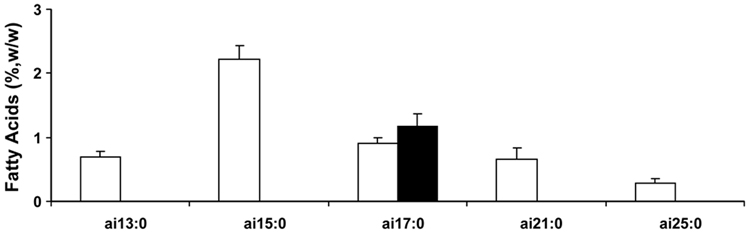
The profile of anteiso BCFA is shown in Figure 3. All five anteiso BCFA detected in vernix are odd carbon numbered. They range from 13 to 25 carbons, and only one, anteiso-17:0, is found in meconium.
Figure 3.
Anteiso BCFA methyl esters in vernix and meconium (means±SEM, n≤18 newborn) listed left to right in order of molecular weight. ai=anteiso. Key: □ vernix; ■ meconium. *p<0.05
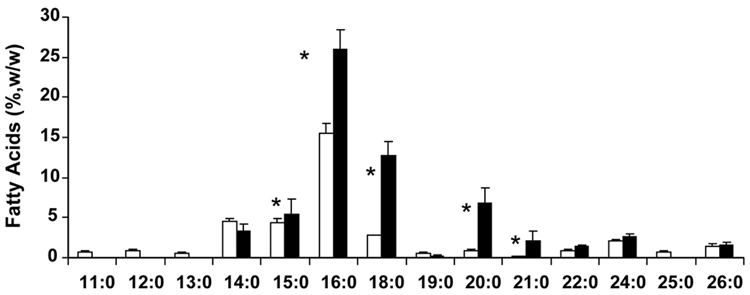
Figure 4 is a graphical summary of the straight chain, n-FA, profiles for vernix and meconium. Vernix normal FA had 11 to 26 carbon atoms, and meconium FA had 14 to 26 carbons, and both contained small amounts of odd chain number FA. Again, meconium BCFA tended to be of greater molecular weight.
Figure 4.
Normal (straight chain) FAME in vernix (means±SEM, n≤18 newborn) listed left to right in order of molecular weight. Key: □ vernix; ■ meconium. *p<0.05.
Discussion
The presence of BCFA in both vernix and meconium of healthy term infants indicates that BCFA are a major component of gut contents of normal term newborns, and their presence in meconium implies that they are present throughout the length of the gut. As such, BCFA are a component of the GI tract milieu present when the first few environmental microorganisms appear in the initial stage of gut colonization during and immediately after parturition or Cesarean section. In meconium, the systematic shift in BCFA profiles to high molecular weights, as well as the absence of most BCFA other than iso-BCFA, indicates that the fetal alimentary canal readily absorbs and metabolizes most BCFA.
There are many reports of BCFA in breastmilk, with the earliest and most extensive, showing 54 BCFA with a cumulative concentration of 1.5%w/w (16). A 1981 paper measured the concentration of BCFA in mature Australian breast milk to be a total of 0.84%w/w and one BCFA, anteiso-17:0 in colostrum at 0.45%w/w of fatty acids, exceeding the concentrations of DHA (0.32%w/w) and ARA (0.40%w/w) in the same mother’s mature breastmilk (17). Chen reported four BCFA with a cumulative concentration of 0.58%, w/w in Canadian breastmilk (32). A study of California women yielded an average of 0.60% BCFA for the four BCFA that were reported (15:0, 16:0, 17:0, 18:0, branch location not reported) (33). Corso found six BCFA in milks of 40 women in southern Italy (34), with anteiso-17:0 ranging from 0.12 to 0.93% of total fatty acids. This variability is not unlike that for DHA; breastmilk DHA ranges between 0.06%w/w and 1.40%w/w (35). Many, but not all, breastmilk fatty acid concentrations are closely linked to the dietary intake of the fatty acid or its precursor, including DHA to which the wide reported range is ascribed (35). It is most likely that the absence of BCFA identification in most breast milk FA papers is due to their low concentration, their appearance in a GC trace amidst the major saturates and monounsaturates in the chromatogram, and the historical absence of a compelling metabolic role for them.
The exposure of the gut to BCFA in utero, and possibly from breastmilk, is greater than any other period of life because BCFA are at trace levels in normal foods. The unique niche represented by BCFA and other components in the human gut may be important for establishing commensal bacteria during colonization. BCFA are prominent membrane components of many bacterial species (36, 37). For instance, BCFA constitute 95% of the FA in several bacilli and lactobacilli, including Sporolactobacillus inulinus, which has very recently been shown to be a probiotic candidate (37). The FA of nine Bifidobacterium strains include BCFA such as iso-14:0, anteiso-15:0, iso-16:0 and iso-18:0 at various levels (0.6–24.6%w/w). iso-14:0 is the second most abundant FA in Bifidobacterium breve with levels as high as 24.6%w/w (38). It is reasonable to hypothesize that the presence of BCFA in the neonatal gut would alter the mix of dominant species, favoring those organisms that use BCFA in their membranes, and we postulate that BCFA are a unique feature of the human fetal gut favoring the growth of commensal bacteria during colonization.
This hypothesis has implications for colonization of the GI tract of very premature infants, and may be a factor in the development of necrotizing enterocolitis (NEC), the etiology and pathogenesis of which is not well understood (39, 40). NEC is one of the major causes of morbidity in premature infants (39) though it is certainly related to pathogen overgrowth (41). Leading hypotheses with empirical support are that NEC is related to prematurity, enteral feeding, and bacterial colonization (40). Importantly, it has not been observed prenatally. NEC risk is higher among lower gestational age infants and is rare in term infants (42). Breast milk consumption is associated with a lower incidence of NEC (40, 43). Although no specific pathogenic bacteria has been associated with NEC (43), supplementation of premature animals and infants with probiotic strains appear to reduce its incidence (39, 44). With these considerations, we hypothesize that BCFA have a role in enhancing proper GI colonization: vernix begins to appear around week 24 of gestation and accumulates as particulates in amniotic fluid toward term (3) thus, the GI tract of very premature infants is not exposed to vernix BCFA prenatally. Postnatally they would be exposed to BCFA if breastfed, but formula-fed preterms would not be exposed to BCFA since they are not a component of preterm formulas. Finally, we note that the incidence of NEC drops as gestational age approaches normal term, therefore later term premature infants would be exposed to some BCFA and may benefit if the hypothesis is correct.
We can estimate the mass of BCFA entering and exiting the alimentary canal. At term, amniotic fluid lipids are about 154 mg/L (45), of which about 52 mg/L are phospholipids that are likely to originate as BCFA-free lung surfactant (3, 10). Thus, the amniotic fluid vernix FA concentration is about 102 mg/L. Of this, our measurements indicate that 57% are FA, to yield 58 mg/L. Our data (Table 2) further indicate that 29% are BCFA, to yield 17 mg/L BCFA. The fetus is estimated to swallow 200 to 500 ml/day of amniotic fluid near term (46, 47), and taking the midpoint of this range, 350 ml/day, 6 mg BCFA per day enter the fetal GI tract amounting to 30×6 = 180 mg BCFA in the last month of gestation. Meconium is the output of the GI tract integrated from about 12 weeks gestation. Total meconium for 27 term infants was reported (48) to be 8.9 g wet weight, averaging 32% dry weight, or 2.8 g. Our data indicate that about 0.55% is BCFA, or about 16 mg average total BCFA in meconium. This value is an order of magnitude lower than our estimate of the BCFA swallowed in the last month of gestation, and suggests that most of the BCFA disappear during transit. The distribution and structural characteristics of BCFA that do appear in meconium reflect processing of vernix by the enterocytes. Our data show that C12–15 BCFA, as well as nearly all BCFA apart from iso-BCFA, are absent from meconium and thus must have been metabolized. The nature of this metabolism remains to be determined, in part because BCFA and their interaction with human enterocytes has not been studied.
Chain elongation is one likely metabolic transformation that would explain the absence of C12–15 BCFA, and preponderance of longer chain BCFA, in meconium. Suggestive evidence in support of this hypothesis is found in the data of Figure 1. The significantly greater level of meconium iso-16:0 compared to vernix iso-16:0, is roughly the sum of vernix iso-14:0 and iso-16:0, consistent with the hypothesis that elongation of vernix iso-14:0 adds to the existing iso-16:0. Similar observations apply to meconium iso-20:0 and vernix iso-18:0 and iso-20:0.
Medium chain fatty acids (C8–C14) are commonly fed to premature infants because they are efficiently absorbed through the gastric mucosa, directly transported to the liver via the portal vein, and oxidized by the immature GI tract. Although the BCFA with 15 or fewer carbons are absent from meconium, figure 4 shows that the FA n-14:0 and n-15:0 are partially excreted. This observation implies that there is selective uptake and retention of BCFA by the fetal GI tract that may not operate as efficiently for the n-FA.
Our measurements of BCFA are in line with previous data. BCFA constituted almost one third of all FA in vernix (11, 49), and the levels of vernix SFA, MUFA and PUFA were within the range encompassed by previous reports (10, 49, 50). We found only odd numbered carbon anteiso BCFA, consistent with some previous reports (49–51), but not with others (10, 11). BCFA averaged 17%w/w of all FA in meconium in our samples. The single previous study showing BCFA in meconium reported only on the free fatty acid fraction and used GC with retention time matching for identification. iso FA with 22 and 24 carbons were identified at 4%w/w and 6%w/w respectively, and nine other iso-BCFA were tentatively assigned (C14–21, 25) with no percent fraction provided.
Though five anteiso BCFA were detected in vernix, anteiso-17:0 was the sole anteiso BCFA detected in meconium, and there is no obvious explanation as to why this was the case. Weanling rats fed 100mg/week anteiso-17:0 in an otherwise fat free diet excreted 8–10% in the feces and stored a similar amount in adipose tissue (52), and apparently also converted a small amount anteiso-15:0. The remaining 80% was metabolized to substances other than anteiso FA. The levels of anteiso-17:0 have been reported to be the highest among all BCFA in at least one study of breastmilk (17), and it is notable that anteiso-17:0 is a major lipids constituent of many bacterial membrane (36).
The combined levels of the middle chain monomethyl and dimethyl BCFA in our vernix samples were similar to the levels reported in a single vernix sample by Nicolaides & Apon (49). In our sample of 18 newborns, the average proportions of dimethyl monomethyl BCFA dominated over middle chain monomethyl BCFA. The first methyl branch in the dimethyl BCFA was located predominantly on the fourth carbon of the chain, consistent with previous findings (49, 50). However, in our study, the second methyl branch in half of the dimethyl BCFA was located on an odd numbered carbon, and in almost all the dimethyl BCFA, this methyl branch was located on the anteiso carbon of the FA chain.
In summary, there are dramatic and systematic differences in BCFA composition between vernix and meconium, indicating that BCFA are actively metabolized in the fetal GI tract. This observation implies that vernix should be considered a nutritional agent, and that BCFA are a normal and quantitatively substantial component of the normal term newborn GI tract. Further studies are warranted to understand the uptake and metabolism of BCFA by enterocytes, and the role of BCFA during bacterial colonization. The absence of vernix, and BCFA, in the GI tract of very premature, formula-fed infants may have a role in the etiology of NEC, among the most devastating conditions facing the preterm infant.
Acknowledgments
Financial Support: This work was supported by NIH grant GM071534
Abbreviations
- BCFA
branched-chain fatty acids
- GC
gas chromatography
- GI
gastrointestinal
- FA
fatty acids
- FAME
fatty acid methyl esters
- MS
mass spectrometry
- MUFA
monounsaturated fatty acids
- SE
sterol esters
- SFA
saturated fatty acids
- TAG
triacylglycerol
- WE
wax esters
Footnotes
Publisher's Disclaimer: Pediatric Research Articles Ahead of Print contains articles in unedited manuscript form that have been peer-reviewed and accepted for publication. As a service to our readers, we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting and review of the resulting proof before it is published in its final definitive form. Please note that during the production process errors may be discovered, which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nicolaides N, Ray T. Skin Lipids. 3. Fatty Chains in Skin Lipids. the Use of Vernix Caseosa to Differentiate between Endogenous and Exogenous Components in Human Skin Surface Lipid. J Am Oil Chem Soc. 1965;42:702–707. doi: 10.1007/BF02540043. [DOI] [PubMed] [Google Scholar]
- 2.Pickens WL, Warner RR, Boissy YL, Boissy RE, Hoath SB. Characterization of vernix caseosa: water content, morphology, and elemental analysis. J Invest Dermatol. 2000;115:875–881. doi: 10.1046/j.1523-1747.2000.00134.x. [DOI] [PubMed] [Google Scholar]
- 3.Narendran V, Wickett RR, Pickens WL, Hoath SB. Interaction between pulmonary surfactant and vernix: a potential mechanism for induction of amniotic fluid turbidity. Pediatr Res. 2000;48:120–124. doi: 10.1203/00006450-200007000-00021. [DOI] [PubMed] [Google Scholar]
- 4.Moore C, Dempsey D, Deitermann D, Lewis D, Leikin J. Fetal cocaine exposure: analysis of vernix caseosa. J Anal Toxicol. 1996;20:509–511. doi: 10.1093/jat/20.6.509. [DOI] [PubMed] [Google Scholar]
- 5.Yoshio H, Tollin M, Gudmundsson GH, Lagercrantz H, Jornvall H, Marchini G, Agerberth B. Antimicrobial polypeptides of human vernix caseosa and amniotic fluid: implications for newborn innate defense. Pediatr Res. 2003;53:211–216. doi: 10.1203/01.PDR.0000047471.47777.B0. [DOI] [PubMed] [Google Scholar]
- 6.Miettinen TA, Luukkainen T. Gas-liquid chromatographic and mass spectrometric studies on sterols in vernix caseosa, amniotic fluid and meconium. Acta Chem Scand. 1968;22:2603–2612. doi: 10.3891/acta.chem.scand.22-2603. [DOI] [PubMed] [Google Scholar]
- 7.Sherman DJ, Ross MG, Day L, Ervin MG. Fetal swallowing: correlation of electromyography and esophageal fluid flow. Am J Physiol. 1990;258:R1386–R1394. doi: 10.1152/ajpregu.1990.258.6.R1386. [DOI] [PubMed] [Google Scholar]
- 8.Hoeger PH, Schreiner V, Klaassen IA, Enzmann CC, Friedrichs K, Bleck O. Epidermal barrier lipids in human vernix caseosa: corresponding ceramide pattern in vernix and fetal skin. Br J Dermatol. 2002;146:194–201. doi: 10.1046/j.1365-2133.2002.04584.x. [DOI] [PubMed] [Google Scholar]
- 9.Nicolaides N, Fu HC, Ansari MN, Rice GR. The fatty acids of wax esters and sterol esters from vernix caseosa and from human skin surface lipid. Lipids. 1972;7:506–517. doi: 10.1007/BF02533016. [DOI] [PubMed] [Google Scholar]
- 10.Rissmann R, Groenink HW, Weerheim AM, Hoath SB, Ponec M, Bouwstra JA. New insights into ultrastructure, lipid composition and organization of vernix caseosa. J Invest Dermatol. 2006;126:1823–1833. doi: 10.1038/sj.jid.5700305. [DOI] [PubMed] [Google Scholar]
- 11.Kaerkkaeinen J, Nikkari T, Ruponen S, Haahti E. Lipids of Vernix Caseosa. J Invest Dermatol. 1965;44:333–338. [PubMed] [Google Scholar]
- 12.Tollin M, Bergsson G, Kai-Larsen Y, Lengqvist J, Sjovall J, Griffiths W, Skuladottir GV, Haraldsson A, Jornvall H, Gudmundsson GH, Agerberth B. Vernix caseosa as a multi-component defence system based on polypeptides, lipids and their interactions. Cell Mol Life Sci. 2005;62:2390–2399. doi: 10.1007/s00018-005-5260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nazzaro-Porro M, Passi S, Boniforti L, Belsito F. Effects of aging on fatty acids in skin surface lipids. J Invest Dermatol. 1979;73:112–117. doi: 10.1111/1523-1747.ep12532793. [DOI] [PubMed] [Google Scholar]
- 14.Nicolaides N. Skin lipids: their biochemical uniqueness. Science. 1974;186:19–26. doi: 10.1126/science.186.4158.19. [DOI] [PubMed] [Google Scholar]
- 15.Jensen RG. Handbook of Milk Composition. San Diego: Academic Press Inc.; 1995. [Google Scholar]
- 16.Egge H, Murawski U, Ryhage R, Gyorgy P, Chatranon W, Zilliken F. Minor constituents of human milk. IV. Analysis of the branched chain fatty acids. Chem Phys Lipids. 1972;8:42–55. doi: 10.1016/0009-3084(72)90042-4. [DOI] [PubMed] [Google Scholar]
- 17.Gibson RA, Kneebone GM. Fatty acid composition of human colostrum and mature breast milk. Am J Clin Nutr. 1981;34:252–257. doi: 10.1093/ajcn/34.2.252. [DOI] [PubMed] [Google Scholar]
- 18.Ahanya SN, Lakshmanan J, Morgan BL, Ross MG. Meconium passage in utero: mechanisms, consequences, and management. Obstet Gynecol Surv. 2005;60:45–56. doi: 10.1097/01.ogx.0000149659.89530.c2. quiz 73-44. [DOI] [PubMed] [Google Scholar]
- 19.Gareri J, Klein J, Koren G. Drugs of abuse testing in meconium. Clin Chim Acta. 2006;366:101–111. doi: 10.1016/j.cca.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 20.Ostrea EM, Jr, Hernandez JD, Bielawski DM, Kan JM, Leonardo GM, Abela MB, Church MW, Hannigan JH, Janisse JJ, Ager JW, Sokol RJ. Fatty acid ethyl esters in meconium: are they biomarkers of fetal alcohol exposure and effect? Alcohol Clin Exp Res. 2006;30:1152–1159. doi: 10.1111/j.1530-0277.2006.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buchanan DJ, Rapoport S. Chemical comparison of normal meconium and meconium from a patient with meconium ileus. Pediatrics. 1952;9:304–310. [PubMed] [Google Scholar]
- 22.Righetti C, Peroni DG, Pietrobelli A, Zancanaro C. Proton nuclear magnetic resonance analysis of meconium composition in newborns. J Pediatr Gastroenterol Nutr. 2003;36:498–501. doi: 10.1097/00005176-200304000-00017. [DOI] [PubMed] [Google Scholar]
- 23.Terasaka D, Clark DA, Singh BN, Rokahr J. Free fatty acids of human meconium. Biol Neonate. 1986;50:16–20. doi: 10.1159/000242556. [DOI] [PubMed] [Google Scholar]
- 24.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 25.Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, Yang R, Colgan SP, Petasis NA. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J Immunol. 2006;176:1848–1859. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- 26.Carballeira NM, Miranda C, Lozano CM, Nechev JT, Ivanova A, Ilieva M, Tzvetkova I, Stefanov K. Characterization of novel methyl-branched chain fatty acids from a halophilic Bacillus species. J Nat Prod. 2001;64:256–259. doi: 10.1021/np000494d. [DOI] [PubMed] [Google Scholar]
- 27.Suutari M, Laakso S. Signature GLC-MS ions in identification of Δ5- and Δ unsaturated iso- and anteiso-branched fatty acids. J Microbiol Methods. 1993;17:39–48. [Google Scholar]
- 28.Karlsson H, Odham G. Studies on feather waxes of birds. VIII. Composition of the wax in the free-flowing secretion from the preen gland of the oystercatcher (Haematopus ostralegus) Ark Kemi. 1969;31:143–158. [Google Scholar]
- 29.Yu QT, Liu BN, Zhang JY, Huang ZH. Location of methyl branchings in fatty acids: fatty acids in uropygial secretion of Shanghai duck by GC-MS of 4,4-dimethyloxazoline derivatives. Lipids. 1988;23:804–810. doi: 10.1007/BF02536225. [DOI] [PubMed] [Google Scholar]
- 30.Apon JM, Nicolaides N. The determination of the position isomers of the methyl branches fatty acids methyl esters by capillary GC/MS. J Chromatogr Sci. 1975;13:467–473. doi: 10.1093/chromsci/13.10.467. [DOI] [PubMed] [Google Scholar]
- 31.Harvey DJ. Identification of long-chain fatty acids and alcohols from human cerumen by the use of picolinyl and nicotinate esters. Biomed Environ Mass Spectrom. 1989;18:719–723. doi: 10.1002/bms.1200180912. [DOI] [PubMed] [Google Scholar]
- 32.Chen ZY, Pelletier G, Hollywood R, Ratnayake WM. Trans fatty acid isomers in Canadian human milk. Lipids. 1995;30:15–21. doi: 10.1007/BF02537037. [DOI] [PubMed] [Google Scholar]
- 33.Aitchison JM, Dunkley WL, Canolty NL, Smith LM. Influence of diet on trans fatty acids in human milk. Am J Clin Nutr. 1977;30:2006–2015. doi: 10.1093/ajcn/30.12.2006. [DOI] [PubMed] [Google Scholar]
- 34.Corso G, Colavita C, Esposito M, Roma R, Napoli C, Zamparelli M, Ansanelli V. Gaschromatography-mass spectrometry analysis of fatty acids in human milk from forty puerperae living in southern Italy. Riv Eur Sci Med Farmacol. 1995;17:215–219. [PubMed] [Google Scholar]
- 35.Brenna JT, Varamini B, Jensen RG, Diersen-Schade DA, Boettcher JA, Arterburn LM. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am J Clin Nutr. 2007;85:1457–1464. doi: 10.1093/ajcn/85.6.1457. [DOI] [PubMed] [Google Scholar]
- 36.Kaneda T. Iso- and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. Microbiol Rev. 1991;55:288–302. doi: 10.1128/mr.55.2.288-302.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang HY, Huang SY, Chen PY, King VA, Lin YP, Tsen JH. Basic characteristics of Sporolactobacillus inulinus BCRC 14647 for potential probiotic properties. Curr Microbiol. 2007;54:396–404. doi: 10.1007/s00284-006-0496-5. [DOI] [PubMed] [Google Scholar]
- 38.Veerkamp JH. Fatty acid composition of Bifidobacterium and Lactobacillus strains. J Bacteriol. 1971;108:861–867. doi: 10.1128/jb.108.2.861-867.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caplan MS, Miller-Catchpole R, Kaup S, Russell T, Lickerman M, Amer M, Xiao Y, Thomson R., Jr Bifidobacterial supplementation reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Gastroenterology. 1999;117:577–583. doi: 10.1016/s0016-5085(99)70450-6. [DOI] [PubMed] [Google Scholar]
- 40.Claud EC, Walker WA. Hypothesis: inappropriate colonization of the premature intestine can cause neonatal necrotizing enterocolitis. FASEB J. 2001;15:1398–1403. doi: 10.1096/fj.00-0833hyp. [DOI] [PubMed] [Google Scholar]
- 41.Hallstrom M, Eerola E, Vuento R, Janas M, Tammela O. Effects of mode of delivery and necrotising enterocolitis on the intestinal microflora in preterm infants. Eur J Clin Microbiol Infect Dis. 2004;23:463–470. doi: 10.1007/s10096-004-1146-0. [DOI] [PubMed] [Google Scholar]
- 42.Beeby PJ, Jeffery H. Risk factors for necrotising enterocolitis: the influence of gestational age. Arch Dis Child. 1992;67:432–435. doi: 10.1136/adc.67.4_spec_no.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990;336:1519–1523. doi: 10.1016/0140-6736(90)93304-8. [DOI] [PubMed] [Google Scholar]
- 44.Hoyos AB. Reduced incidence of necrotizing enterocolitis associated with enteral administration of Lactobacillus acidophilus and Bifidobacterium infantis to neonates in an intensive care unit. Int J Infect Dis. 1999;3:197–202. doi: 10.1016/s1201-9712(99)90024-3. [DOI] [PubMed] [Google Scholar]
- 45.Biezenski JJ, Pomerance W, Goodman J. Studies on the origin of amniotic fluid lipids. I. Normal composition. Am J Obstet Gynecol. 1968;102:853–861. doi: 10.1016/0002-9378(68)90514-0. [DOI] [PubMed] [Google Scholar]
- 46.Pritchard JA. Deglutition by Normal and Anencephalic Fetuses. Obstet Gynecol. 1965;25:289–297. [PubMed] [Google Scholar]
- 47.Pritchard JA. Fetal swallowing and amniotic fluid volume. Obstet Gynecol. 1966;28:606–610. [PubMed] [Google Scholar]
- 48.Friel JK, Matthew JD, Andrews WL, Skinner CT. Trace elements in meconium from preterm and full-term infants. Biol Neonate. 1989;55:214–217. doi: 10.1159/000242919. [DOI] [PubMed] [Google Scholar]
- 49.Nicolaides N, Apon JM. Further studies of the saturated methyl branched fatty acids of vernix caseosa lipid. Lipids. 1976;11:781–790. doi: 10.1007/BF02533404. [DOI] [PubMed] [Google Scholar]
- 50.Nicolaides N. The structures of the branched fatty acids in the wax esters of vernix caseosa. Lipids. 1971;6:901–905. doi: 10.1007/BF02531172. [DOI] [PubMed] [Google Scholar]
- 51.Haahti E, Nikkari T, Salmi AM, Laaksonen AL. Fatty acids of vernix caseosa. Scand J Clin Lab Invest. 1961;13:70–73. doi: 10.3109/00365516109137251. [DOI] [PubMed] [Google Scholar]
- 52.Livingston M, Bell ME, Shorland FB, Gerson T, Hansen RP. The metabolism in the rat of naturally occurring (+)-14-methylhexadecanoic acid. Biochem J. 1957;65:438–440. doi: 10.1042/bj0650438. [DOI] [PMC free article] [PubMed] [Google Scholar]