Abstract
Objective
Lung involvement is the leading cause of morbidity and mortality in systemic sclerosis (SSc; scleroderma), and interstitial lung disease (ILD) is the most common pulmonary manifestation. An abnormal profibrotic Th2/Tc2-polarized T cell response is postulated to mediate tissue damage and fibrosis. The aim of this study was to investigate whether a polarized T cell phenotype in SSc is associated with lung disease or other clinical manifestations of SSc.
Methods
Circulating T cells were characterized by flow cytometry in 62 patients with SSc and 36 healthy control subjects, using antibodies against CD3, CD4, CD8, chemokine receptor CCR5 (Th1/Tc1-specific), and prostaglandin D2 receptor CRTH2 (Th2/Tc2-specific). The ratio between CCR5 and CRTH2 T cell frequencies was used to quantify type 1 (high-ratio) or type 2 (low-ratio) immune polarization.
Results
Patients with SSc exhibited lower CCR5/CRTH2 T cell ratios than those exhibited by control subjects (P < 0.0001), indicating a Th2/Tc2-polarized phenotype. Markedly reduced CCR5/CRTH2 T cell ratios were observed in SSc patients with ILD compared with SSc patients without ILD (P < 0.0001), particularly in patients with active ILD (P < 0.0001) compared with those with stable lung function. Lower CCR5/CRTH2 ratios were strongly associated with a lower value for the percent predicted forced vital capacity (P < 0.0001). In patients with an estimated right ventricular systolic pressure >35 mm Hg, suggestive of pulmonary vascular disease, a lower value for the percent predicted diffusing capacity (DLCO) was associated with higher CCR5/CRTH2 T cell ratios (Th1/Tc1) (P = 0.009), while in those with right ventricular systolic pressure <35 mm Hg, a lower value for the percent predicted DLCO correlated with lower ratios (Th2/Tc2) (P < 0.0001), as observed for ILD.
Conclusion
T cell polarization in SSc is strongly associated with specific manifestations of lung disease. Measurement of T cell polarization may represent a valuable tool to monitor disease activity and predict clinical outcomes in SSc patients with lung disease.
Systemic sclerosis (SSc; scleroderma) is a multi-system autoimmune disease involving the skin and internal organs such as the lungs, heart, gastrointestinal tract, and kidneys (1). It is characterized pathologically by tissue fibrosis, proliferative/obliterative microvascular disease, and immunologic disturbances. Based on the extent of clinically affected skin, 2 major subsets of SSc have been identified: diffuse cutaneous SSc (dcSSc) and limited cutaneous SSc (lcSSc) (2). Currently, interstitial lung disease (ILD) and pulmonary arterial hypertension (PAH), alone or in combination, have the greatest impact on SSc-related morbidity and mortality. Patients with dcSSc and lung involvement have an estimated 5-year survival of <50% (3).
ILD is the most common pulmonary complication in SSc, affecting 25–90% of patients, depending on the method used for diagnosis and the patient’s risk factors, including age at onset, ethnicity, and autoanti-body (i.e., anti–Scl-70) status (4,5). ILD is more frequently associated with dcSSc (40–50%), but patients with lcSSc can be affected as well (30%) (6). Severe progressive pulmonary fibrosis occurs in a substantial subset of patients. Importantly, the extent of the involved skin does not correlate with the severity of lung disease (7). PAH is present in 15–35% of patients with lcSSc and in 25–30% of patients with dcSSc, in whom it is often associated with ILD (7,8).
The clinical course of SSc, particularly that of lung disease, is chronic and highly variable, making it challenging to determine which patients will experience the most severe disease and in whom early aggressive treatment would therefore be justified. Despite our current understanding of the pathogenesis of SSc, clinicians caring for patients with SSc lack reliable biologic markers that characterize disease activity or predict clinical outcomes. An inflammatory mononuclear cell infiltrate is thought to precede the development of end-organ damage and fibrosis, particularly within the 2 major organs affected: the skin and lungs (9,10). CD8+ T cells are seen in the pulmonary interstitium on lung biopsy specimens and are increased in the bronchoalveolar lavage fluid from SSc patients with active lung disease (alveolitis) compared with SSc patients with inactive lung disease or no lung disease (11). These T cells exhibit an activated phenotype (HLA−DR+), and their presence in the bronchoalveolar lavage fluid is quantitatively associated with progressive and more severe ILD (12,13). Similarly, activated T cells are described in skin biopsy specimens from SSc patients who have early active cutaneous involvement (14). Whether T cell activation in SSc is associated with specific immune effectors and mediators is not well studied, nor is it clear whether the prevalence of specific T cell subsets correlates with clinical measures of disease activity or is ultimately associated with distinct clinical phenotypes.
Two functional subsets of T cells with distinct cytokine-secretion profiles are recognized (15). Type 1 (Th1/Tc1) T cells produce predominantly interferon-γ (IFNγ) and interleukin-2 (IL-2) and are involved in delayed-type hypersensitivity and defense against intra-cellular pathogens. Type 2 (Th2/Tc2) T cells secrete IL-4, IL-5, IL-10, and IL-13 and participate in humoral immune (IgE) and antiparasitic responses. Interestingly, these 2 subsets potentially exert opposing roles in regulating tissue remodeling and fibrogenesis. IFNγ suppresses fibroblast activity and the production of extra-cellular matrix proteins (e.g., collagen and fibronectin) (16). In contrast, Th2/Tc2 cytokines (IL-4 and IL-13) activate fibroblasts and collagen production directly or indirectly by inducing secretion of profibrotic cytokines such as transforming growth factor β (17).
Experimental data suggest that a network of potentially profibrotic cellular and humoral mediators is established within the target tissues in patients with SSc (18). Plasma levels of IL-4 and IL-13 are elevated in SSc patients compared with healthy control subjects (19,20). Th2 cytokine expression and secretion are increased in T cells isolated from newly affected SSc skin (21,22). Activated CD8+ T cells in bronchoalveolar lavage fluid from SSc patients with alveolitis have higher type 2 cytokine (IL-4 and IL-5) messenger RNA (mRNA) expression (12,13). These data suggest that in SSc, a specific population of activated T cells exhibiting a profibrotic Th2/Tc2-polarized phenotype may be potentially relevant in mediating tissue fibrosis.
In this study, we sought to investigate the relationship between clinical manifestations of SSc and characteristics of the underlying immune response. We hypothesized that a relative predominance of polarized T cell subsets in the peripheral blood may correlate with specific clinical features, particularly with distinct features of SSc-related lung disease phenotypes. For this purpose, we applied reliable and sensitive tools to measure ex vivo the polarization (type 1 or type 2) of circulating T cells in a well-characterized group of patients with SSc. The accurate characterization and measurement of the immune response in relation to SSc-specific clinical manifestations may provide significant insight into the disease process and lead to the identification of accurate biomarkers of disease activity, predictors of outcome, and, ultimately, novel disease-specific therapeutic targets.
PATIENTS AND METHODS
Patients
Sixty-two consecutive patients presenting at the Johns Hopkins Scleroderma Center were included in the study. All of the patients met the American College of Rheumatology preliminary criteria for the classification of SSc (23) and were subdivided as having dcSSc or lcSSc on the basis of the extent of their skin involvement (2). Control subjects were healthy volunteers recruited through public postings. The study was approved by the Johns Hopkins institutional review board, and written consent was obtained from all participants.
Clinical phenotyping
Detailed demographic data, including age, sex, ethnicity, education, smoking status (current, past, or never), and clinical information about disease duration (estimated from the date of onset of the first non-Raynaud’s symptom), scleroderma subtype, specific organ involvement, and autoantibody status (anti–Scl-70 and anticentromere) were obtained from each patient at the time of his or her visit. Patients with intercurrent acute illnesses, cancer, or other nonscleroderma lung diseases were excluded from the study.
Skin involvement was scored according to the modified Rodnan skin score (range 0–51) (24). Internal organ involvement was assessed using previously published criteria (25). Pulmonary involvement was determined by abnormal results of pulmonary function tests, including measures of the absolute as well as the percent of predicted values for forced vital capacity (FVC) and single-breath carbon monoxide diffusing capacity (DLCO), according to the American Thoracic Society recommendations (26). Values for spirometry were referenced to the National Health and Nutrition Examination Survey and the values described by Hankinson et al (27), and values for DLCO were referenced to those reported by the Burrows group (28). The most recent pulmonary function tests were used for the purpose of the study. Pulmonary vascular disease was considered present if the estimated right ventricular systolic pressure (eRVSP) by Doppler echocardiography was ≥35 mm Hg, and no overt clinical evidence of congestive heart failure or thromboembolic disease was detected. Existing ILD was defined as an FVC <80%. The presence of ILD activity was determined by a >10% decline in FVC results for the 2 most recent consecutive pulmonary function tests. All data were available within 6 months from the study test. Subjects in this subgroup actively assuming any immunosuppressive regimen were excluded from this specific analysis. Heart, gastrointestinal, renal, or musculoskeletal involvement was considered present when the relative score according to the Medsger severity scale was ≥1 (25). The severity of Raynaud’s phenomenon and evidence of sicca complex were determined by clinical criteria (29).
Laboratory procedures
The polarization of circulating T cells in patients with SSc and healthy control subjects was determined using very specific type 1 (Th1/Tc1) or type 2 (Th2/Tc2) surface markers. The chemokine receptor CCR5 has been previously described as an exclusive marker of Th1 cells, while the prostaglandin D2 receptor DP2 (CD294), also referred to as chemoattractant receptor homologous molecule expressed on Th2 cells (CRTH2), recently emerged as a consistent, predictive marker of Th2 cell function in basic and translational studies (30–32).
Peripheral blood samples were obtained from the antecubital veins of the study subjects and collected in BD Vacutainer blood collection tubes containing sodium heparin (Becton Dickinson, Franklin Lakes, NJ). Immediately, 50 μl of blood was placed in polystyrene round-bottomed tubes, and 10 μl of fluorochrome-labeled antibodies or isotype controls (used to detect the presence of nonspecific staining) was added. The reagents used were peridinin chlorophyll A protein–conjugated anti-CD3, fluorescein isothiocyanate–conjugated anti-CD4, allophycocyanin-conjugated anti-CD8, and phycoerythrin-conjugated anti-CCR5 (all from BD Biosciences, San Jose, CA) or anti-CRTH2 antibodies (Miltenyi Biotec, Auburn, CA). The tubes were incubated in the dark for 30 minutes at room temperature. Two milliliters of ACK red blood cell lysing buffer (BioSource International, Camarillo, CA) was then added, and the cells were gently vortexed. After 15 minutes of incubation, 2 washes with phosphate buffered saline were performed, and the samples were immediately analyzed using a 2-laser 4-color flow cytometer (FACSCalibur; BD Biosciences). Calibration for sample acquisition was done using CaliBRITE beads (Becton Dickinson) and AutoCOMP software (Becton Dickinson). Gating for acquisition was set on live lymphocytes and CD3+ cells. The stained samples were analyzed with CellQuest software (Becton Dickinson), and the relative CCR5+ and CRTH2+ T cell frequencies were calculated.
Before starting the investigation, the stability of CCR5 and CRTH2 T cell surface expression was tested in 12 healthy volunteers, at baseline and on days 1, 3, 10, and 25. Using a two-way mixed model, we observed a high consistency (P < 0.001) among the relative frequency of CD3+, CCR5+ and CD3+, CRTH2+ T cell subsets as well as the mean fluorescence intensity for both surface markers. In addition, we also confirmed that T cells identified by CCR5 and CRTH2 exhibit, respectively, a predominant Th1 (IFNγ)– and Th2 (IL-4)–polarized intracellular cytokine profile (32).
Statistical analysis
All variables were examined and transformed when a non-normal distribution was evident. Means and proportions among groups were estimated and tested using analysis of variance (with the least significant difference post hoc test) and cross-tabulation with chi-square tests. To evaluate the consistency of CCR5+ and CRTH2+ T cells across time, interclass correlation coefficients were calculated with a two-way mixed model. Bivariate associations were assessed by calculating Pearson’s correlation coefficients. Linear regression analyses were also used to evaluate relationships between SSc subtypes. Statistical analyses were performed with Stata version 9 software (Stata Corporation, College Station, TX). P values less than or equal to 0.05 were considered significant.
RESULTS
The sociodemographic and clinical characteristics of the study subjects are summarized in Table 1. There were no statistically significant differences between study groups in sex, race/ethnicity, education, and history of smoking. The patients with SSc were predominantly women (84%) age ~50 years; 68% were white, and 22% were African American; 25% reported past smoking, and 9% reported current smoking. Healthy control subjects were younger than patients (P = 0.05). As expected, the modified Rodnan skin score and level of anti–Scl-70 antibodies were significantly higher in the group with dcSSc, while anticentromere antibodies were present only in patients with lcSSc. The 2 patient populations did not differ significantly in terms of disease duration, results of pulmonary function tests (FVC and DLCO), eRVSP, and other internal organ or system involvement. Among patients with renal involvement in the dcSSc group, 2 had a history of hypertensive renal crisis.
Table 1.
Sociodemographic and disease characteristics of patients with limited SSc, patients with diffuse SSc, and healthy control subjects*
| Variable | Limited SSc (n ± 28) |
Diffuse SSc (n ± 34) |
Controls (n ± 36) |
|---|---|---|---|
| Age, years | 51.2 ± 14.4† | 51.1 ± 11.8† | 44.9±12.3 |
| Female sex, % | 85.7 | 82.4 | 82.3 |
| Race/ethnicity, % | |||
| White | 67.9 | 67.2 | 69.4 |
| African American | 21.4 | 23.5 | 22.2 |
| Other | 10.7 | 9.3 | 8.4 |
| Education, % | |||
| High school/trade school | 32.1 | 41.2 | 36.1 |
| College | 67.9 | 58.8 | 63.9 |
| Smoking, % | |||
| Never | 71.4 | 58.8 | 77.8 |
| Past | 21.4 | 29.4 | 16.7 |
| Current | 7.2 | 11.8 | 5.5 |
| Disease duration, years‡ | 9.7 ± 8.2 | 6.9 ± 6.9 | – |
| Rodnan skin score, modified | 3.6 ± 2.9§ | 19.5 ± 11.7 | – |
| Pulmonary function | |||
| FVC, % predicted | 82.9 ± 19.0 | 76.5 ± 18.6 | – |
| DLCO, % predicted | 69.3 ± 22.7 | 66.9 ± 24.0 | |
| eRVSP, mm Hg | 39 ± 18.8 | 38.3 ± 17.5 | – |
| Digital ischemia, % | |||
| Active (Raynaud’s severity score 0–2) | 7 | 6 | – |
| Inactive (Raynaud’s severity score >3) | 93 | 94 | |
| Cardiac involvement, % | 12 | 3.2 | – |
| Gastrointestinal involvement, % | 82.1 | 82.4 | – |
| Kidney involvement, % | 0 | 8 | – |
| Musculoskeletal involvement, % | 46.4 | 35.3 | – |
| Sicca complex, % | 25 | 38.2 | – |
| Autoantibodies, no. (%) | |||
| Anti–Scl-70 | 2 (7)§ | 20 (59) | – |
| Anticentromere | 11 (39)§ | 0 (0) | |
Except where indicated otherwise, values are the mean ± SD. SSc = systemic sclerosis; FVC = forced vital capacity; DLCO = diffusing capacity for carbon monoxide; eRVSP = estimated right ventricular systolic pressure.
P < 0.05 versus controls.
Time from first non-Raynaud’s symptom.
P < 0.0001 versus diffuse SSc.
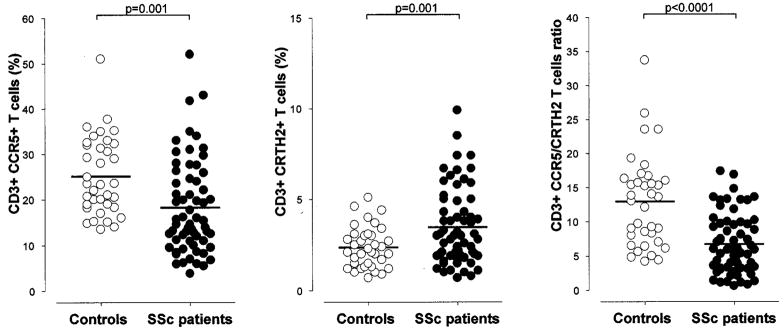
The polarization of circulating T cells was measured in 62 patients with SSc and 36 healthy control subjects. The relative number of CCR5+ T cells (Th1/Tc1) was lower in patients with SSc compared with control subjects (mean ± SD 18.2 ± 10.3 versus 25.1 ± 8.5; P = 0.001), while the number of CRTH2+ T cells (Th2/Tc2) was increased in patients with SSc (3.4 ± 2.0 versus 2.3 ± 1.1; P = 0.001) (Figure 1). The net effect of these opposite frequencies was reflected by a significantly lower CCR5/CRTH2 T cell ratio in patients with SSc (mean ± SD 6.7 ± 4.3 versus 12.9 ± 6.7; P < 0.0001), indicative of a Th2/Tc2-biased immune phenotype. The same differences were detected in CD4+ and CD8+ T cell subsets (data not shown). The subsequent analysis was conducted on SSc patients categorized according to disease subtypes (i.e., lcSSc or dcSSc and the presence of clinical manifestations such as ILD). The observed changes in the CCR5/CRTH2 ratios were consistently the result of CCR5 and CRTH2 expression moving in opposite directions (i.e., lower CCR5+ and higher CRTH2+ T cell subset frequencies for low ratios). Therefore, the CCR5/CRTH2 T cell ratio was adopted as the marker to express the degree of T cell polarization.
Figure 1.
Analysis of CCR5 and CRTH2 surface expression on circulating T cells. The 62 patients with systemic sclerosis (SSc) showed lower CCR5+ and higher CRTH2+ T cells frequencies compared with the 36 healthy control subjects. The net effect was a significantly lower CCR5/CRTH2 T cell ratio in patients with SSc, which is indicative of a Th2/Tc2-polarized immune phenotype. Bars show the mean.
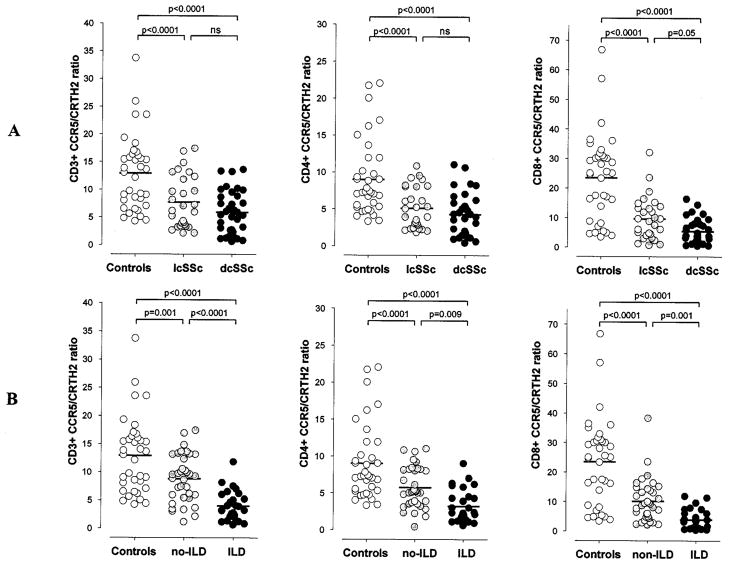
Evaluation of disease subtypes showed that both patients with lcSSc and those with dcSSc exhibited a significantly lower CCR5/CRTH2 ratio in total T cells (CD3+) as well as CD4+ and CD8+ subsets compared with control subjects (P < 0.0001) (Figure 2A). In addition, a trend toward a lower CCR5/CRTH2 ratio was observed in dcSSc compared with lcSSc, with a statistically significant difference only for the CD8+ subset (mean ± SD 5.3 ± 4.2 versus 9.6 ± 7.2; P = 0.05). No association was observed between CCR5/CRTH2 ratios and the modified Rodnan skin score, specific autoantibody subsets (anticentromere or anti–Scl-70), or other clinically relevant SSc-related manifestations, including gastrointestinal disease and renal disease, the severity of Raynaud’s phenomenon, and the presence of active digital ischemia.
Figure 2.
Analysis of circulating T cell polarization (CCR5/CRTH2 T cell ratio) in healthy control subjects (n = 36) and patients with systemic sclerosis (SSc) based on their disease subtype or the presence of interstitial lung disease (ILD). A, Patients with limited cutaneous SSc (lcSSc) (n = 28) and those with diffuse cutaneous SSc (dcSSc) (n = 34) showed lower CCR5/CRTH2 T cell ratios (CD3+, CD4+, and CD8+) compared with control subjects. A trend toward a greater Th2/Tc2 bias was evident in dcSSc compared with lcSSc; this difference was significant for the CD8+ T cell subset. B, SSc patients with ILD (n = 25) exhibited a significantly skewed Th2/Tc2-polarized phenotype, with CCR5/CRTH2 T cell ratios significantly lower than those in patients without ILD (n = 37) and healthy control subjects. Bars show the mean. NS = not significant.
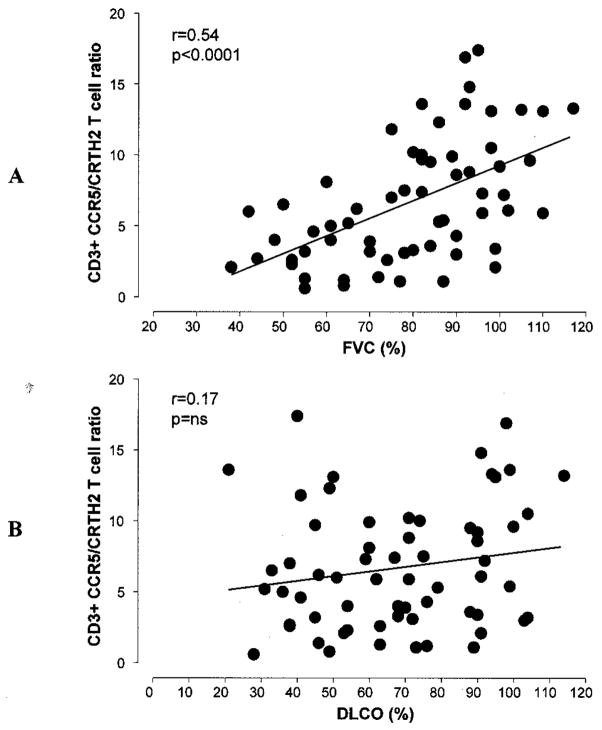
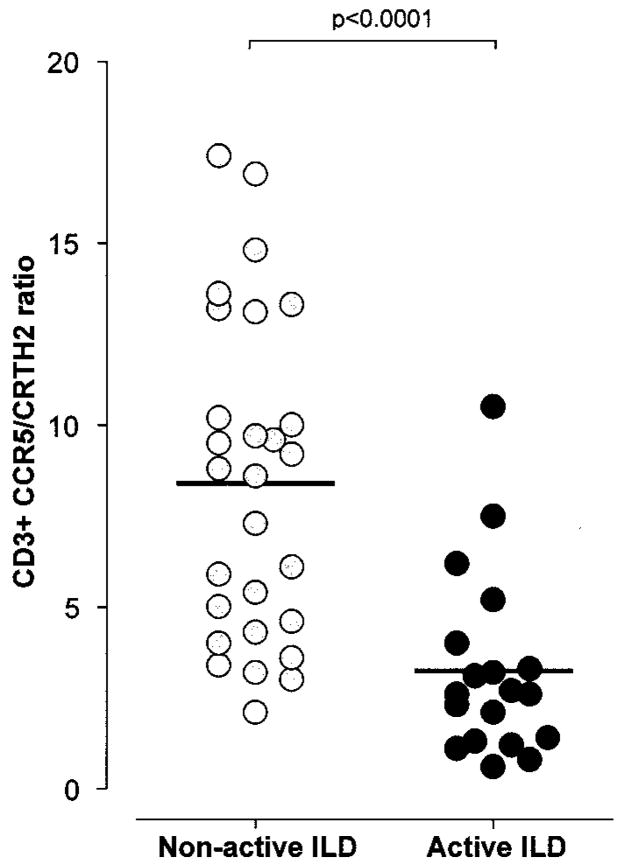
After classifying SSc patients based on the presence or absence of ILD, a significantly lower CCR5/CRTH2 T cell ratio was observed in SSc patients with ILD compared with those without ILD (mean ± SD 4.0 ± 2.6 versus 8.8 ± 4.2; P < 0.0001) and healthy control subjects (4.0 ± 2.6 versus 12.9 ± 6.7; P < 0.0001) (Figure 2B). Both CD4+ and CD8+ T cell subsets exhibited a significantly “skewed” Th2/Tc2-polarized phenotype. To further investigate the strength of this association, we next analyzed the differences in T cell polarization among patients with SSc in relation to the severity of the underlying ILD. As illustrated in Figure 3A, the CCR5/CRTH2 T cell ratio and FVC value (percent of predicted) exhibited a direct positive correlation (r = 0.54, P < 0.0001), suggesting that worse restrictive lung disease is associated with a higher degree of Th2/Tc2 T cell polarization. Similar results were observed for CD4+ and CD8+ T cells, using the absolute FVC value (liters) and the total lung capacity (liters and percent of predicted) as the lung function parameters of reference. In order to consider a possible role of a Th2/Tc2-polarized immune response in the development and progression of SSc lung fibrosis, we divided the patient population into 2 groups: those with a >10% decline in FVC measurements over the 6 months preceding the experimental blood testing and those with stable lung function. The patients with evidence of declining lung volumes (i.e., likely active ILD) at the time of the assay exhibited a significantly lower circulating CCR5/CRTH2 T cell ratio (mean ± SD 3.2 ± 2.5 versus 8.4 ± 4.4; P < 0.0001) (Figure 4).
Figure 3.
Pearson’s correlation between circulating T cell polarization (CCR5/CRTH2 ratio) and forced vital capacity (FVC) or diffusing capacity for carbon monoxide (DLCO) (percent of predicted) in patients with systemic sclerosis. A, Worse restrictive lung disease (lower FVC) was associated with higher degree of Th2/Tc2 T cell polarization. B, No statistically significant relationship was observed between the CCR5/CRTH2 T cell ratio and DLCO.
Figure 4.
Analysis of circulating T cell polarization in patients with systemic sclerosis, based on retrospective evidence of active interstitial lung disease (ILD) (>10% decline in forced vital capacity over the preceding 6 months). A significantly lower CCR5/CRTH2 T cell ratio was detected in patients with active ILD. Bars show the mean.
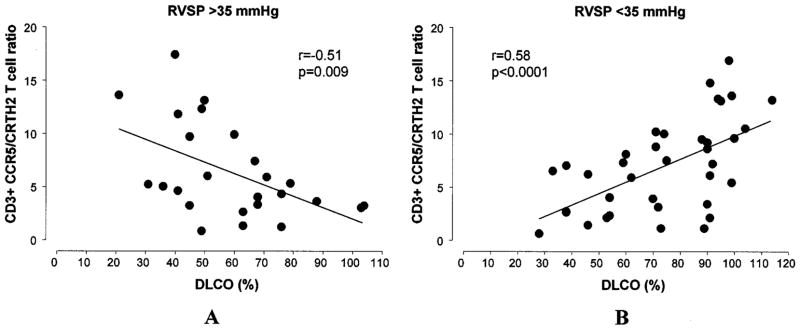
No statistically significant relationship was observed between the CCR5/CRTH2 T cell ratio and the DLCO value (percent of predicted) (r = 0.17) (Figure 3B). However, a U-shaped curve was observed, indicating that a relatively higher CCR5/CRTH2 T cell ratio (Th1/Tc1 polarization) is present in patients with SSc in whom the levels of DLCO are normal or extremely low. Clinically, a significantly and/or disproportionately low DLCO level can be detected when PAH is present. Therefore, we subdivided our study population based on our best available evidence for the presence of pulmonary vascular disease, using an abnormal eRVSP (>35 mm Hg) on the most recent echocardiogram as the discriminating value. Interestingly, an opposite correlation between circulating T cell polarization and the DLCO was observed (Figure 5). In patients with an eRVSP <35 mm Hg, a lower DLCO was significantly associated with lower CCR5/CRTH2 ratios (Th2/Tc2 phenotype) (r = 0.58, P < 0.0001), with a distribution similar to that previously observed for the percent predicted FVC. In contrast, in patients with an eRVSP >35 mm Hg, a negative relationship was measured, with a lower DLCO value associated with higher CCR5/CRTH2 ratios (Th1/Tc1 phenotype) (r = −0.51, P = 0.009).
Figure 5.
Pearson’s correlation between circulating T cell polarization and predicted diffusing capacity for carbon monoxide (DLCO) in patients with systemic sclerosis, based on the presence of pulmonary vascular disease (right ventricular systolic pressure [RVSP] >35 mm Hg). A, In patients with an RVSP >35 mm Hg, a lower DLCO value was associated with higher CCR5/CRTH2 ratios (Th1/Tc1 phenotype). B, In patients with an RVSP <35 mm Hg, a lower DLCO value was significantly associated with lower CCR5/CRTH2 ratios (Th2/Tc2 phenotype).
DISCUSSION
This investigation provides new insights into characteristics of the immune response in scleroderma and is the first to demonstrate a significant correlation between specific T cell subsets and distinct clinical manifestations. We used sensitive markers and specifically the CCR5/CRTH2 ratio of circulating T cells to reliably measure directly ex vivo the polarization of the underlying immune phenotype in a well-characterized group of patients with SSc.
We observed that a significant Th2/Tc2 bias is present among patients with scleroderma compared with healthy control subjects, and that both CD4+ and CD8+ T cells exhibit this “skewed” type 2 phenotype. Moreover, in patients with SSc, Th2/Tc2 polarization was significantly associated with the presence of ILD. This association was further confirmed by a strong linear correlation between a lower CCR5/CRTH2 T cell ratio (Th2/Tc2 bias) and worse FVC values. Although this was a cross-sectional analysis, we did have access to longitudinal lung function studies, allowing us to examine whether low CCR5/CRTH2 ratios were more likely to be observed in patients with active lung disease. Interestingly, the CCR5/CRTH2 T cell ratio was significantly lower in patients with SSc who had experienced a recent decline (>10%) in FVC values, suggestive of lung disease activity at the time of peripheral blood analysis. Although the CCR5/CRTH2 T cell ratio was not significantly associated with the DLCO value, an interesting finding became evident when the presence of pulmonary vascular disease (indicated by an abnormal eRVSP value as measured by echocardiography) was considered. In SSc patients with an eRVSP >35 mm Hg, a lower DLCO value was strongly associated with higher CCR5/CRTH2 ratios, suggestive of Th1/Tc1 T cell polarization, while those with an eRVSP <35 mm Hg exhibited an opposite correlation. Thus, the presence of ILD in our patients with SSc was associated with a Th2/Tc2 bias, with lower CCR5/CRTH2 ratios being even more prevalent in patients with progressive worsening of their lung function. In contrast, the presence of pulmonary vascular disease appears to be characterized by a dominant Th1/Tc1 circulating T cell phenotype.
Notably, although we observed that CCR5/CRTH2 ratios were generally lower in patients with dcSSc compared with patients with lcSSc, the modified Rodnan skin score (degree of skin disease) did not show any correlation with the polarization of T cells. We interpret this finding cautiously because of the cross-sectional design of our study and the high variability of skin involvement over time in scleroderma. In addition, although the lung function test is an excellent measure of ILD and its progression, we acknowledge that the echocardiographic determination of the eRVSP is not a definitive measure of pulmonary vascular disease or PAH. Most of our patients did not undergo right heart catheterization. Nevertheless, evaluating the eRVSP by echocardiography represents a widely accepted and validated screening method for determining the presence of pulmonary vascular disease (33). Thus, the presence of a prevalent Th1/Tc1 T cell phenotype in patients with a high eRVSP may suggest a possible association with emerging or actual PAH, which will need to be further confirmed using direct measures (right heart catheterization) in longitudinal studies.
A dominant Th2 immune response in patients with SSc has been previously reported, but the prevalence of a Th1 or a mixed immune phenotype has also been described (19,20,34,35). Importantly, the methods used to determine polarization of the immune response have been, in most cases, indirect and based on measuring highly variable parameters such as cytokine plasma levels or their mRNA expression within circulating cells or target tissues. In contrast to cytokine genes, CCR5 and CRTH2 are constitutively expressed in type 1 and type 2 T cells, thereby providing a more direct, stable measure of the relative prevalence of either subset. Furthermore, no study has specifically addressed and accurately analyzed the correlation between the polarized T cell phenotype and the underlying SSc-specific clinical manifestations. For these reasons, the clinical and pathogenetic relevance of a polarized immune response in SSc has not yet been fully elucidated.
Our study used a sensitive, reliable, and easily reproducible experimental assay and measured ex vivo the polarization of circulating T cells, using very specific surface markers. The SSc patient population analyzed was relatively large, clinically well characterized, and selected independently of any laboratory study or disease status. These factors confer strength and relevance to our findings and draw attention to the measurement of evolving T cell polarization (the CCR5/CRTH2 T cell ratio) as a potential novel and sensitive approach to monitor the course of disease manifestations in scleroderma and particularly in lung disease. Our findings suggest the possibility that the CCR5/CRTH2 ratio will not change over time in patients with SSc in whom lung disease is stable, while it may decrease, marking a shift toward a higher Th2/Tc2 bias, just before or at the time of onset of active alveolitis. Furthermore, a greater type 2 skewing of circulating T cells at the time of diagnosis or early in the disease course may identify patients who are at higher risk of ILD developing or in whom ILD will progress to more severe disease. Indeed, a group of patients in our study had normal results of pulmonary function tests and exhibited a relatively low CCR5/CRTH2 ratio (Figure 4). It will be of great interest to follow up these patients prospectively and to confirm the predictive value of measuring a polarized T cell response before lung disease becomes manifest.
This is the first report to describe the detection of an opposite pattern of T cell polarization in association with SSc-related ILD or pulmonary vascular disease. The relevance of a Th2/Tc2-polarized immune response in the pathogenesis and progression of SSc lung fibrosis is plausible, although it remains to be confirmed whether such bias is present within early inflammatory lung infiltrates during ILD initiation. With regard to SSc vasculopathy, previous studies have implicated both cellular and humoral immune effector pathways and have demonstrated that T cells can cause microvascular injury directly through cell-mediated cytotoxicity or by inducing proinflammatory molecules on the endothelial cell surface (36,37). Recently, new data have shown that the endothelium and inflammatory cells infiltrating vessel walls in lesional skin and lung biopsy specimens from patients with SSc exhibit up-regulation of IFNγ-inducible molecules that are implicated in vascular smooth muscle migration/proliferation as well as proinflammatory cytokine secretion (38).
Thus, it is possible that a Th1/Tc1 microenvironment is functionally relevant for generation of the vascular disease seen in scleroderma. Further studies are needed to determine whether the polarized immune responses observed in the context of specific SSc lung disease manifestations are causally implicated or whether they result from abnormal lymphocyte trafficking secondary to the specific tissue damage affecting different lung compartments in SSc (i.e., interstitium or vascular bed). Other disease models exist in which extreme immune polarization toward a Th1 or Th2 response can be associated with different disease manifestations and mediate distinct outcomes. For example, in human schistosomiasis an initial acute proinflammatory Th1 response continues until a transition toward a chronic Th2 polarization occurs, with promotion of perioval granuloma formation (39). When this Th1-to-Th2 shift is balanced and controlled, the disease tends to be mild. Conversely, a persistent Th1-type response correlates with a more severe systemic infection, while an excessive Th2-dominated tissue environment is associated with development of severe liver fibrosis (40).
Our study provides new insight into potential pathogenetic pathways in scleroderma and may have important clinical implications, particularly in terms of the management and treatment of lung involvement. Clinicians caring for patients with SSc are challenged to identify those with active lung disease, who predictably will experience progressive loss of lung function. In addition, potentially toxic immunosuppressive treatments are administered and monitored based almost exclusively on crude clinical responses, with significant attendant morbidity and mortality. Measuring the CCR5/CRTH2 T cell ratio in the peripheral blood of patients with SSc in order to quantify polarization of the immune response may prove to be a useful measurable biologic marker to monitor disease activity and allow early identification of patients who will evolve toward a more aggressive and life-threatening disease phenotype. In addition, this assay has the potential of a broader application to other autoimmune conditions associated with ILD, including, for example, the antisynthetase syndrome in myositis, systemic lupus erythematosus lung disease, and mixed connective tissue disorder. Finally, specific interventions to rebalance skewed type 1 or type 2 T cell responses in patients with developing PAH or ILD may represent a novel therapeutic strategy.
In summary, we have demonstrated that specific manifestations of lung disease in patients with SSc are strongly correlated with opposite patterns of circulating T cell polarization, as measured by the CCR5/CRTH2 T cell ratio. A Th2/Tc2-polarized immune response is associated with ILD and particularly with active lung disease. We speculate that polarized T cell effectors may have a crucial role in the development and/or progression of lung disease in SSc. Further characterization and prospective measurement of polarized type 1/type 2 immune effectors in SSc are needed to confirm these data and to allow the identification of more dependable biomarkers of lung disease activity and accurate predictors of outcome, and potentially to open the door to novel disease-specific therapeutic targets.
Acknowledgments
Dr. Boin’s work was supported by an Irvington Institute/Dana Foundation Human Immunology Award and by the Scleroderma Research Foundation. Dr. Rosen’s work was supported by NIH grant P-30-AR-053503. Dr. Casolaro’s work was supported by NIH grant R01-AI-041463.
Footnotes
AUTHOR CONTRIBUTIONS
Dr. Boin had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study design. Boin, Wigley, Rosen, Casolaro.
Acquisition of data. Boin, De Fanis.
Analysis and interpretation of the data. Boin, Wigley, Rosen, Casolaro.
Manuscript preparation. Boin, De Fanis, Wigley, Rosen, Casolaro.
Statistical analysis. Boin, Bartlett.
References
- 1.Wigley FM, Hummers LK. Clinical features of systemic sclerosis. In: Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, editors. Rheumatology. 3. St. Louis (MO): Mosby; 2003. pp. 1463–80. [Google Scholar]
- 2.LeRoy EC, Medsger TA., Jr Criteria for the classification of early systemic sclerosis [review] J Rheumatol. 2001;28:1573–6. [PubMed] [Google Scholar]
- 3.Ioannidis JP, Vlachoyiannopoulos PG, Haidich AB, Medsger TA, Jr, Lucas M, Michet CJ, et al. Mortality in systemic sclerosis: an international meta-analysis of individual patient data. Am J Med. 2005;118:2–10. doi: 10.1016/j.amjmed.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 4.Jacobsen S, Halberg P, Ullman S, Hoier-Madsen M, Petersen J, Mortensen J, et al. A longitudinal study of pulmonary function in Danish patients with systemic sclerosis. Clin Rheumatol. 1997;16:384–90. doi: 10.1007/BF02242456. [DOI] [PubMed] [Google Scholar]
- 5.Schurawitzki H, Stiglbauer R, Graninger W, Herold C, Polzleitner D, Burghuber OC, et al. Interstitial lung disease in progressive systemic sclerosis. Radiology. 1990;176:755–9. doi: 10.1148/radiology.176.3.2389033. [DOI] [PubMed] [Google Scholar]
- 6.Owens GR, Fino GJ, Herbert DL, Steen VD, Medsger TA, Jr, Pennock BE, et al. Pulmonary function in progressive systemic sclerosis: comparison of CREST syndrome variant with diffuse scleroderma. Chest. 1983;84:546–50. doi: 10.1378/chest.84.5.546. [DOI] [PubMed] [Google Scholar]
- 7.Morelli S, Barbieri C, Sgreccia A, Ferrante L, Pittoni V, Conti F, et al. Relationship between cutaneous and pulmonary involvement in systemic sclerosis. J Rheumatol. 1997;24:81–5. [PubMed] [Google Scholar]
- 8.Chang B, Wigley FM, White B, Wise RA. Scleroderma patients with combined pulmonary hypertension and interstitial lung disease. J Rheumatol. 2003;30:2398–405. [PubMed] [Google Scholar]
- 9.Bouros D, Wells AU, Nicholson AG, Colby TV, Polychronopoulos V, Pantelidis P, et al. Histopathologic subsets of fibrosing alveolitis in patients with systemic sclerosis and their relationship to outcome. Am J Respir Crit Care Med. 2002;165:1581–6. doi: 10.1164/rccm.2106012. [DOI] [PubMed] [Google Scholar]
- 10.Prescott RJ, Freemont AJ, Jones CJ, Hoyland J, Fielding P. Sequential dermal microvascular and perivascular changes in the development of scleroderma. J Pathol. 1992;166:255–63. doi: 10.1002/path.1711660307. [DOI] [PubMed] [Google Scholar]
- 11.Yamadori I, Fujita J, Kajitani H, Bandoh S, Tokuda M, Yang Y, et al. Lymphocyte subsets in lung tissues of non-specific interstitial pneumonia and pulmonary fibrosis associated with collagen vascular disorders: correlation with CD4/CD8 ratio in bronchoalveolar lavage. Lung. 2000;178:361–70. doi: 10.1007/s004080000037. [DOI] [PubMed] [Google Scholar]
- 12.Atamas SP, Yurovsky VV, Wise R, Wigley FM, Goter Robinson CJ, Henry P, et al. Production of type 2 cytokines by CD8+ lung cells is associated with greater decline in pulmonary function in patients with systemic sclerosis. Arthritis Rheum. 1999;42:1168–78. doi: 10.1002/1529-0131(199906)42:6<1168::AID-ANR13>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 13.Luzina IG, Atamas SP, Wise R, Wigley FM, Choi J, Xiao HQ, et al. Occurrence of an activated, profibrotic pattern of gene expression in lung CD8+ T cells from scleroderma patients. Arthritis Rheum. 2003;48:2262–74. doi: 10.1002/art.11080. [DOI] [PubMed] [Google Scholar]
- 14.Sakkas LI, Xu B, Artlett CM, Lu S, Jimenez SA, Platsoucas CD. Oligoclonal T cell expansion in the skin of patients with systemic sclerosis. J Immunol. 2002;168:3649–59. doi: 10.4049/jimmunol.168.7.3649. [DOI] [PubMed] [Google Scholar]
- 15.Romagnani S. The Th1/Th2 paradigm [review] Immunol Today. 1997;18:263–6. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 16.Duncan MR, Berman B. Differential regulation of glycosaminoglycan, fibronectin, and collagenase production in cultured human dermal fibroblasts by interferon-α, -β, and - γ. Arch Dermatol Res. 1989;281:11–8. doi: 10.1007/BF00424266. [DOI] [PubMed] [Google Scholar]
- 17.Gurujeyalakshmi G, Giri SN. Molecular mechanisms of antifibrotic effect of interferon γ in bleomycin-mouse model of lung fibrosis: downregulation of TGF-β and procollagen I and III gene expression. Exp Lung Res. 1995;21:791–808. doi: 10.3109/01902149509050842. [DOI] [PubMed] [Google Scholar]
- 18.Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117:557–67. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasegawa M, Fujimoto M, Kikuchi K, Takehara K. Elevated serum levels of interleukin 4 (IL-4), IL-10, and IL-13 in patients with systemic sclerosis. J Rheumatol. 1997;24:328–32. [PubMed] [Google Scholar]
- 20.Needleman BW, Wigley FM, Stair RW. Interleukin-1, interleukin-2, interleukin-4, interleukin-6, tumor necrosis factor α, and interferon-γ levels in sera from patients with scleroderma. Arthritis Rheum. 1992;35:67–72. doi: 10.1002/art.1780350111. [DOI] [PubMed] [Google Scholar]
- 21.Mavalia C, Scaletti C, Romagnani P, Carossino AM, Pignone A, Emmi L, et al. Type 2 helper T-cell predominance and high CD30 expression in systemic sclerosis. Am J Pathol. 1997;151:1751–8. [PMC free article] [PubMed] [Google Scholar]
- 22.Salmon-Ehr V, Serpier H, Nawrocki B, Gillery P, Clavel C, Kalis B, et al. Expression of interleukin-4 in scleroderma skin specimens and scleroderma fibroblast cultures: potential role in fibrosis. Arch Dermatol. 1996;132:802–6. [PubMed] [Google Scholar]
- 23.Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for the classification of systemic sclerosis (scleroderma) Arthritis Rheum. 1980;23:581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 24.Clements P, Lachenbruch P, Siebold J, White B, Weiner S, Martin R, et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol. 1995;22:1281–5. [PubMed] [Google Scholar]
- 25.Medsger TA, Jr, Silman AJ, Steen VD, Black CM, Akesson A, Bacon PA, et al. A disease severity scale for systemic sclerosis: development and testing. J Rheumatol. 1999;26:2159–67. [PubMed] [Google Scholar]
- 26.American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis. 1991;144:1202–18. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 27.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 28.Knudson RJ, Kaltenborn WT, Knudson DE, Burrows B. The single-breath carbon monoxide diffusing capacity: reference equations derived from a healthy nonsmoking population and effects of hematocrit. Am Rev Respir Dis. 1987;135:805–11. doi: 10.1164/arrd.1987.135.4.805. [DOI] [PubMed] [Google Scholar]
- 29.Merkel PA, Herlyn K, Martin RW, Anderson JJ, Mayes MD, Bell P, et al. for the Scleroderma Clinical Trials Consortium. Measuring disease activity and functional status in patients with scleroderma and Raynaud’s phenomenon. Arthritis Rheum. 2002;46:2410–20. doi: 10.1002/art.10486. [DOI] [PubMed] [Google Scholar]
- 30.Messi M, Giacchetto I, Nagata K, Lanzavecchia A, Natoli G, Sallusto F. Memory and flexibility of cytokine gene expression as separable properties of human TH1 and TH2 lymphocytes. Nature Immunol. 2003;4:78–86. doi: 10.1038/ni872. [DOI] [PubMed] [Google Scholar]
- 31.Xue L, Gyles SL, Wettey FR, Gazi L, Townsend E, Hunter MG, et al. Prostaglandin D2 causes preferential induction of proinflammatory Th2 cytokine production through an action on chemoattractant receptor-like molecule expressed on Th2 cells. J Immunol. 2005;175:6531–6. doi: 10.4049/jimmunol.175.10.6531. [DOI] [PubMed] [Google Scholar]
- 32.De Fanis U, Mori F, Kurnat RJ, Lee WK, Bova M, Adkinson NF, et al. GATA3 up-regulation associated with surface expression of CD294/CRTH2: a unique feature of human Th cells. Blood. 2007;109:4343–50. doi: 10.1182/blood-2006-05-025940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan KL, Currie PJ, Seward JB, Hagler DJ, Mair DD, Tajik AJ. Comparison of three Doppler ultrasound methods in the prediction of pulmonary artery pressure. J Am Coll Cardiol. 1987;9:549–54. doi: 10.1016/s0735-1097(87)80047-5. [DOI] [PubMed] [Google Scholar]
- 34.Valentini G, Baroni A, Esposito K, Naclerio C, Buommino E, Farzati A, et al. Peripheral blood T lymphocytes from systemic sclerosis patients show both Th1 and Th2 activation. J Clin Immunol. 2001;21:210–7. doi: 10.1023/a:1011024313525. [DOI] [PubMed] [Google Scholar]
- 35.Fujii H, Hasegawa M, Takehara K, Mukaida N, Sato S. Abnormal expression of intracellular cytokines and chemokine receptors in peripheral blood T lymphocytes from patients with systemic sclerosis. Clin Exp Immunol. 2002;130:548–56. doi: 10.1046/j.1365-2249.2002.02017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choy JC, Cruz RP, Kerjner A, Geisbrecht J, Sawchuk T, Fraser SA, et al. Granzyme B induces endothelial cell apoptosis and contributes to the development of transplant vascular disease. Am J Transplant. 2005;5:494–9. doi: 10.1111/j.1600-6143.2004.00710.x. [DOI] [PubMed] [Google Scholar]
- 37.Kahaleh MB, Fan PS. Mechanism of serum-mediated endothelial injury in scleroderma: identification of a granular enzyme in scleroderma skin and sera. Clin Immunol Immunopathol. 1997;83:32–40. doi: 10.1006/clin.1996.4322. [DOI] [PubMed] [Google Scholar]
- 38.Del Galdo F, Maul GG, Jimenez SA, Artlett CM. Expression of allograft inflammatory factor 1 in tissues from patients with systemic sclerosis and in vitro differential expression of its isoforms in response to transforming growth factor β. Arthritis Rheum. 2006;54:2616–25. doi: 10.1002/art.22010. [DOI] [PubMed] [Google Scholar]
- 39.Wilson MS, Mentink-Kane MM, Pesce JT, Ramalingam TR, Thompson R, Wynn TA. Immunopathology of schistosomiasis. Immunol Cell Biol. 2007;85:148–54. doi: 10.1038/sj.icb.7100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stadecker MJ, Asahi H, Finger E, Hernandez HJ, Rutitzky LI, Sun J. The immunobiology of Th1 polarization in high-pathology schistosomiasis. Immunol Rev. 2004;201:168–79. doi: 10.1111/j.0105-2896.2004.00197.x. [DOI] [PubMed] [Google Scholar]