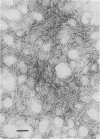
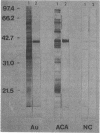
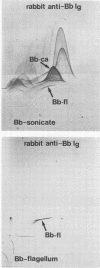
Abstract
The isolation of Borrelia burgdorferi flagella and an enzyme-linked immunosorbent assay (ELISA) for detection of immunoglobulin G (IgG) and IgM to the B. burgdorferi flagellum are described. The diagnostic performance of the flagellum ELISA for serodiagnosis of Lyme disease was compared with the performance of a traditional whole cell B. burgdorferi sonic extract ELISA. We examined sera and cerebrospinal fluid (CSF) from 56 patients with lymphocytic meningoradiculitis (Bannwarth's syndrome), the most frequent secondary-stage manifestation of Lyme disease in Europe. Two hundred healthy individuals and patients with aseptic meningitis, encephalitis, Guillain-Barré syndrome, and syphilis served as controls. The flagellum ELISA was significantly more sensitive than the sonic extract ELISA. The diagnostic sensitivities were increased from 41.1 to 76.8% (P less than 0.01) for IgG and from 35.7 to 67.9% (P less than 0.05) for IgM detection in serum. The increase in sensitivity was most pronounced in patients with a short duration of disease (less than 20 days after onset). The diagnostic specificity increased for IgG detection but was almost unaltered for IgM. The flagellum ELISA did not improve the diagnostic sensitivity of measuring antibodies to borreliae in CSF, most likely owing to the low level of unspecific antibodies in CSF compared with serum. The cross-reactivity of sera and CSF from patients with syphilis decreased significantly. The flagellum antigen of B. burgdorferi shows no strain variation, is easy to purify in sufficient quantity, and is therefore a suitable reference antigen for routine serodiagnosis of Lyme disease.
Full text
PDF

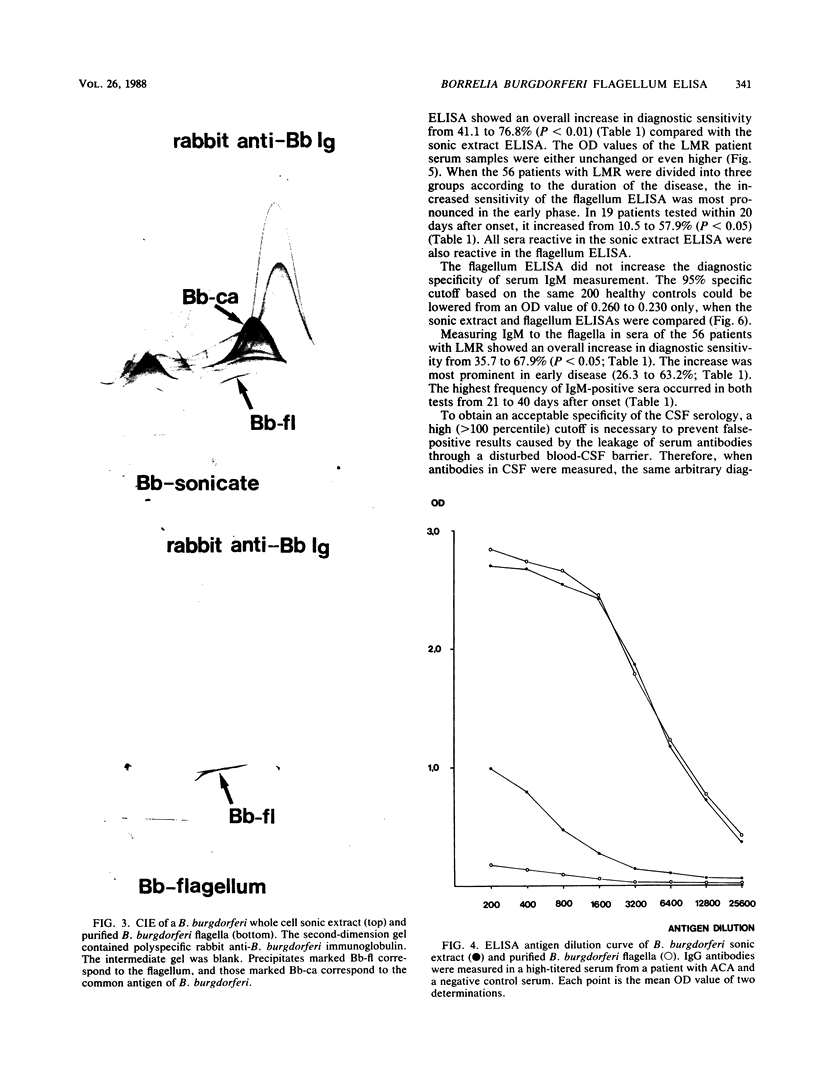
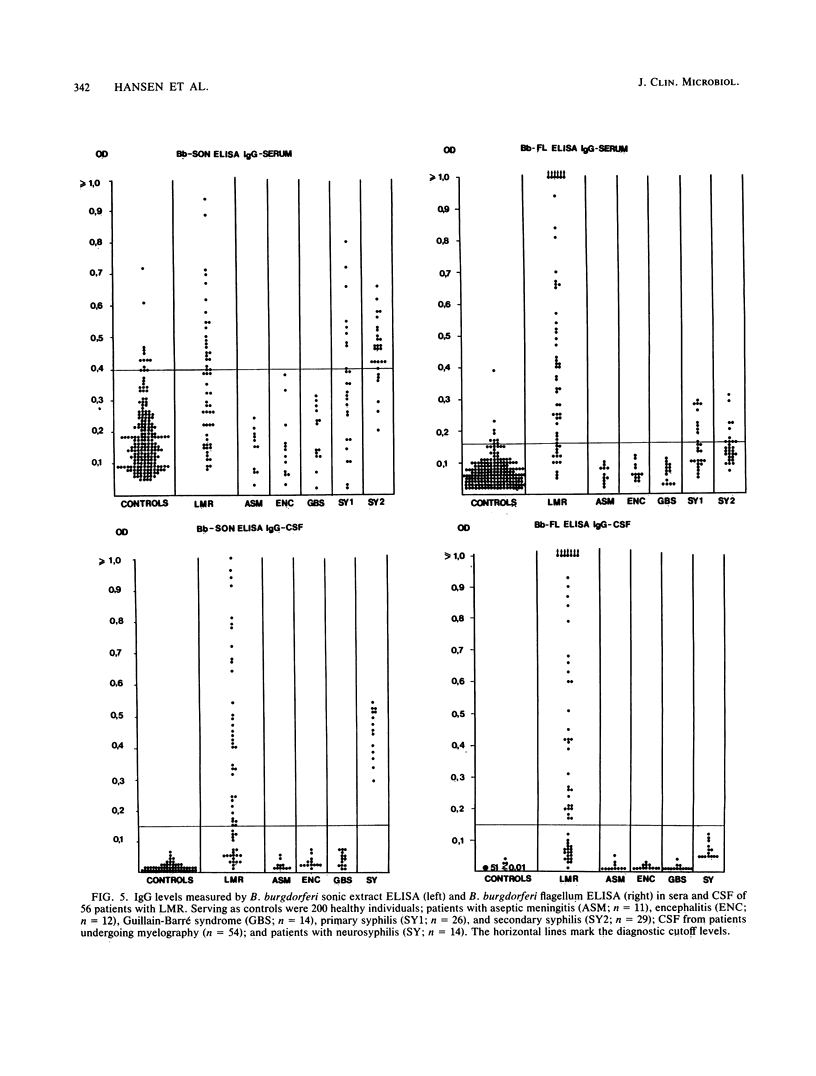
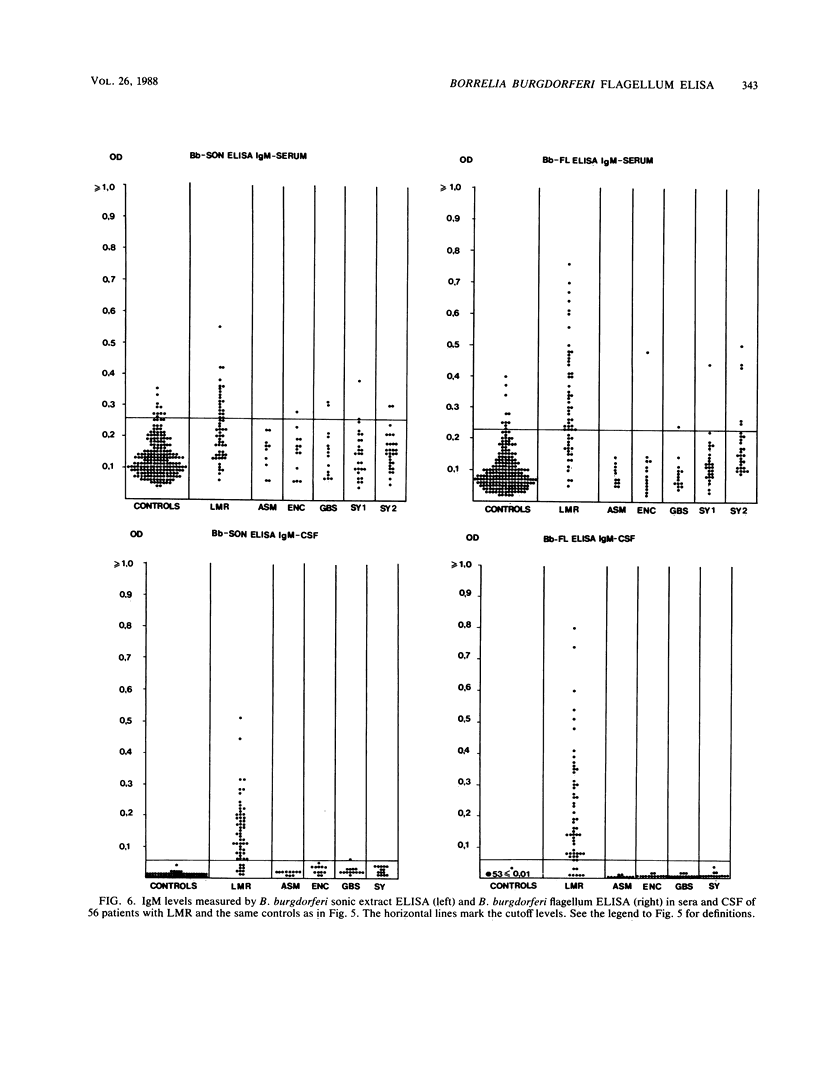
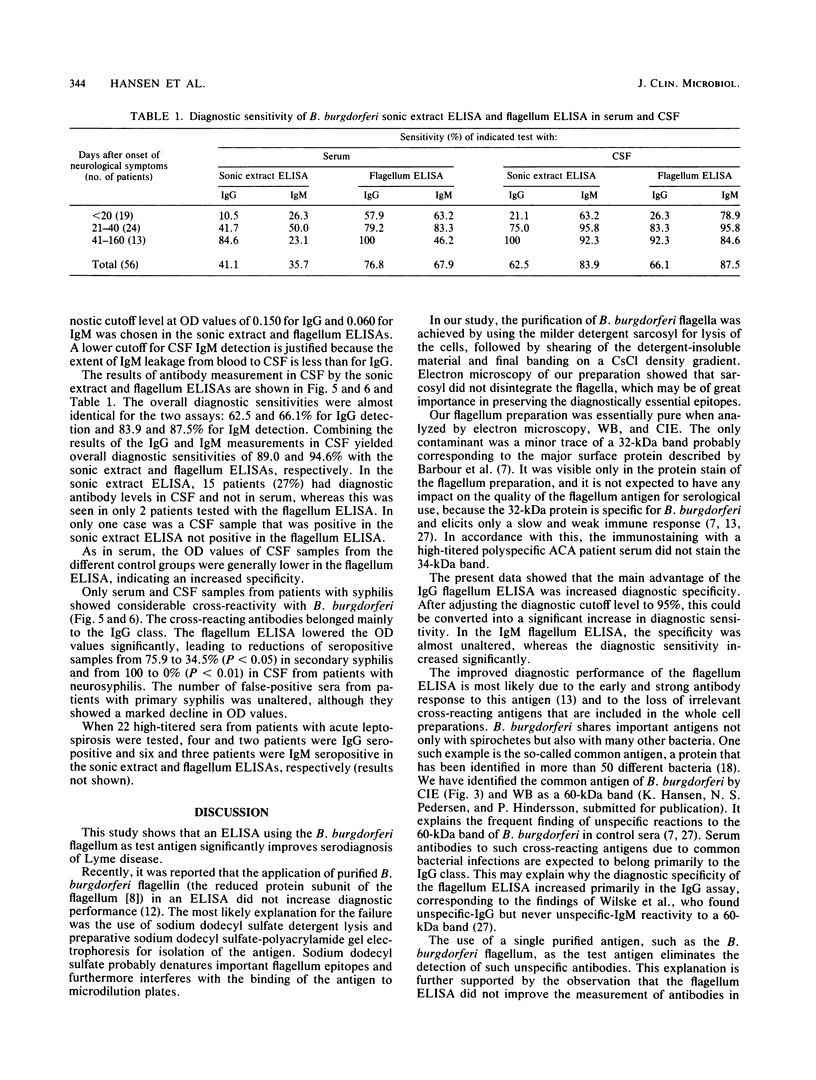


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann R., Hörstrup P., Schmidt R. Tick-borne meningopolyneuritis (Garin-Bujadoux, Bannwarth). Yale J Biol Med. 1984 Jul-Aug;57(4):485–490. [PMC free article] [PubMed] [Google Scholar]
- Ackermann R., Kabatzki J., Boisten H. P., Steere A. C., Grodzicki R. L., Hartung S., Runne U. Spirochäten-Atiologie der Erythema-chronicum-migrans-Krankheit. Dtsch Med Wochenschr. 1984 Jan 20;109(3):92–97. doi: 10.1055/s-2008-1069145. [DOI] [PubMed] [Google Scholar]
- Asbrink E., Hederstedt B., Hovmark A. The spirochetal etiology of erythema chronicum migrans Afzelius. Acta Derm Venereol. 1984;64(4):291–295. [PubMed] [Google Scholar]
- Asbrink E., Hovmark A., Hederstedt B. Serologic studies of erythema chronicum migrans Afzelius and acrodermatitis chronica atrophicans with indirect immunofluorescence and enzyme-linked immunosorbent assays. Acta Derm Venereol. 1985;65(6):509–514. [PubMed] [Google Scholar]
- Asbrink E., Hovmark A., Hederstedt B. The spirochetal etiology of acrodermatitis chronica atrophicans Herxheimer. Acta Derm Venereol. 1984;64(6):506–512. [PubMed] [Google Scholar]
- Barbour A. G., Burgdorfer W., Grunwaldt E., Steere A. C. Antibodies of patients with Lyme disease to components of the Ixodes dammini spirochete. J Clin Invest. 1983 Aug;72(2):504–515. doi: 10.1172/JCI110998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Hayes S. F., Heiland R. A., Schrumpf M. E., Tessier S. L. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect Immun. 1986 May;52(2):549–554. doi: 10.1128/iai.52.2.549-554.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Heiland R. A., Howe T. R. Heterogeneity of major proteins in Lyme disease borreliae: a molecular analysis of North American and European isolates. J Infect Dis. 1985 Sep;152(3):478–484. doi: 10.1093/infdis/152.3.478. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W., Barbour A. G., Hayes S. F., Benach J. L., Grunwaldt E., Davis J. P. Lyme disease-a tick-borne spirochetosis? Science. 1982 Jun 18;216(4552):1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Coleman J. L., Benach J. L. Isolation of antigenic components from the Lyme disease spirochete: their role in early diagnosis. J Infect Dis. 1987 Apr;155(4):756–765. doi: 10.1093/infdis/155.4.756. [DOI] [PubMed] [Google Scholar]
- Craft J. E., Fischer D. K., Shimamoto G. T., Steere A. C. Antigens of Borrelia burgdorferi recognized during Lyme disease. Appearance of a new immunoglobulin M response and expansion of the immunoglobulin G response late in the illness. J Clin Invest. 1986 Oct;78(4):934–939. doi: 10.1172/JCI112683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft J. E., Grodzicki R. L., Steere A. C. Antibody response in Lyme disease: evaluation of diagnostic tests. J Infect Dis. 1984 May;149(5):789–795. doi: 10.1093/infdis/149.5.789. [DOI] [PubMed] [Google Scholar]
- Hardy P. H., Jr, Fredericks W. R., Nell E. E. Isolation and antigenic characteristics of axial filaments from the Reiter Treponeme. Infect Immun. 1975 Feb;11(2):380–386. doi: 10.1128/iai.11.2.380-386.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindersson P., Knudsen J. D., Axelsen N. H. Cloning and expression of treponema pallidum common antigen (Tp-4) in Escherichia coli K12. J Gen Microbiol. 1987 Mar;133(3):587–596. doi: 10.1099/00221287-133-3-587. [DOI] [PubMed] [Google Scholar]
- Hindersson P., Petersen C. S., Pedersen N. S., Høiby N., Axelsen N. H. Immunological cross-reaction between antigen Tp-4 of Treponema pallidum and an antigen common to a wide range of bacteria. Acta Pathol Microbiol Immunol Scand B. 1984 Aug;92(4):183–188. doi: 10.1111/j.1699-0463.1984.tb02818.x. [DOI] [PubMed] [Google Scholar]
- Hovmark A., Asbrink E., Olsson I. The spirochetal etiology of lymphadenosis benigna cutis solitaria. Acta Derm Venereol. 1986;66(6):479–484. [PubMed] [Google Scholar]
- Martínez-Maza O., Fehniger T. E., Ashman R. F. Antibody-secreting cell precursor frequencies among the sheep-erythrocyte-binding cells after immunization. Scand J Immunol. 1983 Apr;17(4):345–354. doi: 10.1111/j.1365-3083.1983.tb00799.x. [DOI] [PubMed] [Google Scholar]
- Muhlemann M. F., Wright D. J., Black C. Serology of Lyme disease. Lancet. 1986 Mar 8;1(8480):553–554. doi: 10.1016/s0140-6736(86)90903-7. [DOI] [PubMed] [Google Scholar]
- Pedersen N. S., Petersen C. S., Vejtorp M., Axelsen N. H. Serodiagnosis of syphilis by an enzyme-linked immunosorbent assay for IgG antibodies against the Reiter treponeme flagellum. Scand J Immunol. 1982 Apr;15(4):341–348. doi: 10.1111/j.1365-3083.1982.tb00657.x. [DOI] [PubMed] [Google Scholar]
- Pfister H. W., Einhäupl K., Preac-Mursic V., Wilske B., Schierz G. The spirochetal etiology of lymphocytic meningoradiculitis of Bannwarth (Bannwarth's syndrome). J Neurol. 1984;231(3):141–144. doi: 10.1007/BF00313682. [DOI] [PubMed] [Google Scholar]
- Russell H., Sampson J. S., Schmid G. P., Wilkinson H. W., Plikaytis B. Enzyme-linked immunosorbent assay and indirect immunofluorescence assay for Lyme disease. J Infect Dis. 1984 Mar;149(3):465–470. doi: 10.1093/infdis/149.3.465. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Grodzicki R. L., Kornblatt A. N., Craft J. E., Barbour A. G., Burgdorfer W., Schmid G. P., Johnson E., Malawista S. E. The spirochetal etiology of Lyme disease. N Engl J Med. 1983 Mar 31;308(13):733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- Stiernstedt G. T., Granström M., Hederstedt B., Sköldenberg B. Diagnosis of spirochetal meningitis by enzyme-linked immunosorbent assay and indirect immunofluorescence assay in serum and cerebrospinal fluid. J Clin Microbiol. 1985 May;21(5):819–825. doi: 10.1128/jcm.21.5.819-825.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilske B., Preac-Mursic V., Schierz G., Busch K. V. Immunochemical and immunological analysis of European Borrelia burgdorferi strains. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Dec;263(1-2):92–102. doi: 10.1016/s0176-6724(86)80108-0. [DOI] [PubMed] [Google Scholar]
- Wilske B., Schierz G., Preac-Mursic V., Weber K., Pfister H. W., Einhäupl K. Serological diagnosis of erythema migrans disease and related disorders. Infection. 1984 Sep-Oct;12(5):331–337. doi: 10.1007/BF01651147. [DOI] [PubMed] [Google Scholar]
- Wilske B., Schierz G., Preac-Mursic V., von Busch K., Kühbeck R., Pfister H. W., Einhäupl K. Intrathecal production of specific antibodies against Borrelia burgdorferi in patients with lymphocytic meningoradiculitis (Bannwarth's syndrome). J Infect Dis. 1986 Feb;153(2):304–314. doi: 10.1093/infdis/153.2.304. [DOI] [PubMed] [Google Scholar]