Abstract
Background
Recent studies have demonstrated a link between the inflammatory response, increased cytokine formation, and neurodegeneration in the brain. The beneficial effects of anti-inflammatory drugs in neurodegenerative diseases, such as Alzheimer's disease (AD), have been documented. Increasing evidence suggests that acetaminophen has unappreciated anti-oxidant and anti-inflammatory properties. The objectives of this study are to determine the effects of acetaminophen on cultured brain neuronal survival and inflammatory factor expression when exposed to oxidative stress.
Methods
Cerebral cortical cultured neurons are pretreated with acetaminophen and then exposed to the superoxide-generating compound menadione (5 μM). Cell survival is assessed by MTT assay and inflammatory protein (tumor necrosis factor alpha, interleukin-1, macrophage inflammatory protein alpha, and RANTES) release quantitated by ELISA. Expression of pro- and anti-apoptotic proteins is assessed by western blots.
Results
Acetaminophen has pro-survival effects on neurons in culture. Menadione, a superoxide releasing oxidant stressor, causes a significant (p < 0.001) increase in neuronal cell death as well as in the release of tumor necrosis factor alpha, interleukin-1, macrophage inflammatory protein alpha, and RANTES from cultured neurons. Pretreatment of neuronal cultures with acetaminophen (50 μM) increases neuronal cell survival and inhibits the expression of these cytokines and chemokines. In addition, we document, for the first time, that acetaminophen increases expression of the anti-apoptotic protein Bcl2 in brain neurons and decreases the menadione-induced elevation of the proapoptotic protein, cleaved caspase 3. We show that blocking acetaminophen-induced expression of Bcl2 reduces the pro-survival effect of the drug.
Conclusion
These data show that acetaminophen has anti-oxidant and anti-inflammatory effects on neurons and suggest a heretofore unappreciated therapeutic potential for this drug in neurodegenerative diseases such as AD that are characterized by oxidant and inflammatory stress.
Background
Considerable evidence implicates neuroinflammation in the pathophysiology of progressive neurodegenerative disorders such as Alzheimer's disease (AD), Parkinson's disease, amyotrophic lateral sclerosis, and multiple sclerosis (MS) [1-3]. A link between increased cytokine formation and neurodegeneration has been demonstrated [4]. The role of non-neuronal cells in the brain, i.e. microglia, astrocytes and endothelial cells, as sources of inflammatory proteins in disorders of the nervous system has been well documented. For example, in AD and Parkinson's disease activated microglia have been identified in the brain regions most affected in these disorders [5]. Astrocytes are an important source of cytokines and chemokines in MS and other diseases of the CNS [6,7]. The cerebral microcirculation of AD patients releases a host of inflammatory proteins including thrombin, tumor necrosis factor-α (TNFα), transforming growth factor-β (TGFβ), interleukin (IL) IL-1β, IL-6, IL-8, macrophage inhibitory protein -1α (MIP-1α) and RANTES [8-11]. In contrast, the role of neurons as a source of inflammatory proteins in the brain has not been examined. A few studies have recently shown that in spinal cord injury all CNS resident cells, including neurons, synthesize and release cytokines [12,13], suggesting that neurons can also be an important source of inflammatory proteins in injury and diseases of the nervous system.
Neurotoxic factors such as amyloid beta (Aβ) evoke oxidative stress and directly injure neurons [14]. The interplay between oxidative stress and inflammatory processes likely contributes to neuronal death in brain injury and disease [15-17]. However, a clear connection between exposure to oxidative stress and release of inflammatory mediators in brain neurons has not been shown. Therapeutic approaches for neurodegenerative disease are focused on reducing oxidative stress and inflammation through diet/life style changes and drug treatment [18-21]. Acetaminophen is a widely used over the counter antipyretic and analgesic drug with unappreciated antioxidant and anti-inflammatory properties. For example, acetaminophen protects hippocampal neurons and PC12 cultures from Aβ peptide-induced oxidative stress through reduction of lipid peroxidation and by lowering cytoplasmic levels of peroxides [22]. Quinolinic acid, a neurotoxic metabolite implicated in the pathogenesis of neurodegenerative disease, is inhibited by administration of acetaminophen [23]. Acetaminophen also protects dopamingeric neurons in vitro from oxidative damage evoked by acute exposure to 6-hydroxydopamine or excessive levels of dopamine [24]. Acetaminophen has been shown to blunt neuronal apoptosis via reduction of the inflammatory transcription factor NF-kappaB [22]. Finally, work from our laboratory also shows that low dose acetaminophen reduces inflammatory protein release from cultured brain endothelial cells exposed to oxidant stress [25].
The objectives of this study are to determine the effects of oxidative stress on the inflammatory response of neurons and whether acetaminophen affects survival and inflammatory protein expression when cultured neurons are exposed to oxidative stress.
Methods
Primary neuronal cultures
Rat cerebral cortical cultures were prepared from cortices of 18-day gestation fetuses, as previously described [26,27]. The cells were seeded on 6-well poly-L-lysine coated plates at a density of 3–5 × 105 cells per ml and incubated in Neurobasal medium containing B-27 supplement, antibiotic/antimycotic, glutamine (0.5 mM) and 5-fluoro-2'-deoxyuridine (20 μg/ml) to inhibit proliferation of glial cells. On day 5, fresh medium without 5-fluoro-2'-deoxyuridine was added. Neuronal cultures were used for experiments after 8–9 days in culture. The primary cerebral cortical cultures utilized were enriched for neurons (>95%). The neuronal identity of the cultures was confirmed using immunofluroescent labeling for beta-3 tubulin, a neuron-specific protein (Promega, Madison, WI) and glial fibrillary acidic protein (Promega).
Assessment of cell viability and apoptosis
Cell viability was assayed using the MTT assay (Promega) as follows. Treatment medium was replaced with fresh treatment medium containing 20 μl/ml of the Cell Titer 96 Aqueous One Solution and incubated for 20 min at 37°C after which optical density was measured at 490 nm using a microplate reader. The quantity of soluble formazan product, as measured by the amount of absorbance, was directly proportional to the number of viable cells. The optical density of untreated controls was set at 100%. The number of viable cells after treatment was determined by measuring optical density and expressing viability as percent of untreated controls.
To assess the effect of acetaminophen-induced Bcl2 expression on neuronal viability, cerebral cortical cultures were incubated with acetaminophen (100 μM) for 32 h. Bcl2 expression was neutralized with 6 μg/ml of anti-human Bcl2 antibody (R & D Systems, Minneapolis, MN) or equivalent amount of mouse IgG for 2 h. The cells were exposed to menadione (5 μM) for 4 h and the effect of the neutralizing antibody on cell survival determined by MTT assay, as described above.
Measurement of cell death by apoptosis was determined using a cell death detection ELISA kit (Roche Diagnostic, Mannheim). Cells were removed from plates, resuspended in lysis buffer and incubated for 30 min at 25°C. The lysates were centrifuged at 200 × g for 10 min and supernatants transferred to streptavidin-coated plates. A mixture of anti-histone-biotin and anti-DNA-peroxidase was added and incubated for 2 h at room temperature. The plates were washed to remove unbound components, and the amount of bound nucleosome determined by spectrophotometer at 405 nm.
Detection of cytokines and chemokines
The cytokines and chemokines MIP-1α (ab9781), RANTES (ab9783), Il-1α (ab1074), IL-1β (ab9787), and TNFα (ab10863) released into the supernatant were quantitated by indirect ELISA. Supernatants (100 μl) collected from cerebral cortical cells after various treatments were coated onto 96-well immulon 2HB (Fisher scientific) flat bottom plates with sodium bicarbonate buffer (0.1 M) and incubated overnight at 4°C. Non-specific sites were blocked with 1% bovine serum albumin solution at 37°C for 45 min. The plates were incubated with primary antibodies (AbCam, Cambridge, MA, dilution 1:1000) in bicarbonate buffer and incubated overnight at 4°C. Following extensive washing of the plate, 200 μl of secondary IgG coupled with horseradish peroxidase (Bio-Rad, Hercules, CA 1/1,000 dilution) was added and incubated for 45 min at 37°C, in the dark. The reaction was developed by adding 200 μl/well of o-phenylene diamine H2O2 (Pierce, Chemicon, CA, USA) for 20 min. Optical density was measured at 450 nm using a microplate ELISA reader (Bio-Rad, Hercules, CA). Samples were assayed in triplicate.
Western blot analysis
Total protein was extracted from neurons using lysis buffer containing 0.1% SDS, 1% Triton X-100 and 0.5% phenylmethyl sulfonylfluoride. Protein was determined by the Bradford method using Bio-Rad protein reagents. Equal amounts of protein were run on a 12% polyacrylamide gel, transferred on to a PVDF membrane, blocked with 5% milk solution (non-fat dry milk in Tris-buffered saline Tween-20) and immuno-blotted with Bcl2 (ab 7973,1:500), cleaved caspase (cell sciences -96645,1:200), and GAPDH (MAB374, 1:1000) primary antibodies and peroxidase-conjugated secondary antibodies. After extensive washing to remove unbound antibodies, membranes were developed with chemiluminescence reagents. Band intensities were quantitified using Quantity One software (Bio-Rad) and graphically expressed as intensity units which reflects the average intensity over the area of the band.
Bcl2 RNA silencing
Primary neuronal cultures were transfected with small interfering RNA (siRNA) for Bcl2 (Sigma-Aldrich, St Louis, MO) or scrambled siRNA (negative control) on day 5 in culture. One hour before transfection, culture media were replaced with media lacking antibiotics or glutamine. Transfection reaction was prepared with 120 pmol/ml of Bcl2 siRNA or scrambled siRNA in 100 μl of Opti-MEM (Invitrogen, Carslbad, CA). Lipofectamine RNAiMAX (Invitrogen, Carslbad, CA) transfection reagent (6 μl) was diluted in 94 μl of OPTI-MEM. The siRNA mixture was slowly added to the lipofectamine and incubated for 20 min at room temperature for complex formation. The neuronal cultures were incubated with the siRNA lipofectamine complex for 6 h, changed to fresh media, and further incubated for 48 h. Bcl2 silencing was detected by RT-PCR. Total RNA extracted from the cells treated with siRNA was reverse transcribed with Bcl2 specific primers and PCR products visualized on a 1.5% agarose gel using UV trans-illumination.
Statistical analysis
Data from each experiment are expressed as mean ± standard deviation (SD). Comparisons between control and treatment groups were conducted using the one-way ANOVA followed by Bonferroni's multiple comparison test for multiple samples. Statistical significance was determined at p < 0.05.
Results
Neuronal survival increases with acetaminophen
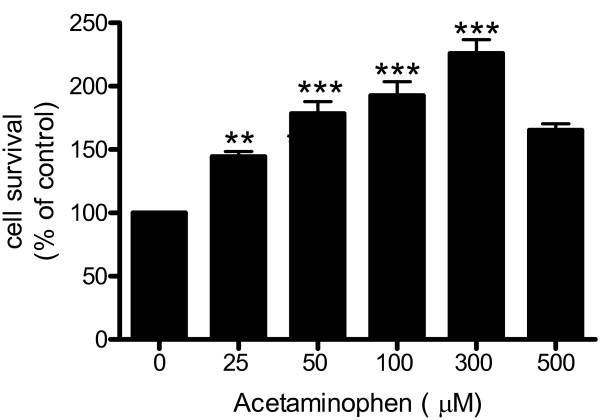
Primary rat neuronal cultures were treated (32 h) with increasing concentrations of acetaminophen (0 – 500 μM) and cell viability assessed by MTT assay. Acetaminophen increased neuronal survival in a dose-dependent manner (Fig. 1). The increase in cell survival was demonstrable at 25 μM (p < 0.01) acetaminophen and highly significant at 50 to 300 μM (p < 0.001).
Figure 1.
Cortical neurons (day 9) were pretreated with acetaminophen (0 – 500 μM) for 32 h. Cell survival was quantitated by MTT assay. The number of control cells, i.e. viable cells not exposed to any treatment, was defined as 100%. Data are mean ± SD from 3 separate experiments. **p < 0.01 vs. control; ***p < 0.001 vs. control.
Acetaminophen protects against neuronal cell death induced by oxidative stress
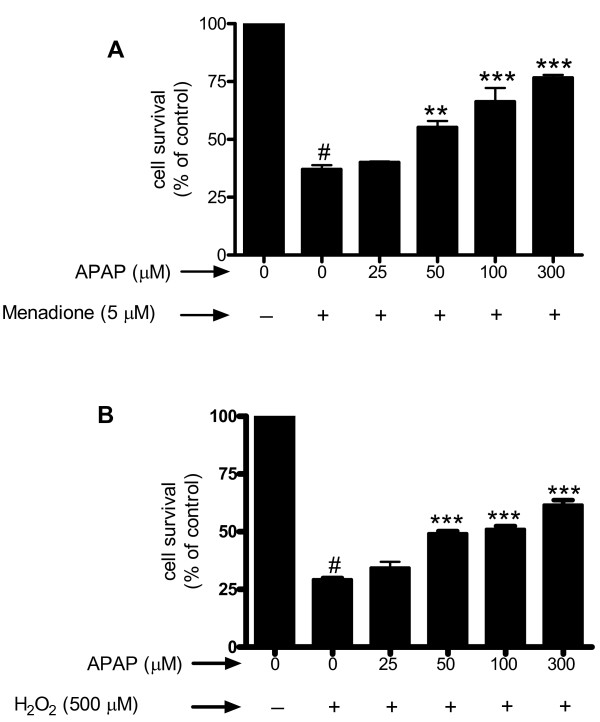
Oxidative stress was induced in cerebral cortical neurons by exposure of cells to either menadione or hydrogen peroxide (H2O2) and cell viability assessed by MTT assay. Treatment of neuronal cultures for 4 h with 5 μM menadione, an agent that releases superoxide [28], evoked marked (63%; p < 0.001) cell death (Fig. 2a). Pretreatment of neurons (32 h) with acetaminophen (50–300 μM), prior to menadione exposure, significantly (p < 0.01 – p < 0.001) increased cell survival. Similarly, treatment of neurons for 4 h with 500 μM H2O2 decreased cell survival by 71% (p < 0.001) and acetaminophen pretreatment was able to significantly (p < 0.001) increase neuronal survival at 50 μM (Fig. 2b).
Figure 2.
Cortical cell cultures were pre-incubated (32 h) with varying doses (0 – 300 μM) of acetaminophen (APAP) for 32 h. The cells were challenged with either menadione (5 μM) (Fig. 2a) or H2O2 (500 μM) (Fig. 2b) for 4 h and cell viability evaluated by MTT assay. Data are mean ± SD from 3 separate experiments. **p < 0.01 vs. menadione alone; ***p < 0.001 vs. menadione or H2O2 alone; #p < 0.001 vs. control.
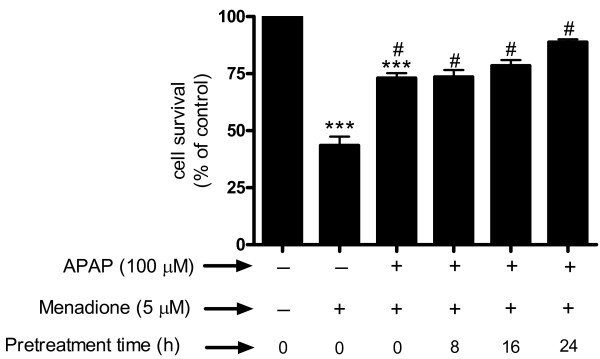
We determined whether pre-incubation with acetaminophen for shorter time periods (8 to 24 h) affected the apoptosis evoked by exposure of neurons to menadione. The results showed that pre-incubation with acetaminophen for 8, 16, or 24 h significantly increased survival compared to menadione alone (Fig. 3). Similarly, co-incubation of acetaminophen with menadione was also capable of increasing cell survival compared to cells treated with menadione alone (p < 0.001) (Fig. 3).
Figure 3.
Cerebral cortical cultures were either co-incubated or pretreated (8, 16, or 24 h) with 100 μM of acetaminophen (APAP) and exposed to 5 μM menadione for 4 h. Apoptosis was measured by ELISA quantitation of nucleosome fragmentation. The number of control cells i.e. viable cells not exposed to any treatment was defined as 100%. Data are mean ± SD from 3 separate experiments. ***p < 0.001 vs. control. #p < 0.001 vs. menadione alone.
Menadione-induced increase in cytokines and chemokines is reduced by acetaminophen
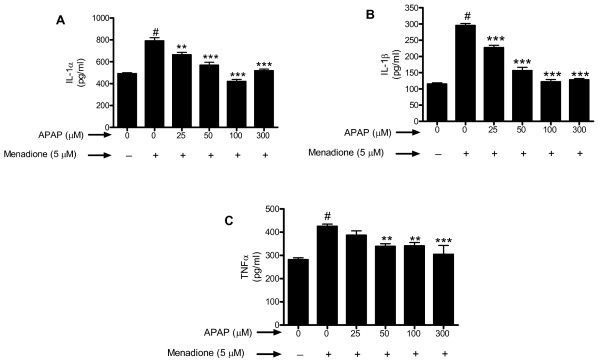
To determine the effect of oxidative stress and acetaminophen on inflammatory protein release from neurons, cerebral cortical cultures were exposed to 5 μM of menadione for 4 h, the supernant collected and assayed by ELISA for the presence of IL-1α, IL-1β, and TNFα. Exposure of neurons to 5 μM menadione resulted in a significant (p < 0.001) increase in release of IL-1α compared to untreated control cells (Fig. 4a). Pre-incubation (32 h) with acetaminophen (25 – 300 μM) significantly (p < 0.01 – 0.001) decreased the release of IL-1α (Fig. 4a).
Figure 4.
Cerebral cortical cultures were pre-incubated for 32 h with acetaminophen (0–300 μM) (APAP) and oxidatively stressed with menadione (5 μM for 4 h). Cytokines (a) IL-1α (b) IL-1β and (c) TNFα released into the supernatants were determined by indirect ELISA. Data are mean ± SD from 3 separate experiments. **p < 0.01 vs. menadione alone; ***p < 0.001 vs. menadione alone; #p < 0.001 vs. control.
Exposure of neuronal cultures to menadione caused an almost 3 fold increase (p < 0.001) in IL-1β release that was significantly (p < 0.001) inhibited by pretreatment (32 h) of cells with acetaminophen (25 – 300 μM) (Fig. 4b). Treatment of neuronal cultures with menadione also caused a significant (p < 0.001) increase in release of TNFα, although the increase was less than that demonstrated for IL-1α or IL-1β (Fig. 4c). Pretreatment of neurons with acetaminophen was able to significantly (p < 0.01 – 0.001) reduce TNFα (Fig. 4c).
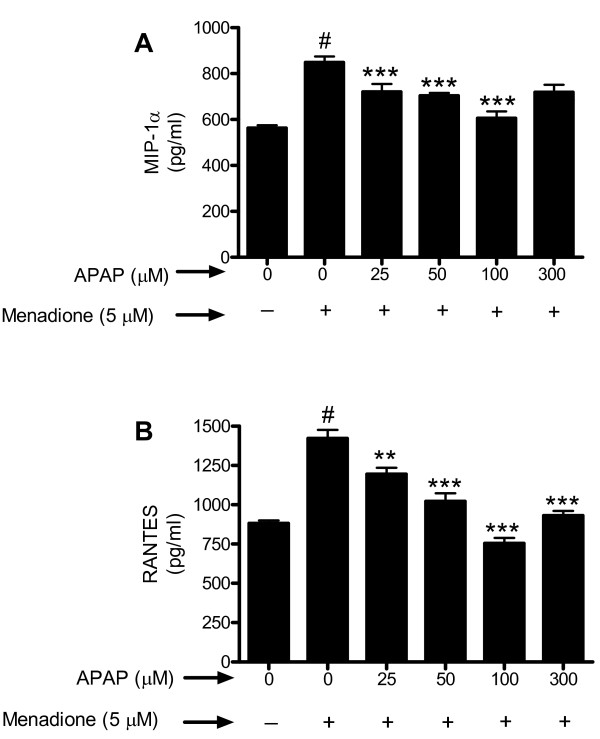
Similar to the results obtained for the cytokines investigated, we showed that menadione caused a significant increase in release of chemokines MIP-1α (Fig. 5a) and RANTES (Fig. 5b). Pretreatment (32 h) of neuronal cultures with acetaminophen (25 – 300 μM) significantly (p < 0.01–0.001) reduced release of both chemokines.
Figure 5.
Cerebral cortical cultures were pre-incubated (32 h) with acetaminophen (0–300 μM) (APAP) and oxidatively stressed with menadione (5 μM for 4 h). Chemokines (a) MIP-1α and (b) RANTES released into the supernatant were determined by indirect ELISA. Data are mean ± SD from 3 separate experiments. #p < 0.001 vs. control; **p < 0.01 vs. menadione alone; ***p < 0.001 vs. menadione alone.
Acetaminophen affects expression of pro and antiapototic proteins in cultured neurons
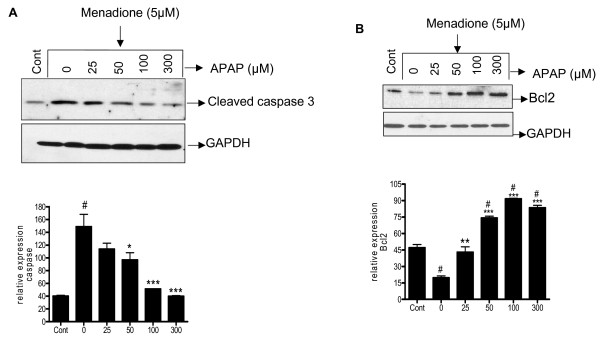
The ability of acetaminophen to affect neuronal apoptosis was assessed by measuring the relative expression of pro- and anti-apoptotic proteins by western blot analysis. Untreated cells express a basal level of pro-apoptotic protein cleaved caspase 3 which increased significantly (p < 0.001) with 5 μM of menadione (Fig. 6a). Acetaminophen significantly (p < 0.05 – 0.001) blunts the expression of this protein at 50 – 300 μM (Fig. 6a).
Figure 6.
Cortical neuron cultures were pre-incubated (32 h) with acetaminophen (0–300 μM) (APAP) and oxidatively stressed with menadione (5 μM for 4 h). Total protein was extracted and western blot analysis performed using specific antibodies for cleaved caspase3 (Fig. 5a) and Bcl2 (Fig. 5b). Blots probed with GAPDH were used to confirm loading equivalency. Relative expression determined from densitometric scans from 3 separate experiments are shown below each blot. #p < 0.001 vs. control; *p < 0.05 vs. 0 μM APAP; **p < 0.01 vs. 0 μM APAP; ***p < 0.001 vs. 0 μM APAP.
Exposure of neurons to menadione (5 μM, 4 h) caused a significant (p < 0.001) down regulation of the anti-apoptotic protein Bcl2. Pre-treatment (32 h) of neurons with acetaminophen (50–300 μM) significantly (p < 0.01–0.001) increased expression of Bcl2 (Fig. 6b).
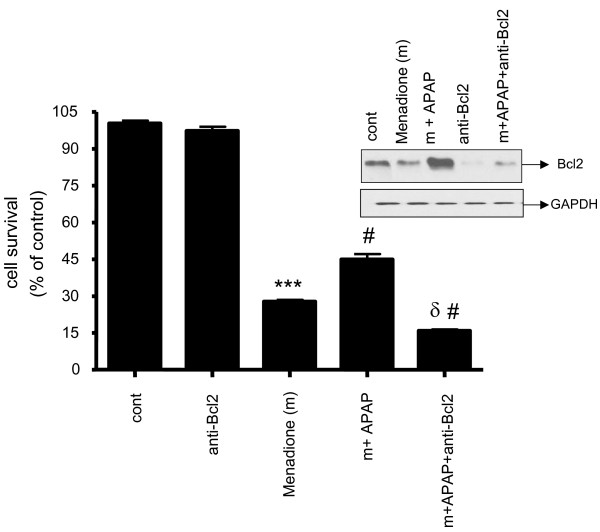
Using neutralizing antibodies to Bcl2 we determined that inhibition of Bcl2 expression prevented the increase in neuronal survival evoked by acetaminophen, suggesting that part of the neuroprotective effect of acetaminophen against menadione injury is mediated by an increase in Bcl2 (Fig. 7).
Figure 7.
Primary cortical neurons were incubated with 100 μM of APAP (32 h) and neutralizing Bcl2 antibody for 2 h and then exposed to oxidative stress (5 μM menadione for 4 h). Cell survival assayed with MTT reagent. In each experiment, the number of control cells i.e. viable cells not exposed to any treatment, was defined as 100%. Inset shows the neutralization of protein expression of Bcl2 with neutralizing anti-human Bcl2 antibody. *** p < 0.001 vs. control. # p < 0.01 vs. menadione. δ p < 0.001 vs. m+APAP (menadione + APAP).
We also examined whether silencing Bcl2 with siRNA had any affect on cytokine and chemokine expression. Our data showed that treatment of neuronal cultures with Bcl2 siRNA significantly (p < 0.05) increased release of TNFα (31%) and MIP-1α (26%) compared to untreated cultures. Bcl2 silencing did not affect the expression of IL-1β, IL-1α, and RANTES (data not shown).
Discussion
In the current study we document that oxidative stress causes release of inflammatory cytokines and chemokines from neurons. A large body of data suggests that communication between oxidative and inflammatory processes drive a deleterious "feed-forward" cycle that results in neuronal cell death [15-17]. This interaction may be especially relevant for understanding the pathogenesis of neurodegenerative diseases. The brain is very susceptible to oxidative stress, in particular the hippocampus, which has limited reactive oxygen species (ROS) scavengers and anti-oxidant capacity [29]. Recent studies demonstrate important neuroprotective effects of acetaminophen that are, in part, mediated by antioxidant actions. Acetaminophen inhibits the formation of the Parkinson's disease toxin 1-methyl-4-phenylpridinium in mitochondria [30,31]. Administration of acetaminophen to rats significantly attenuates superoxide production by the neurotoxin quinolinic acid [23]. Acetaminophen has also been shown to be a potent scavenger of peroxynitrite [32]. In a study utilizing C. elegans as a model system to study neuroprotective effects of various agents, acetaminophen is able to significantly protect dopaminergic neurons from oxidative stress [24]. Taken together, these data are consistent with the results of the current study showing that acetaminophen significantly increases survival of neurons exposed to the oxidant stressor menadione.
Acetaminophen dosage is critical to determining whether the drug evokes toxic or beneficial effects. High doses of acetaminophen have traditionally been associated with necrosis [33,34]. Intake of large doses of acetaminophen (300 mg/kg) results in severe hepatic necrosis [33]. In the brain, overdose of acetaminophen (3 g/kg) causes a dramatic decrease of glutathione levels, ascorbic acid levels and superoxide dismutase activity [35]. High dose acetaminophen (300 mg/kg) can evoke apoptosis via activation of the C-jun N-terminal kinase (JNK) pathway [36]. In contrast, low dose (350 μM) acetaminophen appears to protect cardiac myocytes exposed to reperfusion injury via inhibition of the mitochondrial permeability transition pore and subsequent apoptotic pathway [37]. In the current study we show that acetaminophen at a very low dose, (50 μM or 8 μg/ml) well below toxic dose levels, protects neurons exposed to oxidative stress. The molecular mechanisms of action of acetaminophen are controversial and still poorly understood. Here we document that pretreatment of neurons with acetaminophen increases expression of Bcl2 and decreases cleaved caspase 3 levels. Thus, the ability of acetaminophen to affect both pro- and anti-apoptotic proteins in neurons may represent another mechanism for acetaminophen's beneficial effects.
The relevance of inflammatory processes to neuron dysfunction, pathology and development of neurodegenerative disease is supported by animal studies showing that chronic infusion of the pro-inflammatory protein lipopolysaccharide into the fourth ventricle of the rat brain reproduces many of the pathological changes observed in the AD brain [38]. Administration of NSAID drugs at low concentration for extensive period of time has been shown to reduce neuroinflammation [39,40]. Studies have shown that NSAID's may protect against Alzheimer's by regulating the expression of inflammatory proteins [41]. Despite studies showing that long-term use of NSAIDs is associated with a lower incidence of AD, clinical trials using NSAIDs have been ineffective at improving cognitive function [42]. It is possible that drugs such as NSAIDs that affect primarily or only inflammatory mediators may not provide neuroprotection and that to be neuroprotective therapeutics must target both oxidant and inflammatory pathways.
Acetaminophen has traditionally not been classified as a non-steroidal anti-inflammatory drug (NSAID) because of its weak anti-inflammatory properties. The conclusion that acetaminophen is a weak inhibitor of prostaglandin (PG) synthesis, and thus inflammation, is based on studies in which prostaglandin synthesis is measured in homogenized tissues. However, in cellular systems acetaminophen can inhibit prostaglandin endoperoxide H2 synthase (PGHS) with IC50 values in the range of 4–200 μM [32,43,44]. In addition, as we document in the current study acetaminophen is able to reduce release of inflammatory proteins from neurons. The idea that acetaminophen has beneficial effects in the brain is supported by a recent study showing administration of acetaminophen improves cognitive performance of rodents in the Morris water maze test [45]. The combination of acetaminophen's anti-oxidant and anti-inflammatory properties may render it a very efficacious drug for CNS diseases that are characterized by both oxidant and inflammatory stress.
Conclusion
In the present study we demonstrate that acetaminophen has pro-survival effects on neurons in culture. Pretreatment of cultured brain neurons with acetaminophen significantly increases survival of cells exposed to the oxidant stressor menadione. Also, treatment of cultured neurons with menadione causes release of several inflammatory proteins including RANTES, MIP-1α, TNFα, IL-1α and IL-1β that is diminished by pretreatment of neurons with acetaminophen. We document, for the first time, that acetaminophen increases expression of the anti-apoptotic protein Bcl2 in brain neurons and decreases the elevation of the pro-apoptotic protein caspase 3 evoked by menadione. We show that blocking acetaminophen-induced expression of Bcl2 reduces the pro-survival effect of the drug. These data show that acetaminophen has anti-oxidant and anti-inflammatory effects on neurons and suggest a heretofore unappreciated therapeutic potential for this drug in neurodegenerative diseases such as AD that are characterized by oxidant and inflammatory stress.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
PG conceived the study, participated in its design and coordination, analyzed the data and drafted the manuscript. DT maintained cell cultures and performed biochemical experiments. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
Sources of support: This work was supported in part by grants from the National Institutes of Health (AG15964, AG020569 and AG028367) and McNeil Consumer and Specialty Pharmaceuticals. Dr. Grammas is the recipient of the Shirley and Mildred Garrison Chair in Aging. The authors gratefully acknowledge the secretarial assistance of Terri Stahl.
Contributor Information
Debjani Tripathy, Email: debjani.tripathy@ttuhsc.edu.
Paula Grammas, Email: paula.grammas@ttuhsc.edu.
References
- Skaper SD. The brain as a target for inflammatory processes and neuroprotective strategies. Ann NY Acad Sci. 2007;1122:23–34. doi: 10.1196/annals.1403.002. [DOI] [PubMed] [Google Scholar]
- Infante-Duarte C, Waiczies S, Wuerfel J, Zipp F. New developments in understanding and treating neuroinflammation. J Mol Med. 2008;86:975–985. doi: 10.1007/s00109-007-0292-0. [DOI] [PubMed] [Google Scholar]
- Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29:357–365. doi: 10.1016/j.it.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatsu T, Mogi M, Ichinose H, Togari A. Cytokines in Parkinson's disease. J Neural Transm Suppl. 2000;58:143–151. [PubMed] [Google Scholar]
- Shie FS, Woltjer RL. Manipulation of microglial activation as a therapeutic strategy in Alzheimer's disease. Curr Med Chem. 2007;14:2865–2871. doi: 10.2174/092986707782359981. [DOI] [PubMed] [Google Scholar]
- Nair A, Frederick TJ, Miller SD. Astrocytes in multiple sclerosis: a product of their environment. Cell Mol Life Sci. 2008;65:2702–2720. doi: 10.1007/s00018-008-8059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones MP, Kalkonde Y, Estrada CA, Jimenez F, Ramirez R, Mahimainathan L, Mummidi S, Choudhury GG, Martinez H, Adams L, Mack M, Reddick RL, Maffi S, Haralambous S, Probert L, Ahuja SK, Ahuja SS. Role of astrocytes and chemokine systems in acute TNFalpha induced demyelinating syndrome: CCR2-dependent signals promote astrocyte activation and survival via NF-kappaB Akt. Mol Cell Neurosci. 2008;37:96–109. doi: 10.1016/j.mcn.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammas P, Ovase R. Inflammatory factors are elevated in brain microvessels in Alzheimer's disease. Neurobiol Aging. 2001;22:837–842. doi: 10.1016/s0197-4580(01)00276-7. [DOI] [PubMed] [Google Scholar]
- Grammas P, Ovase R. Cerebrovascular TGF-β contributes to inflammation in the Alzheimer's brain. Am J Pathol. 2002;160:1583–1587. doi: 10.1016/s0002-9440(10)61105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy D, Grammas P. Expression of macrophage inflammatory protein 1-alpha is elevated in Alzheimer's vessels and is regulated by oxidative stress. J Alzheimers Dis. 2007;11:447–455. doi: 10.3233/jad-2007-11405. [DOI] [PubMed] [Google Scholar]
- Tripathy D, Grammas P. RANTES upregulation in the Alzheimer's disease brain: A possible neuroprotective role. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineau I, Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: mulitphasic expression pattern and identification of cell types involved. J Comp Neurol. 2007;500:267–285. doi: 10.1002/cne.21149. [DOI] [PubMed] [Google Scholar]
- Miao P, Madec K, Gong Y, Shen H, Eisenstat D, Melanson M, Gu X, Leong C, Klowak M, Namaka M. Axotomy-induced up-regulation of tumor necrosis factor-alpha in the dorsal root ganglia. Neurol Res. 2008;30:623–631. doi: 10.1179/174313208X289606. [DOI] [PubMed] [Google Scholar]
- Butterfield DA. Amyloid beta-peptide (1–42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer's disease brain. A review. Free Radic Res. 2002;36:1307–1313. doi: 10.1080/1071576021000049890. [DOI] [PubMed] [Google Scholar]
- Reynolds A, Laurie C, Mosley RL, Gendelman HE. Oxidative stress and the pathogenesis of neurodegenerative disorders. Int Rev Neurobiol. 2007;82:297–325. doi: 10.1016/S0074-7742(07)82016-2. [DOI] [PubMed] [Google Scholar]
- Wong Ch, Crack PJ. Modulation of neuro-inflammation and vascular response by oxidative stress following cerebral ischemia-reperfusion injury. Curr Med Chem. 2008;15:1–14. doi: 10.2174/092986708783330665. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Raudensky J. The role of oxidative stress, metabolic compromise, and inflammation in neuronal injury produced by amphetamine-related drugs of abuse. J Neuroimmune Pharmacol. 2008;3:203–217. doi: 10.1007/s11481-008-9121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JY, wen LL, Huang YN, Chen YT, Ku MC. Dual effects of antioxidants in neurodegeneration: direct neuroprotection against oxidative stress and indirect protection via suppression of glia-mediated inflammation. Curr Pharm Des. 2006;12:3521–3533. doi: 10.2174/138161206778343109. [DOI] [PubMed] [Google Scholar]
- Steele M, Stuchbury G, Munch G. The molecular basis of the prevention of Alzheimer's disease through healthy nutrition. Exp Gerontol. 2007;42:28–36. doi: 10.1016/j.exger.2006.06.002. [DOI] [PubMed] [Google Scholar]
- DeLegge MH, Smoke A. Neurodegeneration and inflammation. Nutr Clin Pract. 2008;23:35–41. doi: 10.1177/011542650802300135. [DOI] [PubMed] [Google Scholar]
- Golden TR, Patel M. Catalytic antioxidants and neurodegeneration. Antioxid Redox Signal. 2008 doi: 10.1089/ars.2008.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaglia M, Venezia V, Piccioli P, Stanzione S, Porcile C, Russo C, Mancini F, Milanese C, Schettini G. Acetaminophen protects hippocampal neurons and PC12 cultures from amyloid beta-peptide induced oxidative stress and reduces NF-kappaB activation. Neurochem Int. 2002;41:43–54. doi: 10.1016/s0197-0186(01)00136-x. [DOI] [PubMed] [Google Scholar]
- Maharaj H, Maharaj S, Daya S. Acetylsalicylic acid and acetaminophen protect against oxidative neurotoxicity. Metab Brain Dis. 2006;21:189–199. doi: 10.1007/s11011-006-9012-7. [DOI] [PubMed] [Google Scholar]
- Locke CJ, Fox SA, Caldwell GA, Caldwell KA. Acetaminophen attenuates dopamine neuron degeneration in animal models of Parkinson's disease. Neurosci Lett. 2008;439:129–133. doi: 10.1016/j.neulet.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Tripathy D, Grammas P. Acetaminophen protects brain endothelial cells against oxidative stress. Microvasc Res. 2009 doi: 10.1016/j.mvr.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammas P, Moore P, Weigel PH. Microvessels from Alzheimer's disease brains kill neurons in vitro. Am J Pathol. 1999;154:337–342. doi: 10.1016/S0002-9440(10)65280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirumangalakudi L, Yin L, Rao HV, Grammas P. IL-8 induces expression of matrix metalloproteinases, cell cycle and pro-apoptotic proteins, and cell death in cultured neurons. J Alzheimers Dis. 2007;11:305–311. doi: 10.3233/jad-2007-11307. [DOI] [PubMed] [Google Scholar]
- Warren MC, Bump EA, Medeiros D, Braunhut SJ. Oxidative stress-induced apoptosis of endothelial cells. Free Radic Biol Med. 2000;29:537–547. doi: 10.1016/s0891-5849(00)00353-1. [DOI] [PubMed] [Google Scholar]
- Reiter RJ. Oxidative processes and antioxidative defense mechanisms in the aging brain. FASEB J. 1995;9:526–533. [PubMed] [Google Scholar]
- Maharaj DS, Saravanan KS, Maharaj H, Mohanakumar KP, Daya S. Acetaminophen and aspirin inhibit superoxide anion generation and lipid peroxidation, and protect against 1-methyl-4-phenyl pyridium-induced dopaminergic neurotoxicity in rats. Neurochem Int. 2004;44:355–360. doi: 10.1016/s0197-0186(03)00170-0. [DOI] [PubMed] [Google Scholar]
- Maharaj H, Maharaj DS, Saravanan KS, Mohanakumar KP, Daya S. Aspirin curtails the acetaminophen-induced rise in brain norepinephrine levels. Metab Brain Dis. 2004;19:71–77. doi: 10.1023/b:mebr.0000027418.33772.8b. [DOI] [PubMed] [Google Scholar]
- Schildknecht S, Daiber A, Ghisla S, Cohen RA, Bachschmid MM. Acetaminophen inhibits prostanoid synthesis by scavenging the PGHS-activator peroxynitrite. FASEB J. 2008;22:215–224. doi: 10.1096/fj.06-8015com. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci. 2002;67:322–328. doi: 10.1093/toxsci/67.2.322. [DOI] [PubMed] [Google Scholar]
- James LP, Mayeux PR, Hinson JA. Acetaminophen-induced hepatoxicity. Drug Metab Dispos. 2003;31:1499–1506. doi: 10.1124/dmd.31.12.1499. [DOI] [PubMed] [Google Scholar]
- Nencini C, Giorgi G, Micheli L. Protective effect of silymarin on oxidative stress in rat brain. Phytomedicine. 2007;14:129–135. doi: 10.1016/j.phymed.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Maeda S, Hikiba Y, Ohmae T, Shibata W, Yanai A, Sakamoto K, Ogura K, Nogushci T, Karin M, Ichijo H, Omata M. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting C-Jun N-terminal kinase activation. Gastroenterology. 2008;135:1311–1321. doi: 10.1053/j.gastro.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Hadzimichalis NM, Baliga SS, Golfetti R, Jaques KM, Firestein BL, Merrill GF. Acetaminophen-mediated cardioprotection via inhibition of the mitochondrial permeability transition pore-induced apoptotic pathway. Am J Physiol Heart Circ Physiol. 2007;293:H3348–H3355. doi: 10.1152/ajpheart.00947.2007. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Lukovic L, Bigaud M, Stoeckel ME. Brain inflammatory response induced by intracerebroventricular infusion of lipopolysaccharide: an immunohistochemical study. Brain Res. 1998;794:211–224. doi: 10.1016/s0006-8993(98)00227-3. [DOI] [PubMed] [Google Scholar]
- Rich JB, Rasmusson DX, Folstein MF, Carson KA, Kawas C, Brandt J. Nonsteroidal anti-inflammatory drugs in Alzheimer's disease. Neurology. 1995;45:51–55. doi: 10.1212/wnl.45.1.51. [DOI] [PubMed] [Google Scholar]
- Nilsson SE, Johansson B, Takkinen S, Berg S, Zarit S, McClearn G, Melander A. Does aspirin protect against Alzheimer's dementia? A study in a Swedish population-based sample aged > or = 80 years. Eur J Clin Pharmacol. 2003;59:313–319. doi: 10.1007/s00228-003-0618-y. [DOI] [PubMed] [Google Scholar]
- Zandi PP, Breitner JC. Do NSAIDs prevent Alzheimer's disease? And, if so, why? The epidemiological evidence. Neurobiol Aging. 2001;22:811–817. doi: 10.1016/s0197-4580(01)00297-4. [DOI] [PubMed] [Google Scholar]
- ADAPT Research Group. Martin BK, Szekely C, Brandt J, Piantadosi S, Breitner JC, Craft S, Evans D, Green R, Mullan M. Cognitive function over time in the Alzheimer's Disease Anti-inflammatory Prevention Trial (ADAPT): results of randomized, controlled trial of naproxen and celecoxib. Arch Neurol. 2008;65:896–905. doi: 10.1001/archneur.2008.65.7.nct70006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham GG, Day RO, Milligan MK, Ziegler JB, Kettle AJ. Current concepts of the actions of paracetamol (acetaminophen) and NSAIDs. Inflammopharmacology. 1999;7:255–263. doi: 10.1007/s10787-999-0008-x. [DOI] [PubMed] [Google Scholar]
- Kis B, Snipes JA, Simandle SA, Busija DW. Acetaminophen-sensitive prostaglandin production in rat cerebral endothelial cells. Am J Physiol Regul Integr Comp Physiol. 2005;288:R897–R902. doi: 10.1152/ajpregu.00613.2004. [DOI] [PubMed] [Google Scholar]
- Ishida T, Sato T, Irifune M, Tanaka K, Nakamura N, Nishikawa T. Effect of acetaminophen, a cyclooxygenase inhibitor, on Morris water maze task performance in mice. J Psychopharmacol. 2007;21:757–767. doi: 10.1177/0269881107076369. [DOI] [PubMed] [Google Scholar]