Abstract
BACKGROUND
C-reactive protein (CRP), the prototypic marker of inflammation, is present in atherosclerotic plaques and appears to promote atherogenesis. Also, CRP has been localized to monocytes and tissue macrophages, which are present in the necrotic core of lesions prone to plaque rupture. Leukocyte-derived myeloperoxidase (MPO), primarily hosted in human polymorphonuclear cells (PMNs), has also been shown to be present in human atherosclerotic lesions. Because MPO and CRP concentrations are increased in acute coronary syndrome (ACS) patients and predict poor outcomes, we tested the effect of CRP on MPO release from PMNs and monocytes.
METHODS
We treated human PMNs and monocytes with CRP (25 and 50 mg/L for 6 h) and measured MPO release as total mass and activity in culture supernatants. We also measured nitro-tyrosinylation (NO2-Tyr) of LDL as an indicator of biological activity of CRP-mediated MPO release. Furthermore, we explored the effect of human CRP on MPO release in the rat sterile pouch model.
RESULTS
CRP treatment significantly increased release of MPO (both mass and activity) from human PMNs as well as monocytes (P < 0.05) and caused NO2-Tyr of LDL. Human CRP injection in rats resulted in increased concentrations of MPO in pouch exudates (P < 0.05), thus confirming our in vitro data.
CONCLUSIONS
CRP stimulates MPO release both in vitro and in vivo, providing further cogent data for the proinflammatory effect of CRP. These results might further support the role of CRP in ACS.
C-reactive protein (CRP), the prototypic marker of inflammation, has been shown in numerous studies to predict cardiovascular events (1). CRP induces oxidative stress via superoxide production in various cells involved in the process of atherosclerosis (1, 2). Myeloperoxidase (MPO) is a leukocyte-derived enzyme that catalyzes the formation of a number of reactive oxidant species (3). MPO, primarily hosted in human polymorphonuclear cells (PMNs) (approximately 5% of total proteins), has also been localized to monocytes (approximately 1% of total proteins), and tissue macrophages [reviewed in (3)]. Increased MPO in blood and leukocytes is associated with coronary artery disease and acute coronary syndrome (ACS) (4). Widespread activation of PMNs has been defined in patients with coronary artery disease (5). Previously, pentameric CRP has been reported to modulate the activity of neutrophils (6); however, no data exist indicating an effect of CRP on MPO release in monocytes or in vivo model systems. Thus, we tested the effect of CRP on MPO release from leukocytes both in vitro and in an in vivo system. Additionally, because MPO is expressed in human atherosclerotic lesions, MPO-derived NO2 has emerged as an important source of oxidation and nitro-tyrosinylation (NO2-Tyr) formation in LDL (7). We also tested whether CRP-mediated MPO release results in nitration of LDL.
Development of a valid animal model to test the effects of human CRP (hCRP) has been an important topic of investigation. Pepys and coworkers reported that hCRP administration in rats promotes myocardial as well as cerebral infarcts and have furthermore validated the rat as an appropriate model to test the effect of hCRP by using small-molecule inhibitors of CRP (8). Also, we reported recently that hCRP promotes oxidized-LDL uptake and matrix metalloproteinase-9 release in peritoneal and pouch macrophages in Wistar rats (9). Based on these data, we explored the effect of hCRP on MPO release in vivo in rat pouch exudates.
Human CRP was purified from human ascitic/pleural fluids as described (9). The pentameric configuration of CRP was confirmed by running CRP on nonreducing gel, which showed a single band of 118 kDa. Recently we have shown that our in-house purified, dialyzed CRP mediates its inflammatory effects in toll-like receptor–4 knocked-down cells, providing cogent data that CRP-mediated effects are not due to endotoxin contamination (10). Heparinized blood was used for the isolation of PMNs and monocytes, according to the protocol approved by the institutional review board of the University of California, Davis, by using a 1-step dextran-sodium metrizoate density gradient (11). After centrifugation, 2 distinct layers were obtained. The upper layer consisted of peripheral blood mononuclear cells and the lower layer of PMNs. Both layers were aspirated separately and washed. Approximately 85%–90% of the cells in the lower fraction were found to be neutrophils by differential leukocyte counting. Monocytes were isolated from peripheral blood mononuclear cells by magnetic cell sorting using the negative separation technique (Miltenyi Biotech) (12). Human PMNs and monocytes were separately incubated with CRP (0, 25, and 50 mg/L) for 6 h, and then the culture supernatants were harvested and stored at −70 °C. The cells were lysed for protein measurement. MPO release in culture medium was measured as total mass (Oxis Search) and functional activity (Calbiochem) by commercial ELISA kits. All results were expressed per milligram cell protein. We also investigated whether CRP-mediated MPO release results in nitration of apoB100 of human LDL (200 mg/L) isolated from healthy volunteers and incubated with PMNs. The cells were pretreated with 4-aminobenzoyl hydrazide (ABAH, 20 μmol/L), an irreversible and specific inhibitor of MPO (13), 1 h before CRP treatment to verify MPO-specific effects. ApoB100 was immunoprecipitated from culture medium using antihuman apoB100 (1 mg/L) and protein A Sepharose followed by western blotting and probing for NO2-Tyr using rabbit antihuman NO2-Tyr anti-body (Santa Cruz Biotech). The blot was also probed for apoB100 (Gen Way Biotech) which served as a loading control. We further tested the effect of hCRP on MPO release in Wistar rats using a sterile air-pouch model. The sterile air pouches were formed on the dorsal surface of the rats by use of a slight modification of an established protocol (14) that has been very recently published and validated by us to examine proinflammatory effects of hCRP in rats in vivo (9). hCRP/human serum albumin (25 mg/L intrapouch) was directly injected into the pouch cavities of rats on day 3 after pouch formation, and the rats were killed on the following day. We used the washouts of the pouch for the measurement of rat MPO with a mouse MPO ELISA kit (Hycult Biotechnology), which has cross-reactivity with rat MPO. All experiments were performed at least 3 times in duplicate. The comparisons between group means were analyzed with ANOVA. The experimental results are presented as the mean (SE). Paired t-tests were used to compute differences in the variables, and the level of significance was set at P < 0.05.
Both monocytes and PMNs treated with CRP (25 and 50 mg/L) exhibited significantly increased MPO release compared to controls (Table 1a). Although MPO (mass as well as activity) release in monocytes and PMNs was first seen at 3 h, a significant increase in MPO release was seen at 6 h following CRP treatment. Importantly, the biological activity of CRP for MPO release was as potent as its release from PMNs with lipopolysaccharide (1 mg/L), which is a known activator of leukocytes (Table 1a). Furthermore, MPO activity in the culture supernatants was significantly decreased in cells pretreated with ABAH before CRP exposure (Table 1b). There was increased NO2-Tyr of apoB100 of LDL incubated with PMNs with CRP treatment (Fig. 1A). ABAH pretreatment of PMNs before CRP exposure resulted in decreased NO2-Tyr of apoB100, suggesting that nitration of proteins was specifically due to CRP-mediated MPO release. Importantly, the injection of hCRP compared to human serum albumin (as a control protein) resulted in a significant increase in MPO release in pouch exudates in vivo (Fig. 1B).
Table 1.
Effects on MPO.
| a. MPO release from human PMNs and monocytes following CRP and LPSa treatment. | |||||||
|---|---|---|---|---|---|---|---|
| MPO mass, ng/mg protein |
MPO activity, ng/mg protein |
||||||
| Cell type | Control | CRP, 25 mg/L | CRP, 50 mg/L | Control | CRP, 25 mg/L | CRP, 50 mg/L | LPS 1, mg/L |
| Monocytes | 61 (22) | 118 (27)b | 157 (32)c | 95 (10) | 198 (17)b | 273 (56)c | ND |
| PMNs | 225 (23) | 446 (31) | 563 (77)c | 410 (34) | 723 (78)b | 898 (98)c | 821 (86)b |
| b. Effect of MPO inhibitor (ABAH) on MPO release from human PMNs following CRP treatment | ||
|---|---|---|
| MPO activity, ng/mg protein | ||
| Control | CRP, 25 mg/L | ABAH 3 CRP, 25 mg/L |
| 394 (56) | 842 (107)b | 444 (92)c |
LPS, lipopolysacharide.
P < 0.05 compared to control.
P < 0.03 compared to control.
Fig. 1. (A), Effect of CRP treatment on nitro-tyrosinylation of apoB100 of LDL (200 mg/L) incubated with PMNs in the presence or absence of MPO specific inhibitor (ABAH; 20 μmol/L).
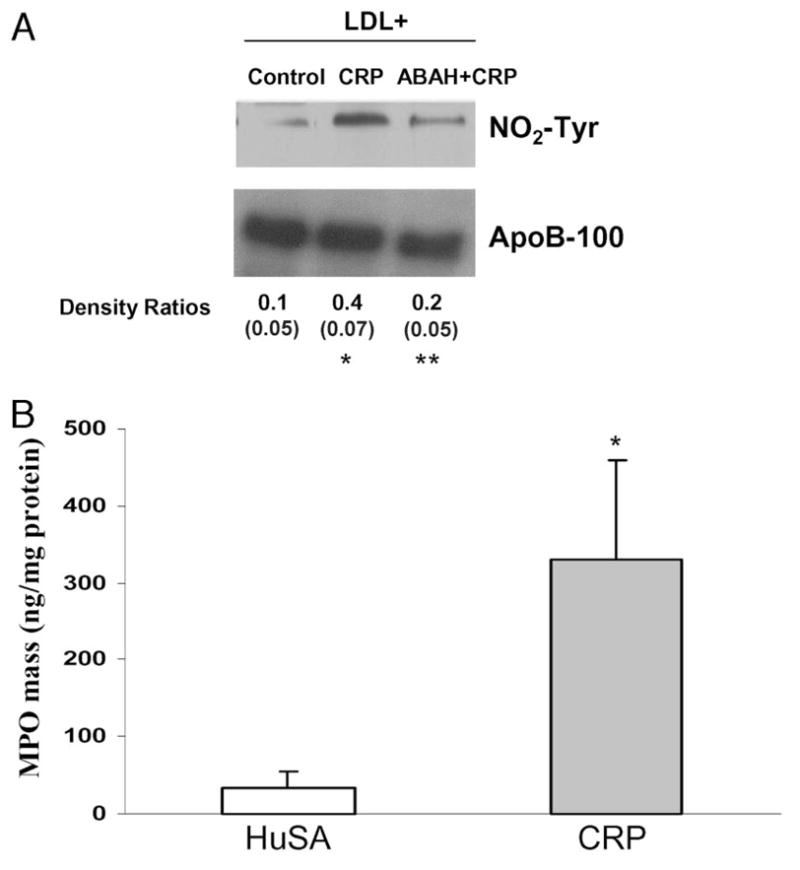
ApoB100 was immunoprecipitated from culture supernatants of PMNs as detailed in the Methods. Immunoprecipitates were electrophoresed and blotted for NO2-Tyr. The blot was also probed for apoB100 to ensure equal loading in each lane. The density ratio is shown for NO2-Tyr/ApoB100. *P < 0.05 compared to control; **P < 0.05 compared to CRP. The gel is representative of 3 different experiments. (B), Effect of hCRP/human serum albumin (HuSA) injection (n = 6 rats per group) (250 μg protein in approximately 5 mL pouch volume; approximately 25 mg/L) in the pouch cavities of Wistar rats on day 3 after the formation of pouch. The rats were killed on the following day. Pouch fluid was aspirated and used for MPO measurement as described in Methods. The results are mean (SE) and are expressed per milligram cell protein. *P < 0.05 compared to HuSA.
Various clinical investigations support the concept that high concentrations of serum CRP are associated with increased risk for CVD (1). Importantly, the colocalization of CRP and macrophages has been demonstrated in advanced human atherosclerotic plaques, (15) and infiltration of PMNs into culprit lesions has been found in ACS (16). Among patients with established ACS, increased MPO concentrations are associated with worse clinical outcomes (4), and on this basis MPO is attractive as an indicator of both prognosis and therapeutic intervention in CVD. Because CRP and MPO have both been reported as powerful markers for coronary artery disease/ACS and both predict poorer outcome at higher concentrations, we hypothesized that CRP induces MPO release from leukocytes, a characteristic that might support its role in ACS. In the present study, CRP induced the release of MPO from human PMNs and monocytes at CRP concentrations ≥25 mg/L. Importantly, CRP concentrations up to 50 mg/L in patients with myocardial infarction have also been reported by numerous investigators (17). Thus, the concentrations of CRP shown to induce MPO release in the current study can clearly be attained in patients, and CRP appears to be a mediator for leukocyte activation and plaque destabilization. Monomeric CRP has been reported to induce IL-8 from human neutrophils (18); however, we report MPO release from human leukocytes with pentameric CRP. Our results are consistent with an earlier report (6). Importantly, increased plasma concentrations of NO2-Tyr are also associated with the presence of CAD (7). Our group and other investigators have previously reported increased nitration of prostacyclin synthase in endothelial cells incubated with CRP [reviewed in (1)]. In this study, we demonstrated increased NO2-Tyr of apoB100 from PMNs treated with CRP compared to control cells. Importantly, Xu et al. (19) have reported that hypochlorous acid treatment of endothelial cells caused nitration of cellular proteins. We further demonstrated that the effect was specifically due to CRP-mediated MPO release, because ABAH pretreatment reversed CRP-mediated NO2-Tyr of apoB100. Importantly, ABAH has been reported to be a specific and irreversible inhibitor of MPO (13), resulting in loss of its activity. In the present investigation, the evidence for inactivation of MPO was confirmed by our results that MPO activity was significantly decreased in culture supernatants from cells treated with ABAH before CRP treatment compared with those from cells treated with CRP alone. Because our in vitro model involved PMNs and monocytes, our use of an in vivo rat sterile pouch model was an ideal choice to confirm our in vitro findings. Importantly, pouch cellular exudates have been shown to have recruitment of PMNs within 3 or 6 h of particular exposure and maintain a relatively constant level through 24 h (14). Our data reveal that results of injection of hCRP were comparable to those of human serum albumin–induced MPO release in pouch exudates of rats. In conclusion, we report the important observation that CRP stimulates MPO release both in vitro and in vivo. This finding could have implications in ACS patients, because higher concentrations of CRP as well as MPO portend a poor prognosis (5, 20). Our future efforts will be directed toward exploring the mechanistic pathways of CRP-mediated MPO release.
Acknowledgments
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, preparation or approval of manuscript.
Footnotes
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors’ Disclosures of Potential Conflicts of Interest: Upon manuscript submission, all authors completed the Disclosures of Potential Conflict of Interest form. Potential conflicts of interest:
Employment or Leadership: None declared.
Consultant or Advisory Role: None declared.
Stock Ownership: None declared.
Honoraria: None declared.
Research Funding: I. Jialal, NIH grant NIHK24 AT00596 as well as RO1 HL074360; U. Singh, American Health Assistance Foundation grant H 2007-030.
Expert Testimony: None declared.
References
- 1.Verma S, Devaraj S, Jialal I. Is C-reactive protein an innocent bystander or proatherogenic culprit? C-reactive protein promotes atherothrombosis. Circulation. 2006;113:2135–50. [PubMed] [Google Scholar]
- 2.Kobayashi S, Inoue N, Ohashi Y, Terashima M, Matsui K, Mori T, et al. Interaction of oxidative stress and inflammatory response in coronary plaque instability: important role of C-reactive protein. Arterioscler Thromb Vasc Biol. 2003;23:1398–404. doi: 10.1161/01.ATV.0000081637.36475.BC. [DOI] [PubMed] [Google Scholar]
- 3.Lau D, Baldus S. Myeloperoxidase and its contributory role in inflammatory vascular disease. Pharmacol Ther. 2006;111:16–26. doi: 10.1016/j.pharmthera.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 4.Baldus S, Heeschen C, Meinertz T, Zeiher AM, Eiserich JP, Munzel T, et al. CAPTURE Investigators. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108:1440–5. doi: 10.1161/01.CIR.0000090690.67322.51. [DOI] [PubMed] [Google Scholar]
- 5.Rudolph V, Steven D, Gehling UM, Goldmann B, Rudolph TK, Friedrichs K, et al. Coronary plaque injury triggers neutrophil activation in patients with coronary artery disease. Free Radic Biol Med. 2007;42:460–5. doi: 10.1016/j.freeradbiomed.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Polevshchikov AV, Nazarov PG, Berestovaia LK. C-reactive protein modulates neutrophil adhesiveness and biocidal activity. Zh Mikrobiol Epidemiol Immunobiol. 1994;1:69–72. [PubMed] [Google Scholar]
- 7.Hazen SL, Zhang R, Shen Z, Wu W, Podrez EA, MacPherson JC, et al. Formation of nitric oxide-derived oxidants by myeloperoxidase in monocytes: pathways for monocyte-mediated protein nitration and lipid peroxidation in vivo. Circ Res. 1999;85:950– 8. doi: 10.1161/01.res.85.10.950. [DOI] [PubMed] [Google Scholar]
- 8.Pepys MB, Hirschfield GM, Tennent GA, Galli-more JR, Kahan MC, Bellotti V, et al. Targeting C-reactive protein for the treatment of cardiovascular disease. Nature (Lond) 2006;440:1217–21. doi: 10.1038/nature04672. [DOI] [PubMed] [Google Scholar]
- 9.Singh U, Dasu MR, Yancey PG, Afify A, Devaraj S, Jialal I. Human C-reactive protein promotes oxidized low-density lipoprotein uptake and matrix metalloproteinase-9. release in Wistar rats J Lipid Res. 2008;49:1015–23. doi: 10.1194/jlr.M700535-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dasu MR, Devaraj S, Du Clos TW, Jialal I. The biological effects of CRP are not attributable to endotoxin contamination: evidence from TLR4 knockdown human aortic endothelial cells. J Lipid Res. 2007;48:509–12. doi: 10.1194/jlr.C600020-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Lum AF, Green CE, Lee GR, Staunton DE, Simon SI. Dynamic regulation of LFA-1 activation and neutrophil arrest on intercellular adhesion molecule 1 (ICAM-1) in shear flow. J Biol Chem. 2002;277:20660–70. doi: 10.1074/jbc.M202223200. [DOI] [PubMed] [Google Scholar]
- 12.Devaraj S, Chan E, Jialal I. Direct demonstration of an anti-inflammatory effect of simvastatin in subjects with the metabolic syndrome. J Clin Endocrinol Metab. 2006;91:4489–96. doi: 10.1210/jc.2006-0299. [DOI] [PubMed] [Google Scholar]
- 13.Kettle AJ, Gedye CA, Winterbourn CC. Mechanism of inactivation of myeloperoxidase by 4-aminobenzoic acid hydrazide. Biochem J. 1997;321:503– 8. doi: 10.1042/bj3210503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies DE, Stevens AJ, Houston JB. Use of the rat air pouch model of inflammation to evaluate regional drug delivery. Agents Actions. 1992 Spec No:C109–11. [PubMed] [Google Scholar]
- 15.Yasojima K, Schwab C, McGeer EG, McGeer PL. Generation of C-reactive protein and complement components in atherosclerotic plaques. Am J Pathol. 2001;158:1039–51. doi: 10.1016/S0002-9440(10)64051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naruko T, Ueda M, Haze K, van der Wal AC, van der Loos CM, Itoh A, et al. Neutrophil infiltration of culprit lesions in acute coronary syndromes. Circulation. 2002;106:2894–900. doi: 10.1161/01.cir.0000042674.89762.20. [DOI] [PubMed] [Google Scholar]
- 17.Pietila KO, Harmoinen AP, Jokinitty J, Pasternack AI. Serum CRP protein concentration in acute myocardial infarction and its relationship to mortality during 24 months of follow-up in patients under thrombolytic treatment. Eur Heart J. 1996;17:1345–9. doi: 10.1093/oxfordjournals.eurheartj.a015068. [DOI] [PubMed] [Google Scholar]
- 18.Khreiss T, József L, Potempa LA, Filep JG. Loss of pentameric symmetry in C-reactive protein induces interleukin-8 secretion through peroxynitrite signaling in human neutrophils. Circ Res. 2005;97:690–7. doi: 10.1161/01.RES.0000183881.11739.CB. [DOI] [PubMed] [Google Scholar]
- 19.Xu J, Xie Z, Reece R, Pimental D, Zou MH. Uncoupling of endothelial nitric oxidase synthase by hypochlorous acid: role of NAD(P)H oxidase-derived superoxide and peroxynitrite. Arterioscler Thromb Vasc Biol. 2006;26:2688–95. doi: 10.1161/01.ATV.0000249394.94588.82. [DOI] [PubMed] [Google Scholar]
- 20.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, et al. Pravastatin Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) investigators. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]


