Abstract
Rationale: Most children with obstructive sleep apnea are able to sustain stable breathing during portions of sleep, despite an anatomic predisposition toward airway collapse. This suggests that additional determinants of airway patency are active, such as neuromuscular compensation.
Objectives/Methods: Using a custom intraoral surface electrode to record pharyngeal dilator muscle activity (the genioglossus [EMGgg]), we evaluated the muscle, ventilatory, and arousal responses to negative-pressure challenges during sleep in 19 healthy control children.
Measurements and Main Results: In response to these challenges, we observed (1) marked variability in individual EMGgg responsiveness (peak EMGgg [mean ± SD], 214 ± 101% baseline), which was consistent within subjects; (2) a relationship between EMGgg activity and inspiratory flow and airway collapsibility; (3) reflex increases in flow (peak flow increase from challenge breaths 1–5 [mean ± SD], 49 ± 41% baseline) and respiratory rate often sufficient to sustain minute ventilation near baseline levels, without arousal; and (4) arousal threshold to be highest in stage 4, intermediate in stage 2, and lowest in REM sleep.
Conclusions: Healthy children have wide variation in upper airway neuromuscular compensatory responses and arousal thresholds that could represent intermediate phenotypes affecting the expression of sleep apnea. Children with robust upper airway neuromuscular responsiveness, or a very high arousal threshold, may be able to sustain minute ventilation when challenged with negative airway pressure.
Keywords: critical closing pressure, genioglossus EMG, intraoral surface electrode
Upper airway patency is dependent on the balance between static pharyngeal mechanics, neuromuscular activity, and intraluminal pressure (1). Genioglossus activity protrudes the tongue, resulting in increased size (2) and decreased collapsibility (3) of the airway. The importance of airway neuromuscular compensation in children is suggested by the following:
Children with obstructive sleep apnea syndrome (OSAS) do not obstruct during wakefulness.
Children with OSAS have an increased genioglossus EMG (EMGgg) during wakefulness compared with normal children (4).
Applying a topical anesthetic to the upper airway of awake children with OSAS decreases their airway size (5).
There is considerable overlap in the airway size between children with and without OSAS (6).
Apnea is predominantly observed during REM and stage 2, but is rare during stage 4 sleep (7).
Even children with severe OSAS are capable of stable breathing during at least a portion of sleep. Thus, neuromuscular reflexes are likely an important determinant of airway patency and stable breathing during sleep.
An important mechanism sustaining airway patency during sleep in children with OSAS is mechanoreceptor-induced activation of pharyngeal dilator muscles. The EMGgg is reduced at sleep onset in both children with and without OSAS (4). In normal children, the EMGgg activity is persistently low during sleep, yet the airway remains patent, likely due to inherent mechanical viscoelastic stability of the airway (8). However, children with moderate/severe OSAS have an airway anatomically predisposed to collapse, and develop airway obstruction at sleep onset, necessitating neuromuscular activation (8). In these children, the EMGgg is actually elevated above the wakeful baseline during periods of stable breathing while asleep. Furthermore, during apneic and hypopneic events, the EMGgg decreases initially, but then may increase over the course of the obstruction (8). This recruitment of EMGgg across an obstructive event may mitigate the decrease in airflow, and thereby allow sustained minute ventilation. However, the response profile of neuromuscular compensation and minute ventilation in normal children exposed to airway negative-pressure challenges during sleep has not been studied.
Arousal has been termed “the forgotten response to respiratory stimuli” (9) and has been variably regarded as an essential life-saving reflex, as well as an adverse epiphenomenon that potentiates obstructive cycling (10). In adults, the resolution of obstructive events during sleep is usually accompanied by an electrocortical arousal, and sleep architecture is commonly disrupted. However, Younes demonstrated that adults with OSAS may on occasion have an increase in flow during induced obstructions sufficient to sustain minute ventilation without arousal (10). In children with OSAS, obstructive events frequently end without a visible electrocortical arousal (11, 12), and the sleep architecture remains intact (7). Mechanisms other than frank arousal therefore must also be operative in children, including reflex increases in pharyngeal dilator activity, as discussed above, and alterations in respiratory timing. The magnitude and relative importance of these mechanisms in the setting of airway collapse are not known.
The objective of the present study was to evaluate the EMGgg responsiveness to upper airway negative-pressure challenges during sleep in normal children. We hypothesized that (1) airway negative-pressure challenges during sleep activate the EMGgg within a few breaths; (2) EMGgg activation is associated with decreases in airway collapsibility and increases in airflow; and (3) in response to negative pressure, normal children are generally capable of sustaining their minute ventilation near baseline levels without arousal. We propose that the aforementioned mechanisms may account for the preponderance of stable breathing during sleep observed in children with sleep apnea.
METHODS
See the online supplement for additional details.
Subjects
The subjects were nonsnoring, asymptomatic children recruited from community fliers. Exclusion criteria included serious medical conditions, previous adenotonsillectomy, or medications known to affect sleep. The study was approved by the institutional review board at Children's Hospital, Boston. Signed, informed consent was obtained from the parent, and assent was received from the child.
Protocol
Subjects underwent a single overnight polysomnogram in the supine position, including an intraoral EMG surface electrode. After stable sleep was established, the negative-pressure challenge protocol was performed repeatedly in stage 2, stage 4, and REM sleep. Insofar as normal, nonsnoring children have a low prevalence of obstructive sleep apnea, baseline polysomnographic data were only obtained from the nonexperimental portions of the single-night study.
EMGgg
A custom intraoral mouthpiece electrode was constructed for each subject to measure EMGgg (see online supplement for details) (4, 13). The signals were amplified, rectified, and integrated on a moving-time-average basis, with a time constant of 200 ms. EMGgg data are presented as a percentage of the EMGgg observed during a maximal tongue thrust maneuver or as a percentage of the baseline sleep period before the negative-pressure challenges.
Negative-Pressure Challenges
Inspiratory flow was measured in response to negative-pressure challenges using previously described techniques (14, 15). Briefly, the subjects breathed through a nasal mask (Respironics, Pittsburgh, PA), which was attached to a pneumotachometer (Hans Rudolph, Kansas City, MO) and pressure transducer (Validyne Engineering, Northridge, CA). End-tidal PCO2 was measured from a port in the mask using an infrared capnometer (Novametrix Cosmo, Pittsburgh, PA). A chin strap ensured that the mouth remained closed. Pressure and flow signals were acquired simultaneously with a Powerlab AD converter (Colorado Springs, CO) and Grass Heritage system (Grass Telefactor, Braintree, MA) before being transferred to Spike (Cambridge Electronic Design, Cambridge, UK) analysis software.
The mask pressure was controlled between +3 and −22 cm H2O using a machine specially modified by Respironics to alternate between positive and negative airway pressure. Subjects were placed on 3 cm H2O of airway pressure at baseline and permitted to sleep. During the negative-pressure challenges, the mask pressure was lowered in 2–cm H2O increments for five breaths, followed by a return to baseline continuous positive airway pressure for 30 s. These pressure drops progressed until flow approached zero or a visible 3-s electrocortical arousal based on standard criteria occurred (16). Data from breaths with visible electrocortical arousals were excluded from analysis.
Data Analysis
Data were evaluated during three intervals: (1) baseline, defined as the 2 to 3 min before the first negative-pressure challenge; (2) negative-pressure challenges of progressively increased magnitude (five breaths); and (3) post-challenge, the 20 s after the completion of the most negative of the pressure challenges not associated with a visible electrocortical arousal. Each breath during these intervals was analyzed for the following parameters: (1) inspiratory EMGgg (area under the curve [AUC], plateau, and the EMGgg value at point of flow response), (2) inspiratory flow (plateau, peak), (3) inspiratory time and expiratory time, and (4) tidal volume and breath-by-breath minute ventilation.
The EEG power spectra ranges (delta frequency of 1–4 Hz, theta of 4–8 Hz, alpha of 8–12 Hz, and sigma/beta of 12–20 Hz) were analyzed for each artifact-free interval (baseline, negative-pressure challenge, post-challenge) using a 2.56-s analysis epoch centered at the start of inspiration. The mean of the 40 2.56-s epochs during the baseline interval was used to determine the control mean ± SD in each spectral range. A breath was defined as associated with a nonvisible electrocortical arousal if the total power of any of the spectral ranges exceeded the 95th percentile of baseline (10).
Statistical Analysis
Analysis of variance (ANOVA) followed by Dunnett's test was used to analyze differences in the EMGgg, flow, and EEG parameters between baseline and breaths 1 through 5. ANOVA followed by Tukey's test was used to compare the slopes of the pressure–flow and pressure–EMGgg regression curves between breaths. A paired t test was used to compare differences between sleep stages. Multiple regression was used to evaluate the AUC EMGgg and peak inspiratory flow (dependent variables) as a function of airway pressure, challenge breath number, EEG arousal (none, delta, theta, alpha, beta), and sleep state (independent variables). Individual subjects were considered class variables. Differences were considered significant if the null hypothesis was rejected at p < 0.05. Statistical analyses were performed using SigmaStat or SPSS (SPSS, Chicago, IL).
RESULTS
Demographic and Baseline Polysomnographic Data
Twenty normal children were recruited for the study. One child could not fall asleep with the intraoral surface electrode in place, and was therefore eliminated from further analysis. All children recruited had no polysomnographic evidence of OSAS. An average of 4.8 ± 2 h (range, 2.6–6.2 h) of undisturbed sleep during the nonexperimental portion of the overnight study was available to evaluate sleep-disordered breathing in the subjects. Demographic and polysomnographic data from the 19 subjects for whom usable EMGgg data were obtained are summarized in Table 1. There were no significant changes in either the oxygen saturation (median, 0%; range, −2 to 1%) or end-tidal carbon dioxide (median, 0 mm Hg; range, 0–2) during the negative-pressure challenges. Baseline levels of EMGgg activity, airflow, and respiratory timing are summarized in Table E1 of the online supplement. There were no significant differences in the inspiratory mean or peak, compared with the AUC EMGgg, which is reported.
TABLE 1.
DEMOGRAPHIC AND POLYSOMNOGRAPHIC DATA OF NORMAL SUBJECTS
| No. subjects | 19 |
| Age, yr (range) | 11.9 ± 1.9 (9.1–16.4) |
| Male, n | 11 |
| BMI, percentile | 70 ± 21 |
| AHI, events/h | 0 ± 0 |
| Average SaO2′ % | |
| Wakefulness | 98.3 ± 1 |
| Stage 2 | 97.7 ± 1.8 |
| Stage 4 | 97.6 ± 1.6 |
| REM | 97.3 ± 1.8 |
| Peak PetCO2′ mm Hg | |
| Wakefulness | 39 ± 1.5 |
| Stage 2 | 42.9 ± 2.4 |
| Stage 4 | 43.6 ± 1.6 |
| REM | 42.7 ± 1.8 |
Definition of abbreviations: AHI = apnea–hypopnea index; BMI = body mass index; PetCO2 = end-tidal PCO2.
Data are presented as mean ± SD unless otherwise specified.
Negative-Pressure Challenges
Negative-pressure challenges were successful in all 19 subjects during both stage 2 and stage 4 sleep. In REM sleep, subjects generally awakened between −4 and −6 cm H2O of negative pressure. Thus, the REM sleep data could be used only for evaluation of arousal threshold, but not flow-limited breathing or neuromuscular compensation. Overall, there were 154 sequential series of negative-pressure challenges, of which 128 ended in a visible electrocortical arousal/awakening and 26 were terminated because V̇min was zero. An average of 8.1 (range, 4–11) sequences of negative-pressure challenges were performed in each subject, with 4.8 individual challenges per sequence (range, 1–11), resulting in an average of 38.7 (range, 25–84; total, 735) five-breath challenges per subject. Overall, 3,135 individual breaths (1,748 stage 4; 1,387 stage 2) were exposed to airway pressure changes resulting in 1,330 (717 stage 2; 613 stage 4) flow-limited breaths and 646 flow-response breaths (339 stage 2; 307 stage 4). The breaths chosen for analysis are described in the individual sections below.
EMGgg Activity during Negative-Pressure Challenges
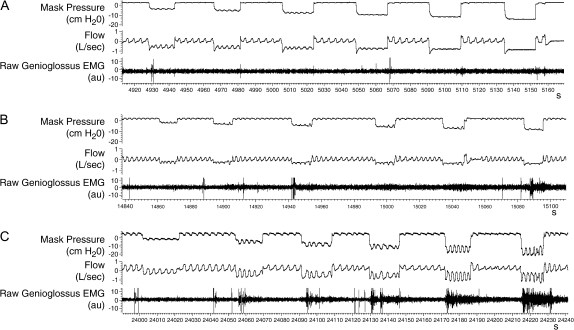
Raw data demonstrating the range of the EMGgg responsiveness to negative-pressure challenges are shown for three subjects in Figure 1. Subject A had little EMGgg activation even with negative-pressure challenges below −16 cm H2O (Figure 1A). Subject B appeared to recruit the EMGgg by breath 3 for challenges below −8 cm H2O (Figure 1B). Subject C had consistently brisk EMGgg responses to all negative-pressure challenges starting at −4.5 cm H2O (Figure 1C). This led to a maintenance of ventilation across the challenge. A detailed view of the phasic increase in the EMGgg and the resultant flow response during a negative-pressure challenge is shown in Figure 2. In this example, the first two breaths during the negative-pressure challenge are flow limited; the third and fourth breaths demonstrate a flow response (see arrows); the fifth breath appears non–flow limited, indicating successful neuromuscular compensation. The qualitative consistency of these responses is demonstrated in Figures E1–E3.
Figure 1.
Raw data from three normal children during stage 4 sleep demonstrating the range of genioglossus EMG (EMGgg) responsiveness during six negative-pressure challenges. Each tracing is approximately 4 min. (A) No increase in the EMGgg or flow is evident during the negative-pressure challenges. (B) Some increase in the EMGgg is noted during challenges 2–6. An increase in inspiratory flow is evident on breaths 4–5 during challenges 2–4. (C) A brisk EMGgg response accompanies challenges 2–6 and is associated with flow at or above baseline without arousal.
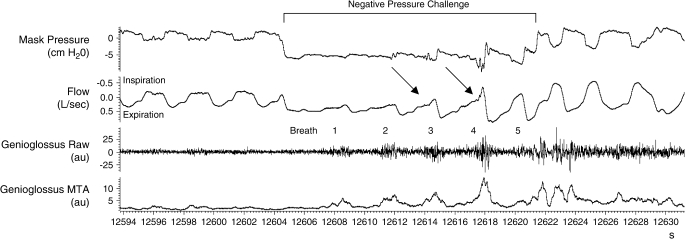
Figure 2.
Raw data from a five-breath negative-pressure challenge demonstrating progressively increasing phasic EMGgg activity during breaths 1–4. Subsequently, the EMGgg decreases in breath 5 after a normal breath flow profile had been reestablished. Inspiratory flow is observed to be flow limited during breaths 1–2, flow limited followed by a flow response (arrows) in breaths 3–4, and non–flow limited in breath 5.
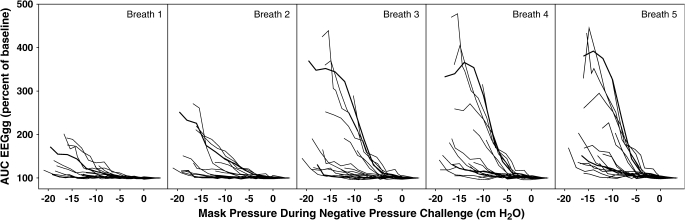
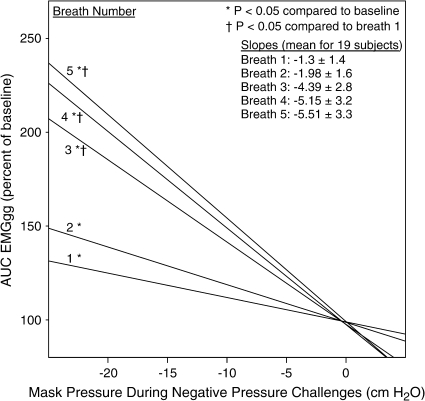
The inspiratory AUC EMGgg data from all 19 subjects during stage 4 sleep for breaths 1 through 5 are shown in Figure 3. Considerable variability in the EMGgg activation induced by negative pressure is evident between subjects. The response appears to be grossly bimodal, with some individuals having AUC EMGgg responses less than 200% of baseline at maximum negative pressure, whereas others have an EMGgg that exceeds 200% at airway pressures below −10 cm H2O. In stage 2 and 4 sleep, the inspiratory AUC EMGgg increased significantly during breaths 3 through 5 of the challenges compared with breath 1 and baseline (p < 0.05). The regression lines of the AUC inspiratory EMGgg data for each of the five breaths in stage 4 are shown in Figure 4. There was no correlation between each subject's EMGgg negative-pressure regression line slope and the following: (1) age, (2) body mass index, or (3) pressure at the time of visible EEG arousal. There was a significant correlation between a subject's slope of the EMGgg negative-pressure regression line and the flow-negative pressure regression slope for breaths 4 and 5 (p < 0.05). That is, children with increased EMGgg responsiveness also had increased flow in response to negative pressure on those breaths.
Figure 3.
Inspiratory area under the curve (AUC) EMGgg data from 19 normal children during progressively increasing negative-pressure challenges lasting five breaths in stage 4 sleep. Each line represents a given subject for each breath across the progressive pressure drops. A marked variability in EMGgg responsiveness is evident between subjects.
Figure 4.
Regression lines for AUC inspiratory EMGgg during each of the five breaths of the negative-pressure challenges in stage 4 sleep. The data demonstrate a marked increase in EMGgg activation as mask pressure is lowered, particularly during breaths 3 to 5.
For flow-limited breaths in both stage 2 and 4 sleep combined, the inspiratory AUC EMGgg increased significantly during breaths 3 through 5 of a challenge compared with breath 1 (p < 0.05), and breaths 4 through 5 were greater than breath 3 (p < 0.05). The peak moving time average (MTA) EMGgg at the point of flow response was positively correlated with decreasing mask pressure (r = 0.44, p < 0.05). That is, higher EMGgg activity was needed to evoke a flow response at more negative airway pressures. For negative-pressure challenges in which there was a flow response on breaths 3, 4, and 5, there was no significant difference in the peak MTA EMGgg at the point of flow response between individual breaths of a challenge.
The inspiratory AUC was elevated compared with baseline for all groups of breaths in the post-challenge intervals (see Table E1; p < 0.05). This activity consisted of a nonphasic increase in tonic EMGgg levels (see Figures 1C and 2).
Flow during Negative-Pressure Challenges
Raw-data examples demonstrating the range of the inspiratory flow responses to negative-pressure challenges are shown for three subjects in Figure 1. In some subjects, inspiratory flow demonstrated gradually increasing flow limitation until the flow approached zero, with increasingly negative airway pressure (Figure 1A). The majority of subjects initially developed a decrease in flow during the negative-pressure challenges, but subsequently developed a flow response by breaths 3 through 5 (Figure 1B). Finally, some subjects were able to sustain, or even increase, inspiratory flow without flow limitation, in response to negative pressure (Figure 1C).
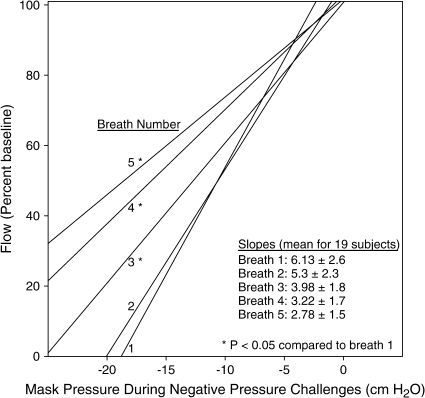
In both stage 2 and stage 4 sleep, the slope of the inspiratory flow–pressure regression lines for pressures at or below the first flow-limited breath was significantly lower (i.e., flatter) for breaths 3 through 5 than for breath 1 (see Figure 5; all p < 0.05), indicating increased flow and decreased upper airway resistance on breaths 3 through 5. Comparison of inspiratory flow between baseline and the post-challenge intervals are shown in Table E1.
Figure 5.
Regression lines for inspiratory flow during each of the five breaths of the negative-pressure challenges in stage 4 sleep. The data demonstrate increased flow on subsequent breaths of a challenge indicating successful compensation.
Tidal Volume and Minute Ventilation during Negative-Pressure Challenges
In both stage 2 and 4 sleep, tidal volume and minute ventilation were significantly increased during breaths 4 and 5 compared with breath 1 of a challenge (p < 0.05). The breath-by-breath minute ventilation data from all 19 subjects during stage 4 sleep are shown in Figure 6. It is evident that by breath 4 of a negative-pressure challenge of up to −8 cm H2O, many breaths without evidence of arousal (Figure 6, solid circles) had a minute ventilation at baseline levels. This indicates successful compensation. In the multiple regression model, there was a significant increase in the inspiratory flow, tidal volume, and minute ventilation in breaths with an increase in the alpha or beta power, but not in breaths with an increased in the delta or theta power.
Figure 6.
Minute ventilation data from 19 normal children during progressively increasing negative-pressure challenges lasting five breaths in stage 4 sleep. Solid circles indicate breaths with no evidence of electrocortical arousals. Open circles indicate breaths with nonvisible electrocortical arousals in the alpha or beta range. By breaths 4 and 5, even without evidence of electrocortical activation, the minute ventilation approached baseline for mask pressures up to −8 cm H2O.
Respiratory Timing during Negative-Pressure Challenges
The inspiratory time (relative to baseline) was not significantly different from baseline on breaths 1, 2, 3, and 4 (118 ± 16, 112 ± 20, 95 ± 18, and 85 ± 17%, respectively), but was decreased on breath 5 (82 ± 16%, p < 0.05). Expiratory time (relative to baseline) on challenge breaths 1 through 5 was 82 ± 18, 79 ± 16, 66 ± 12, 68 ± 15, and 66 ± 13%, which were all significantly decreased from baseline (p < 0.05). Comparison of the inspiratory and expiratory time between baseline and the post-challenge intervals are shown in Table E1.
Arousal Threshold
The mean negative pressure before arousal was significantly lower in stage 4 (−17.2 ± 2.3 cm H2O) compared with stage 2 (−13.5 ± 2.5 cm H2O) or REM sleep (−4.8 ± 1.5 cm H2O; p < 0.05).
DISCUSSION
The primary findings of this study in normal children during sleep are as follows:
There is wide intersubject variability in inspiratory EMGgg activation in response to negative-pressure challenges during non-REM sleep.
The increased EMGgg induced by negative airway pressure is associated with increased flow, increased respiratory rate, and decreased airflow resistance.
During negative-pressure challenges between 0 and −10 cm H2O, the minute ventilation was in the normal range by breath 3 for the majority of breaths, including those without evidence of visible or nonvisible electrocortical arousals.
Breaths during sleep with nonvisible EEG arousals in the alpha and beta range, but not the delta or theta range, were associated with increased EMGgg, flow, and minute ventilation compared with challenge breaths without arousals.
Arousal threshold is highest in stage 4, intermediate in stage 2, and lowest during REM sleep.
We conclude that healthy children have marked variability in upper airway neuromuscular compensatory responses and arousal threshold, which may represent intermediate phenotypes affecting the expression of sleep apnea.
Pharyngeal dilator muscle activity, including the genioglossus, hyoglossus, styloglossus, and other muscles, modulate airflow though the upper airway. Although it is believed that these muscles act in unison, the genioglossus is the most easily measured. Selective activation of the genioglossus has been shown to reduce airway collapsibility in both humans (3) and animal models (17). During wakefulness, children with OSAS have an elevated EMGgg compared with normal control subjects (4), likely a product of negative airway pressure on laryngeal mucosal mechanoreceptors (5). At sleep onset, there is a reduction in the EMGgg in children with and without OSAS (4). Children with severe OSAS will have a rebound increase in the EMGgg during sleep above the wakeful baseline, consistent with a reflex induced by mechano- and/or chemoreceptors (4). In the present study, we document the threshold, time course, and variability of negative-pressure–induced EMGgg activation during sleep in normal children (see Figure 3). These data may account for the wide range of airway collapsibility during sleep previously described in normal children (14), the ability of children with OSAS to sustain stable breathing during part of sleep, and the poor correlation between airway anatomy and OSAS severity (14).
Several previous studies in adults have evaluated the genioglossal response during sleep to both pulse and sustained negative-pressure challenges. Compared with wakefulness, there is a marked reduction during sleep in reflex EMGgg activation to pulses of negative pressure in adults with and without OSAS (18). Nevertheless, Schwartz and colleagues challenged adults with OSAS with sustained reductions in airway pressure lasting three breaths and observed that the phasic EMGgg increased sequentially (19). This response is qualitatively similar to those observed in our normal children in the present study. Interestingly, Schwartz and colleagues observed that despite an increase in EMGgg after a reduction in airway pressure, the airway became more collapsible (increased critical closing pressure). This suggests that nonmuscular factors, such as a reduction in lung volume, increased airway collapsibility. Our study differed from that of Schwartz and colleagues in the following important respects: (1) We studied normal, nonsnoring children (apnea–hypopnea index [AHI] = 0, nonobese) rather than obese adults with OSAS (AHI > 70 events/h, BMI = 41 kg/m2). Thus, our subjects had less collapsible airways than the adults, and were therefore challenged with more negative pressure than the adults. It is quite plausible that the mechanical properties of the obese adult upper airway would supercede neuromuscular control mechanisms by changing the pressure–area curve of the pharynx. (2) The negative-pressure challenges in our study lasted five breaths instead of three, thus permitting more time for EMGgg activation to affect the airway size and collapsibility. (3) Schwartz and colleagues sought to measure collapsibility in the passive airway, and therefore evaluated the flow-limited portion of the breath only. By contrast, our primary objective was to measure the ability of the airway to respond to negative pressure. Therefore, we included in our analysis increases in flow that followed flow-limited breaths in a given challenge sequence. However, if we had restricted our analysis only to the flow-limited portion of the breath, there was still an overall decrease in airway collapsibility observed between breaths 1 through 3 (see Figure 2). This suggests that there is a real difference in the ability of the airway to respond to negative-pressure challenges between normal children and adults with OSAS.
We observed that most normal children can sustain their minute ventilation within a few breaths of induced upper airway collapse, via a combination of pharyngeal dilator muscle activation and increased respiratory rate. Importantly, electrocortical arousal, even based on frequency domain analysis, was not necessary for successful compensation. Thus, our data account for the observation that children with OSAS have obstructive events that frequently terminate without arousal (20) and normal sleep state distribution (7). Furthermore, most patients with OSAS can sustain a stable breathing pattern during sleep without arousal, particularly in stage 4 sleep when the arousal threshold is high. This suggests that the pharyngeal dilators are capable of activation sufficient to open the airway and sustain the minute ventilation, provided the level of mechanoreceptor/chemoreceptor activity is below the arousal threshold. By contrast, REM sleep has a low arousal threshold, and therefore obstructive events are likely to lead to arousal rather than successful compensation. This may partially explain the increased incidence of apneas during REM sleep in children. Because the principal determinants of both arousal and pharyngeal dilator activation are the same, mechanoreceptor and chemoreceptor input, there is often an apparent temporal relationship. Thus, most children challenged with an airway predisposed toward collapse often have robust compensatory reflex mechanisms to maintain adequate ventilation during sleep without arousal.
Flow limitation is a mechanical property of a collapsible tube, such as the pharynx, observed when the downstream pressure is below the closing pressure of the tube (21). The flow profile is further predicated on the “tube law,” which describes the markedly sigmoidal relation between airway pressure and cross-sectional area (22). Consequently, a change of 50% of the maximum airway area may require as little as a 1– to 2–cm H2O change in airway pressure (22, 23). Thus, rapid intrabreath increases in airflow may occur if the pharyngeal musculature shifts the highly compliant portion of the airway pressure–area relationship. When flow-limited airflow is interrupted by a sudden, rapid increase in airflow, we called it a “flow response,” presumably reflecting the sudden opening of the airway. The flow response serves to increase the tidal volume to sustain the minute ventilation. However, it may also result in an overshoot of ventilation leading to cycling of respiratory effort and airway patency. Empirically, it appears that the flow-response breaths without arousal resulted in a minute ventilation near baseline, suggesting a stabilizing influence on breathing during sleep. By contrast, breaths with arousals in the alpha and sigma/beta range frequently resulted in the minute ventilation significantly above baseline (see Figure 6). These data support the conclusion of Younes in adults with OSAS, that nonarousal flow-response mechanisms are sufficient to sustain minute ventilation, in combination with an increased respiratory rate (10). However, a more extensive EEG montage than was applied in this study may have yielded additional evidence for EEG arousal during the negative-pressure challenges.
We observed a persistent augmentation of the tonic EMGgg after the negative-pressure challenges lasting at least 20 s, and anecdotally several minutes. By contrast, the flow and respiratory timing variables returned to baseline between 10 and 15 s after the challenges, with no evidence of an EEG arousal. The fact that the increase in EMGgg appeared without changes in respiration indicates that it was not likely due to a general increase in respiratory drive. It is well established that there is a non–chemoreceptor-driven increase in ventilation that follows induced hyperventilation, termed “post-stimulus potentiation” or “afterdischarge” (24, 25). Furthermore, it is known that airway resistance is decreased during this period (26, 27). A post-stimulus increase in genioglossal activity has been observed during this interval in animal models after intermittent hypoxia (28). In adult humans, the peak inspiratory EMGgg has been observed to return to baseline after four breaths after an induced arousal (29) or spontaneous obstruction (30), but the response of the tonic EMGgg was not reported. However, a persistent tonic elevation of the chin EMG has been reported in adults with OSA after induced obstructions (10). Although the etiology of the persistent increase in tonic EMGgg that we observed is unknown, it has important implications in the pathophysiology of OSAS. A persistent increase in pharyngeal dilator activity may contribute to breathing stability by decreasing airway collapsibility. This might prevent airway closure at the nadir in the oscillation of ventilation that is observed during cyclic breathing in OSAS.
The present data concur with our previous work using progressive, intermittent negative-pressure challenges in sleeping children. These studies indicate the following: (1) Approximately 50% of children will have a Pcrit extrapolated beyond −25 cm H2O, indicating excellent neuromuscular compensation (14, 15). We have now observed a large range of EMGgg responsiveness in children that correlates with changes in collapsibility. (2) Isono and colleagues showed that measurements of collapsibility in children during anesthesia with complete neuromuscular blockade indicate a closing pressure of approximately −8 cm H2O (31). By contrast, in sleeping children using intermittent three-breath challenges, the average collapsibility is –25 cm H2O, indicating neuromuscular compensation (14). More sustained negative-pressure challenges yield a Pcrit on average of –35 cm H2O (14). Our present data confirm substantial neuromuscular compensation during the first three breaths of a negative-pressure challenge, and additional activity during breaths 4 and 5. In infants, a significant increase in the EMGgg during the first two breaths after airway occlusion has also been reported (32, 33). (3) There is frequently a shift to nonflow limited breaths during negative-pressure challenges by breath 4 (14). We observed that the flow response and shift to non–flow-limited breaths was often associated with increases in the EMGgg during breaths 4 and 5 of a negative-pressure challenge. In summary, the EMGgg responsiveness to negative pressure that we observed is consistent with alterations in airflow and collapsibility that have been previously reported.
Limitations
There are a number of limitations of the present study. First, our subjects were older (11.9 ± 1.9 yr; range, 9.1–16.4 yr) than the peak incidence of OSAS in children (aged 2–8 yr), and there is the confounding variable of pubertal status. We do not believe that this adversely affects our conclusions insofar as a previous developmental study indicates that there is little difference in airway collapsibility in this age range (34). Furthermore, only four of our subjects were older than 13 yr, and the EMGgg responsiveness of these subjects was not different from the remainder of the control population. Second, the application of negative pressure to the airway reduces lung volume and thus mechanically increases collapsibility of the upper airway (35, 36). Though we did not measure lung volumes in this study, we did observe a decrease in collapsibility incrementally during the five-breath negative-pressure challenges. Thus, given the reduction in lung volume during the same interval, we may have actually underestimated the ability of pharyngeal dilators to alter airway compliance. Third, the relationship between the EMGgg activity and the force generated by the pharyngeal dilators is likely nonlinear. In addition, we did not measure the activity of other muscles that may be contributing to the compliance of the airway. We do believe, however, that the EMGgg is a reliable measure of neural drive to the upper airway muscles, and therefore our conclusions are qualitatively correct. Fourth, our protocol excluded breaths associated with visible EEG arousals on three EEG leads, but evaluated only a single EEG lead for evidence of spectral changes. Thus, had a more extensive EEG montage been used, we may well have identified additional evidence for subtle arousal from sleep during the negative-pressure challenges. Fifth, we could not obtain reliable measures of collapsibility during REM sleep insofar as arousal occurred on average at −4.8 ± 1.5 cm H2O of negative pressure. Therefore, our results may not be applicable to this state. Sixth, the surface intraoral electrode may not be sensitive enough to record all significant changes in muscle activity. Furthermore, some of the activity recorded by the surface electrode may include additional muscles intrinsic or extrinsic to the genioglossus that may not serve as pharyngeal dilators. Finally, we only studied normal subjects, and it is unclear if these results are applicable to children with OSAS.
Conclusions
We observed a wide range of neuromuscular compensation and arousal threshold during sleep in normal children. Most children were capable of doubling their EMGgg activity during the five-breath negative-pressure challenges without experiencing a visible EEG arousal. We speculate that this robust EMGgg responsiveness in children accounts for the relative paucity of obstructive events in non-REM sleep (7), the tendency for obstructions to terminate without an EEG arousal, and the ability of children with severe OSAS to sustain stable breathing during most of sleep. A high arousal threshold, such as in stage 4 sleep, permits the mechanoreceptor/chemoreceptor input to produce reflexive changes in the activity of pharyngeal dilators sufficient to open the airway, thus sustaining minute ventilation. Thus, we propose that the expression of sleep apnea in a given child is predicated on the balance between at least three intermediate physiologic phenotypes, including airway stiffness/size, neuromuscular compensation, and arousal threshold.
Supplementary Material
Acknowledgments
The authors thank Respironics and Peter Hill for supplying modified continuous positive airway pressure equipment to provide subatmospheric pressure.
Supported by NIH/NHLBI HL073238-01 and by grant MO1 RR02172 to Children's Hospital, Boston, General Clinical Research Center by the National Center for Research Resources, National Institutes of Health (E.S.K.); NIH/NHLBI 58585 (C.L.M.); and NIH/NHLBI 1 P50 HL60292 and NIH RO1 HL048531 (D.P.W.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200509-1450OC on January 26, 2006
Conflict of Interest Statement: E.S.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.L.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.P.W. is a consultant for Respironics, Inc. ($20,000/yr), Alfred E. Mann Foundation ($10,000/yr), Aspire Medical ($10,000/yr), Itamar Medical ($10,000/yr), and WideMed ($4,000/yr). He occasionally consults for Cephalon ($5,000) and Organon ($4,000). He has research grants from Respironics, Inc. (about $200,000/yr), Alfred E. Mann Foundation (about $30,000/yr), Cephalon (about $125,000), and WideMed (about $10,000/yr).
References
- 1.Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol 1978;44:931–938. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi I, Perry A, Rhymer J, Wuyam B, Hughes P, Murphy K, Innes JA, McIvor J, Cheeseman AD, Guz A. Inspiratory co-activation of genioglossus enlarges the retroglossal space in laryngectomized humans. J Appl Phys 1996;80:1595–1604. [DOI] [PubMed] [Google Scholar]
- 3.Oliven A, O'Hearn DJ, Boudewyns A, Odeh M, De Backer W, van de Heyning P, Smith PL, Eisele DW, Allen L, Schneider H, et al. Upper airway response to electrical stimulation of the genioglossus in obstructive sleep apnea. J Appl Phys 2003;95:2023–2029. [DOI] [PubMed] [Google Scholar]
- 4.Katz ES, White DP. Genioglossus activity in children with obstructive sleep apnea during wakefulness and sleep onset. Am J Respir Crit Care Med 2003;168:664–670. [DOI] [PubMed] [Google Scholar]
- 5.Gozal D, Burnside MM. Increased upper airway collapsibility in children with obstructive sleep apnea during wakefulness. Am J Respir Crit Care Med 2004;169:163–167. [DOI] [PubMed] [Google Scholar]
- 6.Arens R, McDonough JM, Costarino AT, Mahboubi S, Tayag-Kier CE, Maislin G, Schwab RJ, Pack AI. Magnetic resonance imaging of the upper airway structure of children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2001;164:698–703. [DOI] [PubMed] [Google Scholar]
- 7.Goh DY, Galster P, Marcus CL. Sleep architecture and respiratory disturbances in children with obstructive sleep apnea. Am J Respir Crit Care Med 2000;162:682–686. [DOI] [PubMed] [Google Scholar]
- 8.Katz ES, White DP. Genioglossus activity during sleep in normal control subjects and children with obstructive sleep apnea. Am J Respir Crit Care Med 2004;170:553–560. [DOI] [PubMed] [Google Scholar]
- 9.Phillipson EA, Sullivan CE. Arousal: the forgotten response to respiratory stimuli. Am Rev Respir Dis 1978;118:807–809. [DOI] [PubMed] [Google Scholar]
- 10.Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med 2004;169:623–633. [DOI] [PubMed] [Google Scholar]
- 11.Mograss M, Ducharme F, Brouillette R. Movement/arousals: description, classification and relationship to sleep apnea in children. Am J Respir Crit Care Med 1994;150:1690–1696. [DOI] [PubMed] [Google Scholar]
- 12.Katz ES, Lutz J. B, Black C, Marcus CL. Pulse transit time as a measure of arousal and respiratory effort in children with sleep-disordered breathing. Pediatr Res 2003;53:580–588. [DOI] [PubMed] [Google Scholar]
- 13.Doble EA, Leiter JC, Knuth SL, Daubenspeck JA, Bartlett D. A noninvasive intraoral electromyographic electrode for genioglossus muscle. J Appl Physiol 1985;58:1378–1382. [DOI] [PubMed] [Google Scholar]
- 14.Marcus CL, Fernandes Do Prado LB, Lutz J, Katz ES, Black CA, Galster P, Carson KA. Developmental changes in upper airway dynamics. J Appl Phys 2004;97:98–108. [DOI] [PubMed] [Google Scholar]
- 15.Marcus CL, Katz ES, Lutz J, Black CA, Galster P, Carson KA. Upper airway dynamic responses in children with the obstructive sleep apnea syndrome. Pediatr Res 2005;57:99–107. [DOI] [PubMed] [Google Scholar]
- 16.Sleep Disorders Atlas Task Force. ASDA report:. EEG arousals: scoring rules and examples. Sleep 1992;15:173–184. [PubMed] [Google Scholar]
- 17.Schwartz AR, Thut DC, Russ B, Seelagy MM, Yuan X, Brower RG, Permutt S, Wise RA, Smith PL. Effect of electrical stimulation of the hypoglossal nerve on airflow mechanics in the isolated upper airway. Am Rev Respir Dis 1993;147:1144–1150. [DOI] [PubMed] [Google Scholar]
- 18.Wheatley JR, Mezzanotte WS, Tangel DJ, White DP. Influence of sleep on genioglossus muscle activation by negative pressure in normal men. Am Rev Respir Dis 1993;148:597–605. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz AR, O'Donnell CP, Baron J, Schubert N, Alam D, Samadi SD, Smith PL. The hypotonic upper airway in obstructive sleep apnea: role of structures and neuromuscular activity. Am J Respir Crit Care Med 1998;157:1051–1057. [DOI] [PubMed] [Google Scholar]
- 20.McNamara F, Issa F, Sullivan C. Arousal pattern following central and obstructive breathing abnormalities in infants and children. J Appl Physiol 1996;81:2651–2657. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz AR, Smith PL, Wise RA, Gold AR, Permutt S. Induction of upper airway occlusion in sleeping individuals with subatmospheric nasal pressure. J Appl Phys 1988;64:535–542. [DOI] [PubMed] [Google Scholar]
- 22.Isono S, Remmers JE, Tanaka A, Sho Y, Sato J, Nishino T. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Phys 1997;82:1319–1326. [DOI] [PubMed] [Google Scholar]
- 23.Isono S, Remmers JE, Tanaka A, Sho Y, Nishino T. Static properties of the passive pharynx in sleep apnea. Sleep 1996;19:S175–S177. [PubMed] [Google Scholar]
- 24.Babcock MA, Badr MS. Long-term facilitation of ventilation in humans during NREM sleep. Sleep 1998;21:709–716. [PubMed] [Google Scholar]
- 25.Eldridge FL. Posthyperventilation breathing: different effects of active and passive hyperventilation. J Appl Phys 1973;34:422–430. [DOI] [PubMed] [Google Scholar]
- 26.Aboubakr SE, Taylor A, Ford R, Siddiqi S, Badr MS. Long-term facilitation in obstructive sleep apnea patients during NREM sleep. J Appl Phys 2001;91:2751–2757. [DOI] [PubMed] [Google Scholar]
- 27.Shkoukani M, Babcock MA, Badr MS. Effect of episodic hypoxia on upper airway mechanics in humans during NREM sleep. J Appl Phys 2002;92:2565–2570. [DOI] [PubMed] [Google Scholar]
- 28.Fuller DD. Episodic hypoxia induces long-term facilitation of neural drive to tongue protruder and retractor muscles. J Appl Phys 2005;98:1761–1767. [DOI] [PubMed] [Google Scholar]
- 29.Carlson DM, Carley DW, Onal E, Lopata M, Basner RC. Acoustically induced cortical arousal increases phasic pharyngeal muscle and diaphragmatic EMG in NREM sleep. J Appl Phys 1994;76:1553–1559. [DOI] [PubMed] [Google Scholar]
- 30.Onal E, Leech JA, Lopata M. Dynamics of respiratory drive and pressure during NREM sleep in patients with occlusive apneas. J Appl Phys 1985;58:1971–1974. [DOI] [PubMed] [Google Scholar]
- 31.Isono S, Shimada A, Utsugi M, Konno A, Nishino T. Comparison of static mechanical properties of the passive pharynx between normal children and children with sleep-disordered breathing. Am J Respir Crit Care Med 1998;157:1204–1212. [DOI] [PubMed] [Google Scholar]
- 32.Gauda EB, Miller MJ, Carlo WA, Difiore JM, Johnsen DC, Martin RJ. Genioglossus response to airway occlusion in apneic versus nonapneic infants. Pediatr Res 1987;22:683–687. [DOI] [PubMed] [Google Scholar]
- 33.Roberts JL, Reed WR, Mathew OP, Thatch BT. Control of respiratory activity of the genioglossus muscle in micrognathic infants. J Appl Physiol 1986;61:1523–1533. [DOI] [PubMed] [Google Scholar]
- 34.Marcus CL, Lutz J, Hamer A, Smith PL, Schwartz AR. Developmental changes in response to subatmospheric pressure loading of the upper airway. J Appl Phys 1999;87:626–633. [DOI] [PubMed] [Google Scholar]
- 35.Heinzner RC, Stanchina ML, Malhotra A, Fogel RB, Patel SR, Jordan AS, Schory K, White DP. Lung volume and continuous positive pressure requirements in obstructive sleep apnea. Am J Respir Crit Care Med 2005;172:114–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van de Graaff WB. Thoracic influence on upper airway patency. J Appl Phys 1988;65:2124–2131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.