Abstract
Rationale: Sleep-disordered breathing recurrent intermittent hypoxia and sympathetic nervous system activity surges provide the milieu for cardiac arrhythmia development.
Objective: We postulate that the prevalence of nocturnal cardiac arrhythmias is higher among subjects with than without sleep-disordered breathing.
Methods: The prevalence of arrhythmias was compared in two samples of participants from the Sleep Heart Health Study frequency-matched on age, sex, race/ethnicity, and body mass index: (1) 228 subjects with sleep-disordered breathing (respiratory disturbance index ⩾ 30) and (2) 338 subjects without sleep-disordered breathing (respiratory disturbance index < 5).
Results: Atrial fibrillation, nonsustained ventricular tachycardia, and complex ventricular ectopy (nonsustained ventricular tachycardia or bigeminy or trigeminy or quadrigeminy) were more common in subjects with sleep-disordered breathing compared with those without sleep-disordered breathing: 4.8 versus 0.9% (p = 0.003) for atrial fibrillation; 5.3 versus 1.2% (p = 0.004) for nonsustained ventricular tachycardia; 25.0 versus 14.5% (p = 0.002) for complex ventricular ectopy. Compared with those without sleep-disordered breathing and adjusting for age, sex, body mass index, and prevalent coronary heart disease, individuals with sleep-disordered breathing had four times the odds of atrial fibrillation (odds ratio [OR], 4.02; 95% confidence interval [CI], 1.03–15.74), three times the odds of nonsustained ventricular tachycardia (OR, 3.40; 95% CI, 1.03–11.20), and almost twice the odds of complex ventricular ectopy (OR, 1.74; 95% CI, 1.11–2.74). A significant relation was also observed between sleep-disordered breathing and ventricular ectopic beats/h (p < 0.0003) considered as a continuous outcome.
Conclusions: Individuals with severe sleep-disordered breathing have two- to fourfold higher odds of complex arrhythmias than those without sleep-disordered breathing even after adjustment for potential confounders.
Keywords: arrhythmia, cohort studies, epidemiology, sleep apnea syndromes
Patients with sleep-disordered breathing (SDB) may be predisposed to arrhythmias because of alterations in sympathetic and parasympathetic nervous system activity occurring with SDB-associated hypoxemia, acidosis, apneas, and arousal (1–3). Mechanisms of arrhythmogenesis involve abnormal automaticity, triggered automaticity, and reentry mechanisms. Abnormal automaticity involves spontaneous cardiac impulse formation and may occur in SDB due to hypoxemia and respiratory acidosis accompanying apneic events (4). Triggered automaticity, pacemaker activity due to a stimulated action potential, may arise in SDB due to enhanced sympathetic nervous system activity associated with respiratory event–related hypoxemia and arousal (5). Reentry mechanisms may occur through the vagal stimulation that results from respiration against a partially occluded airway, which may lead to bradycardia-dependent increased dispersion of atrial repolarization predisposing to intraatrial entry (5–7). Also, SDB-related mechanical effects of negative intrathoracic pressure on the atrial and ventricular free walls promote cardiac stretch, which may predispose to arrhythmias via mechanical-electrical feedback mechanisms (8).
Despite the biological plausibility for SDB-associated hypoxemia, arousals, and autonomic nervous system dysregulation causing generation of abnormal cardiac electrophysiologic impulses, only limited research has rigorously characterized the association between SDB and cardiac arrhythmias. We hypothesized that the prevalence of atrial fibrillation and clinically significant ventricular arrhythmias would be increased with SDB, even after adjusting for potential confounders. In the present investigation, we took advantage of standardized data collection, including electrocardiogram (ECG) data collected during an overnight sleep study, from a large community-based study to examine the association between SDB and cardiac arrhythmias. Some of the results of these studies have been previously reported in the form of an abstract (9).
METHODS
Subjects and Study Design
The Sleep Heart Health Study (SHHS) is a multicenter longitudinal study of 6,441 participants from existing cohorts, aged ⩾ 40 yr, designed to determine the cardiovascular consequences of SDB. The design and objectives of SHHS, and detailed descriptions of its member cohorts, protocols, and quality-control procedures, have been published (10, 11). The SHHS baseline examination was conducted between December 1995 and January 1998 (12). Between 2001 and 2002, all of the original 6,441 participants who were alive and able to be contacted were invited to participate, except for 99 on continuous positive airway pressure therapy. A total of 3,295 agreed to participate in a follow-up exam, which included an overnight polysomnogram (PSG). ECG data for the present analysis were derived during the sleep period from this second PSG, which included lead I bipolar ECG sampled at 250 Hz, a higher frequency than on the baseline PSG.
Because ECG data collected during the PSG needed to be reprocessed and analyzed for this analysis using ECG-specific software, efficiency was optimized by using a nested group-matched exposed and nonexposed design. Exposed subjects were defined as those with a Respiratory Disturbance Index (RDI) ⩾ 30 (SDB), and nonexposed as individuals with an RDI < 5 (non-SDB). Subjects were eligible for inclusion in the “exposed” and “nonexposed” groups if their body mass index (BMI) was between 18 and 40 kg/m2, no use of continuous positive airway pressure was reported, and data were technically satisfactory for the proposed analyses. All “exposed” subjects who met eligibility criteria were included (n = 228). Subjects were selected for the non-SDB group from among the 721 subjects with an RDI < 5 who met eligibility criteria. Group frequency matching to obtain covariate distributions for age (± 2 yr), sex, race/ethnicity, and BMI ± 2 kg/m2, which were balanced with respect to the exposed (SDB) group, yielded a “nonexposed” sample of 338 subjects.
Assuming an arrhythmia prevalence of 10% in the non-SDB group, the study had 88% power to detect a minimum of a twofold increase in prevalence. Ethics approval was obtained from the institutional review board of each SHHS site and coordinating center. Written, informed consent for participation in the SHHS was obtained for all individuals.
Sleep Data
Twelve-lead unattended overnight PSG was accomplished with Compumedics PS (Melbourne, Australia) equipment. Sleep data were centrally scored, as described previously. The RDI is the number of apneas plus hypopneas per hour of sleep. The scoring reliability for the RDI was excellent, as reported (13).
Covariate Data
Covariate data included the following: demographic variables: age, sex, BMI, race/ethnicity; cardiovascular disease (CVD) risk factors: hypertension, diabetes mellitus, total cholesterol and high-density lipoprotein levels, and smoking status; and CVD manifestations: angina, coronary heart disease, congestive heart failure, pacemaker placement, and other cardiac surgery. CVD medications were also considered as covariates.
Outcome Measures
ECG data were analyzed using ECG interpretation software (Somté; CompuMedics). All ECG data were manually reviewed by two observers blinded to respiratory events, with arbitration by an electrophysiologist for any questionable event categorization. ECG data were scored as supraventricular, ventricular ectopic, or normal beats. P-R interval assessments were made using software-based calipers for atrioventricular block evaluation. Ventricular arrhythmias included: premature ventricular contraction, bigeminy, trigeminy, quadrigeminy, nonsustained ventricular tachycardia (defined as ⩾ 3 consecutive ventricular ectopic beats with an average rate ⩾ 100 beats/min), and a summary variable including all complex ventricular ectopy (i.e., bigeminy, trigeminy, quadrigeminy, or nonsustained ventricular tachycardia). Atrial arrhythmias identified were as follows: premature atrial contraction, supraventricular tachycardia, and atrial fibrillation. Conduction delay arrhythmias were coded as first-, second-, and third-degree atrioventricular block; intraventricular conduction delay; and sinus pause (⩾ 3 s). Arrhythmias were coded as present or absent (for dichotomous outcomes) or as continuous measures (number per hour of sleep). The estimated intraclass correlation coefficients for the two observers for a random sample of 20 sleep studies were 0.99 and 0.98 for ventricular ectopic and supraventricular beats, respectively.
Statistical Analysis
Arrhythmia subtypes (ventricular, atrial, and conduction delay) were analyzed as dichotomous outcomes using multiple logistic regression models. SDB was considered the main exposure, and covariates included age, BMI, sex, and coronary heart disease. For complex ventricular ectopy, which occurred at sufficient frequency to allow multivariable adjustments, a forward stepwise modeling approach was used to adjust for possible confounding factors. Specifically, modeling was performed first with demographic variables, then with addition of CVD risk factors, and finally with CVD manifestations, retaining those that were significant or otherwise affected the relationship between SDB and the outcome. Interactions were constructed based on variables in the final main effects model according to statistical and clinical significance.
Atrial, ventricular, and conduction delay arrhythmias per hour were also analyzed as continuous outcomes. Log-transformed values were used in linear regression models and a similar modeling building approach was used as outlined previously.
RESULTS
Primary Analyses
The characteristics of the SDB and non-SDB groups are shown in Table 1. Consistent with the study design, no sex or race differences were observed between groups; however, the SDB group was modestly older and had a higher BMI than the non-SDB group. Although the group differences were statistically significant, they were within the limits for selection. The SDB group also had a statistically lower high-density lipoprotein level, and a higher prevalence of hypertension, coronary angioplasty, coronary artery bypass graft, and myocardial infarction than the unexposed group.
TABLE 1.
SUBJECT CHARACTERISTICS BY SLEEP-DISORDERED BREATHING GROUP
| Characteristic | Exposed, SDB (n = 228) | Unexposed, No SDB (n = 338) | p Value | |||
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, y | ||||||
| Mean ± SD | 70.6 ± 9.72 | 68.6 ± 9.1 | 0.01† | |||
| Median | 71 | 68 | ||||
| Range | 46–98 | 46–91 | ||||
| BMI, kg/m2 | ||||||
| Mean ± SD | 30.1 ± 4.4 | 28.5 ± 4.3 | < 0.001† | |||
| Median | 29.9 | 28.0 | ||||
| Range | 18.7–40.0 | 18.3–39.8 | ||||
| RDI, events/h | ||||||
| Mean ± SD | 44.7 ± 13.1 | 2.7 ± 1.4 | — | |||
| Median | 41.0 | 2.8 | ||||
| Range | 30.1–90.5 | 0–5.0 | ||||
| Sex, | ||||||
| Female, % | 49.12 | 53.0 | 0.37 | |||
| Cardiovascular Disease Risk Factors | ||||||
| Hypertension, % | 58.6 | 39.7 | < 0.0001† | |||
| Diabetes mellitus, % | 19.3 | 13.6 | 0.11 | |||
| Triglycerides, mg/dl | 157.5 ± 89.8 | 155.3 ± 89.8 | 0.90 | |||
| Median | 137 | 123 | ||||
| Range | 37–662 | 35–1528 | ||||
| Cholesterol, mg/dl | 206.9 ± 37.1 | 205.4 ± 39.6 | 0.65 | |||
| Median | 206 | 206.0 | ||||
| Range | 116–363 | 95–348 | ||||
| High-density lipoprotein, mg/dl | 46.8 ± 13.6 | 50.0 ± 15.7 | 0.02† | |||
| Median | 45 | 48 | ||||
| Range | 23–98 | 17–119 | ||||
| ⩾ 20 pack-yr smoking history, % | 49.1 | 52.1 | 0.49 | |||
| Cardiovascular Disease Manifestations | ||||||
| Angina, % | 7.9 | 6.8 | 0.63 | |||
| Coronary angioplasty, % | 9.7 | 3.6 | 0.01† | |||
| Coronary artery bypass graft surgery, % | 7.0 | 3.3 | 0.06† | |||
| Myocardial infarction, % | 13.2 | 6.8 | 0.01† | |||
| Coronary heart disease, %* | 20.2 | 11.0 | 0.002† | |||
| Heart failure, % | 4.0 | 2.1 | 0.19 | |||
| Stroke, % | 4.4 | 4.1 | 0.89 | |||
| Other cardiac surgery, % | 6.6 | 3.0 | 0.04 | |||
| Pacemaker placement, % | 3.1 | 0.9 | 0.05 | |||
Definition of abbreviations: BMI = body mass index; RDI = Respiratory Disturbance Index; SDB = sleep-disordered breathing.
Continuous variable are expressed as mean ± SD, median and range; categoric variables as %. Hypertension in this table is defined only based on antihypertensive medication use.
Missing or unsure data: angina (15 unsure), myocardial infarction (6 unsure), stroke (4 unsure), coronary angioplasty (1 missing), triglycerides (31 missing), high-density lipoprotein (31 missing), cholesterol (14 missing), diabetes mellitus (4 missing), hypertension (4 missing), other cardiac surgery (3 unsure), heart failure (4 unsure), other cardiac surgery (3 missing), smoking (6 missing). Unsure and missing data were considered as absence of the variable in question.
Coronary heart disease is a composite variable representing history of myocardial infarction or coronary artery angioplasty or coronary artery bypass graft surgery.
p value < 0.05.
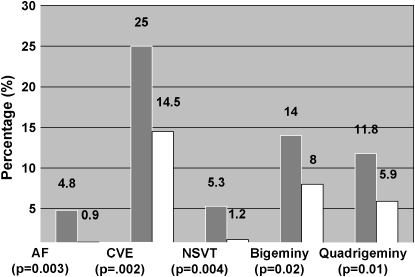
Because the issue of considering CVD as a potential confounding factor versus an intermediate factor in the SDB-arrhythmia pathway is controversial, results are presented for analyses with and without adjustment for CVD. When considering arrhythmias as dichotomous outcomes, the following arrhythmias were more common in the SDB than the non-SDB group: atrial fibrillation, nonsustained ventricular tachycardia, complex ventricular ectopy, bigeminy, and quadrigeminy (Figure 1 and Table 2). As shown in Table 3, after adjusting for age, sex, BMI, and prevalent coronary heart disease, compared with those without SDB, individuals with SDB had four times the odds of atrial fibrillation (odds ratio [OR], 4.02; 95% confidence interval [CI], 1.03–15.74), three times the odds of nonsustained ventricular tachycardia (OR, 3.40; 95% CI, 1.03–11.20), and almost twice the odds of complex ventricular ectopy (OR, 1.74; 95% CI, 1.11–2.74). Also, one-third of subjects (5 of 15) with atrial fibrillation had paroxysmal atrial fibrillation (i.e., atrial fibrillation occurring for only a portion of the sleep record). There were no significant differences between the two groups regarding conduction delay arrhythmias.
Figure 1.
Arrhythmia prevalence (%) according to sleep-disordered breathing (SDB) status. Shaded bars, SDB; open bars, non-SDB. AF, atrial fibrillation; CVE, complex ventricular ectopy; NSVT, nonsustained ventricular tachycardia. n = 228 with SDB and n = 338 without SDB.
TABLE 2.
DISTRIBUTION OF VENTRICULAR, SUPRAVENTRICULAR, AND CONDUCTION DELAY ARRHYTHMIAS ACCORDING TO SLEEP-DISORDERED BREATHING
| SDB, % (n = 228) | No SDB, % (n = 338) | Pearson's χ2 p Value | ||||
|---|---|---|---|---|---|---|
| Ventricular Arrhythmias | ||||||
| Premature Ventricular Contraction (⩾ 5/h) | 35.1 | 21.3 | 0.0003 | |||
| Bigeminy | 14.0 | 8.0 | 0.02 | |||
| Trigeminy | 9.2 | 5.6 | 0.10 | |||
| Quadrigeminy | 11.8 | 5.9 | 0.01 | |||
| Nonsustained ventricular tachycardia | 5.3 | 1.2 | 0.004 | |||
| Complex ventricular ectopy* | 25 | 14.5 | 0.002 | |||
| Supraventricular Arrhythmias | ||||||
| Premature atrial contraction (⩾ 5/h)† | 33.8 | 24.3 | 0.001 | |||
| Atrial fibrillation | 4.8 | 0.9 | 0.003 | |||
| Supraventricular tachycardia | 14.9 | 14.5 | 0.89 | |||
| Conduction Delay Arrhythmias | ||||||
| Sinus pause (⩾ 3 s) | 11.0 | 8.6 | 0.34 | |||
| First-degree atrioventricular block | 25.0 | 22.5 | 0.49 | |||
| Second-degree atrioventricular block type 1 | 1.8 | 0.3 | 0.07 | |||
| Second-degree atrioventricular block type 2 | 2.2 | 0.9 | 0.20 | |||
| Intraventricular conduction delay | 8.9 | 5.3 | 0.11 | |||
Complex ventricular ectopy is defined as bigeminy or trigeminy or quadrigeminy or nonsustained ventricular tachycardia.
Premature atrial contractions analyzed in those studies without atrial fibrillation.
TABLE 3.
ADJUSTED AND UNADJUSTED ODDS RATIOS RELATING ARRHYTHMIA OCCURRENCE AND SLEEP-DISORDERED BREATHING
| Arrhythmia Type | Unadjusted Odds Ratio | Odds Ratio* (95% CI) Adjusted for Age, Sex, BMI | Odds Ratio* (95% CI) Adjusted for Age, Sex, BMI, CHD |
|---|---|---|---|
| Nonsustained ventricular tachycardia | 4.64 (1.48–14.57) | 3.72 (1.13–12.2) | 3.40 (1.03–11.2) |
| Complex ventricular ectopy | 1.96 (1.28–3.00) | 1.81 (1.16–2.84) | 1.74 (1.11–2.74) |
| Atrial fibrillation | 5.66 (1.56–20.52) | 3.85 (1.00–14.93) | 4.02 (1.03–15.74) |
Definition of abbreviations: BMI = body mass index; CHD = coronary heart disease; CI = confidence interval.
Results of logistic regression analysis with SDB as the exposure, n = 228 with SDB and n = 338 without SDB.
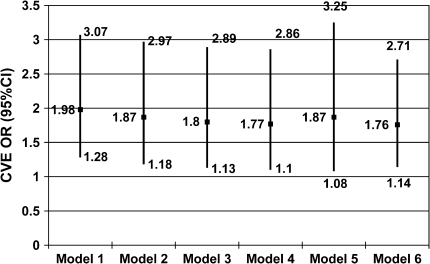
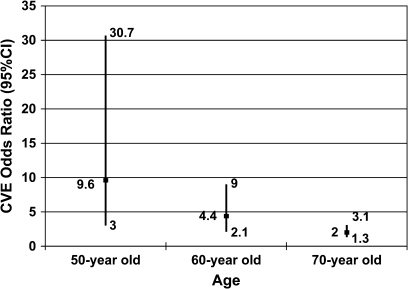
The frequency of complex ventricular ectopy (observed in approximately 24 and 15% of individuals with and without SDB, respectively) allowed for more detailed statistical modeling, with consideration of additional covariates, including CVD risk factors, CVD manifestations, and interaction terms. There were no appreciable changes in the odds ratios for the effect of SDB on complex ventricular ectopy when including alternative sets of covariates, including diabetes mellitus, hypertension, lipid profile, and congestive heart failure (Figure 2). However, in the best-fit final model, which included the covariates age and coronary heart disease, a significant interaction between SDB and age was identified. In this model, the OR for the association of complex ventricular ectopy with SDB had a significant inverse association with age (p = 0.002), with the OR (95% CI) falling from 9.3 (2.8–30.6) at age 50 to 2.0 (1.3–3.1) at age 70 (Figure 3).
Figure 2.
Odds ratios (OR; 95% confidence interval [CI]) of complex ventricular ectopy. Model 1: SDB only. Model 2: SDB and demographics (age, sex, body mass index, and race). Model 3: SDB, demographics, and cardiovascular disease (CVD) risk factors (hypertension, diabetes mellitus, cholesterol, triglycerides, high-density lipoprotein, smoking history). Model 4: SDB, demographics, CVD risk factors, and CVD manifestations (angina, coronary heart disease, congestive heart failure, stroke, pacemaker placement, other cardiac surgery). Model 5: Optimal* reduced model (SDB status, age, and coronary heart disease). Model 6: Optimal reduced model using all available observations (n = 566). Modeling performed with observations for which there was complete covariate data (n = 526). * Optimal model determination was based on statistical and clinical significance of covariates and unsure and missing data were considered as absence of the variable in question.
Figure 3.
ORs (95% CI) of complex ventricular ectopy in subjects with SDB according to age adjusted for coronary artery disease. This graph depicts the ORs (95% CI) of complex ventricular ectopy adjusted for coronary heart disease according to our final model given a 50-, 60-, and 70-yr-old person, respectively.
Similar results were obtained when arrhythmias were characterized as continuous measures (i.e., arrhythmic events/h). Using multivariable linear regression modeling, adjusting for alternative groupings of demographics, CVD risk factors, and CVD manifestations, showed that SDB status was a significant predictor of ventricular ectopic beats/h (log-transformed) in all models (Table 4). For example, in the final model, after adjusting for age, race, triglyceride level, cholesterol, and heart failure, the β coefficient for SDB was 0.56 (SE = 0.18, p = 0.001), indicating that those exposed to SDB were estimated to have a frequency of ventricular ectopic beats/h 75% (95% CI, 24–147%) larger than those without SDB. After excluding the atrial fibrillation studies, there was a nonstatistically significant trend in both adjusted and unadjusted analyses for more supraventricular beats/h in the SDB group (p values for SDB ranged from p = 0.13 for unadjusted to p = 0.14 for adjusted analyses).
TABLE 4.
SLEEP-DISORDERED BREATHING AND VENTRICULAR ECTOPIC BEATS/h USING MULTIVARIABLE LINEAR REGRESSION
| Model | SDB Status β Coefficient | SDB Status SE | SDB Status p Value |
|---|---|---|---|
| Ventricular Ectopic Beats/h (n = 504) | |||
| Unadjusted | 0.89 | 0.22 | < 0.0001 |
| Demographics | 0.79 | 0.21 | 0.0005 |
| CVD risk factors | 0.83 | 0.22 | 0.0002 |
| CVD manifestations | 0.88 | 0.21 | < 0.0001 |
| Demographics + CVD risk factors* | 0.78 | 0.21 | 0.0003 |
| Demographics + CVD risk factors + CVD manifestations† | 0.76 | 0.22 | 0.0004 |
| Demographics + CVD risk factors + CVD manifestations‡ | 0.69 | 0.21 | 0.0008 |
“Model” shows which set of variables used and the resulting effect size and statistical significance for SDB. This analysis is on the subset of patients with complete data on the above variables. The natural logarithm of ventricular ectopic beats/h was used, and to account for values of zero, a constant of 0.05 was added before log transformation.
Age, race, hypertension, triglycerides, and cholesterol kept in the model.
Age, race, triglycerides, cholesterol, and heart failure kept in the model (reduced data, n = 503).
Age, race, triglycerides, cholesterol, and heart failure kept in the model (all data, n = 535).
Secondary Analyses
Within the SDB group only (i.e., RDI ⩾ 30), additional exploratory analyses were performed assessing whether an increased odds (for dichotomous outcomes) or increased frequency of arrhythmias (for continuous outcomes) was observed with increasing severity of SDB as measured by RDl, arousal index, and percentage of sleep time spent below 90% oxygen saturation. Within this group of subjects with severe SDB, no evidence of a dose–response relationship between any of these indices and arrhythmia outcomes were observed across this severity range. Further analyses were performed with sleep efficiency included as a covariate in regression models of SDB and specific arrhythmia type revealing no appreciable change in the strength of SDB–arrhythmia associations.
DISCUSSION
Principal Findings
To our knowledge, this is the first epidemiologic study incorporating rigorous data collection from a large community-based sample to evaluate the association between SDB and nocturnal cardiac arrhythmias. Our primary analyses demonstrate a significant increase in the prevalence of atrial fibrillation, nonsustained ventricular tachycardia, and complex ventricular ectopy among subjects with severe SDB compared with subjects without SDB; these associations persisted after adjustment for potential confounders. Modeling of these dichotomous outcomes was mirrored by parallel findings modeling ventricular ectopic beats as a continuous outcome. Furthermore, when modeling complex ventricular ectopy and extensively adjusting for potential confounders, a significant age–SDB interaction was identified, indicating that, in our sample, SDB is much more strongly associated with complex ventricular ectopy in the younger members of our cohort compared with older cohort members.
It has been controversial whether there is a relation between SDB and nocturnal cardiac arrhythmias. One of the first studies to examine the SDB–arrhythmia relationship reported that 193 of 400 patients (48%) with severe SDB demonstrated cardiac arrhythmias after evaluation by PSG and 24-h Holter monitoring (14). In this study, only patients with severe SDB (mean RDI = 42) were assessed, and there was no referent group or adjustment for potential confounders, including cardiovascular comorbidity. Of note, the arrhythmias in general tended to occur during the sleep period as opposed to periods of wakefulness. Two subsequent studies in clinically referred patient samples yielded conflicting results regarding the SDB–arrhythmia relationship (15, 16). The first involved 173 patients referred for sleep apnea assessment. No differences in the prevalence of ventricular arrhythmias, atrioventricular block, and sinus arrest were noted between the 76 subjects diagnosed with SDB (most of whom had little oxygen desaturation) compared with the remaining referred patients who did not meet criteria for SDB (15). The second study involved a prospective evaluation of 458 patients referred to the sleep clinic, 26.4% of whom had SDB (16). Although the SDB group had a greater frequency of arrhythmias, this report did not provide evidence that group differences were independent of confounding.
A preliminary report provided provocative data indicating that patients with untreated SDB and atrial fibrillation have a higher recurrence of atrial fibrillation after cardioversion than patients without a known SDB diagnosis (17). Although this report was based on a referral sample, their findings are consistent with our fourfold higher prevalence of atrial fibrillation in the SDB group. This group of investigators also found that the proportion of patients with sleep apnea was significantly higher in a group of patients with atrial fibrillation than in a control group (49 vs. 32%, p = 0.0004) (18). In our sample, one-third of the subjects with atrial fibrillation had paroxysmal versus persistent atrial fibrillation. Paroxysmal atrial fibrillation is postulated to be less likely to be associated with structural heart disease, more likely to occur in younger persons, and paroxysmal atrial fibrillation episodes tend to be preceded by fluctuations in autonomic tone, which is consistent with dynamic changes occurring in SDB (19–22).
Two recent studies support a relationship between sleep apnea and fatal and nonfatal cardiovascular events. A recent retrospective study demonstrated that, for people with obstructive sleep apnea, the relative risk of sudden death from cardiac causes from midnight to 6:00 a.m. was 2.57 (95% CI, 1.87–3.52) (23). Patients with untreated severe sleep apnea had a higher incidence of fatal cardiovascular events and nonfatal cardiovascular events than untreated patients with mild–moderate disease and healthy participants in another recent study (24). Our data showing an increased rate of serious nocturnal arrhythmias in individuals with SDB provide one potential explanation for the observed increase in sudden death with sleep apnea. Another recent study demonstrated improvement in ventricular ectopy and sympathetic activation after continuous positive airway pressure treatment in heart failure patients with sleep apnea, thereby supporting a sleep apnea–arrhythmia relationship (25).
The finding of an age–SDB interaction in our model of complex ventricular ectopy prevalence, showing stronger associations in middle-aged than older individuals, is consistent with results from studies of the association of SDB with hypertension and mortality, which also have demonstrated stronger associations in younger versus older individuals (26–28). Specifically, the complex ventricular ectopy OR fell from 9.58 (95% CI, 2.99–30.65) at age 50 to 1.98 (95% CI, 1.26–3.10) at age 70. Potential explanations for a weaker association in older subjects may relate to age-related differences in the nature of SDB or in physiologic responses to SDB. For example, differences in autonomic nervous system responses in older individuals may mitigate their likelihood of developing arrhythmias in response to hypoxia. However, this observed age-dependency may also be a result of survival bias or competitive risks that are difficult to assess in cross-sectional analyses.
Strengths and Limitations
The strengths of the present study include the large community-based sample, collection of detailed covariate data, highly standardized rigorous data collection, and blinded assessment of ECG data. The community-based nature of this study minimizes the possibility of selection bias and increases the generalizability of findings. Accounting for potential confounding variables, including detailed assessment of CVD and CVD risk factors, is a strength of this study. The imbalance of cardiovascular risk factors among the SDB and non-SDB groups was handled by two mechanisms. First, the control subjects were selected to constitute a group that was similar regarding factors that correlate with CVD (age [± 2 yr], BMI [± 2 kg/m2], sex, and race) to match the SDB group. Second, regression and logistic analyses used data on potential confounders in statistical models to further adjust for any residual differences in groups. Although we did model hypertension and diabetes as covariates, we did not attempt to match on these measures since they may represent intermediate pathways linking SDB and arrhythmias.
Several limitations of the present study should be noted. Only a single bipolar lead was used for ECG analysis, which prevented us from evaluating an abnormal axis and ST-T wave abnormalities. We sampled the extremes of the SDB spectrum (SDB defined as RDI ⩾ 30 events/h and non-SDB defined as RDI ⩽ 5), which increased the efficiency of the study, but limited the ability to evaluate an intermediate threshold (between RDI of 5 and 30) associated with an increased prevalence of arrhythmias, and limited our ability to assess dose–response relationships. This study evaluated ECG data from the sleep period only, so we could not evaluate the association between daytime (or wake) and nocturnal (or sleep) rhythm disturbances. Although the sample was community-based, it was not constructed to be a fully representative population-based sample. However, because it is unlikely that the presence of nocturnal arrhythmias influenced willingness to participate in the SHHS, this sample should be free from the referral biases that may be present in clinic samples and should be internally valid. As in any observational study, residual confounding by unmeasured variables cannot be excluded, despite detailed adjustment for CVD and CVD risk factors. Finally, as this was a cross-sectional study, we cannot prove that SDB preceded the development of arrhythmias, although animal models support the biologic plausibility of a causal association between SDB and arrhythmias (29, 30).
Clinical Implications and Future Research Directions
The results of this study have potentially important clinical implications, because they suggest an increased vulnerability to nocturnal cardiac arrhythmias of individuals with SDB and provide an explanation for the observed increase in sudden nocturnal death recently reported with sleep apnea (23). Whether the presence of arrhythmias influences prognosis in SDB is uncertain; if so, this would provide a rationale for systematic arrhythmia detection and reporting in the sleep laboratory (31). Our findings also may help explain the epidemic of atrial fibrillation, because increasing SDB prevalence from increasing obesity may explain a portion of the unexplained rise in atrial fibrillation (32–34). Systematic demonstration of a direct temporal association between each arrhythmia and a preceding respiratory event would enhance causal inferences and is an area of potential future research. Further work is also needed to quantify the population attributable risk of atrial fibrillation and other arrhythmias associated with SDB, the prognostic implications of these arrhythmias, and the ability to prevent or reverse these arrhythmias with SDB treatment.
Supplementary Material
Acknowledgments
The authors thank Lydia Cartar, M.A., and Amy Storfer-Isser, M.S., for their statistical input, and Susan Surovec for her assistance with software issues and data processing. Dr. Mehra had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The Sleep Heart Health Study acknowledges the Atherosclerosis Risk in Communities Study, the Cardiovascular Health Study, the Framingham Heart Study, the Cornell/Mt. Sinai Worksite and Hypertension Studies, the Sleep Heart Health Study, the Tucson Epidemiologic Study of Airways Obstructive Diseases, and the Tucson Health and Environment Study for allowing their cohort members to be part of the Sleep Heart Health Study and for permitting data acquired by them to be used in the study. Sleep Heart Health Study is particularly grateful to the members of these cohorts who agreed to participate in Sleep Heart Health Study as well. Sleep Heart Health Study further recognizes all of the investigators and staff who have contributed to its success. A list of Sleep Heart Health Study investigators, staff, and their participating institutions is available on the Sleep Heart Health Study website, http://www.jhucct.com/shhs/details/sites.htm.
Sleep Heart Health Study Investigators: Arizona: Barbara V. Howard, Medlantic Research Institute (Strong Heart Study—Phoenix Center), Phoenix; Stuart F. Quan, Michael D. Lebowitz, Paul L. Enright, Richard R. Bootzin, Anthony E. Camilli, Bruce M. Coull, Russell R. Dodge, Gordon A. Ewy, Steven R. Knoper, and Linda S. Snyder, University of Arizona, Phoenix (Strong Heart Study—Phoenix Center); California: John A. Robbins and William H. Bonekat, University of California, Davis, Sacramento; Maryland: James P. Kiley and Richard R. Fabsitz, National Heart, Lung, and Blood Institute Project Office, Bethesda; F. Javier Nieto, Jonathan M. Samet, Joel G. Hill, Alan R. Schwartz, Philip L. Smith, and Moyses Szklo, Johns Hopkins University, Washington County; Massachusetts: George T. O'Connor, Sanford H. Auerbach, Emilia J. Benjamin, Ralph B. D'Agostino, Rachel J. Givelber, Daniel J. Gottlieb, and Philip A. Wolf, Boston University, Framingham; Minnesota: Eyal Shahar, Conrad Iber, Mark W. Mahowald, Paul G. McGovern, Lori L. Boland, Sherry Nooyen, Matthew Hill, and Rita Smith, University of Minnesota, Minneapolis; New York: Thomas G. Pickering and Gary D. James, Cornell University, Ithaca; Velvie A. Pogue and Charles K. Francis, Columbia University (Harlem Hospital), New York; David M. Rapoport and Joyce A. Walseben, New York University, New York; Joseph E. Schwartz, State University of New York at Stony Brook, Stony Brook; Ohio: Susan Redline, Carl E. Rosenberg, and Kingman P. Strohl, Sleep Reading Center, Case Western Reserve University, Cleveland; Oklahoma: Elisa T. Lee and J. L. Yeah, University of Oklahoma (Strong Heart Study—Oklahoma Center), Oklahoma City; Pennsylvania: Anne B. Newman and Mark H. Sanders, University of Pittsburgh, Pittsburgh; South Dakota: Thomas K. Welty, Missouri Breaks Research Institute (Strong Heart Study—Dakota Center), Rapid City; Washington: Patricia W. Wahl, Bonnie K. Lind, Vishesh K. Kapur, David S. Siscovick, and Qing Yao, Coordinating Center, University of Washington, Seattle; and Wisconsin: Terry B. Young, University of Wisconsin, Madison.
Supported by National Heart, Lung and Blood Institute cooperative agreements U01HL53940 (University of Washington), U01HL53941 (Boston University), U01HL53938 (University of Arizona), U01HL53916 (University of California, Davis), U01HL53934 (University of Minnesota), U01HL53931 (New York University), U01HL53937 and U01HL64360 (Johns Hopkins University), U01HL63463 (Case Western Reserve University), and U01HL63429 (Missouri Breaks Research).
The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the Indian Health Service.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200509-1442OC on January 19, 2006
Conflict of Interest Statement: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Narkiewicz K, van de Borne PJ, Pesek CA, Dyken ME, Montano N, Somers VK. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation 1999;99:1183–1189. [DOI] [PubMed] [Google Scholar]
- 2.Dimsdale JE, Loredo JS, Profant J. Effect of continuous positive airway pressure on blood pressure: a placebo trial. Hypertension 2000;35:144–147. [DOI] [PubMed] [Google Scholar]
- 3.Narkiewicz K, van de Borne PJ, Cooley RL, Dyken ME, Somers VK. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation 1998;98:772–776. [DOI] [PubMed] [Google Scholar]
- 4.Zipes D. Autonomic modulation of cardiac arrhythmias. In: Jalife J, Zipes DP, editors. Cardiac electrophysiology: from cell to bedside, 2nd ed. Philadelphia: WB Saunders; 1995. pp. 441–454.
- 5.Wit AL, Rosen AR. After depolarizations and triggered activity. In: Fossard HA, Haber E, Jenning RB, Katz M, Morgan E, editors. The heart and cardiovascular system. New York: Raven Press; 1986. pp. 1449–1490.
- 6.Han J, Millet D, Chizzonitti B, Moe GK. Temporal dispersion of recovery of excitability in atrium and ventricle as a function of heart rate. Am Heart J 1966;71:481–487. [DOI] [PubMed] [Google Scholar]
- 7.Conti JB. Cardiac arrhythmias. J Am Coll Cardiol 2005;45:30B–32B. [DOI] [PubMed] [Google Scholar]
- 8.Franz MR. Mechano-electrical feedback in ventricular myocardium. Cardiovasc Res 1996;32:15–24. [PubMed] [Google Scholar]
- 9.Mehra R, Nawabit R, Kirchner HL, Shahar E, Benjamin EJ, Gottlieb DJ, Redline S, Association of Nocturnal Arrhythmias with Sleep-Disordered Breathing. Sleep abstracts. Philadelphia: Associated Professionals Sleep Societies; 2004.
- 10.Quan SF, Howard BV, Iber C, Kiley JP, Nieto FJ, O'Connor GT, Rapoport DM, Redline S, Robbins J, Samet JM, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep 1997;20:1077–1085. [PubMed] [Google Scholar]
- 11.Redline S, Sanders MH, Lind BK, Quan SF, Iber C, Gottlieb DJ, Bonekat WH, Rapoport DM, Smith PL, Kiley JP. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep 1998;21:759–767. [PubMed] [Google Scholar]
- 12.Sleep Heart Health Study. Available from: http://www.jhucct.com/shhs/ (accessed February 7, 2006).
- 13.Whitney CW, Gottlieb DJ, Redline S, Norman RG, Dodge RR, Shahar E, Surovec S, Nieto FJ. Reliability of scoring respiratory disturbance indices and sleep staging. Sleep 1998;21:749–757. [DOI] [PubMed] [Google Scholar]
- 14.Guilleminault C, Connolly SJ, Winkle RA. Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am J Cardiol 1983;52:490–494. [DOI] [PubMed] [Google Scholar]
- 15.Flemons WW, Remmers JE, Gillis AM. Sleep apnea and cardiac arrhythmias; is there a relationship? Am Rev Respir Dis 1993;148:618–621. [DOI] [PubMed] [Google Scholar]
- 16.Hoffstein V, Mateika S. Cardiac arrhythmias, snoring, and sleep apnea. Chest 1994;106:466–471. [DOI] [PubMed] [Google Scholar]
- 17.Kanagala R, Murali NS, Friedman PA, Ammash NM, Gersh BJ, Ballman KV, Shamsuzzaman AS, Somers VK. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003;107:2589–2594. [DOI] [PubMed] [Google Scholar]
- 18.Gami AS, Pressman G, Caples SM, Kanagala R, Gard JJ, Davison DE, Malouf JF, Ammash NM, Friedman PA, Somers VK. Association of atrial fibrillation and obstructive sleep apnea. Circulation 2004;110:364–367. [DOI] [PubMed] [Google Scholar]
- 19.Petersen P, Godtfredsen J. Embolic complications in paroxysmal atrial fibrillation. Stroke 1986;17:622–626. [DOI] [PubMed] [Google Scholar]
- 20.Sherman DG, Goldman L, Whiting RB, Jurgensen K, Kaste M, Easton JD. Thromboembolism in patients with atrial fibrillation. Arch Neurol 1984;41:708–710. [DOI] [PubMed] [Google Scholar]
- 21.Kannel WB, Abbott RD, Savage DD, McNamara PM. Coronary heart disease and atrial fibrillation: the Framingham Study. Am Heart J 1983;106:389–396. [DOI] [PubMed] [Google Scholar]
- 22.Bettoni M, Zimmermann M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation 2002;105:2753–2759. [DOI] [PubMed] [Google Scholar]
- 23.Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med 2005;352:1206–1214. [DOI] [PubMed] [Google Scholar]
- 24.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365:1046–1053. [DOI] [PubMed] [Google Scholar]
- 25.Ryan CM, Usui K, Floras JS, Bradley TD. Effect of continuous positive airway pressure on ventricular ectopy in heart failure patients with obstructive sleep apnoea. Thorax 2005;60:781–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000;283:1829–1836. [DOI] [PubMed] [Google Scholar]
- 27.Lavie P, Herer P, Peled R, Berger I, Yoffe N, Zomer J, Rubin AH. Mortality in sleep apnea patients: a multivariate analysis of risk factors. Sleep 1995;18:149–157. [DOI] [PubMed] [Google Scholar]
- 28.Millman RP, Redline S, Carlisle CC, Assaf AR, Levinson PD. Daytime hypertension in obstructive sleep apnea: prevalence and contributing risk factors. Chest 1991;99:861–866. [DOI] [PubMed] [Google Scholar]
- 29.Sato T, Saito H, Seto K, Takatsuji H. Sleep apneas and cardiac arrhythmias in freely moving rats. Am J Physiol 1990;259:R282–R287. [DOI] [PubMed] [Google Scholar]
- 30.Horner RL, Brooks D, Kozar LF, Gan K, Phillipson EA. Respiratory-related heart rate variability persists during central apnea in dogs: mechanisms and implications. J Appl Physiol 1995;78:2003–2013. [DOI] [PubMed] [Google Scholar]
- 31.Punjabi NM, Welch D, Strohl K. Sleep disorders in regional sleep centers: a national cooperative study. Coleman II Study Investigators. Sleep 2000;23:471–480. [DOI] [PubMed] [Google Scholar]
- 32.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 33.Wattigney WA, Mensah GA, Croft JB. Increased atrial fibrillation mortality: United States, 1980–1998. Am J Epidemiol 2002;155:819–826. [DOI] [PubMed] [Google Scholar]
- 34.Fuster V, Ryden LE, Asinger RW, Cannom DS, Crijns HJ, Frye RL, Halperin JL, Kay GN, Klein WW, Levy S, et al. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: executive summary a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation) developed in collaboration with the North American Society of Pacing and Electrophysiology. Circulation 2001;104:2118–2150. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.