Abstract
Rationale: Treatment of latent tuberculosis (TB) infection with weekly rifapentine and isoniazid is a potentially effective alternative to current therapies.
Objectives: To compare the efficacy of weekly rifapentine/isoniazid to daily rifampin/pyrazinamide in preventing TB in household contacts of patients with pulmonary TB in Brazil.
Methods: Contacts of patients with TB were randomized to rifapentine 900 mg/isoniazid 900 mg once weekly for 12 wk or rifampin 450–600 mg/pyrazinamide 750–1,500 mg daily for 8 wk and followed for at least 2 yr.
Measurements: TB rates, adverse events, and adherence to therapy.
Main Results: A total of 399 household contacts were enrolled, 206 in the rifapentine/isoniazid arm and 193 in the rifampin/pyrazinamide arm. The median age was 34 yr, median weight was 63 kg, 60% of participants were female, and only one patient was HIV infected. Rifapentine/isoniazid was well tolerated, but the trial was halted by the investigators before completion because of unanticipated hepatotoxicity in the rifampin/pyrazinamide arm. Twenty of 193 participants (10%) receiving rifampin/pyrazinamide experienced grade 3 or 4 hepatotoxicity, compared with 2 of 206 participants (1%) on rifapentine/isoniazid (p < 0.001). There were no hospitalizations or deaths due to hepatotoxicity, and all participants' liver enzyme levels returned to normal during follow-up. During follow-up, four cases of active TB developed, three in the rifapentine/isoniazid group and one in the rifampin/pyrazinamide group (1.46 vs. 0.52%; difference, 0.94%; 95% confidence interval, −1.6 to 3.7%).
Conclusions: Rifapentine/isoniazid was better tolerated than rifampin/pyrazinamide and was associated with good protection against TB. Rifapentine/isoniazid weekly for 12 wk is likely a promising therapy for latent TB infection.
Keywords: controlled clinical trial, latent tuberculosis, pyrazinamide, rifampin, rifapentine
Treatment of latent Mycobacterium tuberculosis infection is an important component of tuberculosis (TB) control (1, 2) In Western countries, targeted testing and treatment of latently infected individuals is recommended for those at increased risk of developing active TB, including contacts of infectious cases, individuals with HIV infection and other conditions that reduce host resistance to TB, and immigrants from endemic areas, among others (1). Chemoprophylaxis of high-risk patients using isoniazid (INH) reduces the risk of developing active disease by 60 to 90%, and is the standard of care throughout the world (3). Although INH preventive therapy is highly efficacious and inexpensive, its use may be limited by toxicity and poor adherence with the long duration of treatment required for patients with no symptoms. Consequently, the development of alternative, shorter regimens to treat latent TB infection has become a priority (4).
Rifamycin antibiotics have greater potency against the dormant and semidormant organisms that characterize latent TB infection than INH alone, and may be effective when given for shorter durations than required for INH (5). Several studies of rifampin-based short-course regimens for latent TB have shown similar or superior efficacy to longer courses of INH (6–9). Rifapentine is a long-acting rifamycin with activity comparable to rifampin but with a prolonged half-life that permits weekly dosing (10). Trials of rifapentine given with INH once weekly during the continuation phase of treatment in HIV-uninfected patients with TB showed satisfactory efficacy, particularly in patients with relatively low bacillary burdens (11, 12). Several studies of rifapentine in murine models of chronic or latent TB suggested that weekly treatment in combination with INH was effective (13, 14).
We reasoned that a once-weekly regimen of rifapentine and INH for 12 wk would be efficacious for the treatment of latent TB in high-risk individuals. We therefore conducted a phase 2 trial comparing the safety and efficacy of a weekly dosing regimen of rifapentine and INH to daily rifampin and pyrazinamide, a regimen shown to be equivalent to INH alone in HIV-infected patients with latent TB infection (7–9). Rifampin and pyrazinamide were selected as the control regimen because of their known efficacy, and because we believed that recruitment of patients would be facilitated by having two relatively similar short courses of therapy rather than one short and one long regimen. This study was presented in abstract form at the American Thoracic Society International Conference in May 2005 (15).
METHODS
Households contacts of patients with newly diagnosed pulmonary TB at public clinics in Rio de Janeiro who slept 2 nights or more per week in the same dwelling as the index case were recruited because of their high risk of TB (1). Contacts were tuberculin skin-tested using RT-23 purified protein derivative, read at 2 to 7 d by an experienced reader. Subjects 18 yr or older, with an induration of 5 mm or more, no TB symptoms, and a chest radiograph without evidence of active TB were offered enrollment; patients with comorbid conditions were eligible to enroll, but contacts could have no evidence of liver or renal dysfunction or anemia, and could not ever have received TB drugs for more than 1 mo. Participants signed informed consent, and the protocol was approved by the Johns Hopkins Medicine, Federal University of Rio de Janeiro, and Brazilian Ministry of Health institutional review boards.
The first enrolled contact of an index patients with TB was randomized to a treatment regimen, and subsequent contacts of that index case received the same treatment arm. Allocating all contacts of a given index case to the same treatment arm was done to avoid confusion within households, ensuring that all members of the household were following the same treatment schedule. A computer-generated 1:1 randomization schema with variable block size was used, and allocation was concealed. Treatment was either rifapentine 900 mg and INH 900 mg once weekly for 12 wk or rifampin 450 mg and pyrazinamide 750 mg (weight < 50 kg) or rifampin 600 mg and pyrazinamide 1,500 mg (weight ⩾ 50 kg) once daily for 8 wk (16). Rifapentine/INH ingestion was directly observed in the clinic; daily rifampin/pyrazinamide recipients took one observed dose per week at the clinic and the remainder by self-administration. Participants had complete blood counts and serum chemistries at baseline, and were offered HIV serology by ELISA with Western blot confirmation.
At weekly follow-up visits, participants were questioned about drug toxicity and signs and symptoms of TB. Initially, a 1-mo liver chemistry test was planned, but after the trial began, reports appeared linking rifampin/pyrazinamide to hepatotoxicity, and the protocol was amended to perform serum chemistry tests on all patients every 2 wk during therapy, in accordance with published guidelines (17, 18). After completion of treatment, participants assigned to rifampin and pyrazinamide were seen in the study clinic after 1 mo and then every 3 mo thereafter, whereas participants assigned to rifapentine and INH were seen every 3 mo to ascertain clinical status. Patients reporting any symptoms suggestive of TB were evaluated with chest X-rays and sputum examinations for acid-fast bacilli smear and culture. Those missing follow-up appointments were contacted by outreach workers. TB diagnoses were confirmed by reviewing medical records and health department databases, based on mandatory reporting of all TB in Brazil.
For this phase 2 equivalence study, we assumed the risk of developing TB in untreated contacts would be 8% over 2 yr based on previous research in Rio de Janeiro (19). We estimated that the efficacy of rifampin and pyrazinamide would be 90%, resulting in a case rate of 0.8%. The study was powered to demonstrate that rifapentine and INH would be equivalent to rifampin and pyrazinamide, defined as no more than a 3.2% absolute difference in TB rates. If the rate of TB in the rifapentine and INH arm was 4% or less, efficacy versus no treatment would be 50%, and we reasoned that the convenience of the once-weekly regimen would make such a difference clinically acceptable. Setting power at 80% with a two-tailed α level of 0.05, we planned to enroll a total of 720 participants, with 360 allocated to each treatment arm.
Comparisons of baseline variables in the two treatment groups were performed using Fisher's exact test and t tests, as appropriate. For outcomes, comparisons between study arms were performed using Fisher's exact test, and odds ratios and associated 95% confidence intervals were calculated for TB events. Rates of TB per 100 person-years of follow up were also determined.
RESULTS
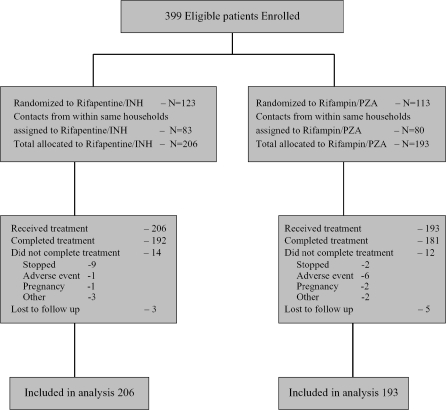
Enrollment began in January 2001. Interim analyses of toxicity were performed twice yearly thereafter, with review by an independent data safety and monitoring board. After the May 2003 interim analysis, the data safety and monitoring board recommended continuation of the study, but enrollment was temporarily suspended at the request of the National Institute of Allergy and Infectious Diseases to permit further institutional review board consideration of higher than expected rates of hepatotoxicity in participants receiving rifampin and pyrazinamide. In July 2003, the study team permanently stopped enrollment into the trial in anticipation of new guidelines from the Centers for Disease Control and Prevention stating that rifampin and pyrazinamide should generally not be used for treating latent TB because of high rates of liver injury and death reported in clinical practice (20). In light of hepatotoxicity occurring among participants receiving rifampin and pyrazinamide in the trial, the investigators concluded that assignment of additional participants to this therapy was unwarranted. Thus, the trial enrolled 399 subjects of an expected sample size of 720. Follow-up of all participants continued through April 2005 for ascertainment of TB, late toxicity, and vital status. Retention in the protocol was 98%, with only eight participants lost to follow-up. Figure 1 shows the disposition of participants enrolled in the trial.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram of patient recruitment and distribution during the trial. INH = isoniazid; PZA = pyrazinamide.
Participants assigned to the two treatment regimens had similar baseline characteristics, shown in Table 1. Over half of participants were female and the mean age was 37 yr. The majority were unemployed, and most had low educational levels. Two-thirds had received bacille Calmette-Guèrin vaccination, which is routinely given in infancy in Brazil. Only one patient had HIV infection, and there were very few other comorbidities associated with risk of TB; thus, these factors were not further analyzed. Baseline laboratory tests were similar in both groups as well (Table 2).
TABLE 1.
BASELINE CHARACTERISTICS BY TREATMENT ARM
| Variable | RPT/INH | RZ | All |
|---|---|---|---|
| No. subjects | 206 | 193 | 399 |
| Index cases, n | 123 | 113 | 236 |
| Mean age | 37.7 | 37.0 | 37.4 |
| Sex, M/F, % | 37/63 | 44/56 | 40/60 |
| Mean PPD size, mm | 12.8 | 13.4 | 13.1 |
| Marital status, % married | 32 | 35 | 33 |
| Race/ethnicity, % | |||
| White | 59.2 | 58.6 | 58.9 |
| Black | 20.8 | 17.6 | 19.2 |
| Mixed | 15.1 | 17.1 | 16.1 |
| Asian | 4.9 | 6.7 | 5.8 |
| Employment, % employed | 52 | 72 | 62 |
| Education | |||
| Completed primary, % | 17 | 7 | 12 |
| Completed secondary, % | 14 | 15 | 14 |
| HIV, % | 0.5 | 0 | |
| BCG vaccination, % | 65 | 66 | 65 |
| Smoker, % | 22 | 25 | 23 |
| Alcohol, % | 34.5 | 38 | 36 |
| Injection drug use, % | 0 | 0.5 | 0.3 |
| Ever in prison, % | 0 | 0 | 0 |
| Ever arrested, % | 1.5 | 0 | 0.8 |
Definition of abbreviations: BCG = bacille Calmette-Guèrin; INH = isoniazid; RPT = rifapentine; RZ = rifampin and pyrazinamide.
TABLE 2.
BASELINE LABORATORY VALUES BY TREATMENT ARM
| Variable | RPT/INH | RZ | All |
|---|---|---|---|
| Mean hematocrit, % | 41.5 | 41.3 | 41.4 |
| Mean white blood cells, per cc | 6,612 | 6,756 | 6,682 |
| Mean AST, IU | 24 | 24 | 24 |
| Mean total bilirubin, mg/dl | 0.69 | 0.60 | 0.65 |
| Mean direct bilirubin, mg/dl | 0.16 | 0.15 | 0.155 |
| Mean indirect bilirubin, mg/dl | 0.59 | 0.46 | 0.48 |
Definition of abbreviations: AST = aspartate aminotransferase; INH = isoniazid; RPT = rifapentine; RZ = rifampin and pyrazinamide.
Of the 193 contacts of 113 index patients who were randomized to receive rifampin and pyrazinamide, 181 completed treatment and 12 stopped treatment before completion. For the 206 contacts of 123 patients randomized to receive rifapentine and isoniazid, 192 completed treatment and 14 terminated before completion (p = 0.82). Adherence was excellent in both arms; among patients who completed the rifapentine and isoniazid arm, all took 12 weekly doses, and among those completing the rifampin and pyrazinamide arm, all took eight weekly supervised doses, and reported adherence to the remainder of treatment was more than 95%. Reasons for premature discontinuation of treatment in the rifapentine arm included adverse drug reactions (1 patient), pregnancy (1 patient), patient preference (9 patients), and other reasons (3 patients). Participants discontinued rifampin and pyrazinamide because of adverse drug reactions (6 patients), pregnancy (2 patients), patient preference (2 patients), and other reasons (2 patients). Participants who discontinued therapy prematurely were monitored according to the study protocol and included in all analyses. The mean follow-up was 2.7 yr in each arm.
The rifapentine and INH regimen was associated with significantly less toxicity than rifampin and pyrazinamide (Table 3). Grade 3 (aspartate aminotransferase [AST] or alanine aminotransferase [ALT] 5–10 times upper limit of normal) or 4 (AST or ALT > 10 times upper limit of normal) hepatotoxicity occurred in 2 of 206 (1%) participants assigned to rifapentine and INH versus 20 of 193 (10%) participants assigned to rifampin and pyrazinamide (p < 0.001). The mean and median AST and ALT in participants assigned to rifapentine remained within normal limits and did not change over the first 8 wk of therapy, whereas mean, but not median, AST and ALT levels rose in participants assigned to rifampin and pyrazinamide (Table 4). Thus, most participants tolerated the rifampin/pyrazinamide regimen with no evidence of liver injury, but a significant minority had substantial elevations in liver enzymes. No patient had symptomatic hepatotoxicity, and none were hospitalized. Hepatotoxicity did not occur before 4 wk, and the majority of cases (13/20) were detected only at Week 8, after treatment was completed. Participants treated with rifampin and pyrazinamide who developed hepatotoxicity had similar baseline characteristics as those who did not, with no differences in sex, age, initial AST or bilirubin levels, or body weight (Table 5). Dosages of pyrazinamide and rifampin did not differ between those with and without liver toxicity. One patient who developed hepatotoxicity while taking rifampin and pyrazinamide also reported heavy alcohol intake, which was believed to contribute to this adverse event. All participants had resolution of hepatotoxicity within 4 to 12 wk. Subsequent testing of 16 of the subjects with hepatotoxicity showed evidence of prior hepatitis A in all (hepatitis A IgG antibody positive), but no evidence of acute viral hepatitis (hepatitis A IgM antibody negative, hepatitis B surface antigen negative). Other participants were not tested for hepatitis A antibodies, which are positive in the majority of Brazilian adults (21, 22).
TABLE 3.
ADVERSE EVENTS DURING FOLLOW-UP
| Variable | RPT/INH | RZ | All |
|---|---|---|---|
| Death | 1 | 3 | 4 |
| Grade 3 hepatotoxicity* | 2 | 14 | 16 |
| Grade 4 hepatotoxicity* | 0 | 11 | 11 |
| Either grade 3 or 4 toxicity* | 2 | 20 | 22 |
| Pregnancy | 1 | 4 | 5 |
Definition of abbreviations: INH = isoniazid; RPT = rifapentine; RZ = rifampin and pyrazinamide.
Grade 3 hepatotoxicity defined as aspartate aminotransferase or alanine aminotransferase 5 to 10 times upper limit of normal; grade 4 hepatotoxicity defined as aspartate aminotransferase or alanine aminotransferase > 10 times upper limit of normal. If initial abnormality was grade 3 but subsequent testing revealed grade 4, patients were counted in both categories.
TABLE 4.
AST AND ALT LEVELS BY TREATMENT ARM THROUGHOUT THE TRIAL
| RPT/INH
|
RZ
|
|||||
|---|---|---|---|---|---|---|
| Variable | Mean | Median | n | Mean | Median | n |
| Week 0 | ||||||
| AST | 23.3 | 22 | 206 | 23.2 | 22 | 193 |
| ALT | 25.5 | 27 | 206 | 20.3 | 19 | 193 |
| Week 2 | ||||||
| AST | 24.3 | 23 | 131 | 25.8 | 24 | 130 |
| ALT | 234 | 20 | 131 | 24.5 | 21 | 130 |
| Week 4 | ||||||
| AST | 24.5 | 23 | 161 | 38.1 | 26 | 154 |
| ALT | 23.1 | 20 | 161 | 48.9 | 25 | 154 |
| Week 6 | ||||||
| AST | 26.4 | 23 | 133 | 43.2 | 27 | 127 |
| ALT | 25.7 | 20 | 133 | 59.0 | 25 | 127 |
| Week 8 | ||||||
| AST | 24.5 | 22 | 150 | 48.6 | 26 | 156 |
| ALT | 25.0 | 19 | 150 | 70.1 | 27.5 | 156 |
| Week 10 | ||||||
| AST | 26.6 | 22 | 64 | 80.0 | 25 | 58 |
| ALT | 26.0 | 18 | 64 | 115.8 | 27 | 58 |
| Week 12 | ||||||
| AST | 23.4 | 22 | 174 | 36.4 | 24 | 176 |
| ALT | 22.7 | 19 | 174 | 48.7 | 20 | 176 |
Definition of abbreviations: ALT = alanine aminotransferase; AST = aspartate aminotransferase; INH = isoniazid; RPT = rifapentine; RZ = rifampin and pyrazinamide.
TABLE 5.
CHARACTERISTICS OF PATIENTS ASSIGNED TO RIFAMPIN/PYRAZINAMIDE WHO DID OR DID NOT DEVELOP GRADE 3 OR 4 HEPATOTOXICITY
| Hepatotoxicity | Yes (n = 20) | No (n = 173) |
|---|---|---|
| Baseline AST, IU | 35.0 | 25.2 |
| Baseline total bilirubin, mg/dl | 0.71 | 0.59 |
| Male sex, % | 45 | 44 |
| Mean age, yr | 40.2 | 36.7 |
| Mean weight, kg | 67.6 | 66.5 |
| Mean pyrazinamide dose, mg/kg/d | 19.1 | 19.3 |
| Mean rifampin dose, mg/kg/d | 9.0 | 9.2 |
Definition of abbreviation: AST = aspartate aminotransferase.
Other adverse events during therapy and follow-up included death in four participants (none related to study drugs or TB) and pregnancy in five participants. None of the pregnancies were judged to be related to study drugs interacting with hormonal contraceptives.
During the follow-up period, four participants developed active TB, diagnosed by culture of sputum in three patients and by clinical findings (exudative pleural effusion with response to TB therapy) in one. Three cases (two pulmonary, one pleural) occurred in the rifapentine and INH arm (1.46%), versus one case in the rifampin and pyrazinamide arm (0.52%; difference = 0.94%; 95% confidence interval [CI], −1.6–3.7%; p = 0.66). The incidence of TB during follow-up was three cases in 564 person-years of follow-up (0.5/100 person-years) for participants taking rifapentine and INH versus one case in 522 person-years of follow-up (0.2/100 person-years) for those taking rifampin and pyrazinamide (relative risk, 2.8; 95% CI, 0.3–26.8; p = 0.66).
DISCUSSION
In this study comparing two short-course, rifamycin-based regimens for the treatment of latent TB infection, we found that rifapentine and INH were significantly better tolerated than rifampin and pyrazinamide. We prematurely terminated our trial because of unexpectedly high rates of hepatotoxicity in the rifampin and pyrazinamide arm. Of participants assigned to this regimen, 10% developed grade 3 or 4 hepatotoxicity, compared with only 1% of individuals taking rifapentine and INH once weekly. This rate of rifampin and pyrazinamide toxicity is comparable to rates reported in recent years from several observational studies and two clinical trials in individuals without HIV infection (23–29), but is substantially higher than rates reported in clinical trials of patients with HIV infection (7–9).
The association of rifampin and pyrazinamide with liver injury during the treatment of latent TB is unexplained and puzzling. Three controlled clinical trials of rifampin and pyrazinamide in HIV-infected adults with latent TB found that this regimen was as well or better tolerated as INH, with extremely low rates of liver toxicity (7–9); yet, two clinical trials in HIV-uninfected adults noted serious hepatotoxicity in 7 to 10% of patients taking this regimen (22, 25). Our results confirm these findings.
We found that both treatment regimens in our study afforded a high degree of protection against active TB in household contacts of infectious cases. Kritski and colleagues, in a study conducted in Rio de Janeiro, reported that 8% of contacts of patients with multidrug-resistant TB developed active disease within 2 yr without preventive treatment (19). A more recent study in Rio de Janeiro also found that 7 to 9% of household contacts with a positive tuberculin skin test developed active disease within 2 yr in the absence of treatment (M. Conde, M.D., personal communication, June 2005). The observed event rates in this study were substantially lower than this, with only 1.46 and 0.52% of patients developing active disease after treatment with rifapentine and INH or rifampin and pyrazinamide, respectively. The incidence rates, 0.5 and 0.2 cases per 100 person-years, are also significantly lower than reported for untreated household contacts of active cases (30). There was no significant difference between the two treatment groups, and the disease rate in the rifapentine and INH arm was well below the acceptable figure of 4%. The upper 95% CI of the difference between the regimens was 3.7%, slightly above our postulated acceptable difference of 3.2% based on a rate in the control arm of 0.8%, reflecting the smaller than planned sample size of the trial due to early discontinuation of enrollment. Without treatment, we would expect to have seen 16 TB cases among the 206 participants in the rifapentine- and INH-treated cohort, whereas only three cases occurred. In addition, rifapentine and INH were well tolerated, with 192 participants completing treatment and only 1% developing liver toxicity. Rifapentine and INH constitute an effective combination therapy for active TB in the continuation phase for patients without cavitation on chest radiographs (12). Its activity in latent TB is therefore not unexpected.
Short-course, rifamycin-based regimens for latent TB have high effectiveness and are easier for patients to comply with than longer courses of INH. Rifapentine and INH given once weekly for 12 wk is an appealing alternative to 9 mo of INH for contacts of TB cases, as it can be incorporated into directly observed therapy programs as part of the management of the index case. A shorter course of rifapentine and INH could improve the cost-effectiveness of preventive therapy even further, as the number of visits and monitoring costs would be reduced (31). TB control programs in developing countries could place a higher priority on treating latent infection in contacts of cases if the treatment could be integrated with curative therapy and an easily administered, short-course regimen were available. Rifapentine and INH given weekly for 12 wk would meet these requirements and could contribute to disease control efforts. Daily rifampin for 3 to 4 mo is another cost-effective option for treating latent TB, although clinical studies are limited (6, 32).
In conclusion, 12 doses of rifapentine and INH given weekly have good activity against latent TB, and are significantly less toxic than rifampin and pyrazinamide coadministration. Our phase 2 trial had limited power because of early discontinuation of enrollment, but demonstrates the potential of this new regimen. Although the overall efficacy of rifapentine and INH will be further described in several ongoing phase 3 trials, this regimen is a likely promising therapy for latent TB infection.
Rifapentine was provided by Aventis, Inc., and isoniazid and rifampin were supplied by the Brazilian Ministry of Health. The authors thank Elizabeth Higgs, M.D., of the National Institute of Allergy and Infectious Diseases (NIAID); Lawrence Moulton, Ph.D., Jonathan Golub, Ph.D., and Timothy Sterling, M.D., of Johns Hopkins University; and Giorgio Roscigno, M.D., and Mr. Paul Sullivan of Aventis, Inc., for support, advice, and assistance during the course of this study. In addition, they thank Solange Cavalcante, M.D., M.P.H., and Betina Durovni, M.D., for assistance with recruitment of participants. The Data Safety and Monitoring Board of the Division of Microbiology and Infectious Diseases of NIAID provided wise and useful oversight of the study. The authors also thank William Bishai, M.D., Ph.D., and Jacques Grosset, M.D., for inspiration and advice.
Supported by National Institutes of Health grants AI 45432, AI 16137, and TW 05574, and Conselho Nacional de Desenvolvimento Científico e Tecnológico.
Originally Published in Press as DOI: 10.1164/rccm.200512-1953OC on February 10, 2006
Conflict of Interest Statement: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.American Thoracic Society/Centers for Disease Control and Prevention. Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR Morb Mortal Wkly Rep 2000;49(RR-6):1–51. [PubMed] [Google Scholar]
- 2.Institute of Medicine. Ending neglect: elimination of tuberculosis in the United States. Washington, DC: National Academy Press; 2000.
- 3.Ferebee SH. Controlled chemoprophylaxis trials in tuberculosis: a general review. Adv Tuberc Res 1969;17:28–106. [PubMed] [Google Scholar]
- 4.Bishai WR, Chaisson RE. Short-course chemoprophylaxis for tuberculosis. Clin Chest Med 1997;18:115–122. [DOI] [PubMed] [Google Scholar]
- 5.Ji B, Truffot-Pernot C, Lacroix C, Raviglione MC, O'Brien RJ, Olliaro P, Roscigno G, Grosset J. Effectiveness of rifampin, rifabutin, and rifapentine for preventive therapy of tuberculosis in mice. Am Rev Respir Dis 1993;148:1541–1546. [DOI] [PubMed] [Google Scholar]
- 6.Hong Kong Chest Service/British Medical Research Council. A double-blind placebo controlled clinical trial of three antituberculosis chemoprophylaxis regimens in patients with silicosis in Hong Kong. Am Rev Respir Dis 1992;145:36–41. [DOI] [PubMed] [Google Scholar]
- 7.Halsey NA, Coberly JS, Desormeaux J, Losikoff P, Atkinson J, Moulton LH, Cantave M, Johnson M, Davis H, Geiter L, et al. Randomised trial of isoniazid versus rifampicin and pyrazinamide for prevention of tuberculosis in HIV-1 infection. Lancet 1998;351:786–792. [DOI] [PubMed] [Google Scholar]
- 8.Mwinga A, Hosp M, Godfrey-Faussett P, Quigley M, Mwaba P, Mugala BN, Nyirenda O, Luo N, Pobee J, Elliott AM, et al. Twice weekly tuberculosis preventive therapy in HIV infection in Zambia. AIDS 1998;12:2447–2457. [DOI] [PubMed] [Google Scholar]
- 9.Gordin F, Chaisson RE, Matts JP, Miller C, de Lourdes Garcia M, Hafner R, Valdespino JL, Coberly J, Schechter M, Klukowicz AJ, et al. Rifampin and pyrazinamide vs isoniazid for prevention of tuberculosis in HIV-infected persons: an international randomized trial. JAMA 2000;283:1445–1450. [DOI] [PubMed] [Google Scholar]
- 10.Burman WJ, Gallicano K, Peloquin C. Comparative pharmacokinetics and pharmacodynamics of the rifamycin antibacterials. Clin Pharmacokinet 2001;40:327–341. [DOI] [PubMed] [Google Scholar]
- 11.Tam CM, Chan SL, Lam CW, Leung CC, Kam KM, Morris JS, Mitchison DA. Rifapentine and isoniazid in the continuation phase of treating pulmonary tuberculosis. Initial report. Am J Respir Crit Care Med 1998;157:1726–1733. [DOI] [PubMed] [Google Scholar]
- 12.Benator D, Bhattacharya M, Bozeman L, Burman W, Cantazaro A, Chaisson R, Gordin F, Horsburgh CR, Horton J, Khan A, et al. Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIV-negative patients: a randomised clinical trial. Lancet 2002;360:528–534. [DOI] [PubMed] [Google Scholar]
- 13.Chapuis L, Ji B, Truffot-Pernot C, O'Brien RJ, Raviglione MC, Grosset JH. Preventive therapy of tuberculosis with rifapentine in immunocompetent and nude mice. Am J Respir Crit Care Med 1994;150:1355–1362. [DOI] [PubMed] [Google Scholar]
- 14.Miyazaki E, Chaisson RE, Bishai WR. Analysis of rifapentine for preventive therapy in the Cornell mouse model of latent tuberculosis. Antimicrob Agents Chemother 1999;43:2126–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaisson RE, Zajdenverg R, Falco G, Barnes GL, Moore RD, Coberly J, Faulhaber JC, Schechter M. Controlled trial of weekly rifapentine/isoniazid for 12 weeks vs. rifampin/PZA for latent TB [abstract]. Proc Am Thorac Soc 2005;2:A20. [Google Scholar]
- 16.Weiner M, Bock N, Peloquin CA, Burman WJ, Khan A, Vernon A, Zhao Z, Weis S, Sterling TR, Hayden K, et al. Pharmacokinetics of rifapentine at 600, 900, and 1,200 mg during once-weekly tuberculosis therapy. Am J Respir Crit Care Med 2004;169:1191–1197. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Update CDC: fatal and severe liver injuries associated with rifampin and pyrazinamide for latent tuberculosis infection, and revisions in American Thoracic Society/CDC recommendations—United States, 2001. MMWR Morb Mortal Wkly Rep 2001;50:733–735. [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Update CDC: fatal and severe liver injuries associated with rifampin and pyrazinamide treatment for latent tuberculosis infection. MMWR Morb Mortal Wkly Rep 2002;51:998–999. [PubMed] [Google Scholar]
- 19.Kritski AL, Marques MJO, Rabahi MF, Vieira MA, Werneck-Barroso E, Carvalho CE, Andrade Gde N, Bravo-de-Souza R, Andrade LM, Gontijo PP, et al. Transmission of tuberculosis to close contacts of patients with multi-drug resistant tuberculosis. Am J Respir Crit Care Med 1996;153:331–335. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Update: adverse event data and revised American Thoracic Society/CDC recommendations against the use of rifampin and pyrazinamide for treatment of latent tuberculosis infection—United States, 2003. MMWR Morb Mortal Wkly Rep 2003;52:735–739. [PubMed] [Google Scholar]
- 21.Santos DC, Souto FJ, Santos DR, Vitral CL, Gaspar AM. Seroepidemiological markers of enterically transmitted viral hepatitis A and E in individuals living in a community located in the North Area of Rio de Janeiro, RJ, Brazil. Mem Inst Oswaldo Cruz 2002;97:637–640. [DOI] [PubMed] [Google Scholar]
- 22.Carrilho FJ, Mendes Clemente C, Silva LC. Epidemiology of hepatitis A and E virus infection in Brazil. Gastroenterol Hepatol 2005;28:118–125. [DOI] [PubMed] [Google Scholar]
- 23.Narita M, Kellman M, Franchini DL, McMillan ME, Hollender ES, Ashkin D. Short-course rifamycin and pyrazinamide treatment for latent tuberculosis infection in patients with HIV infection: the 2-year experience of a comprehensive community-based program in Broward County, Florida. Chest 2002;122:1292–1298. [DOI] [PubMed] [Google Scholar]
- 24.Stout JE, Engemann JJ, Cheng AC, Fortenberry ER, Hamilton CD. Safety of 2 months of rifampin and pyrazinamide for treatment of latent tuberculosis. Am J Respir Crit Care Med 2003;167:824–827. [DOI] [PubMed] [Google Scholar]
- 25.Jasmer RM, Saukkonen JJ, Blumberg HM, Daley CL, Bernardo J, Vittinghoff E, King MD, Kawamura LM, Hopewell PC. Short-course rifampin and pyrazinamide compared with isoniazid for latent tuberculosis infection: a multicenter clinical trial. Ann Intern Med 2002;137:640–647. [DOI] [PubMed] [Google Scholar]
- 26.McNeill L, Allen M, Estrada C, Cook P. Pyrazinamide and rifampin vs isoniazid for the treatment of latent tuberculosis: improved completion rates but more hepatotoxicity. Chest 2003;123:102–106. [DOI] [PubMed] [Google Scholar]
- 27.Tortajada C, Martinez-Lacasa J, Sanchez F, Jimenez-Fuentes A, De Souza ML, Garcia JF, Martinez JA, Cayla JA. Is the combination of pyrazinamide plus rifampicin safe for treating latent tuberculosis infection in persons not infected by the human immunodeficiency virus? Int J Tuberc Lung Dis 2005;9:276–281. [Published erratum appears in Int J Tuberc Lung Dis 2005;9:706.] [PubMed] [Google Scholar]
- 28.Gordin FM, Cohn DL, Matts JP, Chaisson RE, O'Brien RJ. Hepatotoxicity of rifampin and pyrazinamide in the treatment of latent tuberculosis infection in HIV-infected persons: is it different than in HIV-uninfected persons? Clin Infect Dis 2004;39:561–565. [DOI] [PubMed] [Google Scholar]
- 29.van Hest R, Baars H, Kik S, van Gerven P, Trompenaars MC, Kalisvaart N, Keizer S, Borgdorff M, Mensen M, Cobelens F. Hepatotoxicity of rifampin-pyrazinamide and isoniazid preventive therapy and tuberculosis treatment. Clin Infect Dis 2004;39:488–496. [DOI] [PubMed] [Google Scholar]
- 30.Noertjojo K, Tam CM, Chan SL, Tan J, Chan-Yeung M. Contact examination for tuberculosis in Hong Kong is useful. Int J Tuberc Lung Dis 2002;6:19–24. [PubMed] [Google Scholar]
- 31.Diel R, Nienhaus A, Schaberg T. Cost-effectiveness of isoniazid chemoprevention in close contacts. Eur Respir J 2005;26:465–473. [DOI] [PubMed] [Google Scholar]
- 32.Menzies D, Dion MJ, Rabinovitch B, Mannix S, Brassard P, Schwartzman K. Treatment completion and costs of a randomized trial of rifampin for 4 months versus isoniazid for 9 months. Am J Respir Crit Care Med 2004;170:445–449. [DOI] [PubMed] [Google Scholar]