Abstract
Rationale: Although obstructive sleep apnea is strongly associated with obesity, we have little understanding of how obesity may alter the mechanical properties of the pharynx and the role of obesity in the pathogenesis of sleep apnea.
Objectives: The overall objective of this study was to determine the effect of obesity on pharyngeal airway size and pharyngeal wall tissue strain in lean and obese Zucker rats.
Methods: Respiratory-gated magnetic resonance imaging with noninvasive tissue tagging was performed in anesthetized, spontaneously breathing lean (n = 9) and obese (n = 9) Zucker rats. Images acquired during expiration and inspiration of the rostral, mid-, and caudal pharynx were analyzed for airway size and pharyngeal wall tissue strain, using planimetry, optical flow, and finite element analyses. Differences in cross-sectional airway area, lateral and anteroposterior airway diameters, and tissue strain (stretch, compression, and direction of stretch) in the lateral and ventral pharyngeal walls were compared by analysis of variance (significance at p < 0.05).
Measurements and Main Results: Compared with their lean littermates, obese rats had the following significant findings: reduced pharyngeal airway cross-sectional area during inspiration and expiration, smaller increases in airway area during inspiration, and decreased lateral airway dilation during inspiration. Tissue strain in the pharyngeal walls showed no significant differences between obese and lean rats.
Conclusions: These findings suggest that obesity results in a mechanical abnormality that decreases pharyngeal airway size and prevents a normal airway response to a given change in pharyngeal wall tissue strain.
Keywords: magnetic resonance imaging; obstructive sleep apnea; pharynx; rats, Zucker
Although we know that body mass index is the most important predictor of obstructive sleep apnea (OSA) and that patients with OSA have decreased pharyngeal airway size and increased airway collapsibility, we have little understanding of how obesity per se alters the mechanical properties of pharynx (1–4). In addition, we have limited understanding of the mechanical properties of the pharyngeal wall tissues and how they determine changes in airway size (5–8). The Zucker rat is an established model of genetic obesity (9). A particular advantage of this animal model is that the obese phenotype is due to a recessive trait, allowing the lean littermates to be used as control animals. Studies reveal that the mechanical characteristics of the pharyngeal airway in obese Zucker rats are similar to those reported in patients with OSA. The pharyngeal airway is narrower in dead obese Zucker rats compared with their dead lean littermates, and upper airway collapsibility, as measured by critical airway pressure, is increased in anesthetized obese Zucker rats compared with their lean littermates (10).
The purpose of the current study was to determine the relationships between changes in airway size and pharyngeal wall tissue strain during spontaneous breathing in anesthetized, spontaneously breathing obese and lean Zucker rats. As detailed in another publication from this laboratory, magnetic resonance imaging (MRI) with noninvasive tissue tagging was used to measure changes in airway size and pharyngeal wall tissue strain (11). The MRI with tagging sequence produces an evenly spaced grid of dark lines on underlying tissue and a series of MR images acquired just after the line deposition can show distortion of the grid due to tissue motion (12). Analysis of the images, using the grid lines as fiducial markers, yields detailed information about tissue motion in the pharyngeal wall. MRI with noninvasive tagging has been used extensively to study cardiac mechanics (13–18) and tongue movements related to phonation (19–21).
This study was designed to test the following hypotheses: (1) the pharyngeal airway is smaller in obese versus lean rats during expiration; and (2) the increase in airway size during inspiration is decreased in obese rats. We also hypothesized that the differences between obese and lean rats in the phasic changes in airway size during respiration might be explained by group differences in tissue strain in the lateral and ventral pharyngeal walls. Some of the results of this study have been previously reported in abstract form (22).
METHODS
For a detailed description of methods, see the online supplement. With approval of the institutional animal care and use committee, experiments were conducted on nine lean (weight, 386 ± 9.5 [SEM] g) and nine age-matched, obese (weight, 695 ± 34 g) isoflurane-anesthetized, spontaneously breathing Zucker rats. An MRI-compatible pressure catheter (Millar Instruments, Houston, TX) was inserted through the lower ribcage to record intrapleural pressure (for details, see the online supplement). After MRI, euthanasia was performed under anesthesia by intracardiac injection of saturated KCl.
MR imaging was performed in a 4.7-T magnet, using previously reported methods, to obtain 1-mm-thick axial and sagittal images at 22 contiguous locations over the pharyngeal airway (11). Triggered by the nadir of inspiratory pressure, the tagging line pulses were laid down in the first half of expiration and images were acquired in midexpiration and the latter half of inspiration.
The bilateral tympanic bulla was used to align the entire axial series in each rat. The axial images were numbered sequentially, with image 1 proximal to the junction of the hard and soft palates and image 11 at the base of the epiglottis. On the basis of previous work (11, 23), the analysis examined three pharyngeal regions: rostral pharynx (images 1, 4, and 6), midpharynx (images 7–9), and caudal pharynx (images 10 and 11; Figure 1). The oropharyngeal airway was not patent in these spontaneously breathing rats. Therefore, all measurements relate to the nasopharyngeal airway (rostral and midpharynx) and hypopharyngeal airway (caudal pharynx). Cross-sectional area (CSA), anteroposterior (AP) airway diameter, and lateral airway diameter were measured in the axial images, using computer-aided planimetry (NIH-Image, version 1.61/ppc; U.S. National Institutes of Health, Bethesda, MD; http://rsb.info.nih.gov/nih-image/) (11).
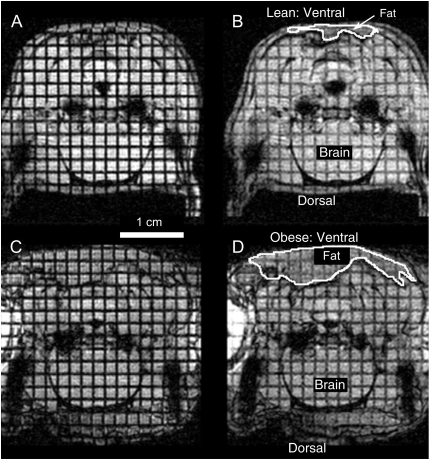
Figure 1.
Midline sagittal images of a supine lean Zucker rat (top) and an obese Zucker rat (bottom) during inspiration. The noninvasive grid lines were applied on these images during the preceding expiration. The transverse lines indicate the planes where the axial images were acquired and among these are eight highlighted lines that indicate the slices analyzed in this study. Axial slices 1, 4, and 6 were identified as rostral pharynx (R); slices 7, 8, and 9 as midpharynx (M); and slices 10 and 11 as caudal pharynx (C). Slices numbered 1, 4, 6, 8, and 10 are annotated for clarity. The magnetic resonance (MR) image of the obese Zucker rat shows large fat deposits near the tongue and surrounding the neck region. Ventral and dorsal sides are noted along with the tongue (Ton), nasopharynx (NP), and brain (BR), and a 1-cm scale is indicated in the image of the obese rat. Selected regions, where fat could be approximately identified in the lean and obese rats, are outlined in the images.
As detailed in a previous publication, optical flow software and two-dimensional strain analysis were used to measure bilateral tissue strain (averaged and expressed as right-sided values) in specific sectors in the lateral and ventral pharyngeal walls, with a sector in brain tissue serving as the control (11) (see Figures E3 and E4 of the online supplement). Respiratory-related movement caused tissue displacement in the control sector, so we analyzed only the strain-related variables that are insensitive to displacement (see Discussion) (12, 15, 17). The following variables were calculated in each sector: tissue stretch (λ1), tissue compression (λ2), direction angle of stretch (β angle) relative to the centroid of the nasopharyngeal airway, and angle of rigid body rotation (α angle), that is, the pure rotation of the sector excluding shape-related changes.
We used a mixed model analysis of variance (ANOVA) (24) to compare airway dimensions in obese and lean Zucker rats during expiration and inspiration and to determine the effect of pharyngeal region (image location) on airway dimensions. The same model was used to test whether there were differences in measures of tissue strain (λ1, λ2, β angle, and α angle) across pharyngeal wall sectors (lateral, ventral, and control) or pharyngeal regions (rostral, mid-, and caudal pharynx) and whether there was an effect of obesity for these comparisons. Post hoc pairwise comparisons were evaluated using Tukey-Kramer adjusted p values (or Bonferroni adjustment) where significance was assumed for p < 0.05 (25).
RESULTS
Intrapleural pressure recordings during imaging revealed significant differences in pleural pressure measurements between lean and obese Zucker rats (see Table 1). Inspiratory and expiratory time were less in the obese rats compared with the lean rats and duty cycle time (inspiratory time/total respiratory cycle time) was increased in the obese Zucker rats. The obese Zucker rats also had a more negative drop in pleural pressure during inspiration.
TABLE 1.
RESPIRATORY MEASUREMENTS MADE FROM PLEURAL PRESSURE RECORDINGS DURING IMAGE ACQUISITION IN SPONTANEOUSLY BREATHING OBESE AND LEAN ZUCKER RATS
| Lean (n = 9)
|
Obese (n = 9)
|
||||
|---|---|---|---|---|---|
| Respiratory Variable | Mean | SEM | Mean | SEM | p Value |
| Respiratory cycle time, ms | 1,267.11 | 80.87 | 868.61 | 91.53 | < 0.005 |
| Expiratory time, ms | 1,036.22 | 75.24 | 680.17 | 87.49 | < 0.007 |
| Inspiratory time, ms | 230.89 | 15.04 | 188.44 | 8.00 | < 0.024 |
| Inspiratory time/respiratory cycle time | 0.19 | 0.01 | 0.23 | 0.01 | < 0.049 |
| Change in pressure from end of expiration to end of inspiration, cm H2O | −14.42 | 1.06 | −19.13 | 1.364 | < 0.015 |
Airway Dimensions
Figure 2 shows representative MRI images during expiration and inspiration in a spontaneously breathing anesthetized lean and obese Zucker rats at matched loci in the caudal pharynx (level 8, i.e., 8 mm caudal from the junction of the hard and soft palates). The images reveal the excess fat deposits surrounding the ventral pharyngeal wall in the obese Zucker rat. Airway size was smaller in the obese rat compared with the lean rat at expiration (obese, 2.66 mm2; lean, 3.74 mm2) and inspiration (obese, 3.17 mm2; lean, 6.52 mm2). In both the obese and lean rats, airway size during inspiration was greater than during expiration.
Figure 2.
Representative MR axial images of the hypopharynx (level 8) in a spontaneously breathing lean Zucker rat (A and B) and obese Zucker rat (C and D). Images in A and C were acquired during midexpiration; images in B and D were acquired just before the nadir of inspiration. The airway is the black area at the midline of each image. The black spaces lateral to the airways in each image are part of the tympanic bulla. Noninvasive grid lines were applied before the expiratory images (A and C) were acquired. Displacement of the grid lines in the subsequent inspiratory images (B and D), particularly in the lean Zucker rat, reveal the tissue strain in the pharyngeal walls. The grid lines provide fiducial markers that were used in the subsequent analysis of tissue strain. Annotations include the following: the 1-cm scale below A, and ventral and dorsal directions indicated for the lean rat in B and similarly for the obese rat in D. Approximate segmentation of typical fat regions in images of the lean rat (B) and obese rat (D) are outlined. The brain region is also noted in B and D.
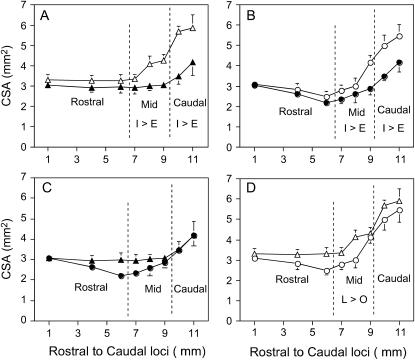
Figure 3 shows the mean ± SEM CSA in nine lean and nine obese Zucker rats during inspiration and expiration. When compared across all regions, mean inspiratory CSA (lean, 4.13 ± 0.17 mm2; obese, 3.58 ± 0.18 mm2) was greater than expiratory CSA (lean, 3.2 ± 0.12 mm2; obese, 2.90 ± 0.12 mm2) in both obese and lean rats (p < 0.0001). In both lean and obese rats inspiratory CSA was also greater than expiratory CSA in specific regions. In the midregion, inspiratory CSA (lean, 2.91 ± 0.13 mm2; obese, 2.48 ± 0.11 mm2) was greater than expiratory CSA (lean, 2.32 ± 0.09 mm2; obese, 2.08 ± 0.09 mm2; p < 0.0001) and, in the caudal region, inspiratory CSA (lean, 3.73 ± 0.20 mm2; obese, 3.46 ± 0.19 mm2) was greater than expiratory CSA (lean, 2.85 ± 0.15 mm2; obese = 2.75 ± 0.13 mm2; p < 0.0001). Differences between inspiratory and expiratory CSA did not reach significance in either group in the rostral region (inspiratory CSA, 2.44 ± 0.09 vs. expiratory CSA, 2.26 ± 0.11 mm2 in lean rats; and inspiratory CSA, 2.22 ± 0.09 vs. expiratory CSA, 2.11 ± 0.08 mm2 in obese rats; p = 0.020, for Bonferroni significance needed at p < 0.0167).
Figure 3.
Mean ± SEM cross-sectional area (CSA) at selected regions of the pharyngeal airway on inspiration (open circles and open triangles) and expiration (solid circles and solid triangles) in nine lean Zucker rats (triangles) and nine obese Zucker rats (circles). Top: Airway area on inspiration and expiration in lean Zucker rats (A) and obese Zucker rats (B). Bottom: Comparison of airway area in lean versus obese rats on expiration (C) and inspiration (D). The sequence of axial images on the abscissa begins with the most rostral image, location 1, proximal to junction of soft and hard palates, and extends to image 11, at the base of the epiglottis (see Figure 2). Overlying vertical dashed lines denote the boundaries of the rostral, mid-, and caudal pharyngeal regions. In A and B, the inspiratory CSA values within the mid- and caudal pharynx are significantly greater than the expiratory CSA in those respective regions (denoted as I > E in the designated region). When differences in region were accounted for, expiratory (C) and inspiratory (D) CSAs of lean rats were significantly greater than those of obese Zucker rats (see text for p values). In D, comparing inspiratory CSAs, the CSA in the midregion was significantly greater in lean Zucker rats than in obese Zucker rats (denoted as L > O, p < 0.05).
Regional differences (rostral, mid-, and caudal pharynx) within both groups of obese and lean Zucker rats showed that CSA during inspiration and the CSA change from expiration to inspiration were significantly different across all three pharyngeal regions in the following order: rostral pharynx < midpharynx < caudal pharynx (p values < 0.0001). Regional differences in expiration within both groups of obese and lean Zucker rats showed that CSA in the rostral and midpharynx was less than that in the caudal pharynx (p < 0.0001), but no differences were detected between the rostral and midpharynx (p = 0.82).
Taking into account differences between regions, ANOVA showed that overall values of CSA were significantly greater in lean than in obese rats during expiration (p < 0.033) and inspiration (p < 0.003). Change in CSA between expiration and inspiration across all regions was also greater in the lean rats (p < 0.046). When results in specific regions were examined, differences in CSA between groups were detected only in the midpharyngeal region, where inspiratory CSA was greater in lean than in obese rats (p < 0.013) with a similar trend for expiratory CSA in the same region (p < 0.055).
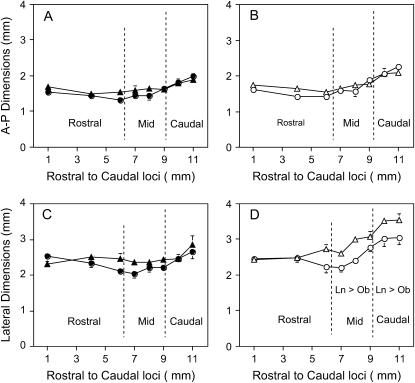
Figure 4 compares AP and lateral airway diameters during expiration and inspiration in lean and obese Zucker rats. There were no significant differences in AP airway dimensions between lean and obese rats during expiration or inspiration. Taking differences in region into account, ANOVA showed that overall lateral diameters during inspiration were significantly greater in lean Zucker rats (2.91 ± 0.06 mm) compared with obese Zucker rats (2.57 ± 0.06 mm; p < 0.046), but no differences were noted during expiration (p = 0.639).
Figure 4.
Comparison of anteroposterior (A-P; top) and lateral (bottom) airway dimensions during expiration (solid symbols in A and C) and inspiration (open symbols in B and D) in nine lean Zucker rats (triangles) and nine obese Zucker rats (circles). Overlying vertical dashed lines denote the boundaries of the rostral, mid-, and caudal pharyngeal regions. Note that lateral dimensions during inspiration were significantly greater in lean Zucker rats compared with obese Zucker rats in the mid- and caudal pharynx (denoted Ln > Ob, p < 0.05).
Pharyngeal Wall Tissue Strain
When tissue sector strain variables (λ1, λ2, and β) were compared between lean and obese Zucker rats, no significant differences between groups emerged. Therefore, the comparisons across regions and sectors reported below were performed with the entire set of rats (n = 18).
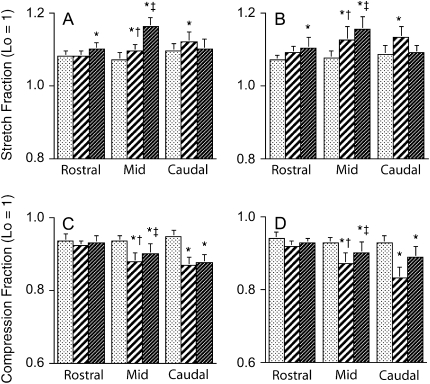
Figures 5A and 5B show the mean ± SEM λ1 (tissue stretch) in each pharyngeal wall sector (lateral, ventral, and control) and pharyngeal region (rostral, mid-, and caudal pharynx) in lean and obese Zucker rats. Taking into account differences in sector and region, ANOVA showed that λ1 was greater in the ventral wall sector than in the control sector in the rostral and midpharynx (p < 0.022) but not in the caudal pharynx (p = 1.0). λ1 was greater in the lateral wall sector than in the control sector in the mid- and caudal pharynx (p < 0.006), but not in the rostral pharynx (p = 0.90). Furthermore, in the midpharynx, λ1 in the ventral wall sector was greater than in the lateral sector (p < 0.0001), but the opposite finding was present in the caudal pharynx (p < 0.027). When λ1 was compared for the same pharyngeal wall sector across regions, the main finding was that the values in the rostral pharynx were significantly less than both mid- and caudal pharyngeal values in both the ventral and lateral wall sectors (p < 0.0001).
Figure 5.
Mean ± SEM λ1 (stretch; A and B) and λ2 (compression; C and D) in each pharyngeal wall sector (control [stippled columns], lateral [hatched columns], and ventral [dark columns]) and pharyngeal region (rostral, mid-, and caudal pharynx) in nine lean Zucker rats (A and C) and nine obese Zucker rats (B and D). Significant differences from control (p < 0.05) within a region are noted by an asterisk. In some regions, there were differences between lateral and ventral sector values, where values noted by (‡) are greater than values noted by (†); p < 0.05. There were no significant differences between lean and obese stretch or compression values, and thus significant differences among lateral ventral and control sectors are represented for both obese and lean groups although the analysis of variance (ANOVA) results were obtained from the combined group data. In the absence of any significantly different tissue stretch or compression within a sector between expiration and inspiration, the respective λ values would reflect only the spurious noise resulting from the sequential MR acquisitions. Lo = unit length (length normalized to 1) of a tissue sector before deformation.
Figures 5C and 5D show λ2 (tissue compression) for lean and obese rats. Taking into account differences in sector and region, ANOVA showed that λ2 values were not significantly different from control in the rostral pharynx (p ⩾ 0.113), but lateral and ventral wall sector values of λ2 were less, that is, tissue sectors were more compressed, than those in the control sector in the mid- and caudal pharynx (p < 0.0001). In the midpharynx, λ2 in the lateral wall sector was less than that in the ventral wall sector (p < 0.001). No such differences were present in the rostral or caudal pharynx (p ⩾ 0.920).
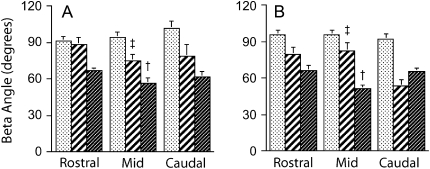
When differences in region and sector were accounted for (considering both lean and obese rats together), lateral and ventral sector β angles (Figure 6) were different from control (p < 0.0001) and overall lateral sector angles were greater than ventral sector β angles (p < 0.0001). In the lateral pharyngeal wall sectors, the β angle declined significantly from 83.47 ± 7.43° in the rostral pharynx to 65.89 ± 8.64° in the caudal pharynx (p < 0.018). Thus, strain in the lateral walls was in a less perpendicular direction (more radially directed) in the more caudal regions. β angles in the ventral sectors did not vary across pharyngeal regions (mean for both lean and obese, 60.79 ± 4.45°) but in the midpharyngeal region, lateral wall sector β (lean, 74.5 ± 5.7°; obese, 82.6 ± 6.2°) was greater than the respective angles in the ventral wall sectors (lean, 56.1 ± 4.7°; obese, 51.0 ± 3.2°) (p < 0.0001). The mean β value in control sectors was 94.07 ± 5.01° and did not vary significantly across regions (p = 0.808). Analysis of the tissue rotation variable α indicated that the maximum value of α in any sector was less than 3°, an order of magnitude that could not be considered significant for group, sector, or regional comparisons.
Figure 6.
Mean ± SEM of the β angle, that is, the direction of tissue stretch (λ1), in each pharyngeal wall sector (control [stippled columns], lateral [hatched columns], and ventral [dark columns]) and each pharyngeal region (rostral, mid-, and caudal pharynx) in nine lean Zucker rats (A) and nine obese Zucker rats (B). There were no significant differences between lean and obese rats, and therefore ANOVA results are reported from the combined group data. Taking into account differences among regions, results showed that lateral β angles were greater than ventral angles and both were significantly different from control (p < 0.0001). Specific contrast analysis showed that in the midregion lateral sector β angles (‡) were greater than the ventral sector angles (†) and different from control (p < 0.05).
DISCUSSION
The objective of the current study was to compare changes in airway size and pharyngeal wall tissue strain during spontaneous breathing in obese versus lean Zucker rats. The results indicate that obesity in Zucker rats is associated with (1) a smaller pharyngeal CSA during both expiration and inspiration, (2) a smaller increase in CSA during inspiration, and (3) decreased lateral airway dilation during inspiration. We did not find significant differences in pharyngeal wall tissue strain between the two groups in the sectors and regions examined. However, analysis of pharyngeal wall tissue strain in all animals revealed striking differences in the magnitude of tissue compression, tissue stretch, and the direction of stretch both within and across pharyngeal regions. Tissue stretch (λ1) in the ventral pharyngeal wall sector was greatest in the midpharynx, whereas stretch in the lateral sector was greatest in the caudal pharynx. The direction of stretch (β angle) in the ventral pharyngeal wall tended to be in a ventral–lateral direction whereas the direction of stretch in the lateral pharyngeal wall tended to be in a more ventral direction in the rostral pharynx, shifting to a more ventral–lateral direction in the caudal pharynx. In general, tissue compression (λ2) in both the ventral and lateral pharyngeal walls decreased in a rostral to caudal direction.
The obese Zucker rat is an established model of obesity and manifests many of the physiologic conditions associated with obesity in humans, including diabetes, hypertension, hyperthyroidism, hyperinsulinemia, and glomerular damage (26–28). Although the obese Zucker rat does not exhibit OSA per se, obesity is the most important predictor of OSA in adult humans (29). Indeed, the pharyngeal airway of obese Zucker rats possesses characteristics that are known risk factors for the pathogenesis of OSA in humans, that is, pharyngeal airway narrowing and increased airway collapsibility (9, 10, 30–34). The anatomy of the rat upper airway differs from that in humans. The rat upper airway is rectilinear compared with the L-shaped airway in humans and the soft palate in rats extends to the epiglottis compared with the separation of these two structures in humans. Despite these anatomic differences, there is considerable homology in pharyngeal muscle anatomy between the two species. In addition, the pharyngeal muscles in rats and humans have similar motor innervation and mechanical actions. The common pathophysiologic and anatomic features between obese rats and humans with OSA make the obese Zucker rat a relevant model to study the relationship of obesity to pharyngeal mechanics.
A previous study from this laboratory in Sprague-Dawley rats details the use of MRI with noninvasive tissue tagging for the study of pharyngeal mechanics (11). In that study, the medial hypoglossal nerve was stimulated to maximally contract the tongue protrudor muscles: the genioglossus and geniohyoid (11). In the spontaneously breathing animals reported in the current study, the oropharyngeal airway was collapsed throughout the respiratory cycle. In contrast, the oropharyngeal airway was patent in the isolated upper airway preparation in our previous study and hypoglossal stimulation caused a large increase in oropharyngeal CSA (11). Because of the differences in oropharyngeal patency in the two studies, the lateral pharyngeal wall sector analyzed in the previous study was more ventral, that is, adjacent to the oropharyngeal airway, compared with the lateral wall sector in the current study, which was selected to be adjacent to the nasopharyngeal airway. Acknowledging these differences, there was an overall increase in nasopharyngeal CSA in both studies that was accompanied by significant tissue stretch (λ1) and tissue compression (λ2). Although it is likely that motor output to the tongue protrudor muscles was not at its maximal level in the spontaneously breathing animals, the similar changes in nasopharyngeal CSA and tissue strain compared with those during hypoglossal stimulation may have been due to simultaneous activation of other pharyngeal muscles in the current experiments.
The potential limitations of the MRI with noninvasive tissue tagging technique are detailed in our previous publication (11). Tissue strain in pharyngeal wall sectors was compared with values obtained in control sectors in the brain to reduce the chance of overestimating positive results because noise artifact in the MR images and optical flow analysis errors would be common to both target and control sectors (11). Displacement of the pharyngeal wall sectors was not reported in the current study because in some experiments there was an unacceptable amount of dorsal–ventral displacement in the brain tissue sector that was attributed to head motion. The finite element analysis algorithm we used for analysis overcomes this problem by computing tissue strain independent of displacement (12, 15, 17). Although the evaluation of strain differences alone between lean and obese rats does not provide a complete explanation for observed differences in CSA and airway lateral diameters, these data demonstrate how the airway walls deform during spontaneous breathing and indicate the direction of stress in a particular sector, which should be parallel to the β angle.
Several factors have the potential to reduce airway size on expiration and inspiration in obese Zucker rats. We do not know whether the stresses acting on the pharyngeal wall due to pharyngeal intralumenal pressure and pharyngeal dilator muscle activation were the same in lean and obese rats. For example, it is known that many anesthetic agents, including isoflurane, can suppress upper airway muscle activity more than diaphragmatic activity (35). Because the current study did not evaluate neural output to pharyngeal and diaphragm muscles or their electromyographic activity, we do not know whether this anesthetic effect differed between the two groups. Even if motor output to the pharyngeal muscles was similar in the obese and lean rats, excess pharyngeal wall fat deposition in the obese rats could conceivably reduce airway size in expiration and alter the mechanical effectiveness of pharyngeal dilator muscles activated during inspiration either directly by mass loading of the pharyngeal wall or indirectly by changing the position of the hyoid bone, an insertion point for many of the pharyngeal muscles.
Differences in respiratory mechanics and pattern of respiration may also have contributed to the pharyngeal differences observed in the obese versus lean animals. Farkas and Schlenker (9) have shown that obese Zucker rats, like obese humans, have decreased functional residual capacity, smaller expiratory reserve volume, reduced total lung capacity, and decreased inspiratory capacity compared with lean Zucker rats. These findings are probably due to mechanical loading of the chest wall by the excess body fat and may explain the different patterns of respiration observed in our anesthetized obese versus lean rats (9). The pleural pressure recordings obtained during MRI revealed a higher respiratory rate and greater change in pleural pressure during inspiration in the obese rats. These respiratory differences are similar to those reported by Nakano and coworkers (32), who found that unanesthetized, obese Zucker rats, of comparable age to the animals in the current study have a more rapid, shallow breathing pattern compared with age-matched lean rats. If muscle-shortening velocity was the same for obese and lean rats, then the reduced CSA from expiration to inspiration in the obese rats in the current study could have resulted from their significantly shorter inspiratory time. Obese Zucker rats have also been shown to have decreased functional residual capacity and smaller tidal volumes than their lean littermates. These lung volume changes can decrease tracheal traction on the pharyngeal airway, another possible explanation for our findings in obese rats of decreased pharyngeal airway size during expiration and reduced airway enlargement during inspiration (36, 37).
Most previous imaging studies of the pharyngeal airway in humans were performed without synchronization to a known phase of the respiratory cycle (38–42). Relatively few imaging studies have employed methods to synchronize images with inspiration and expiration (31, 43–47). Schwab and coworkers (31, 46) performed cine-computed tomographic imaging in awake normal adult humans and patients with OSA to quantify changes in pharyngeal airway CSA during spontaneous breathing. In contrast to the current study, these investigators found that the CSA at the low retropalatal and retroglossal level (analogous to the mid- and caudal regions of the rat pharynx) remains relatively stable during inspiration but enlarges during expiration whereas the smallest airway area occurred at end-expiration, the time in the respiratory cycle when the pharyngeal airway in humans is most vulnerable to collapse. Morell and Badr (45) reported similar findings on fiberoptic imaging of the retropalatal airway in normal adults and patients with OSA during wakefulness and sleep. During wakefulness, retropalatal CSA was greater in early expiration than at the start of inspiration (45). However, during sleep, CSA decreased during inspiration and increased during expiration (45). Morell and Badr (45) found that obese patients had greater phasic CSA changes due to greater narrowing during inspiration and that body mass index was a better predictor of changes in CSA than apnea–hypopnea index. Launois and coworkers (44) performed a similar study in eight awake normal adults but had inconsistent findings.
In contrast to the above studies in humans, studies in spontaneously breathing anesthetized animals report an increase in airway size during inspiration (47, 48). Using computed tomography to image the pharyngeal airway in the English bulldog model of OSA, Veasey and coworkers (47) found that the pharyngeal airway was smallest at end-expiration, but, in accord with our findings, the bulldog's airway dilated in inspiration. Inspiratory pharyngeal airway dilation was also reported in nonimaging studies in spontaneously breathing dogs by Strohl and Fouke (48), where pressure drop measured during inspiration in the isolated upper airway indicated an expansion of the airway.
Several imaging studies report that, compared with normal individuals, the pharyngeal airway in obese patients with OSA has a more circular shape with reduced lateral dimensions (39, 49, 50). We did not find a difference in AP or lateral airway dimension between obese and lean Zucker rats in expiration. However, during inspiration, lateral measurements were greater in lean compared with obese rats and greater lateral widening of the airway could account for the greater respiratory change in CSA in the lean rats. It is interesting to note that whereas the lean rats went to a more elliptical shape (with the long axis in the lateral dimension) during inspiration, the obese rats maintained a more circular-shaped airway, similar to the airway shape reported in patients with OSA (39, 49–51).
In summary, this study contributes new evidence that obesity, the most important predictor of OSA, compromises pharyngeal airway size and function. Compared with their lean littermates, spontaneously breathing, anesthetized, obese Zucker rats have reduced pharyngeal airway CSA during inspiration and expiration, smaller increases in CSA during inspiration, and decreased lateral airway dilation during inspiration. These findings suggest that obesity results in a mechanical abnormality that decreases pharyngeal airway size and prevents a normal airway response to a given change in pharyngeal wall tissue strain.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge consultation with Dr. Lawrence Dougherty and the technical assistance of Kathy Zhang.
Supported by NIH HL-27520 and NIH EB-01780.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200505-705OC on January 26, 2006
Conflict of Interest Statement: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Isono S, Remmers JE, Tanaka A, Sho Y, Sato J, Nishino T. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol 1997;82:1319–1326. [DOI] [PubMed] [Google Scholar]
- 2.Schwab RJ, Pairstein M, Pierson R, Mackley A, Hachadoorian R, Arens R, Maislin G, Pack AI. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med 2003;168:522–530. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz AR, Smith PL, Wise RA, Gold AR, Permutt S. Induction of upper airway occlusion in sleeping individuals with subatmospheric nasal pressure. J Appl Physiol 1988;64:535–542. [DOI] [PubMed] [Google Scholar]
- 4.Smith PL, Gold AR, Meyers D, Haponik EF, Bleecker ER. Weight loss in mildly to moderately obese patients with obstructive sleep apnea. Ann Intern Med 1985;103:850–855. [DOI] [PubMed] [Google Scholar]
- 5.Fregosi RF, Fuller DD. Respiratory-related control of extrinsic tongue muscle activity. Respir Physiol 1997;110:295–306. [DOI] [PubMed] [Google Scholar]
- 6.Fuller DD, Williams PL, Janssen PL, Fregosi RF. Effect of co-activation of tongue protrudor and retractor muscles on tongue movements and pharyngeal airflow mechanics in the rat. J Physiol 1999;519:601–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gleadhill IC, Schwartz AR, Schubert NM, Wise RA, Permutt S, Smith PL. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis 1991;143:1300–1303. [DOI] [PubMed] [Google Scholar]
- 8.Smith PL, Wise RA, Gold AR, Schwartz AR, Permutt S. Upper airway pressure–flow relationships in obstructive sleep apnea. J Appl Physiol 1988;64:789–795. [DOI] [PubMed] [Google Scholar]
- 9.Farkas GA, Schlenker EH. Pulmonary ventilation and mechanics in morbidly obese Zucker rats. Am J Respir Crit Care Med 1994;150:356–362. [DOI] [PubMed] [Google Scholar]
- 10.Magalang UJ, Farkas GA, Najdzionek JS, Nakano H. Obese Zucker rats have narrower upper airway compared to lean litter-mates [abstract]. Am J Respir Crit Care Med 2000;161:A87. [Google Scholar]
- 11.Brennick MJ, Pickup S, Dougherty L, Cater JC, Kuna ST. Pharyngeal airway wall mechanics using tagged magnetic resonance imaging during medial hypoglossal nerve stimulation in rats. J Physiol 2004;561:597–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Axel L, Dougherty L. MR imaging of motion with spatial modulation of magnetization. Radiology 1989;171:841–845. [DOI] [PubMed] [Google Scholar]
- 13.Yuan Q, Axel L, Hernandez EH, Dougherty L, Pila JJ, Scott CH, Ferrari VA, Blom AS. Cardiac–respiratory gating method for magnetic resonance imaging of the heart. Magn Reson Med 2000;43:314–318. [DOI] [PubMed] [Google Scholar]
- 14.Scott CH, Sutton MSJ, Gusani N, Fayad Z, Kraitchman D, Keane MG, Axel L, Ferrai VA. Effect of dobutamine on regional left ventricular function measured by tagged magnetic resonance imaging in normal subjects. Am J Cardiol 1999;83:412–417. [DOI] [PubMed] [Google Scholar]
- 15.Marcus JT, Gotte MJW, VanRossum AC, Kuijer JPA, Heethaar RM, Axel L, Visser CA. Myocardial function in infarcted and remote regions early after infarction in man: assessment by magnetic resonance tagging and strain analysis. Magn Reson Med 1997;38:803–810. [DOI] [PubMed] [Google Scholar]
- 16.Gotte MJW, vanRossum AC, Marcus JT, Kuijer JPA, Axel L, Visser CA. Recognition of infarct localization by specific changes in intramural myocardial mechanics. Am Heart J 1999;138:1038–1045. [DOI] [PubMed] [Google Scholar]
- 17.Axel L, Goncalves RC, Bloomgarden D. Regional heart wall motion: two dimensional analysis and functional imaging with MR imaging. Radiology 1992;183:745–750. [DOI] [PubMed] [Google Scholar]
- 18.Zhou R, Pickup S, Glickson JD, Scott CH, Ferrari VA. Assessment of global and regional myocardial function in the mouse using cine and tagged MRI. Magn Reson Med 2003;49:760–764. [DOI] [PubMed] [Google Scholar]
- 19.Niitsu M, Kumada M, Campeau NG, Niimi S, Riederer SJ, Itai Y. Tongue displacement: visualization with rapid tagged magnetization-prepared MR imaging. Radiology 1994;191:578–580. [DOI] [PubMed] [Google Scholar]
- 20.Napadow VJ, Chen Q, Wedeen VJ, Gilbert RJ. Intramural mechanics of the human tongue in association with physiological deformations. J Biomech 1999;32:1–12. [DOI] [PubMed] [Google Scholar]
- 21.Stone M, Davis EP, Douglas AS, NessAiver M, Gullapalli R, Levine WS, Lundberg A. Modeling the motion of the internal tongue from tagged cine-MRI images. J Acoust Soc Am 2001;109:2974–2982. [DOI] [PubMed] [Google Scholar]
- 22.Brennick MJ, Pickup S, Dougherty L, Kuna ST. Respiratory-gated MRI shows regional pharyngeal airway size is reduced in obese Zucker rats compared to their lean littermates [abstract]. Am J Respir Crit Care Med 2004;169:A431. [Google Scholar]
- 23.Brennick MJ, Trouard TP, Gmitro AF, Fregosi RF. MRI study of pharyngeal airway changes during stimulation of the hypoglossal nerve branches in rats. J Appl Physiol 2001;90:1373–1384. [DOI] [PubMed] [Google Scholar]
- 24.Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS system for mixed models. Cary, NC: SAS Institute; 1996.
- 25.Winer BJ, Brown DR, Michels KM. Statistical principles in experimental design, 3rd ed. McGraw-Hill series in psychology. New York: McGraw-Hill; 1991.
- 26.Kamanna VS, Kirschbaum MA. Association between very low density lipoprotein and glomerular damage in the obese Zucker rat. Am J Nephrol 1993;13:53–58. [DOI] [PubMed] [Google Scholar]
- 27.Katzeff HL, Selgrad C. Impaired peripheral thyroid metabolism in genetic obesity. Endocrinology 1993;132:989–995. [DOI] [PubMed] [Google Scholar]
- 28.Paulson DJ, Tahiliani AG. Cardiovascular abnormalities associated with human and rodent obesity. Life Sci 1992;51:1557–1569. [DOI] [PubMed] [Google Scholar]
- 29.Maislin G, Pack AI, Kribbs NB, Smith PL, Schwartz AR, Kline LR, Schwab RJ, Dinges DF. A survey screen for prediction of apnea. Sleep 1995;18:158–166. [DOI] [PubMed] [Google Scholar]
- 30.Schlenker EH, Farkas GA. Endogenous opioids modulate ventilation in the obese Zucker rat. Respir Physiol 1995;99:97–103. [DOI] [PubMed] [Google Scholar]
- 31.Schwab RJ, Gefter WB, Hoffman EA, Gupta KB, Pack AI. Dynamic upper airway imaging during awake respiration in normal subjects and patients with sleep disordered breathing. Am J Respir Crit Care Med 1993;148:1385–1400. [DOI] [PubMed] [Google Scholar]
- 32.Nakano H, Magalang UJ, Lee S-d, Krasney JA, Farkas GA. Serotonergic modulation of ventilation and upper airway stability in obese Zucker rats. Am J Respir Crit Care Med 2001;163:1191–1197. [DOI] [PubMed] [Google Scholar]
- 33.Magalang UJ, Ray AD, Farkas GA. Effects of hypoglossal nerve stimulation on upper airway mechanics in obese Zucker rats. Am J Respir Crit Care Med 2002;165:A798. [Google Scholar]
- 34.Schwab RJ, Kuna ST, Remmers JE. Anatomy and physiology of upper airway obstruction. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine, 4th ed. Philadelphia, PA: W.B. Saunders; 2005. pp. 840–858.
- 35.Hwang J-C, St John WM, Bartlett D Jr. Respiratory-related hypoglossal nerve activity: influence of anesthetics. J Appl Physiol 1983;55:785–792. [DOI] [PubMed] [Google Scholar]
- 36.Rowley JA, Permutt S, Willey S, Smith PL, Schwartz AR. Effect of tracheal and tongue displacement on upper airway flow dynamics. J Appl Physiol 1996;80:2171–2178. [DOI] [PubMed] [Google Scholar]
- 37.Van de Graff WB. Thoracic influence on upper airway patency. J Appl Physiol 1988;65:2124–2131. [DOI] [PubMed] [Google Scholar]
- 38.Bhattacharyya N, Blake SP, Fried MP. Assessment of the airway in obstructive sleep apnea syndrome with 3-dimensional airway computed tomography. Otolaryngol Head Neck Surg 2000;123:444–449. [DOI] [PubMed] [Google Scholar]
- 39.Ciscar MA, Juan G, Martinez V, Ramon M, Lloret T, Minguez J, Armengot M, Marin J, Basterra J. Magnetic resonance imaging of the pharynx in OSA patients and healthy subjects. Eur Respir J 2001;17:79–86. [DOI] [PubMed] [Google Scholar]
- 40.Jager L, Gunther E, Gauger J. Fluoroscopic MR of the pharynx in patients with obstructive sleep apnea. AJNR Am J Neuroradiol 1998;19:1205–1214. [PMC free article] [PubMed] [Google Scholar]
- 41.Stanford W, Galvin J, Rooholamini M. Effects of awake tidal breathing, swallowing, nasal breathing, oral breathing and the Muller and Valsalva maneuvers on the dimensions of the upper airway. Chest 1988;91:149–154. [DOI] [PubMed] [Google Scholar]
- 42.Stein MG, Gamsu G, deGeer G, Golden JA, Crumley RL, Weeb WR. Cine CT in obstructive sleep apnea. AJR Am J Roentgenol 1987;148:1069–1074. [DOI] [PubMed] [Google Scholar]
- 43.Ell SR, Jolles H, Galvin JR. Cine CT demonstration of nonfixed upper airway obstruction. AJR Am J Roentgenol 1986;146:669–677. [DOI] [PubMed] [Google Scholar]
- 44.Launois SH, Remsburg S, Yang WJ, Weiss JW. Relationship between velopharyngeal dimensions and palatal EMG during progressive hypercapnia. J Appl Physiol 1996;80:478–485. [DOI] [PubMed] [Google Scholar]
- 45.Morrell MJ, Badr MS. Effects of NREM sleep on dynamic within-breath changes in upper airway patency in humans. J Appl Physiol 1998;84:190–199. [DOI] [PubMed] [Google Scholar]
- 46.Schwab RJ, Gefter WB, Pack AI, Hoffman EA. Dynamic imaging of the upper airway during respiration in normal subjects. J Appl Physiol 1993;74:1504–1514. [DOI] [PubMed] [Google Scholar]
- 47.Veasey SC, Panckeri KA, Hoffman EA, Pack AI, Hendricks JC. The effects of serotonin antagonists in an animal model of sleep disordered breathing. Am Rev Respir Dis 1996;153:776–786. [DOI] [PubMed] [Google Scholar]
- 48.Strohl KP, Fouke JM. Dilating forces on the upper airway in anaesthetized dogs. J Appl Physiol 1985;58:452–458. [DOI] [PubMed] [Google Scholar]
- 49.Ryan CF, Love LL. Mechanical properties of the velopharynx in obese patients with obstructive sleep apnea. Am J Respir Crit Care Med 1996;154:806–812. [DOI] [PubMed] [Google Scholar]
- 50.Schwab RJ, Gupta KB, Gefter WB, Hoffman EA, Pack AI. Upper airway soft tissue anatomy in normals and patients with sleep disordered breathing: significance of the lateral pharyngeal walls. Am J Respir Crit Care Med 1995;152:1673–1689. [DOI] [PubMed] [Google Scholar]
- 51.Leiter JC. Upper airway shape: is it important in the pathogenesis of obstructive sleep apnea? Am J Respir Crit Care Med 1996;153:894–898. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.