Abstract
Rationale: Tumor necrosis factor (TNF) mediates a spectrum of airway inflammatory responses, including those to air pollutants, and is an asthma candidate gene. One TNF promoter variant (G–308A) affects expression of TNF and has been associated with inflammatory diseases; however, studies of asthma have been inconsistent. Because ozone produces oxidative stress, increased airway TNF, and inflammation, the associations of the −308 TNF polymorphism with asthma may vary by ozone exposure and variants of oxidant defense genes glutathione-S-transferase (GST) M1 and GSTP1.
Objectives: To investigate the association of TNF G–308A with asthma and wheezing and to determine whether these associations vary with ozone exposure and GSTM1 and GSTP1 genotype.
Methods: We studied associations of TNF–308 genotype with lifetime and current wheezing and asthma among 3,699 children in the Children's Health Study. We examined differences in associations with community ozone and by GSTM1 null and GSTP1 105 Ile/Val (A105G) genotype.
Results: Children with TNF–308 GG had decreased risk of asthma (odds ratio, 0.8; 95% confidence interval, 0.7–0.9) and lifetime wheezing (odds ratio, 0.8; 95% confidence interval, 0.7–0.9). The protective effects of GG genotype on wheezing outcomes were of greater magnitude in lower compared with higher ozone communities. These findings were replicated in the two cohorts of fourth-grade children recruited in 1993 and 1996. The reduction of the protective effect from the −308 GG genotype with higher ozone exposure was most marked in the GSTM1 null and GSTP1 Ile/Ile groups.
Conclusions: The TNF–308 GG genotype may have a protective role in asthma pathogenesis, depending on airway oxidative stress levels.
Keywords: child, genetic epidemiology, lung
Asthma is a common complex disease with multiple determinants that include genetic variation, environmental exposures, and gene–environment interactions (1–4). Tumor necrosis factor (TNF)-α has a recognized role in asthma pathophysiology, and variation in the locus that affects expression of this cytokine, especially in response to inhaled air pollutants, may contribute to asthma occurrence (5–11).
TNF-α plays a central role in the initiation of airway inflammation and the generation of airway hyperreactivity (12–14). Genetic variants may affect TNF levels in the airways and thereby modulate asthma and wheezing occurrence. Many studies of respiratory conditions have focused on the G–308A polymorphism in the TNF gene 5′ untranslated region because of its associations with inflammation and diseases, including asthma (5, 9, 15–26). The studies of asthma have been inconsistent in the direction of effects, and some have found no significant association (24–26). A number of methodologic issues may contribute to these inconsistencies among studies. Alternatively, variation in asthma and wheezing may reflect differences in the magnitude of this inflammatory response, which depends on exposures that produce oxidative stress, airway antioxidant defenses, and TNF responses. Differences in genetic background and environmental exposures that interact with TNF could also contribute to differences in results among studies.
Ozone, a common air pollutant that has been associated with asthma incidence and exacerbation, is a strong oxidant that produces an inflammatory response (27–31). It has been suggested that ozone may modify the effect of the TNF−308 polymorphism on asthma and wheezing (32). The level of oxidative stress produced by ozone drives TNF-mediated inflammation; however, the level of oxidative stress is likely to depend on airway antioxidant defenses and ozone levels. Airway antioxidant defenses are mediated in part by enzymatic antioxidants, including glutathione-S-transferases (GSTs) (33). GSTs function in antioxidant defenses through reactive oxygen species metabolism, repair of reactive oxygen species damage, and detoxification of xenobiotics (34, 35). We investigated the modifying effects of GSTM1 and GSTP1 genotypes on TNF–ozone associations because these genes are expressed in the respiratory tract, are involved in antioxidant defenses, and have common functional alleles that result in the total absence or a marked alteration in the enzyme's activity (4, 33, 36–44).
To further investigate the role of TNF in asthma occurrence, we examined the associations TNF G–308A polymorphism with asthma and wheezing at entry into the Children's Health Study (CHS) (44, 45). The CHS is a population-based study of school-aged children from 12 southern California communities representing a wide range of exposures to ambient air pollution. We investigated the effects of the TNF−308 polymorphism on asthma and wheezing because we focused on variation in associations of this functional change with outcomes in high and low ambient ozone environments and in relationship with GSTM1 null and GSTP1 Ile105Val genotypes. Some of the results presented in this article have been reported in an abstract (46).
METHODS
The design and methods for the CHS have been described in detail (45, 47). The CHS recruited fourth-, seventh-, and tenth-grade students from schools in 12 southern California communities in 1993 and recruited a second group of fourth-grade students from the same schools in 1996. Information on sociodemographics, asthma risk factors, household exposures, asthma, and wheeze outcomes was collected by questionnaire completed at study entry by the parents or guardian of each CHS participant. An air pollution monitoring network was established in 1994, and levels were measured continuously in each of the 12 communities included in the CHS. Children and a sample of their parents provided buccal cells as the DNA resource for genotyping several years after study entry. The study protocol was approved by the institutional review board for human studies at the University of Southern California, and written, informed consent was provided by a parent or legal guardian for all participants.
Questionnaire
Children's asthma and wheezing histories were characterized using questionnaire responses. Asthma status was defined as any reported lifetime history of a physician diagnosis of asthma at study entry. Lifetime and past–12-mo wheezing symptoms were provided by the participant's parent or guardian at CHS entry. Risk factors and possible confounders were collected in the questionnaire, including gestational age, birth weight, race and Hispanic ethnicity, level of parental education, insurance coverage, in utero exposure to maternal smoking and second-hand exposure to tobacco smoke, and family history of asthma and allergy.
Air Pollution Data
Air pollution monitoring stations were established in each of the 12 study communities beginning in 1994 (45, 47). Each station measured average hourly levels of ozone. We computed the annual average of the ozone levels obtained from 10:00 a.m. to 6:00 p.m. (the 8-h daytime average) in each community in 1995. Community-level ozone exposures were grouped into low and high subcategories using a 50-ppb cutoff. The mean annual ambient ozone level was 37.5 ppb in low-ozone communities and 57.8 ppb in high-ozone communities.
DNA Collection and Genotyping
Participants and a sample of their parents provided samples of genomic DNA beginning in 1998 using standard buccal cell collection procedures (44). Because a number of subjects had left the study in the years between enrollment and DNA collection, TNF genotyping results were available for 3,699 children (87 individuals with DNA samples had undetermined TNF genotyping results). Among the 3,699 individuals with TNF genotyping results, 553 individuals had undetermined GSTM1 genotype, and 88 had undetermined GSTP1 genotype (26 missing results for both GSTs). The DNA samples not collected were largely the result of residential moves required from changes in parent employment. Children in the cohort who did not have genotyping results showed modest differences in socioeconomic-related characteristics from children who had the genotyping results (see Table E1 of the online supplement).
Buccal scrapes were collected using standard protocols, and genomic DNA was isolated using a Purgene DNA isolation kit (Gentra Systems, Minneapolis, MN). The polymorphisms were identified by real-time polymerase chain reaction (PCR) using allele-specific minor groove binder (MGB) probes on an ABI Prism 7700 Sequence Detector (Applied Biosystems, Foster City, CA). Each genotype was validated by using PCR/restriction fragment length polymorphism methods (48). The sequences of primers and probes we used is listed in Table E2.
Statistical Analysis
Unconditional logistic regression was used to estimate the association of the TNF–308 genotype with lifetime and current asthma and with wheezing outcomes. Genetic models were chosen based on available biological evidence from mechanistic and association studies. For TNF–308, we grouped genotypes into GG versus AG or AA because we hypothesized that the GG genotype is protective due to reduced inflammatory response. Confounding by a priori identified covariates was assessed by examining changes in TNF-effect estimates from the models with and without the potential confounder. To assess confounding by admixture, we examined genotype associations in child–parent trios using a logistic regression model that included indicators of parent mating type (49). In addition, we stratified our study population by non-Hispanic and Hispanic whites (two major ethnic groups) to assess whether effects of TNF and ozone differed by ethnicity. To determine whether our findings could be replicated in an independent group of subjects, we fit models in two independent groups of children, one group recruited in 1993 and another group of children recruited in 1996.
To investigate whether ambient ozone level modulated the associations of TNF genotype with asthma and wheezing outcomes, we fitted stratified models restricted to the 2,727 fourth graders who resided in communities with lower or higher ozone levels. We restricted this analysis to fourth graders because valid exposure data at the same age (approximate age, 10 yr) were available to estimate annual averages in the year before the questionnaire responses. Annual averages were also determined for the year before enrollment in the study. When considering the effects of GSTM1 genotype on the TNF associations, we stratified participants by GSTM1 genotype (null versus present) or GSTP1 105 genotypes using a dominant model (Ile/Ile vs. Ile/Val or Val/Val) and assessed TNF associations in low- and high-ozone areas.
To evaluate the effects of lifetime ozone exposure on lifetime history of asthma and wheezing, we restricted our analysis to a subgroup of 878 fourth graders who had lived in the same community from birth until study entry. All analyses were conducted using SAS software version 9.1 (SAS Institute, Cary, NC).
RESULTS
Selected characteristics for CHS participants with TNF genotyping information are presented in Table 1. The majority of the participants were non-Hispanic whites. More than 16% of children in the study were exposed in utero to maternal smoking. Second-hand smoke exposure was common (about 33% lifetime and 18% current) in the study population. The average prevalence for lifetime asthma and wheezing was 15 and 34%, respectively. The TNF alleles were in Hardy-Weinberg equilibrium in each ethnic group and the GG genotype varied from 71.2% in non-Hispanic whites to 87.3% in Asians (Table 2). The GSTM1 null and the GSTP1 105 Ile/Ile genotypes were observed for 47.6 and 39.6% of children, respectively.
TABLE 1.
SELECTED CHARACTERISTICS OF CHILDREN'S HEALTH STUDY PARTICIPANTS AND A SUBSET OF FOURTH GRADERS
| Fourth Graders (n = 2,727)
|
All CHS Participants
|
|||
|---|---|---|---|---|
| n | % | n | % | |
| Demographic information | ||||
| Sex | ||||
| Girls | 1,407 | 51.6 | 1,978 | 53.5 |
| Boys | 1,320 | 48.4 | 1,721 | 46.5 |
| Age at study entry, yr | ||||
| 8–9 | 1,939 | 71.2 | 1,939 | 52.5 |
| 10–11 | 787 | 28.8 | 787 | 21.3 |
| 12–13 | 1 | 0.0 | 552 | 14.9 |
| 14–18 | 0 | 0.0 | 421 | 11.3 |
| Ethnicity | ||||
| Non-Hispanic White | 1,574 | 57.7 | 2,182 | 59.0 |
| Hispanic | 772 | 28.3 | 989 | 26.7 |
| African American | 120 | 4.4 | 156 | 4.2 |
| Asian | 110 | 4.1 | 157 | 4.2 |
| Other | 151 | 5.5 | 215 | 5.8 |
| Gestational age | ||||
| Full term | 2,335 | 85.6 | 3,189 | 86.2 |
| < 4 wk early | 197 | 7.2 | 254 | 6.9 |
| ⩾ 4 wk early | 125 | 4.6 | 160 | 4.3 |
| Missing | 70 | 2.6 | 99 | 2.7 |
| In utero exposure to maternal smoking | ||||
| Yes | 445 | 16.8 | 603 | 16.9 |
| Any lifetime SHS exposure | ||||
| Yes | 866 | 33.1 | 1,230 | 34.6 |
| Current SHS exposure | ||||
| Yes | 473 | 17.9 | 650 | 18.1 |
| Ozone exposure | ||||
| High (⩾ 50 ppb) | 1,605 | 58.9 | 2,166 | 58.6 |
| Lifetime residence | ||||
| Yes | 878 | 32.2 | 1,271 | 34.4 |
| Respiratory outcomes | ||||
| Ever asthma | 403 | 15.1 | 551 | 15.2 |
| Ever wheezing | 886 | 34.3 | 1,222 | 34.8 |
| Current wheezing* | 493 | 22.5 | 704 | 23.6 |
| Medication for wheezing* | 296 | 14.9 | 401 | 14.9 |
Definition of abbreviations: CHS = Children's Health Study; SHS = second-hand smoke.
Based on self-report for the past 12 mo.
TABLE 2.
TNF G-308A GENOTYPES BY ETHNICITY IN CHILDREN'S HEALTH STUDY PARTICIPANTS (n = 3,699)
| TNF (G-308A)
|
|||||||
|---|---|---|---|---|---|---|---|
| GG
|
GA
|
AA
|
|||||
| Ethnicity | Total | n | % | n | % | n | % |
| Non-Hispanic white | 2,182 | 1,553 | 71.2 | 572 | 26.2 | 57 | 2.6 |
| Hispanic | 989 | 814 | 82.3 | 171 | 17.3 | 4 | 0.4 |
| African American | 156 | 122 | 78.2 | 33 | 21.2 | 1 | 0.6 |
| Asian | 157 | 137 | 87.3 | 18 | 11.5 | 1 | 1.3 |
| Other | 215 | 171 | 79.5 | 40 | 18.6 | 4 | 1.9 |
Definition of abbreviation: TNF = tumor necrosis factor.
Hardy-Weinberg equilibrium holds for TNF alleles in each ethnicity.
Children who were homozygous for the TNF–308 G showed reduced risk for asthma and wheezing (Table 3). For example, children with the GG genotype had a lower prevalence of lifetime asthma (odds ratio [OR], 0.8; 95% confidence interval [CI], 0.7–0.9) compared with children carrying at least one −308 A allele (e.g., GA or AA). Current and lifetime wheezing occurred less frequently in children who were −308 G homozygotes. The associations of the −308 A allele were replicated in the two cohorts of fourth graders independently recruited in 1993 and 1996 (Table E3). The effect estimates were qualitatively similar based on analyses of the 338 complete trios, indicating that our estimates for all CHS children are not due to bias from population stratification. The association for TNF did not substantially differ between non-Hispanic and Hispanic whites. GSTM1 and GSTP1 genotypes were not significantly associated with the outcomes in this analysis (Table E5).
TABLE 3.
ASSOCIATIONS OF TNF G-308A WITH ASTHMA AND WHEEZING OUTCOMES IN CHILDREN AND TRIOS AT CHILDREN'S HEALTH STUDY ENTRY
| CHS
|
Complete Trios
|
||||||
|---|---|---|---|---|---|---|---|
| Outcomes | TNF | Ca/Co | OR | 95% CI | Ca/Co | OR | 95% CI |
| Ever asthma | GA/AA | 399/2,331 | 1.0 | 29/102 | 1.0 | ||
| GG | 152/733 | 0.8 | 0.7–0.9 | 62/285 | 0.6 | 0.2–1.3 | |
| Ever wheezing | GA/AA | 884/1,762 | 1.0 | 54/72 | 1.0 | ||
| GG | 338/523 | 0.8 | 0.7–0.9 | 132/210 | 0.5 | 0.3–1.1 | |
| Current wheezing | GA/AA | 499/1,758 | 1.0 | 30/72 | 1.0 | ||
| GG | 205/523 | 0.7 | 0.6–0.9 | 80/210 | 0.6 | 0.3–1.6 | |
| Medication for wheezing | GA/AA | 279/1,761 | 1.0 | 22/72 | 1.0 | ||
| GG | 122/523 | 0.7 | 0.5–0.8 | 54/210 | 0.5 | 0.2–1.4 | |
Definition of abbreviations: Ca/Co = number of cases/number of controls; CHS = Children's Health Study; CI = confidence interval; OR = odds ratio; TNF = tumor necrosis factor.
Models are adjusted for age, sex, race/ethnicity, town, lifetime residence, grade, and smoking exposure (in utero and second-hand).
We found that the protective effects of the GG genotype on wheezing outcomes were stronger for children living in low-ozone communities than in high-ozone communities (Table 4). Compared with children who had the GA or AA genotypes, those with the GG genotype had a marked reduction of ever wheezing with low ozone exposure (OR, 0.5; 95% CI, 0.4–0.7); however, this reduction was not observed in children living in high-ozone communities (OR, 1.0; 95% CI; 0.8–1.3). This difference in genotypic effects between low- and high-ozone environments was statistically significant (interaction p = 0.003). Similarly, the protective associations of the −308 GG genotype with current wheezing and medication for wheezing were of significantly greater magnitude for those exposed to lower compared with higher ozone (interaction p = 0.04 and 0.02 for current wheezing and medication for wheezing, respectively). The association of TNF with any lifetime history of asthma was similar in the low- and high-ozone communities (OR, 0.8 and 0.9; Table 4). We found no direct associations of ozone with asthma or wheezing outcomes (data not shown). In addition, we found no substantial differences in the effect of the −308 GG genotype in children in relation to exposure to the other monitored air pollutants in the CHS (PM10, PM2.5, NO2, and acid vapor levels) or second-hand smoke exposure (data not shown). The variation in the protective effect of the −308 GG genotype on lifetime asthma and wheezing in high- and low-ozone communities was similar in analyses restricted to the subgroup of lifetime residents (data not shown). We found that the variations in the associations of the −308 A allele by community ozone were observed in the two cohorts of fourth graders independently recruited in 1993 and 1996 (Table E3).
TABLE 4.
THE ASSOCIATION OF TNF G-308A WITH ASTHMA AND WHEEZING OUTCOMES IN FOURTH-GRADE CHILDREN'S HEALTH STUDY PARTICIPANTS IN LOWER AND HIGHER OZONE COMMUNITIES*
| Low Ozone
|
High Ozone
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Outcomes | TNF | Ca/Co | OR | 95% CI | Ca/Co | OR | 95% CI | p Value for Interaction |
| Ever asthma | GA/AA | 44/195 | 1.0 | 63/339 | 1.0 | |||
| GG | 122/726 | 0.8 | 0.5–1.1 | 174/1002 | 0.9 | 0.7–1.2 | 0.51 | |
| Ever wheezing | GA/AA | 106/126 | 1.0 | 137/252 | 1.0 | |||
| GG | 256/575 | 0.5 | 0.4–0.7 | 387/745 | 1.0 | 0.8–1.3 | 0.003 | |
| Current wheezing | GA/AA | 61/126 | 1.0 | 78/252 | 1.0 | |||
| GG | 147/574 | 0.5 | 0.4–0.8 | 207/743 | 0.9 | 0.6–1.2 | 0.04 | |
| Medication for wheezing | GA/AA | 39/126 | 1.0 | 49/252 | 1.0 | |||
| GG | 84/574 | 0.4 | 0.3–0.7 | 124/745 | 0.9 | 0.6–1.2 | 0.02 | |
For definition of abbreviations,see Table 3.
Models are adjusted for age, sex, race/ethnicity, town, lifetime residence, grade, and smoking exposure (in utero and second-hand).
Two ozone strata were defined as less than and greater than 50-ppb ozone average.
To assess the role of GSTM1 null genotype on the protective effect of the −308 GG genotype on wheezing in low-ozone communities, we fitted models stratifying subjects by their GSTM1 genotypes (present or null, 352 excluded for missing genotypes). We found that the difference in the −308 GG genotype effect between low and high ozone exposure was more marked in the GSTM1 null compared with the GSTM1 present group (Table 5).
TABLE 5.
THE ASSOCIATION OF TNF G-308A WITH WHEEZING OUTCOMES IN FOURTH-GRADE CHILDREN'S HEALTH STUDY PARTICIPANTS, STRATIFIED BY GSTM1 GENOTYPE AND OZONE EXPOSURE*
| GSTM1 Null
|
GSTM1 Present
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low Ozone
|
High Ozone
|
Low Ozone
|
High Ozone
|
||||||||||||
| Outcomes | TNF | Ca/Co | OR | 95% CI | Ca/Co | OR | 95% CI | Ca/Co | OR | 95% CI | Ca/Co | OR | 95% CI | ||
| Ever wheezing | GA/AA | 47/57 | 1.0 | 50/111 | 1.0 | 50/58 | 1.0 | 63/105 | 1.0 | ||||||
| GG | 107/245 | 0.5 | 0.3–0.8 | 152/292 | 1.1 | 0.7–1.5 | 126/275 | 0.5 | 0.4–0.8 | 176/332 | 0.9 | 0.6–1.2 | |||
| p = 0.009† | p = 0.10† | ||||||||||||||
| Current wheezing | GA/AA | 30/57 | 1.0 | 27/111 | 1.0 | 27/58 | 1.0 | 36/105 | 1.0 | ||||||
| GG | 59/245 | 0.4 | 0.2–0.6 | 87/291 | 0.9 | 0.6–1.5 | 77/274 | 0.7 | 0.4–1.1 | 93/331 | 0.7 | 0.5–1.2 | |||
| p = 0.009† | p = 0.75† | ||||||||||||||
| Medication for wheezing | GA/AA | 18/57 | 1.0 | 17/111 | 1.0 | 17/58 | 1.0 | 21/105 | 1.0 | ||||||
| GG | 33/245 | 0.3 | 0.1–0.6 | 48/292 | 0.8 | 0.5–1.4 | 45/274 | 0.5 | 0.3–1.0 | 54/332 | 0.8 | 0.4–1.3 | |||
| p = 0.02† | p = 0.42† | ||||||||||||||
For definition of abbreviations,see Table 3.
Models are adjusted for age, sex, race/ethnicity, town, lifetime residence, grade, and smoking exposure (in utero and second-hand).
Two ozone strata were defined as less than and greater than 50-ppb ozone average (Figure 1).
Testing of interaction between TNF and ozone within GSTM1 genotypes.
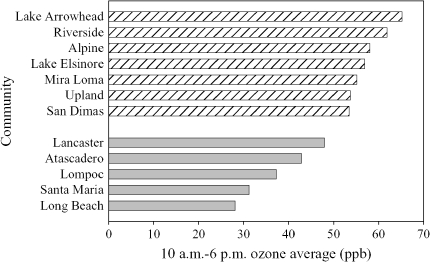
Figure 1.
Average ozone in 12 Children's Health Study communities. Hatched bars: higher ozone; mean, 57.8 ppb. Shaded bars: lower ozone; mean, 37.5 ppb.
Similarly, the difference in the effect of TNF between low and high ozone exposure was greater in those with the GSTP1 Ile/Ile genotype compared with the GSTP1 Ile/Val and Val/Val genotypes (Table 6). We had insufficient sample size to jointly stratify on GSTM1 and GSTP1 genotype and examine the interaction of TNF genotype with ozone levels.
TABLE 6.
THE ASSOCIATION OF TNF G-308A WITH WHEEZING OUTCOMES IN FOURTH-GRADE CHILDREN'S HEALTH STUDY PARTICIPANTS, STRATIFIED BY GSTP1 Ile105Val AND OZONE EXPOSURE*
| GSTP1 Ile/Ile (n = 1,054)
|
GSTP1 Ile/Val or Val/Val (n = 1,605)
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low Ozone
|
High Ozone
|
Low Ozone
|
High Ozone
|
||||||||||||
| Outcomes | TNF | Ca/Co | OR | 95% CI | Ca/Co | OR | 95% CI | Ca/Co | OR | 95% CI | Ca/Co | OR | 95% CI | ||
| Ever wheezing | GA/AA | 63/76 | 1.0 | 81/130 | 1.0 | 38/46 | 1.0 | 50/110 | 1.0 | ||||||
| GG | 150/348 | 0.6 | 0.3–0.9 | 227/431 | 1.1 | 0.8–1.7 | 101/216 | 0.5 | 0.4–0.8 | 153/302 | 0.9 | 0.6–1.2 | |||
| p = 0.04† | p = 0.07† | ||||||||||||||
| Current wheezing | GA/AA | 37/76 | 1.0 | 49/130 | 1.0 | 23/46 | 1.0 | 25/110 | 1.0 | ||||||
| GG | 86/348 | 0.5 | 0.3–0.9 | 122/431 | 1.2 | 0.7–2.0 | 58/215 | 0.5 | 0.3–0.8 | 84/300 | 0.7 | 0.5–1.1 | |||
| p = 0.06† | p = 0.28† | ||||||||||||||
| Medication for wheezing | GA/AA | 21/76 | 1.0 | 35/130 | 1.0 | 16/46 | 1.0 | 13/110 | 1.0 | ||||||
| GG | 52/348 | 0.4 | 0.2–0.8 | 76/431 | 1.3 | 0.7–2.6 | 30/215 | 0.5 | 0.3–0.9 | 47/302 | 0.6 | 0.4–0.9 | |||
| p = 0.02† | p = 0.53† | ||||||||||||||
For definition of abbreviations,see Table 3.
Models are adjusted for age, sex, race/ethnicity, town, lifetime residence, grade, and smoking exposure (in utero and second-hand).
Two ozone strata were defined as less than and greater than 50-ppb ozone average (Figure 1).
Testing of interaction between TNF and ozone within GSTP1 genotypes.
DISCUSSION
A growing number of studies have provided evidence that expression of genetic determinants of asthma and wheezing depends on exposure to environmental stressors (1, 2, 42–44). In this study, we show that the TNF−308 GG genotype is protective for asthma and wheezing but that protection depends on outdoor ozone levels in the community of residence. Children living in high-ozone communities were not protected from increased asthma and wheezing by the GG genotype. These associations were replicated in two cohorts of fourth-grade children who were independently recruited in 1993 and 1996, indicating that the associations were unlikely to arise by chance. In addition to ozone, two common variants in unlinked genes involved in antioxidant defenses, GSTM1 and GSTP1, may affect the expression of the protective effect of the TNF–308 GG genotype, as indicated by the less marked protection in high-ozone communities among groups with the GSTM1 null or GSTP1 105 Val allele. We have previously reported that asthma occurrence may depend on epistatic relationships between antioxidant and inflammatory genes (4). We now provide evidence for a role of gene–environment interactions and genetic interactions in the occurrence of childhood asthma and wheezing.
Our results are consistent with the majority of studies reporting that the allele G of the TNF–308 polymorphism is associated with decreased risk of asthma and wheezing (9, 18, 20–23). However, a number of studies on the association of TNF–308 polymorphism with asthma have reported conflicting results (5, 9, 18, 20–26). Based on our findings, the inconsistency among studies may be due to differences in exposures or genetic background among the populations studied.
Although we did not directly study the mechanisms for the effects of the TNF–308 GG variant or the effects of ozone exposure on TNF-mediated asthma and wheezing in children, we speculate that TNF–308 GG promoter genotype is associated with less intense inflammatory responses to oxidant stressors and that this reduced responsiveness results in reduced risk for asthma and wheezing. TNF–308 genotype has been shown to influence the rate of transcription and protein translation of TNF with the G allele resulting in lower expression (50). High levels of TNF have been observed in the bronchoalveolar lavage fluid, serum, and bronchial submucosa of patients with asthma (51). Because TNF levels are associated with airway inflammation, increased bronchial hyperresponsiveness, and atopy, the GG allele has a biologically plausible role in the occurrence of asthma and wheezing. A modifying effect of ozone exposure on the effects of the TNF G–308A polymorphism on lung function has also been reported from studies of experimental ozone exposures in which the −308 G was associated with lung function changes after acute ozone exposure (52). However, this genetic association with lung function is in the opposite direction of the effect of the GG genotype on asthma and wheezing we report in this study. This difference may reflect a different mechanism producing the findings for lung function deficits than for asthma and wheezing. For example, acute lung function deficits from ozone are not well correlated with cellular inflammatory responses, suggesting another function of TNF in producing deficits on ozone exposure. However, the reasons for the opposite effects on acute change in lung function are not clear and may warrant further study.
The protective effect of the TNF–308 GG genotype seems to depend on the level of oxidative stress, reflecting oxidant exposure and adequacy of antioxidant defenses. Oxidative stress is intimately involved in multiple inflammation processes that are regulated in part by cytokines such as TNF (53, 54). The TNF–308 A allele may alter gene expression and amplify the intensity of the inflammatory response to oxidants, leading to increased risk and severity of asthma and wheezing in susceptible groups. Ozone, an ambient photochemical oxidant, is capable of producing pulmonary inflammation and injury. Exposure to ozone results in the release of inflammatory cytokines, including TNF (8). Pretreatment of rats with antibody to TNF can reduce ozone-induced inflammation and lung damage, and acute ozone-induced airway hyperreactivity is reduced in TNF receptor−deficient mice (55). The findings in this study suggest that the effects of the −308 G on transcription are overcome by higher levels of oxidative stress. These results are consistent with studies of effects of GSTM1 and antioxidant vitamins in children exposed to high levels of ozone. We suggest that sequence variants that affect expression of genes participating in inflammatory pathways may show variable penetrance in the setting of high ozone exposure and low antioxidant defenses.
Interpretation of our results requires consideration of several limitations. We conducted a cross-sectional study of school-aged children. A limitation of this approach is the potential for selection bias. Because our study was based in a cohort, we were able to examine the potential for selection bias in the cross-sectional study. Of the total number of eligible subjects, approximately 40% did not provide buccal cell samples for this study, which may give rise to selection bias. Although those with and without genotypes did modestly differ in socioeconomic status and ethnicity, adjustment for these variables in the health models had little effect on estimates, making selection bias an unlikely explanation for our findings.
A second important limitation was the phenotypic definition of participants' asthma and wheezing status, which used parental report of physician-diagnosed asthma and wheezing symptoms. The use of physician diagnosis of asthma has been widely used in epidemiologic studies, and self-report has been found to accurately reflect physician diagnosis (56). Asthma is a variable clinical syndrome characterized by recurrent symptoms of wheezing, breathlessness, chest tightness, and coughing. There are no gold standard tests to diagnose asthma. Some researchers advocate using objective tests for detecting airway hyperresponsiveness or atopy to define asthma. However, the sensitivity of these tests is low, and studies have shown that these tests are not superior to clinical history information for asthma diagnosis. Another concern in using physician-diagnosed asthma to classify asthma status is that limited access to health care may result in underdiagnosis of asthma. In our sample, more than 80% of children had medical insurance, which suggests that access to medical care was not limited. To assess this potential source of misclassification of asthma status, we considered the effects of health insurance, income, and education on the risk estimates for wheezing and found little change in the adjusted risk estimates. Because asthma and wheezing status was defined without the knowledge of genotype, differential misclassification of asthma status by TNF genotype is probably not a major source of bias that accounts for our results. Therefore, although there is likely to be nondifferential misclassification of asthma and wheezing status, such misclassification of asthma and wheezing status would not account for the genetic and environmental associations we observed.
In addition, ozone exposure assessment was based on ambient levels measured at central site monitors in each community. We dichotomized exposure into high- and low-ozone communities to account for uncertainties in indoor levels and time–activity patterns of the study participants. Although these estimates have associated measurement error, it is likely that the groups provided substantial contrasts in average ozone exposure. However, we only observed an association with ozone in a susceptible subgroup. We previously reported that children playing three or more team sports in a high-ozone environment had increased risk for developing asthma (29). Due to sample size limitations, we were unable to consider team sports as an additional effect modifier in this genetic analysis.
Other inflammatory exposures and conditions have the potential to confound the relationship between the TNF–308 polymorphisms and asthma. Some that have been well described in the literature include active personal smoking, exposure to second-hand smoke, indoor allergens, allergic diseases, and family history of asthma. Second-hand smoke and active smoking were taken into account by adjusting for these exposures; adjusting for cats, dogs, allergy, and family history of asthma individually and in combination resulted in negligible changes in the associations. Exposure to tobacco smoke was assessed using questionnaire responses about household sources and was not validated by objective measurements such as cotinine levels. However, the validity of exposure estimates based on questionnaire responses has been investigated and found to provide reasonably valid estimates of exposure for adjustment for confounding (57–59).
Conducting a candidate gene association study to assess the role of the TNF–308 polymorphism and susceptibility to asthma requires careful interpretation in light of the potential for population stratification and linkage disequilibrium in the chromosomal region that contains the TNF locus. In our analyses of asthma, TNF genotype, ozone, GSTM1, and GSTP1, we considered the effects of ethnicity and found the risk pattern of asthma and wheezing showed neither important confounding by ethnicity nor substantial differences in effects by ethnic status. We also examined the associations using parent–child trios and found associations consistent with the associations in all the children. Population stratification is unlikely to explain our findings. We note that the region spanning the TNF loci has long-range linkage disequilibrium (over 45 kb), and the associations with the −308 G allele may be due to other linked variants in this region (60). The TNF region is comprised of fewer than 20 common haplotypes. The −308 G variant occurs on two long-range haplotypes in populations with European ancestry, one of which is the most common haplotype in the linked region (60). Association studies have been conducted that have examined the TNF loci haplotypes and have found associations with haplotypes containing the −308 G variant (10). Based on the available information on locus region linkage structure, the associations with the −308 polymorphism reflect a comparison of haplotypes containing the −308 G variant to the remaining haplotypes across the 45-kb region and indicate that the −308 G variant or another variant on these haplotypes underlie the increased susceptibility for asthma and the loss of protection in high-ozone communities in the context of variant genotypes. Although studies of the TNF region using extended haplotypes capture the variation across the major histocompatibility complex region, splitting the −308 polymorphism across several haplotypes may require larger sample sizes to detect the same magnitude of effect for a functional polymorphism. Because the −308 polymorphism seems to be functional, our assessment of gene–environment interactions focused on assessing this genetic variant.
In conclusion, the protective association of TNF–308 GG genotype in children with low ozone exposures or protective GSTM1 or GSTP1 genotype suggests that this relatively common genetic polymorphism, or haplotype marked by this polymorphism, plays a protective role in asthma pathogenesis among children depending on airway oxidative stress levels.
Supplementary Material
Acknowledgments
The authors thank Christine Tidwell for assisting in the production and format of this manuscript. The authors acknowledge the hard work of the study field team and the cooperation of the 12 communities, the school principals, the many teachers, the students and their parents, and the staff at the participating air quality districts.
Supported by the Southern California Environmental Health Sciences Center (grant no. 5P30ES007048), the National Institute of Environmental Health Sciences (grant no. 5P01ES011627), the Children's Environmental Health Center (grant nos. 5P01ES009581, R826708-01, and RD831861-01), the Environmental Protection Agency, the National Heart, Lung and Blood Institute (grant nos. 5R01HL61768 and 5R01HL076647), and the Hastings Foundation.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200508-1256OC on March 2, 2006
Conflict of Interest Statement: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Kleeberger SR, Peden D. Gene-environment interactions in asthma and other respiratory diseases. Annu Rev Med 2005;56:383–400. [DOI] [PubMed] [Google Scholar]
- 2.Colilla S, Nicolae D, Pluzhnikov A, Blumenthal MN, Beaty TH, Bleecker ER, Lange EM, Rich SS, Meyers DA, Ober C, et al. Evidence for gene-environment interactions in a linkage study of asthma and smoking exposure. J Allergy Clin Immunol 2003;111:840–846. [DOI] [PubMed] [Google Scholar]
- 3.Miller RL. Breathing freely: the need for asthma research on gene-environment interactions. Am J Public Health 1999;89:819–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Millstein J, Conti DV, Gilliland FD, Gauderman WJ. A testing framework for identifying susceptibility genes in the presence of epistasis. Am J Hum Genet 2006;78:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albuquerque RV, Hayden CM, Palmer LJ, Laing IA, Rye PJ, Gibson NA, Burton PR, Goldblatt J, Lesouef PN. Association of polymorphisms within the tumour necrosis factor (TNF) genes and childhood asthma. Clin Exp Allergy 1998;28:578–584. [DOI] [PubMed] [Google Scholar]
- 6.Babu KS, Davies DE, Holgate ST. Role of tumor necrosis factor alpha in asthma. Immunol Allergy Clin North Am 2004;24:583–597 (v–vi.). [DOI] [PubMed] [Google Scholar]
- 7.Liebhart J, Cembrzynska-Nowak M, Bienkowska M, Liebhart E, Dobek R, Zaczynska E, Panaszek B, Obojski A, Malolepszy J. Relevance of the selected cytokine release (TNF-alpha, IL-6, IFN-gamma, and IFN-alpha) to the exacerbation of bronchial asthma from airway mycotic infections: predominant role of TFN-alpha? J Investig Allergol Clin Immunol 2002;12:182–191. [PubMed] [Google Scholar]
- 8.Paulesu L, Luzzi E, Bocci V. Studies on the biological effects of ozone: 2. Induction of tumor necrosis factor (TNF-alpha) on human leucocytes. Lymphokine Cytokine Res 1991;10:409–412. [PubMed] [Google Scholar]
- 9.Winchester EC, Millwood IY, Rand L, Penny MA, Kessling AM. Association of the TNF-alpha-308 (G→A) polymorphism with self-reported history of childhood asthma. Hum Genet 2000;107:591–596. [DOI] [PubMed] [Google Scholar]
- 10.Randolph AG, Lange C, Silverman EK, Lazarus R, Weiss ST. Extended haplotype in the tumor necrosis factor gene cluster is associated with asthma and asthma-related phenotypes. Am J Respir Crit Care Med 2005;172:687–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bilolikar H, Nam AR, Rosenthal M, Davies JC, Henderson DC, Balfour-Lynn IM. Tumour necrosis factor gene polymorphisms and childhood wheezing. Eur Respir J 2005;26:637–646. [DOI] [PubMed] [Google Scholar]
- 12.Shah A, Church MK, Holgate ST. Tumour necrosis factor alpha: a potential mediator of asthma. Clin Exp Allergy 1995;25:1038–1044. [DOI] [PubMed] [Google Scholar]
- 13.Carroll MC, Katzman P, Alicot EM, Koller BH, Geraghty DE, Orr HT, Strominger JL, Spies T. Linkage map of the human major histocompatibility complex including the tumor necrosis factor genes. Proc Natl Acad Sci USA 1987;84:8535–8539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi IW, Sun K, Kim YS, Ko HM, Im SY, Kim JH, You HJ, Lee YC, Lee JH, Park YM, et al. TNF-alpha induces the late-phase airway hyperresponsiveness and airway inflammation through cytosolic phospholipase A(2) activation. J Allergy Clin Immunol 2005;116:537–543. [DOI] [PubMed] [Google Scholar]
- 15.Di Somma C, Charron D, Deichmann K, Buono C, Ruffilli A. Atopic asthma and TNF-308 alleles: linkage disequilibrium and association analyses. Hum Immunol 2003;64:359–365. [DOI] [PubMed] [Google Scholar]
- 16.Mitsuta K, Matsuse H, Fukushima C, Kawano T, Tomari S, Obase Y, Goto S, Urata Y, Shimoda T, Kondo T, et al. Production of TNF-alpha by peripheral blood mononuclear cells through activation of nuclear factor kappa B by specific allergen stimulation in patients with atopic asthma. Allergy Asthma Proc 2003;24:19–26. [PubMed] [Google Scholar]
- 17.Noguchi E, Yokouchi Y, Shibasaki M, Inudou M, Nakahara S, Nogami T, Kamioka M, Yamakawa-Kobayashi K, Ichikawa K, Matsui A, et al. Association between TNFA polymorphism and the development of asthma in the Japanese population. Am J Respir Crit Care Med 2002;166:43–46. [DOI] [PubMed] [Google Scholar]
- 18.Witte JS, Palmer LJ, O'Connor RD, Hopkins PJ, Hall JM. Relation between tumour necrosis factor polymorphism TNFalpha-308 and risk of asthma. Eur J Hum Genet 2002;10:82–85. [DOI] [PubMed] [Google Scholar]
- 19.Finotto S, Ohno I, Marshall JS, Gauldie J, Denburg JA, Dolovich J, Clark DA, Jordana M. TNF-alpha production by eosinophils in upper airways inflammation (nasal polyposis). J Immunol 1994;153:2278–2289. [PubMed] [Google Scholar]
- 20.Moffatt MF, Cookson WO. Tumour necrosis factor haplotypes and asthma. Hum Mol Genet 1997;6:551–554. [DOI] [PubMed] [Google Scholar]
- 21.Chagani T, Pare PD, Zhu S, Weir TD, Bai TR, Behbehani NA, Fitzgerald JM, Sandford AJ. Prevalence of tumor necrosis factor-alpha and angiotensin converting enzyme polymorphisms in mild/moderate and fatal/near-fatal asthma. Am J Respir Crit Care Med 1999;160:278–282. [DOI] [PubMed] [Google Scholar]
- 22.Li Kam Wa TC, Mansur AH, Britton J, Williams G, Pavord I, Richards K, Campbell DA, Morton N, Holgate ST, Morrison JF. Association between -308 tumour necrosis factor promoter polymorphism and bronchial hyperreactivity in asthma. Clin Exp Allergy 1999;29:1204–1208. [DOI] [PubMed] [Google Scholar]
- 23.Moffatt MF, James A, Ryan G, Musk AW, Cookson WO. Extended tumour necrosis factor/HLA-DR haplotypes and asthma in an Australian population sample. Thorax 1999;54:757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louis R, Leyder E, Malaise M, Bartsch P, Louis E. Lack of association between adult asthma and the tumour necrosis factor alpha-308 polymorphism gene. Eur Respir J 2000;16:604–608. [DOI] [PubMed] [Google Scholar]
- 25.Buckova D, Holla LI, Vasku A, Znojil V, Vacha J. Lack of association between atopic asthma and the tumor necrosis factor alpha-308 gene polymorphism in a Czech population. J Investig Allergol Clin Immunol 2002;12:192–197. [PubMed] [Google Scholar]
- 26.Lin YC, Lu CC, Su HJ, Shen CY, Lei HY, Guo YL. The association between tumor necrosis factor, HLA-DR alleles, and IgE-mediated asthma in Taiwanese adolescents. Allergy 2002;57:831–834. [DOI] [PubMed] [Google Scholar]
- 27.Hiltermann JT, Lapperre TS, van Bree L, Steerenberg PA, Brahim JJ, Sont JK, Sterk PJ, Hiemstra PS, Stolk J. Ozone-induced inflammation assessed in sputum and bronchial lavage fluid from asthmatics: a new noninvasive tool in epidemiologic studies on air pollution and asthma. Free Radic Biol Med 1999;27:1448–1454. [DOI] [PubMed] [Google Scholar]
- 28.Peden DB. Air pollution in asthma: effect of pollutants on airway inflammation. Ann Allergy Asthma Immunol 2001;87:12–17. [DOI] [PubMed] [Google Scholar]
- 29.McConnell R, Berhane K, Gilliland F, London SJ, Islam T, Gauderman WJ, Avol E, Margolis HG, Peters JM. Asthma in exercising children exposed to ozone: a cohort study. Lancet 2002;359:386–391. [DOI] [PubMed] [Google Scholar]
- 30.McDonnell WF, Abbey DE, Nishino N, Lebowitz MD. Long-term ambient ozone concentration and the incidence of asthma in nonsmoking adults: the AHSMOG Study. Environ Res 1999;80:110–121. [DOI] [PubMed] [Google Scholar]
- 31.Bascom R, Bromberg P, Costa D, Devlin R, Dockery D, Frampton M, Lambert W, Samet J, Speizer F, Utell M. Health effects of outdoor air pollution. Am J Respir Crit Care Med 1996;153:3–50.8542133 [Google Scholar]
- 32.Saxon A, Diaz-Sanchez D. Air pollution and allergy: you are what you breathe. Nat Immunol 2005;6:223–226. [DOI] [PubMed] [Google Scholar]
- 33.Gilliland FD, McConnell R, Peters J, Gong H Jr. A theoretical basis for investigating ambient air pollution and children's respiratory health. Environ Health Perspect 1999;107:403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol 1995;30:445–600. [DOI] [PubMed] [Google Scholar]
- 35.Strange RC, Jones PW, Fryer AA. Glutathione S-transferase: genetics and role in toxicology. Toxicol Lett 2000;112–113:357–363. [DOI] [PubMed] [Google Scholar]
- 36.Talalay P, Fahey JW, Holtzclaw WD, Prestera T, Zhang Y. Chemoprotection against cancer by phase 2 enzyme induction. Toxicol Lett 1995;82–83:173–179. [DOI] [PubMed] [Google Scholar]
- 37.Gilliland FD, Li Y-F, Saxon A, Diaz-Sanchez D. Glutathione-S-Transferase M1 and P1 genotypes protect against xenobiotic enhancement of allergic responses. Lancet 2004;363:119–125. [DOI] [PubMed] [Google Scholar]
- 38.Lee YL, Lin YC, Lee YC, Wang JY, Hsiue TR, Guo YL. Glutathione S-transferase P1 gene polymorphism and air pollution as interactive risk factors for childhood asthma. Clin Exp Allergy 2004;34:1707–1713. [DOI] [PubMed] [Google Scholar]
- 39.Piirila P, Wikman H, Luukkonen R, Kaaria K, Rosenberg C, Nordman H, Norppa H, Vainio H, Hirvonen A. Glutathione S-transferase genotypes and allergic responses to diisocyanate exposure. Pharmacogenetics 2001;11:437–445. [DOI] [PubMed] [Google Scholar]
- 40.Spiteri MA, Bianco A, Strange RC, Fryer AA. Polymorphisms at the glutathione S-transferase, GSTP1 locus: a novel mechanism for susceptibility and development of atopic airway inflammation. Allergy 2000;55:15–20. [DOI] [PubMed] [Google Scholar]
- 41.Fryer AA, Bianco A, Hepple M, Jones PW, Strange RC, Spiteri MA. Polymorphism at the glutathione S-transferase GSTP1 locus: a new marker for bronchial hyperresponsiveness and asthma. Am J Respir Crit Care Med 2000;161:1437–1442. [DOI] [PubMed] [Google Scholar]
- 42.Kabesch M, Hoefler C, Carr D, Leupold W, Weiland SK, von Mutius E. Glutathione S transferase deficiency and passive smoking increase childhood asthma. Thorax 2004;59:569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romieu I, Sienra-Monge JJ, Ramirez-Aguilar M, Moreno-Macias H, Reyes-Ruiz NI, Estela del Rio-Navarro B, Hernandez-Avila M, London SJ. Genetic polymorphism of GSTM1 and antioxidant supplementation influence lung function in relation to ozone exposure in asthmatic children in Mexico City. Thorax 2004;59:8–10. [PMC free article] [PubMed] [Google Scholar]
- 44.Gilliland FD, Li YF, Dubeau L, Berhane K, Avol E, McConnell R, Gauderman WJ, Peters JM. Effects of glutathione S-transferase M1, maternal smoking during pregnancy, and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med 2002;166:457–463. [DOI] [PubMed] [Google Scholar]
- 45.Peters JM, Avol E, Navidi W, London SJ, Gauderman WJ, Lurmann F, Linn WS, Margolis H, Rappaport E, Gong H, et al. A study of twelve Southern California communities with differing levels and types of air pollution. I. Prevalence of respiratory morbidity. Am J Respir Crit Care Med 1999;159:760–767. [DOI] [PubMed] [Google Scholar]
- 46.Gilliland FD, Li Y-F, Tsai W, Dubeau L, Avol E, Peters JM. TNFα −308 genotype and ozone effects on asthma and wheezing: results from the Children's Health Study (CHS). Am J Respir Crit Care Med 2003;167:A580. [Google Scholar]
- 47.Peters JM, Avol E, Gauderman WJ, Linn WS, Navidi W, London SJ, Margolis H, Rappaport E, Vora H, Gong H Jr, et al. A study of twelve Southern California communities with differing levels and types of air pollution: II. Effects on pulmonary function. Am J Respir Crit Care Med 1999;159:768–775. [DOI] [PubMed] [Google Scholar]
- 48.Huang SL, Su CH, Chang SC. Tumor necrosis factor-alpha gene polymorphism in chronic bronchitis. Am J Respir Crit Care Med 1997;156:1436–1439. [DOI] [PubMed] [Google Scholar]
- 49.Gauderman WJ, Witte JS, Thomas DC. Family-based association studies. J Natl Cancer Inst Monogr 1999;26:31–37. [DOI] [PubMed] [Google Scholar]
- 50.Wilson AG, di Giovine FS, Blakemore AI, Duff GW. Single base polymorphism in the human tumour necrosis factor alpha (TNF alpha) gene detectable by NcoI restriction of PCR product. Hum Mol Genet 1992;1:353. [DOI] [PubMed] [Google Scholar]
- 51.Bradding P, Roberts JA, Britten KM, Montefort S, Djukanovic R, Mueller R, Heusser CH, Howarth PH, Holgate ST. Interleukin-4, -5, and -6 and tumor necrosis factor-alpha in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. Am J Respir Cell Mol Biol 1994;10:471–480. [DOI] [PubMed] [Google Scholar]
- 52.Yang IA, Holz O, Jorres RA, Magnussen H, Barton SJ, Rodriguez S, Cakebread JA, Holloway JW, Holgate ST. Association of tumor necrosis factor-alpha polymorphisms and ozone-induced change in lung function. Am J Respir Crit Care Med 2005;171:171–176. [DOI] [PubMed] [Google Scholar]
- 53.Churg A, Wang RD, Tai H, Wang X, Xie C, Dai J, Shapiro SD, Wright JL. Macrophage metalloelastase mediates acute cigarette smoke-induced inflammation via tumor necrosis factor-alpha release [comment]. Am J Respir Crit Care Med 2003;167:1083–1089. [DOI] [PubMed] [Google Scholar]
- 54.Zhang J, Liu Y, Shi J, Larson DF, Watson RR. Side-stream cigarette smoke induces dose–response in systemic inflammatory cytokine production and oxidative stress. Exp Biol Med 2002;227:823–829. [DOI] [PubMed] [Google Scholar]
- 55.Shore SA, Schwartzman IN, Le Blanc B, Murthy GG, Doerschuk CM. Tumor necrosis factor receptor 2 contributes to ozone-induced airway hyperresponsiveness in mice. Am J Respir Crit Care Med 2001;164:602–607. [DOI] [PubMed] [Google Scholar]
- 56.Burr ML. Diagnosing asthma by questionnaire in epidemiological surveys. Clin Exp Allergy 1992;22:509–510. [DOI] [PubMed] [Google Scholar]
- 57.Committee on the Assessment of Asthma and Indoor Air. Clearing the air: asthma and indoor exposures. Washington, DC: National Academy of Sciences; 2000.
- 58.California Environmental Protection Agency. Health effects of exposure to environmental tobacco smoke. Sacramento (CA): California Environmental Protection Agency; 1997. [DOI] [PMC free article] [PubMed]
- 59.Respiratory health effects of passive smoking: lung cancer and other disorders. Washington, DC: U.S. Environmental Protection Agency; 1992.
- 60.Allcock RJ, Windsor L, Gut IG, Kucharzak R, Sobre L, Lechner D, Garnier JG, Baltic S, Christiansen FT, Price P, et al. High-density SNP genotyping defines 17 distinct haplotypes of the TNF block in the Caucasian population: implications for haplotype tagging. Hum Mutat 2004;24:517–525. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.