Abstract
Rationale: Inflammation is now recognized as an integral part of the pathogenesis of chronic obstructive pulmonary disease (COPD). In contrast to the sterile airways of normal lungs, bacterial pathogens are often isolated from the airways in stable COPD. This “colonization” of the tracheobronchial tree, currently believed to be innocuous, could serve as an inflammatory stimulus, independent of current tobacco smoke exposure.
Objective: To test the hypothesis that bacterial colonization is associated with airway inflammation in stable COPD.
Methods: Bronchoscopy with bronchoalveolar lavage (BAL) was performed in three groups of subjects: 26 ex-smokers with stable COPD (COPD), 20 ex-smokers without COPD (ex-smokers), and 15 healthy nonsmokers (nonsmokers). Quantitative bacterial cultures, cell counts, chemokine, cytokine, proteinase/antiproteinase, and endotoxin levels in the BAL fluid were compared.
Results: Potentially pathogenic bacteria were recovered at ⩾ 100 cfu/ml in 34.6% of COPD, 0% of ex-smokers, and in 6.7% of nonsmokers (p = 0.003). All values are expressed as median (interquartile range). Subjects with colonized COPD had significantly greater relative (12.0 [28.4] vs. 3.0 [7.8]%, p = 0.03) and absolute (4.98 [5.26] × 104/ml vs. 3.04 [2.82] × 104/ml, p = 0.02) neutrophil counts, interleukin 8 (33.8 [189.8] vs. 16.9 [20.1] pg/ml, p = 0.005), active matrix metalloproteinase-9 (2.16 [4.30] vs. 0.84 [0.99] U/ml, p = 0.03), and endotoxin (36.0 [72.6] vs. 3.55 [7.17] mEU/ml, p = 0.004) levels in the BAL than the subjects with noncolonized COPD. These inflammatory constituents of BAL were also significantly elevated in subjects with colonized COPD when compared with ex-smokers and nonsmokers.
Conclusions: Bacterial colonization is associated with neutrophilic airway lumen inflammation in ex-smokers with COPD and could contribute to progression of airway disease in COPD.
Keywords: bacterial colonization, chronic obstructive pulmonary disease, neutrophilic inflammation
Tobacco smoke is undoubtedly the major stimulus for the inflammatory process in chronic obstructive pulmonary disease (COPD). However, several observations suggest that other stimuli or perpetuating mechanisms exist. Only 15% of smokers develop COPD, suggesting either an inherent susceptibility or an additional inflammatory stimulus (1). A persistent inflammatory process is seen in the central and small airways of ex-smokers, which is indistinguishable from inflammation seen in current smokers (2–4). Ongoing inflammation is present in bronchoalveolar lavage (BAL) fluid and in bronchial biopsies among ex-smokers with COPD (5). Lymphoid follicles and inflammatory mucus exudates have been demonstrated recently in ex-smokers with advanced COPD, with the speculation that these findings are related to chronic microbial colonization (6, 7). Although the rate of decline of lung function in early COPD normalizes after smoking cessation, this may not occur in older smokers with more severe airway obstruction (8). Persistent symptoms and recurrent exacerbations are seen in ex-smokers with moderate to severe COPD, suggesting that persistent inflammation has clinical consequences.
One potential explanation for this persistent airway inflammation is a chronic microbial infection of the lungs in COPD, which has the potential to alter the host inflammatory response to tobacco smoke and/or serve as an additional inflammatory stimulus (7, 9). The healthy human airway is sterile, with several innate immune mechanisms acting in coordination to maintain this sterility. Smoking appears to disrupt these innate immune mechanisms, and as a consequence, microbial pathogens are able to persist in the lower airway in COPD. Among the pathogens implicated are Adenovirus, Chlamydia pneumoniae, Pneumocystis jiroveci, and bacterial pathogens (9–13). Of the bacterial pathogens that “colonize” the lower airways in COPD, nontypeable Haemophilus influenzae is the most frequently isolated (9, 14).
There is evidence that bacterial “colonization” of the lower airways in stable COPD is not innocuous. Bacterial colonization has been associated with greater levels of airway inflammation measured in sputum, increased frequency of exacerbations, and an accelerated decline in lung function (15–19). Sputum largely reflects the processes in the trachea and major bronchi. Whether bacterial colonization is detrimental in the peripheral tracheobronchial tree, which is the major site of airway obstruction in COPD, requires bronchoscopic sampling. Bacterial colonization is prevalent among healthy smokers (20). Furthermore, the proinflammatory effects of smoking overlap those induced by bacterial infection, especially neutrophilic infiltration of the airway (21, 22). Therefore, to reliably distinguish the proinflammatory effects of bacterial colonization from those related to active smoking requires limiting study to only ex-smokers.
Our hypothesis was that bacterial colonization is an inflammatory stimulus in the peripheral tracheobronchial tree in COPD independent of current tobacco smoke exposure. To test this hypothesis, we compared airway inflammation in BAL fluid obtained from ex-smokers with COPD with ex-smokers who did not have COPD and with nonsmoking control subjects and determined the contribution of bacterial colonization to this inflammation. Some of the results of these studies have been previously reported in the form of abstracts (23–25).
METHODS
Study Subjects
The study protocol was approved by the Veterans Affairs Western New York Healthcare System institutional review board. Three groups of volunteer subjects were enrolled. Inclusion criteria for group 1, ex-smokers with COPD, included evidence of airway obstruction on spirometry and 20 pack-years or more of cigarette smoking but cessation of smoking at least 1 yr before enrollment. Inclusion criteria for group 2, ex-smokers without COPD, were the same as for group 1 except for absence of lung disease by clinical evaluation, chest X-ray, and spirometry. Inclusion criteria for group 3, healthy nonsmokers, were the same as for group 2 except these participants were life-long nonsmokers, defined as no tobacco smoke exposure or remote exposure of fewer than 5 pack-years. In all three groups, no antibiotic or systemic steroid exposure in the 4 wk preceding enrollment was allowed. (Detailed inclusion criteria are provided in the online supplement.)
Study Procedures
After obtaining informed consent, clinical evaluation, carbon monoxide screening, spirometry, electrocardiogram, and chest X-ray were performed. Bronchoscopies were conducted under conscious sedation with midazolam and fentanyl and topical anesthesia to the nasal and pharyngeal mucosae. A sequential two-scope procedure was performed, in which the glottis was anesthetized with the first bronchoscope and another bronchoscope with an uncontaminated channel was used to complete the procedure. The right middle lobe was lavaged with 50 × 3 ml of saline.
BAL Processing
The lavage fluid was placed on ice and processed within 30 min. Mucus was removed by filtering the lavage fluid through sterile gauze. Quantitative cultures were performed on the BAL fluid to determine frequency of colonization by potentially pathogenic bacteria (PPB). PPB included Haemophilus spp., Streptococcus pneumoniae, Moraxella catarrhalis, Staphylococcus aureus, Pseudomonas aeruginosa, and gram-negative enteric bacteria (20). All other species isolated were classified as non-PPB. A threshold concentration of 100 (102) cfu/ml was used to define significant growth of PPB (26). (Details are provided in the online supplement.)
A total cell count was obtained with a hemacytometer and cell viability determined by trypan blue exclusion. Duplicate cytospin slides were stained with Giemsa and 400 cells were counted by a blinded observer to obtain a differential cell count. The rest of the BAL fluid was centrifuged at 500 g for 10 min. The cell-free supernatant was stored at −80°C for further analysis.
Mediator Measurements
Aliquots of BAL fluid were concentrated with Centricon filters (Millipore, Billerica, MA), and levels of interleukin 1β (IL-1β), tumor necrosis factor α, IL-6, IL-8, IL-10, and IL-12 were determined by a multiplex flow cytometric assay (27). (Details are in the online supplement.) Leukotriene B4 (LTB4), matrix metalloproteinase-9 (MMP-9), tissue inhibitor of matrix metalloproteinase-1 (TIMP-1), and neutrophil elastase/anti–neutrophil elastase complex (NE-A1AT) were measured with commercial ELISA kits. Active MMP-9 was estimated by gelatin zymography. (Details provided in the online supplement.) Endotoxin levels were measured by a limulus ameobocyte assay (Associates of Cape Cod, East Falmouth, MA).
Statistical Analysis
Demographic data were normally distributed and are expressed as mean ± SEM and analyzed with analysis of variance with Fisher's test for multiple comparisons. BAL fluid data were not normally distributed and are reported as median (interquartile range) and analyzed with nonparametric statistics. Frequency of colonization among the groups was compared with χ2 analysis. Whether age and cumulative smoking were independently related to BAL fluid measurements was determined by multivariate regression analysis after adjustment for lung function (FEV1% predicted). The relationships between inflammatory mediators and cell counts in BAL fluid were ascertained by determining the Spearman correlation coefficients. In all tests, a p value of less than 0.05 was considered significant.
RESULTS
Subject Demographics
A total of 86 subjects were enrolled. Of these, 23 did not complete the study: seven decided not to have the bronchoscopy, six had a comorbid illness making bronchoscopy risky, six had an FEV1 of less than 35% or hypercapnia, three had concomitant fibrotic lung disease, and in one subject an inconsistent smoking history was obtained. One bronchoscopy in a nonsmoker was not completed because of transient laryngospasm. Another nonsmoker had purulent tracheobronchial secretions and symptoms of acute bronchitis that he had failed to inform us about before the bronchoscopy. Both of these subjects were excluded from further analysis.
Of the 61 subjects included in the subsequent analyses, 26 had COPD (group 1, COPD), 20 were ex-smokers with normal lung function (group 2, ex-smokers), and 15 were nonsmokers (group 3, non-smokers). Demographic data are presented in Table 1. As expected, subjects with COPD had significantly worse spirometric parameters than the other two groups. Global Initiative for Obstructive Lung Disease (GOLD) classification of severity of airflow obstruction among the subjects with COPD was as follows: five subjects were stage 1, 15 subjects were stage 2, and six subjects were stage 3 (28). Subjects with COPD were also older than control subjects and had more cumulative tobacco exposure (Table 1). Ex-smokers differed only in their cumulative tobacco smoke exposure from the nonsmokers. Subjects with COPD were on a variety of respiratory medications, whereas none of the group 2 or 3 subjects were on any respiratory medication.
TABLE 1.
SUBJECT DEMOGRAPHICS
| Group 1 (COPD) | Group 2 (Ex-Smokers) | Group 3 (Nonsmokers) | p Value, ANOVA | |
|---|---|---|---|---|
| Number | 26 | 20 | 15 | |
| Age, yr | 64.7 ± 1.7*† | 56.0 ± 2.2* | 51.1 ± 4.2† | 0.001 |
| Sex, M/F | 20/6 | 11/9 | 9/6 | 0.26§ |
| Smoking, pack-yr | 66.0 ± 6.3*† | 31.7 ± 1.8*‡ | 0.3 ± 0.2†‡ | < 0.001 |
| FEV1, L | 1.89 ± 0.13*† | 2.67 ± 0.13* | 3.10 ± 0.21† | < 0.001 |
| FEV1, % predicted | 59.8 ± 4.1*† | 90.3 ± 2.9* | 98.8 ± 12.9† | < 0.001 |
| FVC, L | 3.37 ± 0.17 | 3.49 ± 0.18 | 3.94 ± 0.24 | 0.12 |
| FVC, % predicted | 84.5 ± 3.9*† | 94.8 ± 3.4* | 103.1 ± 4.2† | 0.006 |
| FEV1/FVC ratio | 55.3 ± 2.5*† | 76.9 ± 1.1* | 78.5 ± 1.3† | < 0.001 |
Definition of abbreviation: ANOVA = analysis of variance; COPD = chronic obstructive pulmonary disease.
Data are presented as mean ± SEM.
p < 0.05 between groups 1 and 2, Fisher's protected least significant difference (PLSD) test.
p < 0.05 between groups 1 and 3, Fisher's PLSD test.
p < 0.05 between groups 2 and 3, Fisher's PLSD test.
χ2 p value.
BAL Culture Results
PPB were recovered from BAL fluid in 9 of 26 (34.6%) subjects with COPD compared with 0 of 20 (0%) ex-smokers and 3 of 15 (20%) nonsmokers (p = 0.01). At a threshold concentration of 100 bacteria/ml or greater, PPB were recovered in 9 of 26 (34.6%) subjects with COPD compared with 0 of 20 (0%) ex-smokers and 1 of 15 (6.7%) nonsmokers (p = 0.003; Table 2). Haemophilus spp. were the most frequent isolates, in 26.9% of subjects with COPD.
TABLE 2.
RECOVERY OF POTENTIAL PATHOGENIC BACTERIA FROM BRONCHOALVEOLAR LAVAGE FLUID
| Group | Positive for PPB, % (n)* | Positive for PPB at Least 102/ml, % (n)† | Pathogens Isolated | Titers of pathogens (cfu/ml) |
|---|---|---|---|---|
| 1 (COPD) | 34.6% (9/26) | 34.6% (9/26) | HH and HP | 3 × 102 and 2 × 102 |
| NTHI and SP | 7 × 104 and 2 × 104 | |||
| HP | 1 × 102 | |||
| PA | 6 × 102 | |||
| SA | 1 × 102 | |||
| NTHI | 1.5 × 104 | |||
| NTHI | 5 × 105 | |||
| HP | 6.5 × 105 | |||
| HP | 3 × 105 | |||
| 2 (Ex-smokers) | 0% (0/20) | 0% (0/20) | ||
| 3 (Nonsmokers) | 20% (3/15) | 6.7% (1/15) | SP | 1 × 101 |
| HP | 1 × 101 | |||
| HP | 2.5 × 102 |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; HH = Haemophilus hemolyticus; HP = Haemophilus parainfluenzae; NTHI = nontypeable Haemophilus influenzae; PA = Pseudomonas aeruginosa; PPB = potential pathogenic bacteria; SA = Staphylococcus aureus; SP = Streptococcus pneumoniae.
Comparison between groups, p value = 0.01, χ2.
Comparison between groups, p value = 0.003, χ2.
Non-PPB were isolated from BAL fluid in 13 of 26 (50%) subjects with COPD compared with 7 of 20 (35%) ex-smokers and 10 of 15 (67%) nonsmokers (p = not significant). At a threshold concentration of 100 bacteria/ml or greater, non-PPB were recovered in 6 of 26 (23%) subjects with COPD compared with 4 of 20 (20%) ex-smokers and 6 of 15 (40%) nonsmokers (p = not significant). Non-PPB were predominantly α hemolytic Streptococci, Micrococcus, and Neisseria species.
BAL Recovery and Cell Counts
Data are presented in Table 3. The volume of BAL fluid recovered as well as cell viability was lower in subjects with COPD than the other two groups (Table 3). A relative and absolute neutrophilia was seen among subjects with COPD when compared with the other two groups, with a corresponding relative but not absolute decrease in macrophages. Ex-smokers had significantly higher absolute lymphocyte counts compared with the subjects with COPD.
TABLE 3.
BRONCHOALVEOLAR LAVAGE RECOVERY AND CELL COUNTS
| Group 1 (COPD) | Group 2 (Ex-Smokers) | Group 3 (Nonsmokers) | p Value, Kruskal-Wallis | |
|---|---|---|---|---|
| Volume, ml | 49.5 (33)*† | 75 (26.5)* | 72 (29.5)† | < 0.001 |
| Cell viability, % | 81 (16)*† | 90 (12.6)* | 90.1 (15.2)† | 0.04 |
| Total cells, × 104/ml | 58.2 (132.0) | 145.0 (167.8) | 78.1 (201.7) | 0.25 |
| Neutrophils, % | 7.0 (10.2)*† | 1.2 (1.5)* | 1.2 (2.9)† | < 0.001 |
| Macrophages, % | 82.7 (18.7)*† | 90.5 (8.5)* | 93.5 (14.9)† | 0.03 |
| Eosinophils, % | 0 (0.2) | 0 (0) | 0 (0.5) | 0.39 |
| Lymphocytes, % | 5.4 (6.9) | 10 (8.5) | 3.7 (11.1) | 0.24 |
| Neutrophils, × 104/ml | 3.21 (3.14)*† | 1.14 (1.74)* | 0.45 (1.17)† | 0.009 |
| Macrophages, × 104/ml | 41.3 (130.4) | 134.6 (163.8) | 76.1 (153.6) | 0.11 |
| Eosinophils, × 104/ml | 0.0 (0.10) | 0.0 (0.0) | 0.0 (0.51) | 0.34 |
| Lymphocytes, × 104/ml | 2.67 (5.78)* | 8.3 (13.4)* | 2.34 (19.5) | 0.04 |
Definition of abbreviation: COPD = chronic obstructive pulmonary disease.
Data are presented as median (interquartile range).
p < 0.05 between groups 1 and 2, Mann-Whitney U rank test.
p < 0.05 between groups 1 and 3, Mann-Whitney U rank test.
Inflammatory Mediators in BAL
Data are presented in Table 4. Subjects with COPD demonstrated increased levels of IL-8 and LTB4 in the BAL fluid compared with the other two groups. Subjects with COPD also had increased levels of proteinase and proteinase inhibitors, including NE-A1AT complex, MMP-9, and TIMP-1. NE-A1AT and MMP-9 levels were elevated in ex-smokers in comparison to nonsmokers, although to a lesser degree than seen in subjects with COPD.
TABLE 4.
INFLAMMATORY MEDIATORS IN BRONCHOALVEOLAR LAVAGE
| Group 1 (COPD) | Group 2 (Ex-Smokers) | Group 3 (Nonsmokers) | P Value, Kruskal-Wallis | |
|---|---|---|---|---|
| IL-8, pg/ml | 20.6 (26.2)*† | 7.5 (11.1)* | 8.3 (4.9)† | 0.001 |
| TNF-α, pg/ml | 0.06 (0.11) | 0 (0.11) | 0.08 (0.25) | 0.35 |
| IL-1β, pg/ml | 0.14 (0.39) | 0.09 (0.11) | 0 (0.11) | 0.10 |
| IL-10, pg/ml | 0.04 (0.34) | 0 (0.03) | 0 (0.22) | 0.23 |
| IL-12, pg/ml | 0 (0) | 0 (0.13) | 0 (0.15) | 0.73 |
| IL-6, pg/ml | 1.32 (2.84) | 1.02 (1.51) | 0.69 (0.63) | 0.07 |
| LTB4, pg/ml | 2.7 (2.5)*† | 0.1 (0.4)* | 0.8 (1.5)† | < 0.001 |
| NE-A1AT, ng/ml | 33.1 (41.6)*† | 17.0 (15.6)*‡ | 12.4 (7.9)†‡ | 0.002 |
| MMP-9, ng/ml | 0.50 (1.09)*† | 0.23 (0.40)*‡ | 0.10 (0.17)†‡ | < 0.001 |
| TIMP-1, ng/ml | 0.45 (0.35)*† | 0.22 (0.19)* | 0.20 (0.23)† | 0.012 |
| MMP-9 active, U/ml | 1.03 (1.73)† | 0.70 (0.85)‡ | 0.33 (0.28)†‡ | < 0.001 |
| Endotoxin, mEU/ml | 5.57 (30.51) | 5.29 (11.89) | 7.60 (19.34) | 0.62 |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; IL = interleukin; LTB4 = leukotriene B4; MMP-9 = matrix metalloproteinase-9; TIMP-1 = tissue inhibitor of matrix metalloproteinase-1; TNF-α =tumor necrosis factor α.
Data presented as median (interquartile range).
p < 0.05 between groups 1 and 2, Mann-Whitney U rank test.
p < 0.05 between groups 1 and 3, Mann-Whitney U rank test.
p < 0.05 between groups 2 and 3, Mann-Whitney U rank test.
Relationship of Age and Cumulative Smoking (pack-years) to BAL Constituents
Several of the BAL measurements were related in a univariate regression to age and pack-years (data not shown). However, in multivariate regression after controlling for FEV1% predicted, only relative (but not absolute) neutrophil count still demonstrated a significant relationship with age (p = 0.04). None of the BAL constituent levels were related to pack-years in the multivariate analysis.
Effect of Bacterial Colonization on Airway Inflammation in COPD
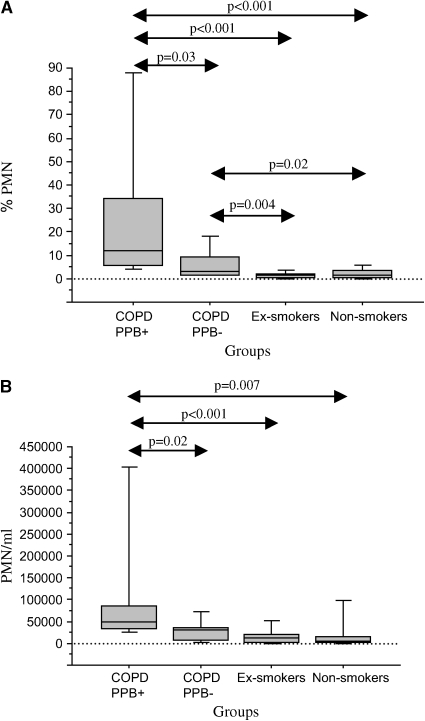
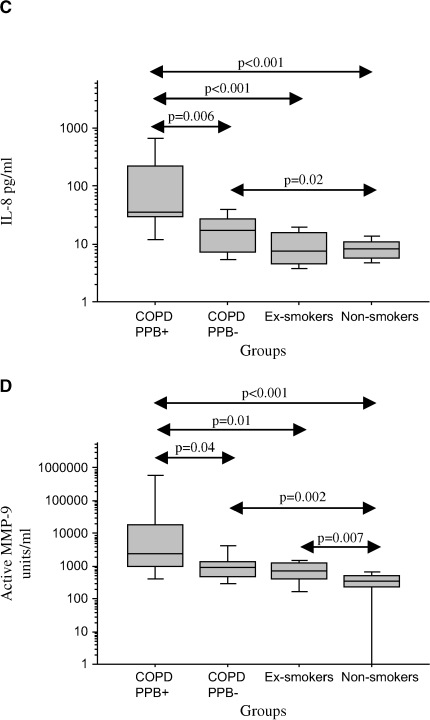
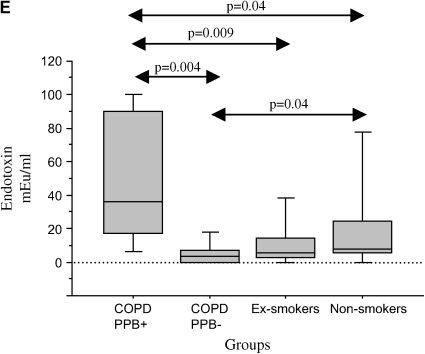
To ascertain whether bacterial colonization of the bronchial lumen is an inflammatory stimulus in stable COPD, we compared BAL findings among subjects with COPD that were colonized (n = 9) with those not colonized with PPB (n = 17). There were no differences in age, pack-years of smoking, and any of the spirometric parameters between colonized and noncolonized patients (Table 5). BAL recovery and total cell counts also did not differ with bacterial colonization (Table 6). However, the relative and absolute concentrations of neutrophils were elevated with bacterial colonization (Table 6; Figure 1). A significant elevation of IL-8 was seen with bacterial colonization, with a trend for IL-10 (p = 0.06) also to be elevated (Table 7; Figure 1). Among the proteinases, active MMP-9 was significantly elevated with colonization (Table 7; Figure 1). Endotoxin levels were also elevated with colonization (Table 7; Figure 1).
TABLE 5.
DEMOGRAPHIC VARIABLES WITH GROUP 1 (EX-SMOKERS WITH CHRONIC OBSTRUCTIVE PULMONARY DISEASE) SPLIT BY THE PRESENCE AND ABSENCE OF POTENTIAL PATHOGENIC BACTERIA IN BRONCHOALVEOLAR LAVAGE CULTURE
| Group 1 (COPD PPB+) | Group 1 (COPD PPB−) | Group 2 (Ex-Smokers) | Group 3 (Nonsmokers) | p Value, ANOVA | |
|---|---|---|---|---|---|
| Number | 9 | 17 | 20 | 15 | |
| Age, yr | 61.2 ± 3.1* | 66.5 ± 1.9‡§ | 56.0 ± 2.2*‡ | 51.1 ± 4.2§ | 0.002 |
| Sex, M/F | 6/3 | 14/3 | 11/9 | 9/6 | 0.34‖ |
| Smoking, pack-yr | 70.0 ± 13.0*† | 63.9 ± 7.0‡§ | 31.7 ± 1.8*‡ | 0.3 ± 0.2†§ | < 0.001 |
| FEV1, L | 1.92 ± 0.22*† | 1.87 ± 0.16‡§ | 2.67 ± 0.13*‡ | 3.10 ± 0.21†§ | < 0.001 |
| FEV1, % predicted | 59.3 ± 7.8*† | 60.0 ± 4.9‡§ | 90.3 ± 2.9*‡ | 98.8 ± 12.9†§ | < 0.001 |
| FVC, L | 3.40 ± 0.28 | 3.35 ± 0.21 | 3.49 ± 0.18 | 3.94 ± 0.24 | 0.24 |
| FVC, % predicted | 82.4 ± 6.7† | 85.6 ± 4.9§ | 94.8 ± 3.4 | 103.1 ± 4.2†§ | 0.016 |
| FEV1/FVC ratio | 55.5 ± 3.8*† | 55.2 ± 3.3‡§ | 76.9 ± 1.1*‡ | 78.5 ± 1.3†§ | < 0.001 |
Definition of abbreviations: ANOVA = analysis of variance; COPD = chronic obstructive pulmonary disease; PPB+ = presence of potential pathogenic bacteria; PPB− = absence of potential pathogenic bacteria.
p < 0.05 between groups 1 PPB+ and 2, Fisher's protected least significant difference (PLSD) test.
p < 0.05 between groups 1 PPB+ and 3, Fisher's PLSD test.
p < 0.05 between groups 1 PPB− and 2, Fisher's PLSD test.
p < 0.05 between groups 1 PPB− and 3, Fisher's PLSD test.
χ2 p value.
TABLE 6.
BRONCHOALVEOLAR LAVAGE RECOVERY AND CELL COUNTS WITH GROUP 1 (EX-SMOKERS WITH CHRONIC OBSTRUCTIVE PULMONARY DISEASE) SPLIT BY THE PRESENCE AND ABSENCE OF POTENTIALLY PATHOGENIC BACTERIA IN BRONCHOALVEOLAR LAVAGE CULTURE
| Group 1 (COPD PPB+) | Group 1 (COPD PPB−) | Group 2 (Ex-Smokers) | Group 3 (Nonsmokers) | p Value, Kruskal-Wallis | |
|---|---|---|---|---|---|
| Volume, ml | 43.0 (30.2)*† | 54.0 (27.5)§‖ | 75 (26.5)*§ | 72 (29.5)†‖ | < 0.001 |
| Cell viability, % | 80 (14.2) | 82 (14.5) | 90 (12.6) | 90.1 (15.2) | 0.09 |
| Total cells, × 104/ml | 55.4 (32.6) | 61.0 (188.0) | 145.0 (167.8) | 78.1 (201.7) | 0.40 |
| Neutrophils, % | 12.0 (28.4)*†‡ | 3.0 (7.8)‡§‖ | 1.2 (1.5)*§ | 1.2 (2.9)†‖ | < 0.001 |
| Macrophages, % | 80.2 (27.0)*† | 85.0 (22.7) | 90.5 (8.5)* | 93.5 (14.9)† | 0.02 |
| Eosinophils, % | 0 (0.9) | 0 (0.1) | 0 (0) | 0 (0.5) | 0.30 |
| Lymphocytes, % | 6.7 (8.0) | 5.2 (6.7) | 10 (8.5) | 3.7 (11.1) | 0.42 |
| Neutrophils, × 104/ml | 4.98 (5.26)*†‡ | 3.04 (2.82)‡ | 1.14 (1.74)* | 0.45 (1.17)† | 0.004 |
| Macrophages, × 104/ml | 38.0 (46.0)* | 44.5 (170.2) | 134.6 (163.8)* | 76.1 (153.6) | 0.17 |
| Eosinophils, × 104/ml | 0.0 (0.55) | 0.0 (0.02) | 0.0 (0.0) | 0.0 (0.51) | 0.26 |
| Lymphocytes, × 104/ml | 2.84 (4.50) | 2.49 (6.65)§ | 8.3 (13.4)§ | 2.34 (19.5) | 0.11 |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; PPB+ = presence of potential pathogenic bacteria; PPB− = absence of potential pathogenic bacteria.
Data are presented as median (interquartile range).
p < 0.05 between groups 1 PPB+ and 2, Mann-Whitney U rank test.
p < 0.05 between groups 1 PPB+ and 3, Mann-Whitney U rank test.
p < 0.05 between groups 1 PPB+ and 1 PPB−, Mann-Whitney U rank test.
p < 0.05 between groups 1 PPB− and 2, Mann-Whitney U rank test.
p < 0.05 between groups 1 PPB− and 3, Mann-Whitney U rank test.
Figure 1.
Comparison of bronchoalveolar lavage (BAL) fluid measurements among patients with chronic obstructive pulmonary disease (COPD) colonized (COPD, PPB+) and not colonized with potential pathogenic bacteria (COPD, PPB−), ex-smokers, and nonsmokers. The horizontal lines represent median values, the boxes represent 25th–75th quartiles, and the vertical lines represent 10th–90th percentile values. Significant differences between groups are represented by double-sided arrows with associated p values by Mann-Whitney U rank test. (A) Relative neutrophil count (PMN%); (B) absolute neutrophil count (PMN); (C) interleukin-8 (IL-8) level (pg/ml); (D) active matrix metalloproteinase-9 (MMP-9) level (U/ml); (E) endotoxin level (mEU/ml).
TABLE 7.
INFLAMMATORY MEDIATORS IN BRONCHOALVEOLAR LAVAGE WITH GROUP 1 (EX-SMOKERS WITH CHRONIC OBSTRUCTIVE PULMONARY DISEASE) SPLIT BY THE PRESENCE AND ABSENCE OF POTENTIALLY PATHOGENIC BACTERIA IN BRONCHOALVEOLAR LAVAGE CULTURE
| Group 1 (COPD PPB+) | Group 1 (COPD PPB−) | Group 2 (Ex-Smokers) | Group 3 (Nonsmokers) | p Value, Kruskal-Wallis | |
|---|---|---|---|---|---|
| IL-8, pg/ml | 35.8 (189.8)*†‡ | 16.9 (20.1)‡§ | 7.5 (11.1)* | 8.3 (4.9)†§ | < 0.001 |
| TNF-α, pg/ml | 0.09 (0.75) | 0.06 (0.08) | 0 (0.11) | 0.08 (0.25) | 0.28 |
| IL-1β, pg/ml | 0.22 (2.27) | 0.14 (0.14) | 0.09 (0.11) | 0 (0.11) | 0.21 |
| IL-10, pg/ml | 0.43 (1.76) | 0 (0.17) | 0 (0.03) | 0 (0.22) | 0.08 |
| IL-12, pg/ml | 0 (0) | 0 (0.13) | 0 (0.13) | 0 (0.15) | 0.59 |
| IL-6, pg/ml | 2.19 (3.92)*† | 1.03 (1.87) | 1.02 (1.51)* | 0.69 (0.63)† | 0.04 |
| LTB4, pg/ml | 2.43 (2.16)*† | 2.73 (2.82)§‖ | 0.06 (0.35)*‖ | 0.78 (1.51)†§ | < 0.001 |
| NE-A1AT, ng/ml | 50.9 (758.1)† | 28.7 (30.6)§ | 17.0 (15.6) | 12.4 (7.9)†§ | 0.006 |
| MMP-9, ng/ml | 1.20 (1.39)*† | 0.39 (0.69)§ | 0.23 (0.40)* | 0.10 (0.17)†§ | 0.001 |
| TIMP-1, ng/ml | 0.49 (0.62)† | 0.33 (0.29)§ | 0.22 (0.19) | 0.20 (0.23)†§ | 0.03 |
| MMP-9 active, U/ml | 2.30 (17.39)*†‡ | 0.91 (0.84)‡§ | 0.70 (0.85)* | 0.33 (0.28)†§ | < 0.001 |
| Endotoxin, mEU/ml | 36.0 (72.6)*†‡ | 3.55 (7.17)‡§ | 5.29 (11.89)* | 7.60 (19.34)†§ | 0.008 |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; IL = interleukin; LTB4 = leukotriene B4; MMP-9 = matrix metalloproteinase-9; PPB+ = presence of potential pathogenic bacteria; PPB− = absence of potential pathogenic bacteria; TIMP-1 = tissue inhibitor of matrix metalloproteinase-1; TNF-α =tumor necrosis factor α.
Data presented as median (interquartile range).
p < 0.05 between groups 1 PPB+ and 2, Mann-Whitney U rank test.
p < 0.05 between groups 1 PPB+ and 3, Mann-Whitney U rank test.
p < 0.05 between groups 1 PPB+ and 1PPB−, Mann-Whitney U rank test.
p < 0.05 between groups 1 PPB− and 3, Mann-Whitney U rank test.
p < 0.05 between groups 1 PPB− and 2, Mann-Whitney U rank test.
Significant elevations of relative and absolute neutrophil counts, IL-8, IL-6, active MMP-9, and endotoxin were also seen among the subjects with colonized COPD compared with the ex-smokers and nonsmokers (Tables 6 and 7; Figure 1). In addition, LTB4, IL-10, NE-A1AT, MMP-9, and TIMP-1 were increased among subjects with colonized COPD in comparison to ex-smokers and/or nonsmokers (Table 7).
Subjects with noncolonized COPD demonstrated more neutrophil %, IL-8, LTB4, NE-A1AT, MMP-9, TIMP-1, and active MMP-9 in comparison to nonsmokers (Table 7; Figure 1). Increased neutrophil % and LTB4 were the only significant differences observed between subjects with noncolonized COPD and ex-smokers (Table 6; Figure 1).
Correlation between BAL Cellular Content and Inflammatory Mediators
Of the various cytokines/chemokines in the BAL, IL-8 was the only mediator that demonstrated a significant relationship with BAL neutrophil content (rho = 0.30, p = 0.02) with a trend for IL-10 (rho = 0.32, p = 0.06). BAL neutrophil content was correlated with BAL content of NE-AIAT (rho = 0.38, p = 0.004), MMP-9 (rho = 0.38, p = 0.004), active MMP-9 (rho = 0.34, p = 0.01), and TIMP-1 (rho = 0.28, p = 0.03). Such relationships were not observed for the BAL macrophage count.
DISCUSSION
Although previous studies have demonstrated the association between bacterial colonization and airway inflammation in COPD, they have been limited to sampling the central tracheobronchial tree by induced sputum and/or by the confounding effect of active smoking (15, 17, 20).
This study, by restricting inclusion to volunteers who were ex-smokers and by bronchoscopic sampling, demonstrates a clear association between bacterial pathogen isolation and neutrophilic airway inflammation in the peripheral bronchial tree in COPD. Furthermore, IL-8 appears to be a key chemokine mediator of this process. Elevated levels of MMP-9 and NE-AIAT that are correlated with neutrophil counts suggest that these neutrophils are a source of destructive proteinases that could contribute to progression of COPD (1).
The key role of IL-8 and neutrophils in the development of COPD has been suggested by several recent observations. Tanino and colleagues studied asymptomatic smokers and found that increased levels of IL-8 in BAL fluid distinguished emphysematous smokers from those without emphysema (29). Bronchial lumen neutrophilia among subjects with chronic bronchitis has been associated with worse lung function and more sputum production (21). Peripheral airway dysfunction in COPD, measured by high-resolution computed tomography scanning, has been significantly associated with neutrophil counts in induced sputum (30). Fuke and colleagues used laser-capture microdissection and found increased IL-8 expression in the bronchiolar epithelium in early COPD (31). The combination of these various observations with our observations that IL-8 and neutrophil counts are related to bacterial colonization indicates the potential of bacterial colonization of the lower respiratory tract to contribute to airway inflammation and progressive airway obstruction in COPD.
Although our study was confined exclusively to ex-smokers, bacterial colonization could contribute in a similar manner to COPD pathogenesis among active smokers. In fact, bacterial colonization occurs early in this disease, as demonstrated by Soler and coworkers, who found that 8 of 18 smokers were colonized with PPB in protected brush specimens obtained by bronchoscopy (20). In the present study, bacterial colonization was observed in only a third of patients with COPD. This could mean that a subgroup of patients with COPD is prone to colonization and the consequent damaging effects, whereas the other two-thirds are resistant to bacterial colonization, which therefore has little if any role in their disease. However, this was a cross-sectional study and bacterial colonization in COPD is an intermittent phenomenon (32). Therefore, it is likely that many of the subjects with noncolonized COPD in this study would be colonized if they were to be studied again at a different point in time. One can therefore speculate that the level of airway inflammation is not constant over time in COPD but changes depending on whether the airway is sterile or colonized with a PPB.
Alternative explanations of the findings of this study need to be considered. Enhanced airway neutrophilic inflammation from another source could damage bacterial clearance mechanisms and allow bacterial colonization to occur in COPD. Although this possibility cannot be excluded, bacterial antigens such as endotoxin, peptidoglycan, and membrane proteins have potent inflammatory effects in vitro and in other settings by signaling through Toll-like receptors that are central to the innate host defense (33, 34). It would be very surprising if similar effects were not exerted by these bacterial antigens in the airway. Furthermore, chronic infection and associated inflammation are now considered to play a role in the pathogenesis of several previously chronic noninfectious diseases. These include Pseudomonas and cystic fibrosis, Helicobacter pylori and peptic ulcer disease, gut flora and inflammatory bowel disease, and C. pneumoniae and coronary artery disease (35–38).
Limitations of this study are related to the challenge of recruiting volunteers for research based on an invasive procedure and the restriction of enrollment to ex-smokers. These limitations include the cross-sectional design, the modest number of subjects, and the differences in age and cumulative tobacco exposure between the groups. Absence of a significant association among age, pack-years, and airway inflammation, and the substantial relationship of biologically linked inflammatory mediators and cells with bacterial colonization should allay concerns about these limitations.
The findings of this study have several important implications. If bacterial colonization contributes substantially to the pathogenesis of COPD, it is likely that susceptibility to bacterial colonization could explain a proportion of the “susceptibility” to tobacco smoke among smokers. The preponderance of subjects in this study had mild to moderate COPD. The incidence of bacterial colonization may increase with increasing severity of COPD (39). Therefore, the importance of bacterial colonization–induced inflammation could increase as the airflow obstruction characteristic of COPD becomes more severe. Furthermore, this process could underlie the continued deterioration often seen in ex-smokers with COPD with established disease. Another important implication is that research involving measurement of bronchial lumen inflammation in COPD, especially interventional studies with repeated measurements in the same patient, should take into account the colonization status of the patient for proper interpretation of the findings.
Determination of the cellular sources of the inflammatory mediators and the pathways by which bacterial pathogens induce production of these mediators are important questions that need to be addressed in future research. Whether a similar process of inflammation driven by bacterial colonization exists in current smokers needs to be determined. Inflammation in COPD is compartmentalized, and whether a relationship exists between inflammation in the bronchial wall and the lung parenchyma with the presence of bacteria in the lumen or the tissues warrants investigation. Whether bronchial bacterial colonization can drive systemic inflammation in COPD would be another fertile area of research. In an otherwise difficult and often frustrating disease to treat, the development of novel methods to reduce or eradicate bacterial colonization represents unique opportunities of future intervention.
Supplementary Material
Acknowledgments
The authors thank Timothy F. Murphy, M.D., and Brydon J. B. Grant, M.D., for helpful comments, and Adeline Thurston for secretarial support.
Supported by NHLBI RO1 HL066549.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200509-1525OC on February 10, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med 2000;343:269–280. [DOI] [PubMed] [Google Scholar]
- 2.Turato G, Di Stefano A, Maestrelli P, Mapp CE, Ruggieri MP, Roggeri A, Fabbri LM, Saetta M. Effect of smoking cessation on airway inflammation in chronic bronchitis. Am J Respir Crit Care Med 1995;152:1262–1267. [DOI] [PubMed] [Google Scholar]
- 3.Wright JL, Lawson LM, Pare PD, Wiggs BR, Kennedy S, Hogg JC. Morphology of peripheral airways in current smokers and ex-smokers. Am Rev Respir Dis 1983;127:474–477. [DOI] [PubMed] [Google Scholar]
- 4.Mullen JBM, Wright JL, Wiggs BR, Pare PD, Hogg JC. Structure of central airways in current smokers and ex-smokers with and without mucus hypersecretion: relationship to lung function. Thorax 1987;42:843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutgers SR, Postma DS, ten Hacken NHT, Kauffman HF, van der Mark TW, Koeter GH, Timens W. Ongoing airway inflammation in patients with COPD who do not currently smoke. Thorax 2000;55:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645–2653. [DOI] [PubMed] [Google Scholar]
- 7.Barnes PJ. Small airways in COPD. N Engl J Med 2004;350:2635–2637. [DOI] [PubMed] [Google Scholar]
- 8.Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med 2002;166:675–679. [DOI] [PubMed] [Google Scholar]
- 9.Sethi S, Murphy TF. Bacterial infection in chronic obstructive pulmonary disease in 2000: a state-of-the-art review. Clin Microbiol Rev 2001;14:336–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vitalis TZ, Kern I, Croome A, Behzad H, Hayashi S, Hogg JC. The effect of latent adenovirus 5 infection on cigarette smoke-induced lung inflammation. Eur Respir J 1998;11:664–669. [PubMed] [Google Scholar]
- 11.Matsuse T, Hayashi S, Kuwano K, Keunecke H, Jefferies WA, Hogg JC. Latent adenoviral infection in the pathogenesis of chronic airways obstruction. Am Rev Respir Dis 1992;146:177–184. [DOI] [PubMed] [Google Scholar]
- 12.von Hertzen L, Alakarppa H, Koskinen R, Liippo K, Surcel H-M, Leinonen M, Saikku P. Chlamydia pneumoniae infection in patients with chronic obstructive pulmonary disease. Epidemiol Infect 1997;118:155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris A, Sciurba FC, Lebedeva IP, Githaiga A, Elliott WM, Hogg JC, Huang L, Norris KA. Association of chronic obstructive pulmonary disease severity and pneumocystis colonization. Am J Respir Crit Care Med 2004;170:408–413. [DOI] [PubMed] [Google Scholar]
- 14.Murphy TF, Brauer AL, Schiffmacher AT, Sethi S. Persistent Colonization by Haemophilus influenzae in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2004;170:266–272. [DOI] [PubMed] [Google Scholar]
- 15.Banerjee D, Khair OA, Honeybourne D. Impact of sputum bacteria on airway inflammation and health status in clinical stable COPD. Eur Respir J 2004;23:685–691. [DOI] [PubMed] [Google Scholar]
- 16.Wilkinson TM, Patel IS, Wilks M, Donaldson GC, Wedzicha JA. Airway bacterial load and FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003;167:1090–1095. [DOI] [PubMed] [Google Scholar]
- 17.Hill AT, Campbell EJ, Hill SL, Bayley DL, Stockley RA. Association between airway bacterial load and markers of airway inflammation in patients with stable chronic bronchitis. Am J Med 2000;109:288–295. [DOI] [PubMed] [Google Scholar]
- 18.Patel IS, Seemungal TA, Wilks M, Lloyd-Owen SJ, Donaldson GC, Wedzicha JA. Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax 2002;57:759–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bresser P, Out TA, van Alphen L, Jansen HM, Lutter R. Airway inflammation in nonobstructive and obstructive chronic bronchitis with chronic Haemophilus influenzae airway infection: comparison with noninfected patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;162:947–952. [DOI] [PubMed] [Google Scholar]
- 20.Soler N, Ewig S, Torres A, Filella X, Gonzalez J, Zaubet A. Airway inflammation and bronchial microbial patterns in patients with stable chronic obstructive pulmonary disease. Eur Respir J 1999;14:1015–1022. [DOI] [PubMed] [Google Scholar]
- 21.Thompson AB, Daughton D, Robbins RA, Ghafouri MA, Oehlerking M, Rennard SI. Intraluminal airway inflammation in chronic bronchitis: characterization and correlation with clinical parameters. Am Rev Respir Dis 1989;140:1527–1537. [DOI] [PubMed] [Google Scholar]
- 22.Sun G, Stacey MA, Vittori E, Marini M, Bellini A, Kleimberg J, Mattoli S. Cellular and molecular characteristics of inflammation in chronic bronchitis. Eur J Clin Invest 1998;28:364–372. [DOI] [PubMed] [Google Scholar]
- 23.Sethi S, Maloney J, Grove L, Smigiera J, Berenson C. Colonization of the lower respiratory tract and airway neutrophilia in ex-smokers with stable COPD. American Thoracic Society International Conference, Seattle, WA, May 2003.
- 24.Sethi S, Maloney J, Grove L, Wrona C, Berenson CS. Bacterial colonization of the lower respiratory tract is associated with airway inflammation in ex-smokers with stable COPD. American Thoracic Society International Conference, Orlando, FL, May 2004.
- 25.Sethi S, Wrona C, Maloney J, Berenson C. Bacterial colonization is associated with increased matrix metalloproteinase-9 (MMP-9) in bronchoalveolar lavage of ex-smokers with stable COPD. American Thoracic Society International Conference, San Diego, CA, May 2005.
- 26.Rosell A, Monso E, Soler N, Torres F, Angrill J, Riise G, Zalacain R, Morera J, Torres A. Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease. Arch Intern Med 2005;165:891–897. [DOI] [PubMed] [Google Scholar]
- 27.Earley M Jr, ogt R, Shapiro H, Mandy FF, Keller K, Bellisario R, Pass K, Marti G, Stewart C, Hannon W. Report from a workshop on multianalyte microsphere assays. Cytometry 2002;50:239–242. [DOI] [PubMed] [Google Scholar]
- 28.National Institutes of Health, National Heart, Lung, and Blood Institute, World Health Organization. Executive summary: global strategies for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Bethesda, MD: National Institutes of Health, National Heart, Lung, and Blood Institute, and World Health Organization; 2004. [PubMed]
- 29.Tanino M, Betsuyaku T, Takeyabu K, Tanino Y, Yamaguchi E, Miyamoto K, Nishimura M. Increased levels of interleukin-8 in BAL fluid from smokers susceptible to pulmonary emphysema. Thorax 2002;57:405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Donnell RA, Peebles C, Ward JA, Daraker A, Angco G, Broberg P, Pierrou S, Lund J, Holgate ST, Davies DE, et al. Relationship between peripheral airway dysfunction, airway obstruction, and neutrophilic inflammation in COPD. Thorax 2004;59:837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuke S, Betsuyaku T, Nasuhara Y, Morikawa T, Katoh H, Nishimura M. Chemokines in bronchiolar epithelium in the development of chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 2004;31:405–412. [DOI] [PubMed] [Google Scholar]
- 32.Sethi S, Evans N, Grant BJB, Murphy TF. Acquisition of a new bacterial strain and occurrence of exacerbations of chronic obstructive pulmonary disease. N Engl J Med 2002;347:465–471. [DOI] [PubMed] [Google Scholar]
- 33.Sha Q, Truong-Tran AQ, Plitt JR, Beck LA, Schleimer RP. Activation of airway epithelial cells by Toll-like receptor agonists. Am J Respir Cell Mol Biol 2004;31:358–364. [DOI] [PubMed] [Google Scholar]
- 34.Berenson CS, Murphy TF, Wrona CT, Sethi S. Outer membrane protein P6 of nontypeable Haemophilus influenzae is a potent and selective inducer of human macrophage proinflammatory cytokines. Infect Immun 2005;73:2728–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med 2003;168:918–951. [DOI] [PubMed] [Google Scholar]
- 36.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984;1:1311–1315. [DOI] [PubMed] [Google Scholar]
- 37.Saikku P, Leinonen M, Mattila K, Ekman MR, Nieminen MS, Makela PH, Huttunen JK, Valtonen V. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet 1988;2:983–986. [DOI] [PubMed] [Google Scholar]
- 38.Podolsky DK. Inflammatory bowel disease. N Engl J Med 2002;347:417–429. [DOI] [PubMed] [Google Scholar]
- 39.Zalacain R, Sobradillo V, Amilibia J, Barron J, Achotegui V, Pijoan JI, Llorente JL. Predisposing factors to bacterial colonization in chronic obstructive pulmonary disease. Eur Respir J 1999;13:343–348. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.