Abstract
Rationale: Cyclosporin A (CsA) is known to preserve cardiac contractile function during endotoxemia, but the mechanism is unclear. Increased nitric oxide (NO) production and altered mitochondrial function are implicated as mechanisms contributing to sepsis-induced cardiac dysfunction, and CsA has the capacity to reduce NO production and inhibit mitochondrial dysfunction relating to the mitochondrial permeability transition (MPT).
Objectives: We hypothesized that CsA would protect against endotoxin-mediated cardiac contractile dysfunction by attenuating NO production and preserving mitochondrial function.
Methods: Left ventricular function was measured continuously over 4 h in cats assigned as follows: control animals (n = 7); LPS alone (3 mg/kg, n = 8); and CsA (6 mg/kg, n = 7), a calcineurin inhibitor that blocks the MPT, or tacrolimus (FK506, 0.1 mg/kg, n = 7), a calcineurin inhibitor lacking MPT activity, followed in 30 min by LPS. Myocardial tissue was then analyzed for NO synthase-2 expression, tissue nitration, protein carbonylation, and mitochondrial morphology and function.
Measurements and Main Results: LPS treatment resulted in impaired left ventricular contractility, altered mitochondrial morphology and function, and increased protein nitration. As hypothesized, CsA pretreatment normalized cardiac performance and mitochondrial respiration and reduced myocardial protein nitration. Unexpectedly, FK506 pretreatment had similar effects, normalizing both cardiac and mitochondrial parameters. However, CsA and FK506 pretreatments markedly increased protein carbonylation in the myocardium despite elevated manganese superoxide dismutase activity during endotoxemia.
Conclusions: Our data indicate that calcineurin is a critical regulator of mitochondrial respiration, tissue nitration, protein carbonylation, and contractile function in the heart during acute endotoxemia.
Keywords: LPS, mitochondria, multiple organ dysfunction syndrome, nitration, sepsis
Sepsis is a leading cause of death in the United States (1), and sepsis-related mortality is highest in the subset of patients who develop septic shock. Cardiac contractile dysfunction is common during septic shock, and attendant reductions in systemic oxygen delivery are believed to contribute to organ failures and death (2, 3). However, the precise molecular and cellular mechanisms of sepsis-related cardiac contractile inhibition are complex and incompletely understood.
During severe infection, bacterial components (e.g., endotoxins) initiate an intense primary immune response characterized by the release of potent proinflammatory cytokines. These early mediators of the sepsis syndrome promote inducible nitric oxide synthase-2 (NOS2) expression, resulting in accelerated nitric oxide (NO) production. In this context, selective NOS2 inhibition attenuates myocardial depression during exposure to endotoxin in rodents (4, 5), whereas nonselective inhibition of NOS isoforms causes refractory shock and reduced cardiac output in a subset of patients with severe sepsis (6). Considered together, there is reason to believe that NOS2 induction participates directly in the pathogenesis of cardiac dysfunction during sepsis.
Evidence indicates that mitochondria are important targets of injury during sepsis. Mitochondria are the major source of cellular energy, providing more than 90% of the ATP required for cell work. When mitochondria are rendered dysfunctional, as occurs in vital organs in the context of sepsis (7–9), bioenergetic limitation of cell and organ function is expected. Yet, other complications, such as increased mitochondrial reactive oxygen species (ROS) production or the release of mitochondrial inducers of programmed cell death (i.e., apoptosis), may be more serious events. The degree to which mitochondrial damage contributes to heart failure during sepsis is currently unknown; however, investigations by Fauvel and coworkers indicate that cyclosporin A (CsA), a potent inhibitor of the mitochondrial permeability transition (MPT) (10), normalizes heart function and apoptosis during conditions modeling sepsis (11). In addition to blocking the MPT, CsA also inhibits calcineurin-dependent signaling pathways, thereby attenuating the expression of NOS2 (12). The situation is further complicated by the capacity of NO to induce the MPT (13) and to inhibit mitochondrial electron transport (14).
On the basis of these observations, we hypothesized that pharmacologic inhibition of calcineurin signaling pathways would prevent NO-induced mitochondrial damage and myocardial contractile dysfunction during acute endotoxemia. A normotensive feline endotoxemia model in which systemic alterations of mitochondrial morphology and function have been previously demonstrated (8, 15, 16) was employed. Relevant cardiac parameters, including contractility, mitochondrial morphology and function, and myocardial NOS2 expression, protein nitration, and carbonylation, were assessed in animals randomly treated with either LPS alone or after a 30-min pretreatment with CsA or tacrolimus (FK506), a calcineurin inhibitor lacking affinity for the MPT pore (17), and in time-matched control animals. The comparison of pretreatment with CsA to an equipotent dose of FK506 isolated the effects of calcineurin inhibition from those associated with inhibiting the MPT pore. The results of these investigations provide novel insights into the pathogenesis of myocardial dysfunction in the context of sepsis. Some of the results of these studies have been previously reported in the form of abstracts (18, 19).
METHODS
Animal Preparation
All experiments were approved by the Ohio State University (Columbus, OH) Institutional Laboratory Animal Care and Use Committee in accordance with National Institutes of Health (Bethesda, MD) guidelines and employed a feline model that has been described previously (8). Details of the surgical preparation are described in the online supplement.
Reagents
LPS from Escherichia coli (serotype 0127:B8) was obtained from Sigma (St. Louis, MO), dissolved in buffered isotonic saline, and used at a concentration of 1.0 mg/ml. CsA (Sandimmune, 10 mg/ml; Novartis Pharma AG, Basel, Switzerland) and tacrolimus (FK506, Prograf, 5 mg/ml; Fujisawa Healthcare, Inc., Deerfield, IL) were dissolved in buffered isotonic saline and administered as described below.
Experimental Protocol
On completion of the surgical preparations, and after a 30-min stabilization period, baseline measurements, detailed elsewhere (8) and in the online supplement, were performed. The animals were then randomly assigned to receive either buffered isotonic saline vehicle (control; n = 7) or intravenous LPS (3.0 mg/kg; n = 8) alone or 30 min after prior treatment with CsA (6 mg/kg, intravenous; n = 7) or FK506 (0.1 mg/kg, intravenous; n = 7) and monitored experimentally over the subsequent 4 h. The doses of CsA and FK506 were chosen on the basis of the findings of previous experiments (16) and are explained in the online supplement.
Cardiac Parameter Measurements
Hemodynamic and ventilatory parameters were determined at baseline and maintained within normal limits throughout the experimental 4-h period. Left ventricular (LV) pressure was monitored with a 2F Millar Mikro-Tip catheter (model SPC-320; Millar Instruments, Houston, TX) placed via the left carotid artery before baseline measurements. The analog signal derived from the catheter was amplified, digitized, and processed with a CA Recorder Series II system (DISS, Pinckney, MI). Cardiac contractility was assessed using the first derivative of LV pressure generation (dP/dT, mm Hg/s) at its maximal point and at a submaximal point (25 mm Hg LV generated pressure). LV relaxation time (time in milliseconds required for the maximum negative dP/dT to recover 50% back toward 0 mm Hg/s) was also evaluated. Each measurement was determined from the mean value recorded over a 10-min steady state period and expressed as a percentage of a similar determination at baseline (because of animal-to-animal variability).
Computerized Analysis of Mitochondrial Ultrastructural Morphology
At 4 h post-treatment, LV tissue samples were obtained and processed for ultrastructural (i.e., electron microscopy) evaluation, as described elsewhere (8, 15) and in the online supplement. Electron photomicrographs were then converted to digital images with a high-resolution flatbed scanner and digitally analyzed, as described previously (20) (see the online supplement for additional details).
Cardiac Mitochondrial Respiration
Cardiac mitochondria were isolated by a standard procedure based on differential centrifugation, as detailed previously (8, 21) and in the online supplement. All steps were performed at 0 to 4°C and without delay, to minimize the potential for degradation during the isolation procedure. Mitochondrial protein concentration was determined spectrophotometrically by biuret assay and comparing with standards of known concentration. Respiration rates of cardiac mitochondria were determined immediately after isolation with a Clark O2 electrode and an oxygen monitor, as described elsewhere (8) and in the online supplement.
Immunohistochemistry and Image Analysis
At 4 h post-treatment, LV tissue samples were obtained, formalin fixed and later paraffin embedded, sectioned, and processed for histologic analysis. Cross-sections of myocardial tissue (5 μm) were then prepared for immunohistochemical evaluation according to standard procedures (22), as described in the online supplement. Images were captured with a Polaroid DMC high-resolution digital camera (Polaroid, Cambridge, MA), mounted on an Olympus BX-40 microscope (Olympus America, Melville, NY), and analyzed with Image-Pro 5.0 software (MediaCybernetics, Silver Spring, MD). Immunoprevalence of NOS2 and 3-nitrotyrosine (3-NT) was evaluated by color threshold analysis as detailed elsewhere (22) and in the online supplement.
Quantification of Protein Carbonylation
Carbonyl groups formed by oxidation were quantified according to the method described by Levine and coworkers (23), with slight modifications. Briefly, after solubilization of 1.0 mg of protein in a solution containing 1% Lubrol, 150 mM KCl, and 3 mM N-2-hydroxyethylpiperazine-N′-ethane sulfonic acid (pH 7.4), contaminating nucleic acids were removed by centrifugation after the addition of 20% streptomycin sulfate in 5 mM N-2-hydroxyethylpiperazine-N′-ethane sulfonic acid (pH 7.2). Proteins were precipitated with 20% trichloroacetic acid then incubated with 10 mM 2,4-dinitrophenylhydrazine in 2 M HCl and allowed to stand at room temperature for 1 h, with vortexing every 10–15 min. After reprecipitation and centrifugation with 20% trichloroacetic acid, the pellets were washed three times with ethyl acetate–ethanol (1:1, vol/vol) to remove any residual 2,4-dinitrophenylhydrazine. The protein pellet was then redissolved in 6 M guanidine (pH 2.3). Carbonyl content was calculated from the maximum absorbance at 366 nm, using a molar absorption coefficient of 22,000/M/cm.
Tissue Superoxide Dismutase Activity
Tissue superoxide dismutase (SOD) activity was determined spectrophotometrically with an SOD assay kit (Cayman Chemical Co., Ann Arbor, MI). The kit uses a tetrazolium salt for the detection of superoxide radicals generated by xanthine oxidase and hypoxanthine (24). Briefly, cardiac tissue samples were homogenized as described above and subjected to low-speed centrifugation, conducted at 1,500 × g for 5 min at 4°C. The resultant supernatants were applied to the SOD assay kit according to the manufacturer's instructions. The addition of 1 to 3 mM potassium cyanide, which inhibits CuZnSOD, allowed for the detection of MnSOD activity. Absorbance was read at 450 nm, and reaction rates were calculated on the basis of the linearized SOD standard rate.
Tissue Manganese Superoxide Dismutase and 3-Nitrotyrosine Expression
The presence of MnSOD, actin, and 3-NT in LV homogenates was determined by Western blot analysis, using standard techniques as described previously (16) and in the online supplement. The primary antibodies employed were a rabbit anti-rat polyclonal anti-MnSOD (diluted 1:2000; Abcam, Inc., Cambridge, MA), a mouse anti-chicken monoclonal anti-actin (0.5 μg/ml; Chemicon International, Inc., Temecula, CA), and a rabbit anti-mouse polyclonal anti-nitrotyrosine (diluted 1:1000; Upstate Cell Signaling Solutions, Lake Placid, NY). Horseradish peroxidase–linked anti-rabbit or anti-mouse secondary antibodies (diluted 1:10,000; Amersham Pharmacia, Little Chalfont, UK) were used for MnSOD and 3-NT, or actin, respectively.
Direct Effect of FK506 on NOS2 Activity
NOS2-catalyzed conversion of l-[14C]arginine to l-[14C]citrulline was performed in the presence of FK506 in a dose–response fashion as described previously (25) and in the online supplement.
Myeloperoxidase Staining and Quantification
At 4 h post-treatment, LV tissue samples were obtained, formalin fixed and later paraffin embedded, sectioned, and processed for histologic analysis.
Cross-sections of myocardial tissue (4 μm) were then prepared for immunohistochemical evaluation according to standard procedures (22), described in the online supplement. Positively stained cells were visualized and counted per high-power field (magnification, ×100) at multiple randomly selected fields for each tissue analyzed.
Data Analysis and Statistics
All data are expressed as means ± SEM. SigmaPlot 7.0 and SigmaStat 5.0 software (SPSS, Chicago, IL) were used to fit the data and carry out the statistical analyses. Comparisons of physiologic parameters were made using one-way analyses of variance (treatment) with repeated measures (time or replicates). Additional simultaneous comparisons of LV myocardial function, mitochondrial morphologic and functional assessments, NOS2 and 3-NT immunoprevalence, protein carbonylation, tissue SOD activity, and tissue MnSOD and 3-NT expression were performed by one-way analyses of variance. Post hoc analyses between group means were performed by Fisher's test for least significant difference or the Newman-Keuls test, where applicable. Spearman's nonparametric correlation analysis was employed to test for significant associations between parameters. Statistical significance was based on a value of p ⩽ 0.05.
RESULTS
Cardiac Function
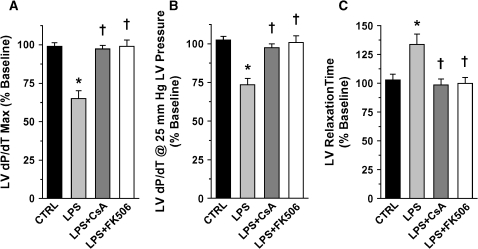
As illustrated in Figure E1 of the online supplement, physiologic parameters were maintained within normal limits throughout the experimental time course. To normalize for individual animal variation, assessments of LV myocardial function at 4 h post-treatment were expressed as percentages of similar measurements made at baseline for all groups (Figure 1). At 4 h post-LPS, significant reductions in the maximum dP/dT and dP/dT measured at 25 mm Hg LV pressure were observed. Pretreatment with CsA and FK506 protected against these LPS-induced impairments in cardiac contractility (Figures 1A and 1B). LV relaxation time, defined as the time for the maximum negative dP/dT to recover 50% toward dP/dT = 0 (RT50), increased dramatically after LPS treatment. This prolonged RT50 was likewise attenuated with CsA and FK506 pretreatments (Figure 1C). Thus, pretreatment with either CsA or FK506 protected against both systolic and diastolic myocardial dysfunction in LPS-treated animals.
Figure 1.
Assessment of left ventricular (LV) myocardial function at 4 h post-treatment as a percentage of similar measurements made at baseline for all treatment groups (values represent means ± SEM): (A) maximum LV dP/dT; (B) LV dP/dT at 25 mm Hg LV-generated pressure, and (C) LV relaxation time (time for maximum negative dP/dT to recover 50% back to 0 [RT50]). Both contractility measurements demonstrated significant reductions from baseline values after LPS administration (*p < 0.01 vs. control [CTRL]). In addition, the RT50 increased dramatically relative to baseline in association with LPS treatment (*p < 0.01 vs. control). Pretreatment with cyclosporin A (CsA) and FK506 protected against these LPS-induced systolic and diastolic impairments in left ventricular function (†p < 0.01 vs. LPS-treated alone).
Mitochondrial Ultrastructure and Respiratory Function
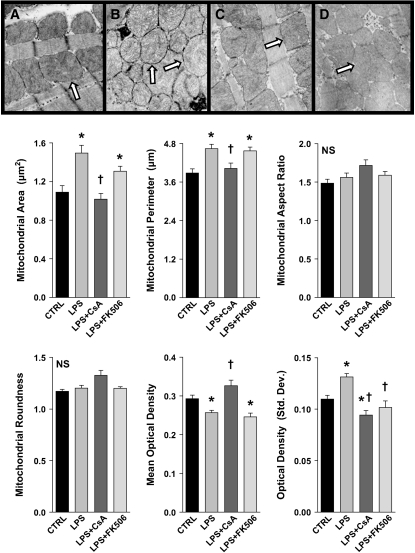
Electron microscopic evaluation of LV myocardial samples derived from LPS-treated animals demonstrated significant changes in mitochondrial morphology within 4 h, including high-amplitude swelling, as evidenced by increases in mitochondrial area and perimeter (Figure 2). Mitochondrial shape, as reflected by roundness and aspect ratio determinations, did not change significantly after LPS administration; however, the optical density of heart mitochondria was altered in LPS-treated animals, suggesting a decrease in the protein-to-fluid ratio within the mitochondria consistent with swelling (Figure 2). CsA and FK506 pretreatments demonstrated differential protective effects in this setting. CsA-pretreated animals exhibited mitochondrial area and perimeter determinations similar to those of control animals, and conferred protection against changes in the optical density parameters typically observed after LPS. By contrast, although morphologically similar in appearance to those of control animals, objective assessment of mitochondria from FK506-pretreated animals demonstrated little or no protection from LPS-induced alterations (Figure 2).
Figure 2.
Top: Representative electron photomicrographs of cardiac mitochondria (arrows) 4 h post-treatment: (A) Control; (B) LPS; (C) LPS + CsA; (D) LPS + FK506. Bottom: Objective evaluation of mitochondrial morphology determined by digital image analysis, as described in Methods (values represent means ± SEM). LPS treatment resulted in mitochondrial swelling as evidenced by increases in mitochondrial area and perimeter and a decrease in intramitochondrial mean optical density (*p < 0.05 vs. control). By contrast, parameters of mitochondrial shape, roundness, and aspect ratio demonstrated no significant changes after LPS. CsA pretreatment prevented all the LPS-induced mitochondrial ultrastructural changes (†p < 0.05 vs. LPS-treated alone), whereas FK506 pretreatment failed to confer protection against these alterations. (Staining, uranyl acetate and lead citrate; original magnification, ×55,000).
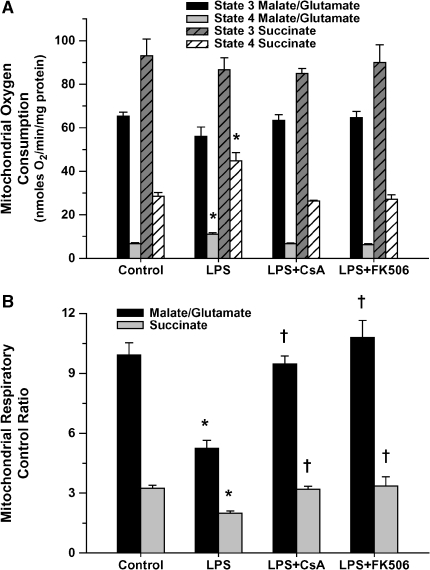
Correspondingly, mitochondrial respiratory function was significantly altered 4 h post-LPS, as indicated by a decreased respiratory control ratio (Figure 3B) resulting primarily from a notable increase in state 4 (ADP-independent) respiration (Figure 3A). This effect was observed for both respiratory substrates used. In addition, uncoupling of mitochondrial respiration, as reflected by a lower P:O ratio, was demonstrated in the LPS-treated group (1.79 ± 0.04 vs. 1.37 ± 0.07, p < 0.05; control vs. LPS, using succinate as the respiratory substrate). Pretreatment with CsA and FK506 normalized mitochondrial respiratory function after LPS administration (Figure 3) with P:O ratios unchanged relative to control animals (1.77 ± 0.02 and 1.75 ± 0.09 for the CsA and FK506-pretreated groups, respectively).
Figure 3.
Assessment of myocardial mitochondrial respiratory function 4 h post-treatment for all treatment groups (values represent means ± SEM), using either malate/glutamate or succinate as the respiratory substrate. (A) State 3 (ADP dependent) and state 4 (ADP independent) respiratory rates. LPS treatment was associated with elevated state 4 respiration regardless of substrate (*p < 0.01 vs. matching control), which was normalized with CsA or FK506 pretreatment. (B) Respiratory control ratio (RCR). The RCR decreased dramatically in conjunction with the LPS-induced increase in state 4 respiration (*p < 0.01 vs. matching control). Pretreatment with either CsA or FK506 prevented any LPS-induced decline in the RCR (†p < 0.01 vs. matching LPS-treated alone).
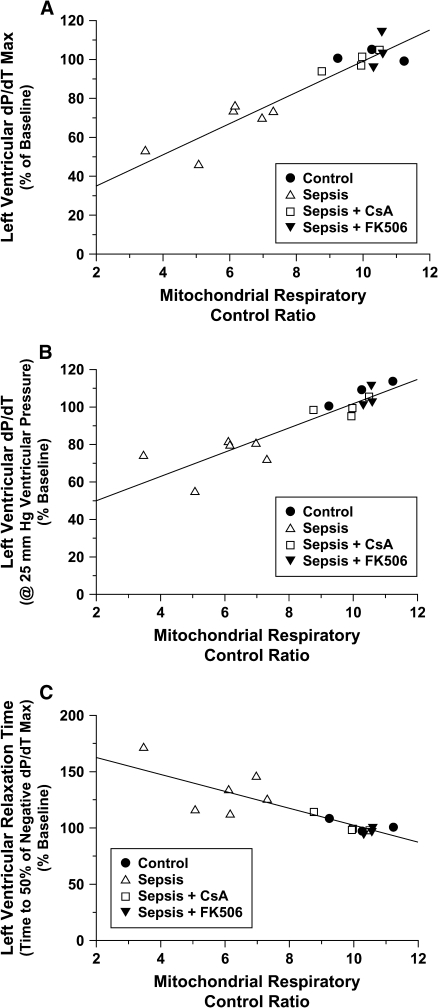
Significant and strong correlations exist between the assessment of mitochondrial respiratory efficiency (i.e., respiratory control ratio) and determinations of myocardial function (i.e., contractility and relaxation rates; Figure 4).
Figure 4.
Relationship between the mitochondrial RCR, using malate/glutamate as the respiratory substrate, and (A) maximum LV dP/dT; (B) LV dP/dT at 25 mm Hg LV-generated pressure, and (C) LV relaxation time (RT50). Significant correlations were demonstrated between mitochondrial respiratory function and all measurements of LV myocardial performance at 4 h post-treatment (A: r = 0.95; B: r = 0.90; and C: r = 0.85; p < 0.05).
Immunohistochemistry
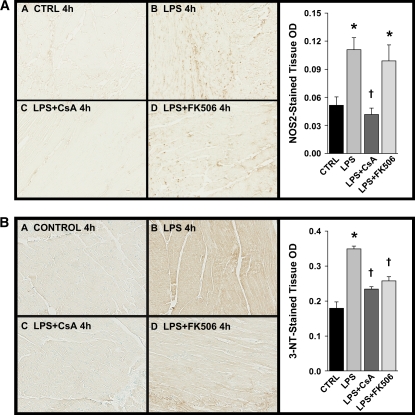
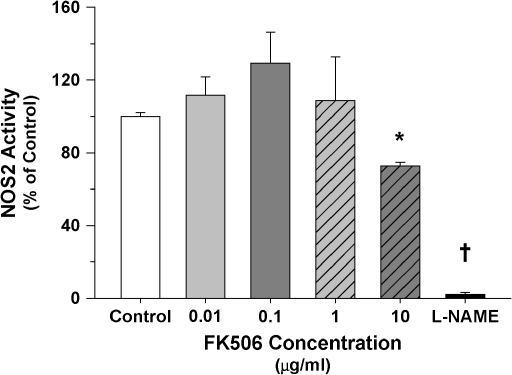
As expected, on the basis of previous investigations (26), immunohistochemical analyses of cardiac tissue derived from LPS-treated animals demonstrated significantly increased protein nitration, as reflected by 3-NT immunostaining (Figure 5B). Pretreatment with the calcineurin inhibitors CsA and FK506 attenuated myocardial nitration, which was apparently unrelated to the expression of NOS2 immunoprevalence (Figure 5A) or, in the case of FK506, a lack of any direct inhibition of NOS2 activity (Figure 6).
Figure 5.
Representative light photomicrographs of cardiac ventricular tissue 4 h post-treatment for all groups after immunohistochemical staining for (A) immunoprevalence of nitric oxide synthase-2 (NOS2) induction and (B) immunoprevalence of 3-nitrotyrosine (3-NT). NOS2 induction was observed in cardiac tissues 4 h after LPS treatment (*p < 0.01 vs. control). Pretreatment with CsA attenuated this increase (†p < 0.01 vs. LPS-treated alone), but FK506 pretreatment did not protect against LPS-induced increase in cardiac NOS2 immunoprevalence (A). In addition, LPS treatment was associated with significant increases in cardiac tissue nitration (*p < 0.01 vs. control). Pretreatment with either CsA or FK506 prevented this increase in 3-NT immunoprevalence after LPS (†p < 0.01 vs. LPS-treated alone) (B).
Figure 6.
Relationship of NOS2 activity to increasing concentrations of FK506 (NOS2 activity presented as a percentage of measurements made in the absence of FK506; values represent means ± SEM). FK506 did not demonstrate any direct inhibitory effect on NOS2 activity over a concentration range relevant to the present study. Some inhibition did occur at the higher pharmacologic dose of 10 μg/ml (*p < 0.01 vs. control). By comparison, NOS2 activity was nearly completely blocked by L-NAME, a known inhibitor of NOS (†p < 0.01 vs. all doses).
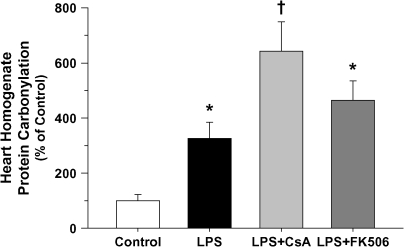
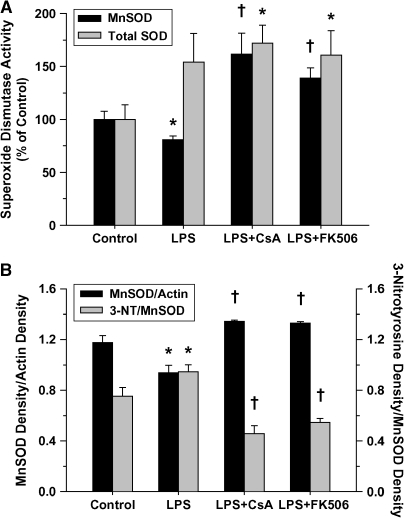
In contrast to myocardial nitration, calcineurin inhibition was shown to significantly increase tissue protein carbonylation (Figure 7), presumably because of increased oxidant production, rather than inhibition of critical antioxidant enzymes such as superoxide dismutase (Figure 8A). Compared with LPS treatment alone, MnSOD activity was increased in both groups receiving calcineurin inhibitor pretreatment (i.e., CsA or FK506), apparently because of the combined effects of increased relative expression and reduced nitration of tissue MnSOD (Figure 8B) (27). By contrast, nitration of other mitochondrial proteins, including mitochondrial complex I, cytochrome c, and actin, was not influenced by LPS in the presence or absence of calcineurin inhibitors (data not shown) compared with control animals. Myocardial inflammation, as reflected by neutrophil infiltration, was unaffected by LPS treatment or calcineurin inhibition (see Figure E2). As such, this variable is unlikely to account for the changes in nitration or carbonylation observed after calcineurin inhibitor pretreatment compared with receiving LPS alone.
Figure 7.
Protein carbonylation of cardiac ventricular homogenates as a percentage of similar measurements made in matching control animals at 4 h post-treatment for all treatment groups (values represent means ± SEM). Increased oxidant stress was evident after LPS treatment (*p < 0.05 vs. control) and further elevated when combined with calcineurin inhibition (†p < 0.05 vs. control and LPS-treated alone).
Figure 8.
(A) Superoxide dismutase (SOD) activity of cardiac ventricular homogenates as a percentage of similar measurements made in matching control animals at 4 h post-treatment for all treatment groups (values represent means ± SEM). Relative to control animals, LPS treatment alone was associated with reduced MnSOD activity and increased total SOD activity in the ventricular tissue (*p < 0.05 vs. matching control), indicating that SOD activity was selectively reduced in the mitochondrial compartment. Pretreatment with either CsA or FK506 resulted in significantly increased activities for both the total tissue SOD (*p < 0.05 vs. matching control) and MnSOD (†p < 0.05 vs. matching control and LPS-treated alone). (B) Relative protein expression and nitration of MnSOD in cardiac ventricular homogenates at 4 h post-treatment for all treatment groups (values represent means ± SEM). Significantly decreased relative tissue expression (normalized to actin) and notably increased nitration of MnSOD were demonstrated in the ventricular tissue after LPS treatment alone (*p < 0.05 vs. matching control). By contrast, relative tissue MnSOD expression was dramatically increased and MnSOD nitration significantly reduced in both groups receiving calcineurin inhibitor pretreatment (†p < 0.01 vs. matching control and LPS-treated alone).
DISCUSSION
The major findings of these investigations were interesting in that calcineurin was demonstrated to be a primary regulator of myocardial contractile function, mitochondrial respiration, and carbonyl protein formation during acute endotoxemia. In concordance with our hypothesis, CsA effectively inhibited the expression of NOS2 and prevented tissue nitration; however, questions relating to the primary source of NO and the roles of NO-independent mechanisms in the pathogenesis of organ failure remain unanswered. The observed dissociation of NOS2 expression with myocardial contractile function and with increased cardiac tissue nitration in the FK506 pretreatment group (Figure 5) was unexpected and may be explained by several possible scenarios, but direct inhibition of NOS2 by calcineurin inhibitors (i.e., FK506) is not supported by our data (Figure 6). Alternatively, calcineurin inhibitors are known to influence the activity of calcium-dependent NOS isoforms (e.g., endothelial NOS) by post-translational modifications (28) and by reducing their expression (29). Furthermore, calcineurin can influence cardiac contractility independent of NOS (30). The observation that calcineurin inhibition effectively normalizes mitochondrial respiratory function and prevents cardiac contractile dysfunction at the expense of increased myocardial protein carbonylation has important implications for the pathogenesis of organ damage in the context of sepsis.
The mechanisms by which NO regulates myocardial contractility are complex, and a comprehensive discussion is beyond the scope of this article. As noted in a review of this topic by Massion and coworkers, NO normally regulates cardiac function at many levels, including autonomic signaling and excitation–contraction coupling, and by matching mitochondrial energy production to myocardial demands (31). Under pathologic conditions, such as during endotoxemia, the heart generates abnormally high levels of NO and other oxidants, including superoxide, which favors the formation of peroxynitrite (26). Peroxynitrite, in turn, inhibits mitochondrial electron transport at complex I (32, 33) and interrupts the function of myocardial contractile units by altering calcium transients (34) and directly modifying actin (35). Although it was not feasible to comprehensively monitor the diverse actions of NO in the heart, we observed enhanced protein nitration in the heart of endotoxemic animals, which was attenuated by pretreatment with calcineurin inhibitors (Figure 5). With the exception of MnSOD, relevant targets of nitration in the cytoplasmic and mitochondrial compartments (i.e., actin, complex I, and cytochrome c) demonstrated no significant change in relative nitration, indicating that peroxynitrite-mediated protein modifications occur selectively in the setting of endotoxemia. Alternative targets include enzymes regulating mitochondrial lipid metabolism (36) or proteins localized to the myocardial endothelium (37). Moreover, it is possible that NO directly inhibits cardiac contractile function (38), and that isoforms of NOS other than NOS2 contribute significantly to NO formation in this model.
The specific role of mitochondria in the pathogenesis of sepsis-induced heart failure remains controversial. In 1994, Solomon and colleagues (39) demonstrated no change in basal high-energy phosphate (i.e., ATP) stores in the hearts of septic dogs, despite obvious mitochondrial swelling and impaired contractile function. The authors concluded that impaired mitochondrial ATP formation does not contribute to myocardial contractile dysfunction during sepsis. However, it is interesting to note that the septic animals were severely limited in terms of their capacity to increase myocardial energy metabolism in response to catecholamines (39), suggesting that the heart was operating at or near maximum capacity during sepsis and implying a loss of cardiac reserve. The results of the current investigations support the notion that putative mediators of the sepsis syndrome simultaneously inhibit mitochondrial function and cardiac contractility and that calcineurin is instrumental in this process. Moreover, impaired myocardial function in the LPS treatment group, as reflected by decreased cardiac contractility and inhibition of mitochondrial function, was associated with an overall reduction in protein carbonylation, relative to matching animals pretreated with calcineurin inhibitors (Figure 7). In this regard, protein carbonylation is a common by-product of oxidative stress due to a wide spectrum of ROS, and compared with protein nitration, carbonyl proteins are more prevalent (40) and are not readily reversible (41), which makes them the preferred indicators of oxidative stress (40). The lower level of protein carbonylation in the LPS-treated animals relative to those pretreated with calcineurin inhibitors was not explained by enhanced SOD activity (Figure 8A). Instead, it is likely that the combined effects of reduced cardiac work (i.e., decreased cardiac contractility) and increased NO formation resulted in the observed decrease in protein carbonyl formation in favor of protein nitration in the LPS treatment group compared with the groups pretreated with calcineurin inhibitors.
The contribution of the mitochondrion to the pathogenesis of sepsis-induced oxidant stress is a topic of particular interest. Mitochondrial respiration is a major source of oxidant production during muscle contraction (42), and mitochondrial ROS production is accelerated during endotoxemia (43). This could explain why mitochondrial proteins are susceptible to oxidative modification during endotoxemia (44). Enhanced mitochondrial ROS formation may result from an imbalance between SOD and catalase (45) or a diversion of electrons away from the electron transport chain at complex II (43). With respect to the latter, it is expected that uncoupling of mitochondria, such as occurs during endotoxemia, will reduce the electrochemical gradient across the inner mitochondrial membrane, thereby reducing mitochondrial ROS production (46). Thus, partial uncoupling of mitochondrial respiration is another factor that may contribute to lower levels of protein carbonyl formation in the LPS group compared with those pretreated with calcineurin inhibitors.
Support for the concept that mitochondrial dysfunction contributes directly to impaired cardiac contractility under conditions modeling sepsis is provided by the present investigation. A strong correlation between altered mitochondrial respiratory function and impaired cardiac contractile function is demonstrated in Figure 4. However, the mechanisms by which calcineurin regulates mitochondrial function during endotoxemia are unclear. CsA pretreatment was observed to inhibit mitochondrial swelling in the heart (Figure 2) and in other vital organs during endotoxemia, suggesting that sepsis-induced mitochondrial swelling is a manifestation of the MPT (16, 47). Fauvel and colleagues reported that CsA effectively preserves myocardial contractility in endotoxemic rats, leading them to speculate that inhibition of the MPT was the primary mechanism of protection (11). The MPT pore spans the inner mitochondrial membrane and is normally found in its “closed” orientation, during which the pore is impermeable to most molecules and proteins. In its open configuration, the pore is permeable to small proteins (< 1.5 kD) and ions, leading to mitochondrial swelling and dissolution of the electrochemical gradient required for ATP production, which is designated the MPT. Under these conditions, the mitochondria release factors promoting programmed cell death and lysosomal mitochondrial clearance (48), which may contribute to mitochondrial depletion in the heart (49). The observation that pretreatment with FK506 preserved cardiac function but had no significant impact on mitochondrial swelling implies that acute mitochondrial swelling in the heart, presumably caused by transient opening of the MPT pore, given that it is blocked by CsA, is not the primary cause of cardiac dysfunction in the early phases of endotoxemia in our model. However, at higher doses of endotoxin, such as those employed in studies by Fauvel and coworkers, induction of the MPT appears to override the protective effects of calcineurin inhibition, and mitochondrial cell death pathways are activated (11). The mechanism by which calcineurin regulates both mitochondrial function and cardiac contractility is not obvious from these investigations, but changes in calcium flux in mitochondria (50) and at the level of the sarcoplasmic reticulum (30) may be responsible and are supported by these investigations. With respect to the latter, investigations by Santana and coworkers indicate that calcineurin inhibition augments excitation–contraction coupling in the heart by increasing the conductivity of calcium channels located in the sarcoplasmic reticulum (30).
Another potential explanation for the cardioprotection conferred by calcineurin inhibitors in these experiments could relate to the immunomodulatory effects of the calcineurin inhibitors. CsA and FK506 are immunosuppressive compounds, believed to exert their action through binding to small intracellular regulatory proteins, the cyclophilins or FK-binding proteins, also known as immunophilins (51). When complexed with CsA and FK506, the immunophilins lead to the inhibition of calcineurin (52) and subsequent inhibition of T-cell activation (53). In terms of in vitro T-cell inhibition, CsA is 50 to 100 times less potent than FK506 (54), and at the doses used for these experiments no significant reduction in the innate immune response (as reflected by circulating tumor necrosis factor-α levels) was detected (16), and myocardial tissue inflammation was minimal (see Figure E2) and not significantly different in any of the treatment groups. Thus, it is unlikely that calcineurin inhibitors significantly reduced the production of extracellular ROS (e.g., by leukocytes), thereby reducing myocardial protein nitration in this model. Further investigation is needed to determine the actual source of myocardial ROS production, especially NO and superoxide anion, in the acute phase of sepsis.
Conclusions
These investigations only partially support our hypothesis that the calcineurin inhibitors CsA and FK506 preserve myocardial function by inhibiting NO production and preventing mitochondrial damage in the heart during acute endotoxemia. Myocardial tissue nitration was attenuated in the calcineurin pretreatment groups; however, the source of NO, the targets of nitration, and the consequences of enhanced NO production in terms of myocardial contractility remain to be determined. Furthermore, inhibition of superoxide production does not explain the reduced protein nitration after calcineurin inhibitor pretreatment. In fact, calcineurin inhibition in conjunction with LPS treatment was shown to increase protein carbonyl formation despite increased SOD activity. At the dose of LPS used for these investigations, opening of the MPT pore, perhaps only transiently (55), appears to contribute to mitochondrial swelling in the heart, but mitochondrial swelling was insufficient to explain impaired cardiac performance. Although the mechanism remains to be determined, it is apparent that calcineurin is a critical regulator of cardiac metabolism, contractility, and ROS formation during the acute phase of endotoxemia. It remains to be determined whether calcineurin-mediated depression of cardiac contractility and mitochondrial function, and associated changes in ROS formation, are adaptive or maladaptive in the context of sepsis.
Supplementary Material
Acknowledgments
The authors thank Kathleen S. Wolken and the Campus Microscopy and Imaging Facility of Ohio State University Medical Center for technical proficiency and help in preparing the tissue samples for electron microscopy analysis.
Supported by American Lung Association grant CI-002-N, American Heart Association grant 0051013B, and National Institutes of Health grants HL04335, HL59791, and HL63067.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200411-1507OC on January 19, 2006
Conflict of Interest Statement: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003;348:1546–1554. [DOI] [PubMed] [Google Scholar]
- 2.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy collaborative group. N Engl J Med 2001;345:1368–1377. [DOI] [PubMed] [Google Scholar]
- 3.Parker MM, Shelhamer JH, Bacharach SL, Green MV, Natanson C, Frederick TM, Damske BA, Parrillo JE. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med 1984;100:483–490. [DOI] [PubMed] [Google Scholar]
- 4.Ullrich R, Scherrer-Crosbie M, Bloch KD, Ichinose F, Nakijima H, Picard MH, Zapol WM, Quezado ZMN. Congenital deficiency of nitric oxide synthase 2 protects against endotoxin-induced myocardial dysfunction in mice. Circulation 2000;102:1440–1446. [DOI] [PubMed] [Google Scholar]
- 5.Ichinose F, Hataishi R, Wu JC, Kawai N, Rodrigues AC, Mallari C, Post JM, Parkinson JF, Picard MH, Bloch KD, et al. A selective inducible NOS dimerization inhibitor prevents systemic, cardiac, and pulmonary hemodynamic dysfunction in endotoxemic mice. Am J Physiol Heart Circ Physiol 2003;285:H2524–H2530. [DOI] [PubMed] [Google Scholar]
- 6.Lopez A, Lorente JA, Steingrub J, Bakker J, McLuckie A, Willatts S, Brockway M, Anzueto A, Holzapfel L, Breen D, et al. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med 2004;32:21–30. [DOI] [PubMed] [Google Scholar]
- 7.Brealey D, Karyampudi S, Jacques TS, Novelli M, Stidwell R, Taylor V, Smolenski RT, Singer M. Mitochondrial dysfunction in a long-term rodent model of sepsis and organ failure. Am J Physiol Regul Integr Comp Physiol 2004;286:R491–R497. [DOI] [PubMed] [Google Scholar]
- 8.Crouser ED, Julian MW, Blaho DV, Pfeiffer DR. Endotoxin-induced mitochondrial damage correlates with impaired respiratory activity. Crit Care Med 2002;30:276–284. [DOI] [PubMed] [Google Scholar]
- 9.Gellerich FN, Trumbeckaite S, Opalka JR, Gellerich JF, Chen Y, Neuhof C, Redl H, Werdan K, Zierz S. Mitochondrial dysfunction in sepsis: evidence from bacteraemic baboons and endotoxaemic rabbits. Biosci Rep 2002;22:99–113. [DOI] [PubMed] [Google Scholar]
- 10.Broekemeier KM, Dempsy ME, Pfeiffer DR. Cyclosporin A is a potent inhibitor of the inner membrane permeability transition in liver mitochondria. J Biol Chem 1989;264:7826–7830. [PubMed] [Google Scholar]
- 11.Fauvel H, Marchetti P, Obert G, Joulain O, Chopin C, Formstecher P, Neviere R. Protective effects of cyclosporine A from endotoxin-induced myocardial dysfunction and apoptosis in rats. Am J Respir Crit Care Med 2002;165:449–455. [DOI] [PubMed] [Google Scholar]
- 12.Dusting GJ, Akita K, Hickey H, Smith M, Gurevich V. Cyclosporin A and tacrolimus (FK506) suppress expression of inducible nitric oxide synthase in vitro by different mechanisms. Br J Pharmacol 1999;128:337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solenski NJ, Kostecki VK, Dovey S, Periasamy A. Nitric-oxide-induced depolarization of neuronal mitochondria: implications for neuronal death. Mol Cell Neurosci 2003;24:1151–1169. [DOI] [PubMed] [Google Scholar]
- 14.Carreras MC, Franco MC, Peralta JG, Poderoso JJ. Nitric oxide, complex I, and the modulation of mitochondrial reactive species in biology and disease. Mol Aspects Med 2004;25:125–139. [DOI] [PubMed] [Google Scholar]
- 15.Crouser ED, Julian MW, Dorinsky PM. Ileal Vo2–Do2 alterations induced by endotoxin correlate with severity of mitochondrial injury. Am J Respir Crit Care Med 1999;160:1347–1353. [DOI] [PubMed] [Google Scholar]
- 16.Crouser ED, Julian MW, Huff JE, Joshi MS, Bauer JA, Gadd ME, Wewers MD, Pfeiffer DR. Abnormal permeability of inner and outer mitochondrial membranes contributes independently to mitochondrial dysfunction in the liver during acute endotoxemia. Crit Care Med 2004;32:478–488. [DOI] [PubMed] [Google Scholar]
- 17.Connern CP, Halestrap AP. Recruitment of mitochondrial cyclophilin to the mitochondrial inner membrane under conditions of oxidative stress that enhance the opening of a calcium-sensitive non-specific channel. Biochem J 1994;302:321–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crouser ED, Julian MW, Huff JE, Joshi MS, Bauer JA, Pfeiffer DR. Mitochondrial damage is an early manifestation of sepsis-induced ventricular dysfunction [abstract]. Am J Respir Crit Care Med 2003;167:A555. [Google Scholar]
- 19.Joshi MS, Julian MW, Crouser ED, Bauer JA. Cyclosporin A prevents left ventricular dysfunction in a feline model of acute sepsis: relation to NOS2 induction, protein nitration and mitochondrial injury [abstract]. Am J Respir Crit Care Med 2004;169:A637. [Google Scholar]
- 20.Joshi MS, Crouser ED, Julian MW, Schanbacher BL, Bauer JA. Digital imaging analysis for the study of endotoxin-induced mitochondrial ultrastructure injury. Anal Cell Pathol 2000;21:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobus WE, Tiozzo R, Lugli G, Lehninger AL, Carafoli E. Aspects of energy-linked calcium accumulation by rat heart mitochondria. J Biol Chem 1975;250:7863–7870. [PubMed] [Google Scholar]
- 22.Crouser ED, Julian MW, Weinstein DM, Fahy RJ, Bauer JA. Endotoxin-induced ileal mucosal injury and nitric oxide dysregulation are temporally dissociated. Am J Respir Crit Care Med 2000;161:1705–1712. [DOI] [PubMed] [Google Scholar]
- 23.Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 1990;186:464–478. [DOI] [PubMed] [Google Scholar]
- 24.Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 1971;44:276–287. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida M, Xia Y. Heat shock protein 90 as an endogenous protein enhancer of inducible nitric-oxide synthase. J Biol Chem 2003;278:36953–36958. [DOI] [PubMed] [Google Scholar]
- 26.Khadour FH, Panas D, Ferdinandy P, Schulze C, Csont T, Lalu MM, Wildhirt SM, Schulz R. Enhanced NO and superoxide generation in dysfunctional hearts from endotoxemic rats. Am J Physiol Heart Circ Physiol 2002;283:H1108–H1115. [DOI] [PubMed] [Google Scholar]
- 27.Guo W, Adachi T, Matsui R, Xu S, Jiang B, Zuo MH, Kirber M, Lieberthal W, Cohen RA. Quantitative assessment of tyrosine nitration of manganese superoxide dismutase in angiotensin II-infused rat kidney. Am J Physiol Heart Circ Physiol 2003;285:H1396–H1403. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Steiner JP. Nitric oxide synthase, immunophilins and poly(ADP-ribose) synthetase: novel targets for the development of neuroprotective drugs. Neurol Res 1995;17:285–288. [DOI] [PubMed] [Google Scholar]
- 29.Ritter O, Schuh K, Brede M, Rothlein N, Burkard N, Hein L, Neyses L. AT2 receptor activation regulates myocardial eNOS expression via the calcineurin–NF–AT pathway. FASEB J 2003;17:283–285. [DOI] [PubMed] [Google Scholar]
- 30.Santana LF, Chase EG, Votaw VS, Nelson MT, Greven R. Functional coupling of calcineurin and protein kinase A in mouse ventricular myocytes. J Physiol 2002;544:57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Massion PB, Feron O, Dessy C, Belligand JL. Nitric oxide and cardiac function: ten years after, and continuing. Circ Res 2003;93:388–398. [DOI] [PubMed] [Google Scholar]
- 32.McMahon TJ, Moon RE, Luschinger BP, Carraway MS, Stone AE, Stolp BW, Gow AJ, Pawloski JR, Watke P, Singel DJ, et al. Nitric oxide in the human respiratory cycle. Nat Med 2002;8:711–717. [DOI] [PubMed] [Google Scholar]
- 33.Poderoso JJ, Carreras MC, Lisdero C, Riobo N, Schopfer F, Boveris A. Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondria and submitochondrial particles. Arch Biochem Biophys 1996;328:85–92. [DOI] [PubMed] [Google Scholar]
- 34.Brunner F, Wolkart G. Peroxynitrite-induced cardiac depression: role of myofilament desensitization and cGMP pathway. Cardiovasc Res 2003;60:355–364. [DOI] [PubMed] [Google Scholar]
- 35.Borbely A, Toth A, Edes I, Virag L, Papp JG, Varro A, Paulus WJ, van der Velden J, Steinen GJ, Papp Z. Peroxynitrite-induced α-actinin nitration and contractile alterations in isolated human myocardial cells. Cardiovasc Res 2005;67:225–233. [DOI] [PubMed] [Google Scholar]
- 36.Marcondes S, Turko IV, Murad F. Nitration of succinyl-CoA:3-oxoacid CoA-transferase in rats after endotoxin administration. Proc Natl Acad Sci USA 2001;98:7146–7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kooy NW, Lewis SJ, Royall JA, Ye YZ, Kelly DR, Beckman JS. Extensive tyrosine nitration in human myocardial inflammation: evidence for the presence of peroxynitrite. Crit Care Med 1997;25:812–819. [DOI] [PubMed] [Google Scholar]
- 38.Shoji H, Takahashi S, Okabe E. Intracellular effects of nitric oxide on force production and Ca2+ sensitivity of cardiac myofilaments. Antioxid Redox Signal 1999;1:509–521. [DOI] [PubMed] [Google Scholar]
- 39.Solomon MA, Correa R, Alexander HR, Koev LA, Cobb JP, Kim DK, Roberts WC, Hoffman WD, Bacher J, Yatsiv I, et al. Myocardial energy metabolism and morphology in a canine model of sepsis. Am J Physiol Heart Circ Physiol 1994;266:H757–H768. [DOI] [PubMed] [Google Scholar]
- 40.Dalle-Donne I, Giustarini D, Colombo R, Rossi R, Milzani A. Protein carbonylation in human disease. Trends Mol Med 2003;4:169–176. [DOI] [PubMed] [Google Scholar]
- 41.Kuo WN, Kanadia RN, Shanbhag VP, Toro R. Denitration of peroxynitrite-treated proteins by ‘protein nitrases’ from rat heart and brain. Mol Cell Biochem 1999;201:11–16. [DOI] [PubMed] [Google Scholar]
- 42.Bejma J, Ji LL. Aging and acute exercise enhance free radical generation in rat skeletal muscle. J Appl Physiol 1999;87:465–470. [DOI] [PubMed] [Google Scholar]
- 43.Taylor DE, Ghio AJ, Piantadosi CA. Reactive oxygen species produced by liver mitochondria of rats in sepsis. Arch Biochem Biophys 1995;316:70–76. [DOI] [PubMed] [Google Scholar]
- 44.Suliman HB, Welty-Wolf KE, Carraway M, Tatro L, Piantadosi CA. Lipopolysaccharide induces oxidative cardiac mitochondrial damage and biogenesis. Cardiovasc Res 2004;64:279–288. [DOI] [PubMed] [Google Scholar]
- 45.Ritter C, Andrades ME, Reinke A, Menna-Barreto S, Moreira JC, Dal-Pizzol F. Treatment with N-acetylcysteine plus deferoxamine protects rats against oxidative stress and improves survival in sepsis. Crit Care Med 2004;32:342–349. [DOI] [PubMed] [Google Scholar]
- 46.Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett 1997;416:15–18. [DOI] [PubMed] [Google Scholar]
- 47.Crouser ED, Julian MW, Joshi MS, Bauer JA, Wewers MD, Hart JM, Pfeiffer DR. Cyclosporin A ameliorates mitochondrial ultrastructural injury in the ileum during acute endotoxemia. Crit Care Med 2002;30:2722–2728. [DOI] [PubMed] [Google Scholar]
- 48.Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, Crowe RA, Cascio WE, Bradham CA, Brenner DA, et al. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta 1998;1366:177–196. [DOI] [PubMed] [Google Scholar]
- 49.Watts JA, Kline JA, Thornton LR, Grattan RM, Brar SS. Metabolic dysfunction and depletion of mitochondria in hearts of septic rats. J Mol Cell Cardiol 2004;36:141–150. [DOI] [PubMed] [Google Scholar]
- 50.Smaili SS, Stellato KA, Burnett P, Thomas AP, Gaspers LD. Cyclosporin A inhibits inositol 1,4,5-triphosphate-dependent Ca2+ signals by enhancing Ca2+ uptake into the endoplasmic reticulum and mitochondria. J Biol Chem 2001;276:23329–23340. [DOI] [PubMed] [Google Scholar]
- 51.Galat A. Peptidylproline cis-trans-isomerases: immunophilins. Eur J Biochem 1993;216:689–707. [DOI] [PubMed] [Google Scholar]
- 52.Liu J, Farmer JD, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin–cyclosporin A and FKBP–FK506 complexes. Cell 1991;66:807–815. [DOI] [PubMed] [Google Scholar]
- 53.Clipstone NA, Crabtree GR. Identification of calcineurin as a key signaling enzyme in T-lymphocyte activation. Nature 1992;357:695–697. [DOI] [PubMed] [Google Scholar]
- 54.Andersson J, Nagy S, Groth CG, Andersson U. Effects of FK506 and cyclosporine A on cytokine production studied in vitro at a single-cell level. Immunology 1992;75:136–142. [PMC free article] [PubMed] [Google Scholar]
- 55.Simpson PB. The local control of cytosolic Ca2+ as a propagator of CNS communication: integration of mitochondrial transport mechanisms and cellular responses. J Bioenerg Biomembr 2000;32:5–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.