Abstract
Rationale: Interactions of nontypeable Haemophilus influenzae (NTHI) with macrophages are implicated in the pathogenesis of chronic obstructive pulmonary disease (COPD). However, the immunologic mechanisms that mediate NTHI-macrophage inflammation are poorly understood. Outer membrane protein (OMP) P6 and lipooligosaccharide (LOS) of NTHI are potent immunomodulators. We theorized that alveolar macrophages in COPD possess fundamental immune defects that permit NTHI to evade host responses.
Objective: To test this hypothesis, we obtained human alveolar and blood macrophages from exsmokers with COPD, exsmokers without COPD, and nonsmokers.
Methods: Alveolar and blood macrophages from each donor were incubated with purified LOS and OMP P6 and with OMP P2 and the total outer membrane preparation (0.1–1 μg/ml).
Measurements: Supernatants (24 h) were assayed for IL-1β, TNF-α, IL-10, IL-12, and IL-8 by multianalyte multiplexed flow cytometry.
Results: Comparative induction of COPD and non-COPD alveolar macrophages by LOS and OMP P6 revealed diminished IL-8, TNF-α, and IL-1β responses of COPD alveolar macrophages (p ⩽ 0.03 for each). COPD alveolar macrophages also had diminished responses to total outer membrane (p ⩽ 0.03 for each). In contrast, COPD blood macrophages had no significant differences among donor groups in IL-8, TNF-α, or IL-1β responsiveness to NTHI antigens. Diminished IL-12 responses of COPD blood macrophages to NTHI antigens, compared with nonsmokers, could not be independently dissociated from group differences in age and pack-years.
Conclusions: These findings support a paradigm of defective immune responsiveness of alveolar macrophages, but not blood macrophages, in COPD.
Keywords: chronic obstructive pulmonary disease; macrophage, alveolar; nontypeable Haemophilus influenzae; phagocytosis
Nontypeable Haemophilus influenzae (NTHI) are a major cause of respiratory infections with proclivity for people with chronic obstructive pulmonary disease (COPD) (1, 2). Not only are NTHI the most common pathogenic bacteria isolated from the airways of patients with COPD in stable and exacerbated states, but NTHI interactions with macrophages are implicated in the pathogenesis of COPD (3).
Bacterial colonization is associated with advanced airway inflammation, increased frequency of exacerbations, and accelerated decline in lung function, which suggests that bacterial colonization of the lower airways in COPD induces chronic airway inflammation and contributes to progressive airway obstruction (4–7). Once initiated, chronic inflammation of the lower airways may promote the progression of COPD (3). Although increasing evidence supports a key role for interactions of macrophages and NTHI in chronic lung pathology, the precise nature of the interaction between alveolar macrophages and NTHI has not been defined (8, 9). Although NTHI isolates may persist and remain viable intracellularly after phagocytosis by macrophages, failure to induce a strong proinflammatory response from alveolar macrophages would promote survival of pathogens, potentially providing a source for ongoing inflammatory stimuli for nonmacrophage immune mediators. Findings of previous studies have relied upon nonalveolar and nonhuman macrophages, and the significance of some findings is obscured by different responses of alveolar macrophages compared with blood macrophages. For example, studies have shown alveolar macrophages to be selectively up-regulated by cytokines and LPS compared with same-donor blood monocytes (10, 11). However, the role of antigens of respiratory bacteria on this process has not been explored.
As the resident mononuclear phagocytes of the lung, alveolar macrophages function as key defenses against inhaled pathogens (12, 13). Phagocytosis of NTHI by alveolar macrophages of smokers has been accompanied by increased bactericidal activity compared with alveolar macrophages of nonsmokers (14). However, inclusion of active smokers adds a potentially confounding variable to the modulation of macrophage function.
We previously found outer membrane protein (OMP) P6, a highly conserved 16-kD lipoprotein of NTHI, to be a potent and selective immune regulator of human macrophages, retaining proinflammatory properties at far lower concentrations than NTHI lipooligosaccharide (LOS). OMP P6 stimulation of macrophages was predominantly mediated through induction of TNF-α, IL-8, and IL-10 (15). However, in related studies, T-lymphocyte proliferative responses to OMP P6 were diminished among donors who had exacerbations of COPD (16). Although findings of both studies are limited to in vitro responses of human peripheral blood cells, the latter study identifies impaired immunologic responses in select individuals with COPD. Furthermore, no studies have examined the comparative responses of alveolar macrophages and extrapulmonary macrophages to antigens of respiratory bacteria.
Although interactions between human macrophages and NTHI are critical to the pathogenesis of lung diseases, including COPD, failure to evoke a strong proinflammatory response from alveolar macrophages might impair host clearance of respiratory pathogens and perpetuate colonization. We theorized that distinct immunologic responses of alveolar macrophages in COPD, not mediated by active smoking, permit strains of NTHI to avoid triggering proinflammatory responses in alveolar macrophages, contributing to disease in COPD. We further posited that alveolar and blood macrophages of the same individual have distinct responses to NTHI antigens and performed experiments to test these hypotheses. Some results of this study have been previously reported in abstract form (17, 18).
METHODS
Purification of Outer Membrane Components
Preparations of total outer membrane (OM), LOS, OMP P2, and OMP P6 were purified from NTHI1479 and quantitated as previously detailed (15). Each constituent's purity was confirmed by SDS-PAGE, silver stain, and Limulus amebocyte lysate assay (Associates of Cape Cod Inc., East Falmouth, MA) (15).
OMP P2 and P6 Quantitation
To determine the concentrations of OMPs P6 and P2 in clinical samples, densitometric quantitation was performed. A total protein extract was prepared from NTHI1479 (19). Fractions (0.02–0.06%), extracted from 2.5–7.5 × 105 bacteria, were visualized on SDS-PAGE (15). A standard protein curve (0.5–10 μg) was constructed for two-dimensional analytical scanning of gels (Molecular Dynamics/Amersham, Sunnyvale, CA). Densitometric volumes of OMPs P2 and P6 were quantitated in relation to standard curve values (20). Several measurements were averaged for each OMP and corrected for numbers of bacteria from which each was extracted.
Recruitment of Subjects
All study procedures received prior Institutional Review Board approval. After giving informed consent, volunteers were screened for inclusion into one of three groups: Group 1, exsmokers with COPD; Group 2, exsmokers without COPD; and Group 3, nonsmokers. All subjects underwent clinical assessment, routine spirometry, and chest X-rays. Strict inclusion and exclusion criteria are given in Figure E1 in the online supplement.
Purification of Human Blood Monocyte–Derived Macrophages
Whole blood was obtained from each participant. Blood mononuclear cells were purified by density centrifugation and seeded (106 cells/well) onto 96-well Linbro tissue culture plates (Hampton Research, Aliso Viejo, CA) as previously detailed (21). After incubation (7–10 d), nonadherent cells were removed. Percent viability of blood macrophages (mean ± SEM), by trypan blue exclusion, for Groups 1, 2, and 3 was 93.3 ± 0.58, 94.5 ± 0.85, and 95.1 ± 1.5, respectively (p > 0.1).
Purification of Human Alveolar Macrophages
All participants underwent bronchoalveolar lavage (BAL) to obtain alveolar macrophages within 72 h of blood monocyte purification. Details are given in Figure E2. Human alveolar macrophages were purified from BAL suspensions by centrifugation and seeded onto 96-well tissue culture plates (105 cells/ml). After incubation (24 h), nonadherent cells were removed. Remaining alveolar macrophages were consistently 98–100% esterase positive (15, 21). Percent viability of alveolar macrophages (mean ± SEM), by trypan blue exclusion, was 77.3 ± 2.5, 82.5 ± 2.9, and 85.1 ± 1.8 for Groups 1, 2, and 3, respectively (p > 0.1).
Macrophage-NTHI Antigen Incubation
Adherent human alveolar and blood macrophages were incubated with each NTHI outer membrane constituent (0.1–10 μg/ml; 8–48 h) as previously described (15). All experiments included control wells treated with Escherichia coli K235 LPS (1 μg/ml) (Sigma Chemical Co., St. Louis, MO) or with buffer diluents of each antigen. Details are given in Figure E3.
Cytokine Analysis
Cytokine concentrations were determined by multianalyte microsphere assay and analyzed by flow cytometry (22). Samples were measured in duplicate; blank values were subtracted from all readings. Final concentrations were corrected for interexperiment differences in macrophage numbers. Details, including analysis of cytokine data, are given in Figure E4.
Statistical Analysis
Demographic data were normally distributed and were analyzed with ANOVA with Fisher's test for multiple comparisons. Demographic data are expressed as means ± SEM (Table 1). Induced cytokine data among groups were not normally distributed and are reported as median (interquartile range [IQR]) as determined by the Kruskal-Wallis and Mann-Whitney U rank tests. For all comparisons, p < 0.05 was considered significant. To confirm whether age and cumulative smoking were independently related to macrophage cytokine output, measurements were determined by multivariate regression analysis after adjustment for lung function (FEV1, % predicted).
TABLE 1.
DEMOGRAPHIC CHARACTERISTICS OF PARTICIPANTS
| Characteristics | Group 1* (n = 22) | Group 2 (n = 15) | Group 3 (n = 9) | p Value |
|---|---|---|---|---|
| Age, yr | 65.3 ± 1.7† | 54.9 ± 2.5 | 43.3 ± 1.7 | < 0.01‡§‖ |
| Sex, M/F | 17/5 | 9/6 | 5/4 | |
| Pack-years smoking | 68.9 ± 6.9 | 32.7 ± 2.2 | 0.3 ± 0.3 | < 0.0001‡§‖ |
| FEV1, L | 1.83 ± 0.13 | 2.66 ± 0.10 | 3.13 ± 0.28 | < 0.0001‡§ |
| FEV1, % predicted | 58.55 ± 4.44 | 88.47 ± 3.42 | 99.22 ± 11.87 | < 0.0001‡§ |
| FVC, L | 3.35 ± 0.19 | 3.50 ± 0.13 | 3.92 ± 0.33 | 0.21 |
| FVC, % predicted | 84.68 ± 4.43 | 94.67 ± 3.69 | 104.0 ± 6.55 | 0.03¶ |
| FEV1/FVC | 53.91 ± 2.49 | 76.07 ± 1.26 | 79.77 ± 1.554 | < 0.0001‡§ |
Group 1, chronic obstructive pulmonary disease; Group 2, exsmokers; Group 3, nonsmokers.
Values are given as mean ± SEM unless otherwise noted.
p < 0.005, Group 1 versus Group 2.
p < 0.005, Group 1 versus Group 3.
p < 0.005, Group 2 versus Group 3.
p < 0.05, Group 1 versus Group 3.
Data analysis is by ANOVA and by post hoc analysis with Fisher's protected least significant difference.
RESULTS
Recruitment of Participants
Recruitment efforts yielded 70 volunteers. Of these, 20 were eliminated: six were of excessively high risk, six later decided against participation, and eight did not meet entry criteria. Of the 50 qualified participants recruited, 48 underwent successful bronchoscopies. One bronchoscopy was terminated prematurely because of transient laryngospasm; another was terminated because the subject had purulent endobronchial exudates and had active symptoms of bronchitis that he had failed to inform us of before bronchoscopy. Both were nonsmokers (Group 3). Forty-six bronchoscopies yielded adequate alveolar macrophages to perform the required experiments.
In total, 34 men and 16 women were recruited. The 46 subjects whose data were evaluable for this study included 30 men and 16 women. Group 1 (referred to as “COPD”) included 22 participants; Group 2 (referred to as “exsmokers”) included 15 participants; Group 3 (“nonsmokers”) included nine participants. Demographic data are presented in Table 1. The subjects in Group 1 had significantly worse spirometric parameters (FEV1 and FEV1, % predicted) than Groups 2 and 3. Subjects with COPD were also older than control subjects and had more cumulative tobacco exposure (Table 1). Exsmokers without COPD (Group 2) also differed from nonsmoking healthy control subjects in their cumulative tobacco smoke exposure and age. Multivariate regression analyses were used to determine the independent impact of group disparities in FEV1, cumulative pack-years, and age on immunologic outcomes.
No participants were taking systemic steroids or antibiotics. Fifteen of the 22 members of Group 1 were taking bronchoactive medications, including inhaled corticosteroids (n = 6), β agonists (n = 15), anticholinergic medications (n = 12), and theophylline preparations (n = 3). No members of Groups 2 or 3 were taking any medications of these classes.
Quantitation of OMPs P2 and P6 in Clinical Samples
To determine the concentrations of OMPs P6 and P2 in clinical respiratory secretions, a standard curve was constructed. Whole bacterial lysate fractions (0.02–0.06%) extracted from 2.5 × 105 and 7.5 × 105 bacteria were visualized on SDS-PAGE (15). Densitometric quantitation was performed in relation to known standards and revealed 95.2 ± 17.7 μg of OMP P2 and 4.09 ± 0.37 μg of OMP P6 per 107 bacteria. In related studies, we have quantified NTHI in the sputum of patients with COPD and found a median value of 107 (interquartile range 102) NTHI/ml for 535 separate clinical isolates (unpublished data). Therefore, our SDS-PAGE quantities represent sputum concentrations of OMPs P2 and P6 in COPD of 95.2 ± 17.7 μg/ml (2.3 ± 0.4 μM) and 4.09 ± 0.37 μg/ml (0.26 ± 0.02 μM), respectively. This range was used to choose concentrations of both OMPs for the current study that are within the range found in clinical COPD samples when NTHI is present.
TNF-α Induction
To investigate the relative immunologic responsiveness of alveolar macrophages of each group to NTHI antigens, alveolar macrophages were incubated with each NTHI constituent (0.1 μg/ml for 24 h), and supernatants were assayed for TNF-α (Figure 1). Median baseline values (buffer-treated cells) for TNF-α were 0 (IQR, 6.3), 0.3 (IQR, 2.9), and 4.0 (IQR, 4.6) (p > 0.05) and for LPS-treated controls (1 μg/ml) were 807.0 (IQR, 939.0), 433.3 (IQR, 833.0), and 613.1 (IQR, 1,638.4) (p > 0.1) for Groups 1, 2, and 3, respectively.
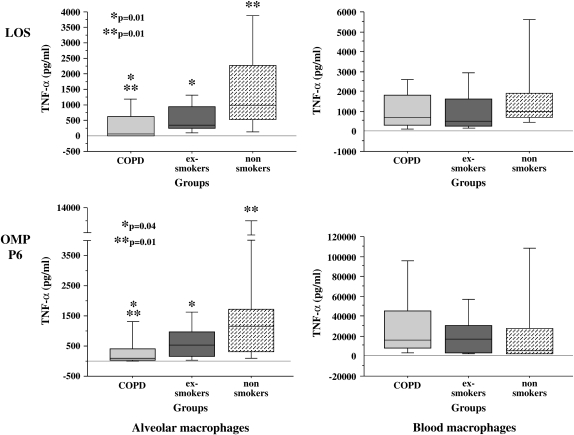
Figure 1.
Lipooligosaccharide (LOS) and outer membrane protein (OMP) P6 induction of human alveolar and blood macrophage TNF-α. Alveolar (left column) and blood macrophages (right column) were obtained from former smokers with chronic obstructive pulmonary disease (COPD) (Group 1, light gray shading), former smokers without COPD (Group 2, dark gray shading), and nonsmokers (Group 3, stippled shading). Cells were incubated with LOS (top graphs) and OMP P6 (lower graphs) each at 0.1 μg/ml. Supernatant TNF-α concentration was measured at 24 h. Results are shown as box plots for each group. Each box encompasses the 25th to 75th interquartile range, with the horizontal line in each box representing median values. Each vertical bar encompasses the 10th to 90th percentile ranges. Values correspond with data given in Table 2.
The greatest overall difference between groups was between alveolar macrophages of exsmokers with COPD and exsmokers without COPD (Figure 1 and Table 2). LOS induction of TNF-α for the COPD group was significantly diminished compared with alveolar macrophages of the exsmoker group (p = 0.01) and compared with nonsmoker alveolar macrophages (p = 0.01). TNF-α induction by OMP P6 was also reduced in alveolar macrophages of the COPD group compared with the exsmoker group (p = 0.04) and the nonsmoker group (p = 0.01) (Figure 1). A less pronounced reduction in TNF-α level occurred with alveolar macrophages of the COPD and nonsmoker groups incubated with total OMs (Table 2). TNF-α elicited with OMP P2 was relatively low for alveolar macrophages of all groups, as previously reported (15). In contrast, blood macrophages of all three groups exhibited no statistically significant differences in TNF-α induction by OMP P6, LOS, OMP P2, or the total OMs (p > 0.2 for each) (Figure 1 and Table 2).
TABLE 2.
NONTYPEABLE Haemophilus influenzae ANTIGEN INDUCTION OF HUMAN ALVEOLAR AND BLOOD MACROPHAGE TNF-α*
| LOS | P6 | P2 | Total OM | |
|---|---|---|---|---|
| Alveolar macrophages | ||||
| Group 1† | 71.5 (619.6)‡§ | 107.0 (367.0)‡§ | 3.3 (10.0) | 32.3 (34.4)§ |
| Group 2 | 332.4 (688.5) | 546.0 (826.4) | 5.5 (3.5) | 123.5 (231.1) |
| Group 3 | 990.9 (1728.5) | 1,147.5 (1,406.1) | 10.4 (17.9) | 657.6 (1,070.5) |
| Kruskal-Wallis p value | 0.006 | 0.028 | 0.35 | 0.039 |
| Blood macrophages | ||||
| Group 1 | 694.0 (1,539.0) | 956.9 (1,514.5) | 16.0 (116.3) | 918.8 (1,811.8) |
| Group 2 | 493.6 (1,357.8) | 777.5 (824.3) | 36.3 (97.2) | 634.3 (1,320.6) |
| Group 3 | 989.7 (1,209.3) | 1,070.3 (1,379.8) | 54.0 (179.1) | 1,028.5 (876.2) |
| Kruskal-Wallis p value | 0.26 | 0.35 | 0.33 | 0.59 |
Definition of abbreviations: LOS = lipooligosaccharide; OM = outer membrane.
Alveolar and blood macrophages of each donor were treated with 0.1 μg/ml of each nontypeable H. influenzae antigen. Values correspond with data in Figure 2. Each value is expressed as median pg/ml (interquartile range).
Group 1, chronic obstructive pulmonary disease; Group 2, exsmokers; Group 3, nonsmokers.
p < 0.05, Group 1 versus Group 2 by Mann Whitney U rank test.
¶p < 0.05, Group 1 versus Group 3 by Mann Whitney U rank test.
IL-8 Induction
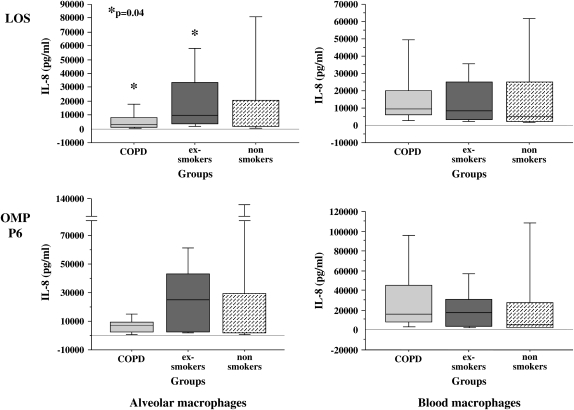
To determine if the relative immunologic hyporesponsiveness of alveolar macrophages of exsmokers with COPD included induction of chemokines, supernatants of alveolar macrophages, incubated with each NTHI constituent (0.1 μg/ml for 24 h), were assayed for IL-8 (Figure 2 and Table 3). Median baseline values (buffer-treated cells) for IL-8 were 1,147.8 (IQR, 2,650.0), 718.4 (IQR, 1,388.6), and 1,421.3 (IQR, 604.2) (p > 0.1) and for LPS-treated control subjects were 22,021 (IQR, 41,463), 33,739 (IQR, 32,691), and 3,565 (IQR, 49,034) (p > 0.1) for Groups 1, 2, and 3, respectively.
Figure 2.
LOS and OMP P6 induction of human alveolar and blood macrophage IL-8. Alveolar (left column) and blood macrophages (right column) were obtained from former smokers with COPD (Group 1, light gray shading), former smokers without COPD (Group 2, dark gray shading), and nonsmokers (Group 3, stippled shading). Supernatant concentrations for TNF-α, elicited by LOS (top graphs) and OMP P6 (lower graphs) at 0.1 μg/ml, are as detailed in Figure 1. Data are represented by box plots for each group as detailed in Figure 1. Values correspond with data given in Table 3.
TABLE 3.
NONTYPEABLE Haemophilus influenzae ANTIGEN INDUCTION OF HUMAN ALVEOLAR AND BLOOD MACROPHAGE IL-8*
| LOS | P6 | p2 | Total OM | |
|---|---|---|---|---|
| Alveolar macrophages | ||||
| Group 1† | 3271.9 (6893.1)‡ | 6,781.9 (7,286.6) | 857.7 (2,105.7) | 2,268.6 (5,594.1) |
| Group 2 | 9229.8 (30057.6) | 24,832.6 (40,906.2) | 1,851.8 (1,187.9) | 7,126.9 (8,754.1) |
| Group 3 | 2028.5 (18749) | 2,184.5 (27,430.6) | 1,738.6 (1,351.1) | 2,334.7 (3,730) |
| Kruskal Wallis p value | 0.029 | 0.3 | 0.76 | 0.22 |
| Blood macrophages | ||||
| Group 1 | 9,685.3 (13,500.2) | 16,424.2 (37,274.9) | 2,415.3 (2,987.8) | 10,339.1 (21,597.6) |
| Group 2 | 8,354.7 (21,933.6) | 16,469.4 (27,022.6) | 2,347.2 (9,513.4) | 4,924.9 (19,710.1) |
| Group 3 | 5,098.7 (22,403.3) | 4,897.2 (25,556.2) | 2,085.0 (4,119.7) | 4,619.7 (5,122.6) |
| Kruskal Wallis p value | 0.65 | 0.25 | 0.85 | 0.13 |
Definition of abbreviations: LOS = lipooligosaccharide; OM = outer membrane.
Alveolar and blood macrophages of each donor were treated with 0.1 μg/ml of each nontypeable H. influenzae antigen. Values correspond with data in Figure 1. Each value is expressed as median pg/ml (interquartile range).
Group 1, chronic obstructive pulmonary disease; Group 2, exsmokers; Group 3, nonsmokers.
p < 0.05, Group 1 versus Group 2 by Mann Whitney U rank test.
NTHI LOS induction of IL-8 for the COPD group was reduced compared with LOS induction of IL-8 for alveolar macrophages of exsmokers (p = 0.04) (Figure 2). OMP P6 (0.1 μg/ml) induction of IL-8 was not statistically different between the COPD and exsmoker groups. No statistical differences in IL-8 induction with the total OM preparation could be detected among groups. As in our previous experience with blood macrophages, OMP P2 purified from NTHI 1,479 was not as potent at IL-8 induction as was the same concentration of OMP P6 or LOS (15).
As with TNF-α induction, blood macrophages of all three groups exhibited no statistically significant differences in IL-8 induction by OMP P6, LOS, OMP P2, or the total OM preparation (p > 0.1 for each) (Figure 2 and Table 3). Thus, IL-8 induction by all NTHI antigens revealed a striking reduction between LOS responsiveness of alveolar macrophages of exsmokers with COPD and those of exsmokers without COPD that was not a feature of blood macrophages.
IL-1β Induction
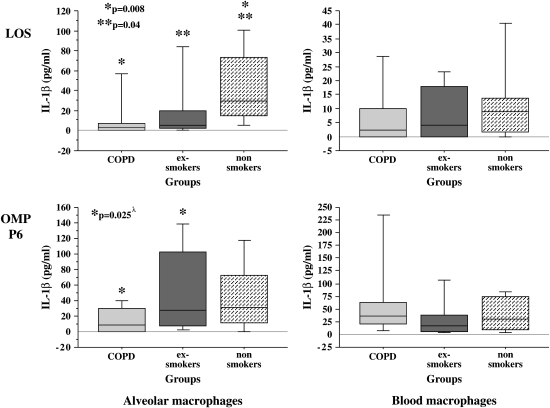
To determine if the relative immunologic hyporesponsiveness of alveolar macrophages of exsmokers with COPD included additional proinflammatory mediators, supernatants of alveolar macrophages, incubated with each NTHI constituent (24 h), were assayed for IL-1β (Figure 3). Median baseline values (buffer-treated cells) for IL-1β were 0 (IQR, 1.3), 0 (IQR, 0), and 0 (IQR, 1.1) (p > 0.05) and for LPS-treated control subjects were 19.1 (IQR, 9.1), 22.4 (IQR, 24.3), and 25.3 (IQR, 102.0) (p > 0.1) for Groups 1, 2, and 3, respectively. The optimal concentration of NTHI antigens for IL-1β response was determined to be 1 μg/ml. Therefore, results are presented for IL-1β elicited with 1 μg/ml of each NTHI constituent.
Figure 3.
LOS and OMP P6 induction of human alveolar and blood macrophage IL-1β. Alveolar (left column) and blood macrophages (right column) were obtained from former smokers with COPD (Group 1), former smokers without COPD (Group 2), and nonsmokers (Group 3). Shading denoting each individual group and supernatant concentrations for IL-1β elicited by OMP P6 (top graphs) and LOS (lower graphs) at 1 μg/ml are as detailed in Figure 1. Data are represented by box plots for each group as detailed in Figure 1. Values correspond with data given in Table 4. λ Multivariate regression analysis showed age as an independent determinant of OMP P6 induction of IL-1β from alveolar macrophages.
Overall results show a significant reduction of induced IL-1β of alveolar macrophages of the COPD group. LOS induction of IL-1β was diminished in alveolar macrophages of the COPD group compared with those of the nonsmoker group (p = 0.008) and of the exsmoker group (p = 0.04) (Figure 3 and Table 4). OMP P6 induction of IL-1β for COPD alveolar macrophages was also reduced compared with alveolar macrophages of the exsmoker group (p = 0.025) (Figure 3). Although multivariate regression analysis showed age to be an independent predictor of OMP P6 induction of IL-1β (see below), no other demographic features were independent predictors of any other alveolar macrophage cytokine responses. Diminished IL-1β induction also occurred with alveolar macrophages of each group incubated with the total OM preparation (Table 4). Although statistical differences are present between groups for alveolar macrophages incubated with OMP P2, overall IL-1β values are relatively low in all groups (16).
TABLE 4.
NONTYPEABLE Haemophilus influenzae ANTIGEN INDUCTION OF HUMAN ALVEOLAR AND BLOOD MACROPHAGE IL-1β*
| LOS | P6 | P2 | Total OM | |
|---|---|---|---|---|
| Alveolar macrophages | ||||
| Group 1† | 3.0 (6.9)‡ | 9.0 (29.7)‖ | 0.0 (2.3) | 2.6 (14.6)‡‖ |
| Group 2 | 5.2 (17.3)§ | 27.1 (94.8) | 0.0 (0.0) | 14.5 (31.5)§ |
| Group 3 | 29.7 (58.1) | 30.7 (61.0) | 3.7 (5.1) | 87.4 (105.4) |
| Kruskal Wallis p value | 0.02 | 0.04†† | —¶ | 0.001 |
| Blood macrophages | ||||
| Group 1 | 2.4 (9.8) | 35.8 (42.3) | 0.0 (4.4) | 4.2 (31.1) |
| Group 2 | 4.0 (17.8) | 17.1 (31.9) | 3.5 (13.8) | 11.0 (19.0) |
| Group 3 | 8.9 (12.3) | 29.7 (64.3) | 4.1 (9.7) | 19.4 (30.4) |
| Kruskal Wallis p value | 0.42 | 0.21 | 0.15 | 0.20 |
Definition of abbreviations: LOS = lipooligosaccharide; OM = outer membrane.
Alveolar and blood macrophages of each donor were treated with 1 μg/ml of each nontypeable H. influenzae antigen. Values correspond with data in Figure 3. Each value is expressed as median pg/ml (interquartile range).
Group 1, chronic obstructive pulmonary disease; Group 2, exsmokers; Group 3, nonsmokers.
p < 0.05, Group 1 versus Group 3 by Mann Whitney U rank test.
p < 0.05, Group 2 versus Group 3 by Mann Whitney U rank test.
p < 0.05, Group 1 versus Group 2 by Mann Whitney U rank test.
Age was an independent determinant of OM protein P6 induction of IL-1β (p = 0.04).
**p < 0.05, but values were considered too low for biological significance.
As with IL-8 and TNF-α induction, blood macrophages of all three groups exhibited no statistically significant differences in IL-1β induction by OMP P6, by LOS, or by the total OM preparation (p ⩾ 0.2 for each) (Figure 3). In summary, IL-1β induction by all NTHI antigens revealed diminished responsiveness of alveolar macrophages of donors with COPD compared with exsmokers without COPD in response to NTHI antigens.
IL-12 Induction
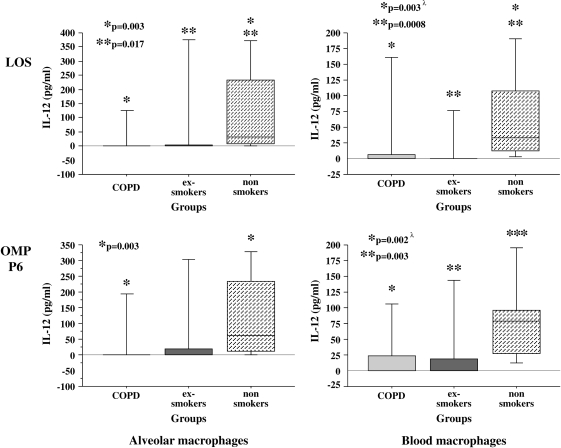
To further explore the relative immunologic hyporesponsiveness of alveolar macrophages of exsmokers with COPD, supernatants of alveolar macrophages were assayed for IL-12 (Figure 4). Median baseline values (buffer-treated cells) for IL-12 were 0 (IQR, 0), 0 (IQR, 0), and 0 (IQR, 2.3) (p > 0.1) and for LPS-treated control subjects were 4.7 (IQR, 62.5), 0 (IQR, 127.9), and 54.5 (IQR, 184.5) (p > 0.1) for Groups 1, 2, and 3, respectively.
Figure 4.
LOS and OMP P6 induction of human alveolar and blood macrophage IL-12. Alveolar (left column) and blood macrophages (right column) were obtained from former smokers with COPD (Group 1), former smokers without COPD (Group 2), and nonsmokers (Group 3). Shading denoting each individual group and supernatant concentrations for IL-12 elicited by LOS (top graphs) and OMP P6 (lower graphs) at 0.1 μg/ml are as detailed in Figure 1. Data are represented by box plots for each group as detailed in Figure 1. Values correspond with data given in Table 5. λ Multivariate regression analysis showed cumulative pack-years to be an independent determinant of LOS induction of IL-12 and age as an independent determinant of OMP P6 induction of IL-12 from blood macrophages only. No demographic variables were independent determinants for alveolar macrophage IL-12 responses.
IL-12 induction by all NTHI antigens predominantly revealed a reduced response of alveolar macrophages of subjects with COPD and those of exsmokers compared with nonsmokers (Figure 4 and Table 5). Diminished IL-12 responses occurred in alveolar macrophages of the COPD and exsmoker groups (Figure 4). Alveolar macrophages of the exsmoker group also had a diminished response to LOS and OMP P6 compared with alveolar macrophages of nonsmokers (Figure 4). Diminished IL-12 responses also occurred with alveolar macrophages of the COPD and exsmoker groups to the total OM preparation (Table 5). In contrast to results with TNF-α, IL-8, and IL-1β, which showed unimpaired responsiveness of COPD blood macrophages, IL-12 induction of blood macrophages was reduced in the COPD and exsmoker groups compared with the nonsmoker group. Multivariate regression analyses of showed cumulative pack-years and age to be independent determinants of blood macrophage IL-12 responses (see below). Age and cumulative pack-years were not independent predictors of any alveolar macrophage IL-12 responses.
TABLE 5.
NONTYPEABLE Haemophilus influenzae ANTIGEN INDUCTION OF HUMAN ALVEOLAR AND BLOOD MACROPHAGE IL-12
| LOS | P6 | P2 | Total OM | |
|---|---|---|---|---|
| Alveolar macrophages | ||||
| Group 1† | 0.0 (0.0)‡ | 0.0 (0.0)‡ | 0.0 (0.0) | 0.0 (0.8)‡ |
| Group 2 | 0.0 (5.6)§ | 0.0 (18.7) | 0.0 (0.0) | 0.0 (4.9)§ |
| Group 3 | 32.9 (226.0) | 63.7 (219.9) | 0.0 (33.9) | 23.3 (145.3) |
| Kruskal Wallis p value | 0.005 | 0.017 | 0.14 | 0.042 |
| Blood macrophages | ||||
| Group 1 | 0.0 (6.4)‡ | 0.0 (22.7)‡ | 0.0 (0.0) | 0.0 (14.8)‡ |
| Group 2 | 0.0 (0.0)§ | 0.0 (18.5)§ | 0.0 (0.0) | 0.0 (7.6)§ |
| Group 3 | 32.5 (94.9) | 78.8 (69.2) | 8.6 (42.8) | 53.6 (116.8) |
| Kruskal Wallis p value | 0.009‖¶ | 0.003¶ | —** | 0.01‖ |
Definition of abbreviations: LOS = lipooligosaccharide; OM = outer membrane.
*Alveolar and blood macrophages of each donor were treated with 0.1 μg/ml of each nontypeable H. influenzae antigen. Values correspond with data in Figure 4. Each value is expressed as median pg/ml (interquartile range).
Group 1, chronic obstructive pulmonary disease; Group 2, exsmokers; Group 3, nonsmokers.
p < 0.05, Group 1 versus Group 3 by Mann Whitney U rank test.
p < 0.05, Group 2 versus Group 3 by Mann Whitney U rank test.
Cumulative pack-years was an independent determinant of total OM induction of IL-12 (p = 0.04) and LOS induction of IL-12 (p = 0.01) for blood macrophages.
Age was an independent determinant of OMP P6 induction of IL-12 (p = 0.04) and of total OM induction of IL-12 (p = 0.03).
p < 0.05, but values were too low for biological significance.
IL-10 Induction
To further determine if the relative immunologic hyporesponsiveness of alveolar macrophages of exsmokers with COPD applied to down-regulatory cytokines, supernatants of alveolar macrophages incubated with each NTHI constituent (1 μg/ml for 24 h) were assayed for IL-10 (Figure 5). Median baseline values (buffer-treated cells) for IL-10 were 0 (IQR, 0), 0 (IQR, 0), and 0 (IQR, 0) (p > 0.1) and for LPS-treated control subjects were 12.1 (IQR, 11.1), 9.8 (IQR, 15.4), and 6.8 (IQR, 63.7) (p > 0.1) for Groups 1, 2, and 3, respectively. The optimal concentration of NTHI antigens for IL-10 response was determined to be 1 μg/ml. Therefore, results are presented for IL-10 elicited with 1 μg/ml of each NTHI constituent.
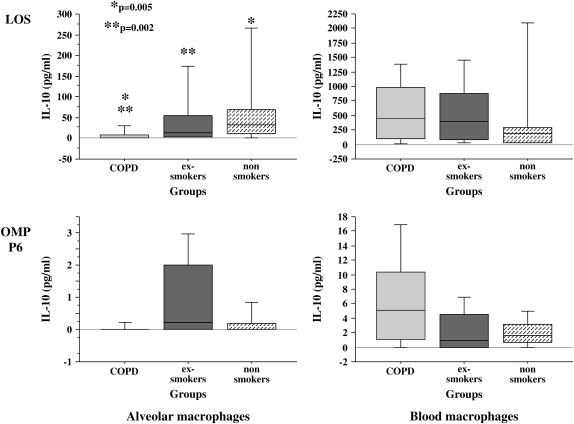
Figure 5.
LOS and OMP P6 induction of human alveolar and blood macrophage IL-10. Alveolar (left column) and blood macrophages (right column) were obtained from former smokers with COPD (Group 1), former smokers without COPD (Group 2), and nonsmokers (Group 3). Shading denoting each individual group and supernatant concentrations for IL-10 elicited by LOS (top graphs) and OMP P6 (lower graphs) at 1 μg/ml are as detailed in Figure 1. Data are represented by box plots for each group as detailed in Figure 1. Values correspond with data given in Table 6.
OMP P6 was not a potent inducer of IL-10 from alveolar or blood macrophages of any group. However, with LOS, a significant impairment of IL-10 induction occurred for alveolar macrophages of the COPD group compared with the exsmoker and the nonsmoker groups (p = 0.002) (Figure 5 and Table 6). OMP P2 was not a potent stimulus for IL-10 from alveolar macrophages of any group. As with IL-8 and TNF-α induction, no significant differences between groups were elicited from blood macrophages treated with any NTHI antigens (Table 6).
TABLE 6.
NONTYPEABLE Haemophilus influenzae ANTIGEN INDUCTION OF HUMAN ALVEOLAR AND BLOOD MACROPHAGE IL-10*
| LOS | P6 | P2 | Total OM | |
|---|---|---|---|---|
| Alveolar macrophages | ||||
| Group 1† | 2.1 (8.7)‡§ | 0.0 (0.0) | 0.0 (0.0) | 0.0 (34.3)‡§ |
| Group 2 | 12.1 (51.4) | 0.2 (2.0) | 0.1 (2.6) | 26.4 (52.8) |
| Group 3 | 32.2 (58.1) | 0.0 (0.2) | 0.0 (0.7) | 81.0 (138.3) |
| Kruskal Wallis p value | 0.002 | —‖ | —‖ | 0.0009 |
| Blood macrophages | ||||
| Group 1 | 438.4 (887.7) | 5.0 (9.2) | 45.1 (65.6) | 859.0 (1,393.7) |
| Group 2 | 400.2 (796) | 1.0 (4.5) | 45.5 (167.7) | 470.4 (1,513.5) |
| Group 3 | 180.9 (257.9) | 1.7 (2.5) | 36.1 (79.6) | 453.4 (569.6) |
| Kruskal Wallis p value | 0.45 | 0.06 | 0.62 | 0.56 |
Definition of abbreviations: LOS = lipooligosaccharide; OM = outer membrane.
Alveolar and blood macrophages of each donor were treated with 1 μg/ml of each nontypeable H. influenzae antigen. Values correspond with data in Figure 5. Each value is expressed as median pg/ml (interquartile range).
Group 1, chronic obstructive pulmonary disease; Group 2, exsmokers; Group 3, nonsmokers.
p < 0.05, Group 1 versus Group 2 by Mann Whitney U rank test.
p < 0.05, Group 1 versus Group 3 by Mann Whitney U rank test.
p < 0.05, but values were considered too low for biological significance.
In summary, IL-10 induction by all NTHI antigens was reduced in alveolar macrophages of the COPD group compared with those of exsmokers without COPD and with nonsmokers, predominantly in response to LOS. No group differences in responsiveness of blood macrophages to any NTHI antigens were observed.
Multivariate Regression Analyses
Because demographic differences existed between groups (Table 1), multivariate regression analyses were performed to determine the independent relationship of age and cumulative smoking on cytokine induction after adjustment for lung function (FEV1, %predicted). For this analysis, p < 0.05 was considered significant. In all analyses of alveolar macrophages, cumulative pack-years was not an independent determinant for alveolar macrophage cytokine responses. Except for significant association of age with OMP P6 induction of IL-1β (p = 0.04), age was also not an independent predictor of alveolar macrophage cytokine responses.
Multivariate regression analyses of blood macrophage induction of IL-12 (Figure 4) showed cumulative pack-years to be an independent determinant of total OM induction of IL-12 (p = 0.04) and LOS induction of IL-12 (p = 0.01). Age was an independent determinant of OMP P6 induction of IL-12 (p = 0.04) and of total OM induction of IL-12 (p = 0.03). Therefore, age and cumulative smoking have minimal effects on alveolar macrophage results but significantly confound the results of group differences of blood macrophage IL-12 concentrations.
DISCUSSION
This study is the first to demonstrate a fundamental immunologic defect of alveolar macrophages in COPD and the first to investigate comparative immunologic responses of same-donor alveolar and blood macrophages. The presence of impaired responsiveness of alveolar macrophages, but not blood macrophages, in COPD donors suggests that different intracellular signaling pathways may be activated by the same antigens in macrophages of different compartments and highlights the need for using alveolar macrophages for study of interactions with respiratory pathogens. The only significant difference among groups in blood macrophage induction was for IL-12, and in each instance, multivariate regression analyses showed cumulative pack-years and age to be independent determinants of NTHI antigen induction. In contrast, aside from age being an independent predictor of OMP P6 induction of IL-1β of alveolar macrophages, no other demographic features were independent predictors of any other alveolar macrophage cytokine responses.
Alveolar macrophage function in COPD was comparatively diminished in response to different NTHI antigens in our study. For example, OMP P6 and LOS induction of IL-8 demonstrated impaired responsiveness of COPD alveolar macrophages. However, OMP P6 and LOS induction of TNF-α not only demonstrated a difference between COPD and exsmoker (non-COPD) alveolar macrophages, but also between COPD and nonsmoker alveolar macrophages. In both instances, exsmoker alveolar macrophages displayed immunologic properties that were more like alveolar macrophages of nonsmokers. The relatively weak overall responsiveness to OMP P2 is consistent with our previous studies and supports specificity of macrophage responses to different antigens (15). Although OMP P6 and LOS were potent cytokine inducers, alveolar macrophage responsiveness to LOS was a better discriminator between COPD and non-COPD alveolar macrophages than was responsiveness to OMP P6. Although alveolar macrophages of exsmokers displayed an immunologic profile that was more like that of nonsmokers than of COPD donors for TNF-α, IL-8, and IL-10, fine differences are seen for the induction of IL-12 and IL-1β, where alveolar macrophages of exsmokers without COPD had diminished responses compared with those of nonsmokers. Therefore, a less pronounced immunologic defect than the defect in COPD macrophages exists in alveolar macrophages of former smokers who have not progressed to COPD compared with nonsmokers.
Because NTHI is a strictly human pathogen, human macrophages were used as the model for these studies. Because NTHI is a predominant respiratory pathogen, alveolar macrophages provided clinically relevant target cells (23). We chose concentrations of NTHI outer membrane antigens that were clinically relevant to those of respiratory samples of COPD donors. OMP P6, a conserved lipoprotein expressed by NTHI in vivo, is a potent immunomodulator of human macrophages and a promising vaccine candidate (24, 25). OMP P2 is a well characterized porin protein and is the predominant OMP of NTHI (26, 27). Because endotoxins are potent modulators of macrophages, we purified the specific LOS of NTHI (28). Our total NTHI OM preparation contained not only LOS but also peptidoglycan and numerous immunoactive OM molecules. Thus, it was not surprising that the total OM preparation should globally stimulate macrophage cytokine output, further confirming dysfunction of alveolar macrophages in COPD.
NTHI is the most frequently recovered pathogen from sputum in stable and acutely exacerbated COPD and is associated with more frequent exacerbations and accelerated decline in lung function (4, 5, 29). The relationship of NTHI with progressive disease in COPD is underscored by an increased risk of exacerbation associated with the acquisition of new respiratory strains of NTHI (2). The vicious circle hypothesis has been proposed to explain the contribution of bacterial colonization of the lower airways to the pathogenesis of COPD (3). Once initiated, bacterial colonization may perpetuate inflammation of the lower airways, contributing to the progression of COPD (3). However, an accumulating body of evidence has identified impaired immunologic responsiveness in COPD. For example, proliferative T-lymphocyte responses to NTHI OMP P6 are diminished in exacerbations of COPD (16). Although findings are limited to in vitro study of peripheral blood cells, they highlight impaired immunologic responses in select individuals with COPD. Intrinsic dysfunction of alveolar macrophages in COPD is further exemplified by the diminished expression of histone deacetylase, a nuclear enzyme that regulates chromatin structure, in alveolar macrophages of patients with COPD compared with other subjects (30). Our current findings of impaired responsiveness of alveolar macrophages in COPD to immunoregulatory products of respiratory pathogens support a paradigm of impaired innate immune responses in COPD. Failure to evoke a strong innate immune response by alveolar macrophages in COPD would likely impair the clearance of bacterial pathogens, providing a source for ongoing induction of inflammation, consistent with the vicious cycle hypothesis. Our findings suggest a nonmacrophage source of ongoing inflammation.
Results of studies of immune dysregulation in COPD may be obscured by the use of murine and human nonalveolar macrophages, by the concurrent use of glucocorticoids, and by active smoking. For example, although peripheral monocytes of COPD donors had increased IL-6 and MCP-1 levels in response to exogenous LPS compared with controls, it is not known whether this also occurred in the airways and lungs (31). Although alveolar macrophages in mild and moderate COPD displayed increased expression of NF-κB and related signaling molecules and macrophages of induced sputum of COPD donors had diminished lipopolysaccharide induction of TNF-α, cell donors included active smokers, some of whom required glucocorticoids (32, 33). In fact, studies with TNF-α receptor–deficient mice support a role for TNF-α–mediated inflammation in the development of COPD, supporting the extension of studies to evaluate human immune cell function in COPD (34). Some alveolar macrophage dysfunction in COPD may be attributed to active smoking (14). The impact of cigarette smoke on increased induction of chemokines has been demonstrated in murine models and in the sputum of patients with COPD (35, 36). In fact, increased concentrations of IL-8 in BAL fluid has served as a distinguishing marker of COPD in active smokers (37). Our study omitted active smokers to remove this potentially confounding variable and confirms dysfunction of alveolar macrophages in COPD independent of active smoking.
There are limitations to our study resulting from the recruitment of volunteers for research that entails an invasive procedure and the elimination of active smokers. Limitations are reflected in the cross-sectional design, the moderate number of subjects in each group, and the differences in age and cumulative tobacco exposure between the groups. However, the absence of a statistically significant association between age and pack-years, with the group-related differences of biologically induced inflammatory mediators in alveolar macrophages, should allay concerns about these limitations.
Persistent exposure to bacterial antigens can regulate the expression of immunoregulatory surface molecules and result in relative tolerance of alveolar macrophages (38, 39). Numerous mechanisms may control tolerance in alveolar macrophages, including regulation of TLR and CD14 surface receptors (40–42). Down-regulatory cytokines, including elicited IL-10, may promote tolerance in some instances. However, our finding of impaired LOS induction of alveolar macrophage IL-10 in the COPD group does not support this as a mechanism for our model (43, 44). One study of macrophage tolerance to LPS in a murine model determined that cells of different compartments have different susceptibilities to tolerance (38). Bronchoalveolar cells were relatively resistant to LPS tolerance. Although these findings support our finding of compartmentalization of macrophage hyporesponsiveness, they may suggest further limitations to applying mechanisms derived from animal models to our current study.
Our discovery of similar patterns of impaired cytokine responsiveness in response to different purified antigens suggests that intrinsic alveolar macrophage impairment in COPD is not limited to a single intracellular signaling pathway. Exploring the underlying immunologic factors that regulate destructive processes and impaired responsiveness of macrophages in COPD will be a key direction of our future studies.
In summary, alveolar macrophages of exsmokers with COPD have a distinct immunologic profile of impaired responsiveness to NTHI antigens, which is not a feature of COPD blood macrophages and further distinguishes them from alveolar macrophages of exsmokers without COPD. These results support an immunologic basis for the persistence of NTHI in COPD.
Supplementary Material
Acknowledgments
The authors are grateful for scientific advice offered by Timothy F. Murphy, M.D., for advice on statistical analysis provided by Brydon J. Grant, M.D., and for the technical assistance of Jane M. Smigiera for these studies.
Supported by research grant 1RO1HL6654901 (C.S.B., S.S.) from the National Institutes of Health and by the Department of Veterans Affairs.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200509-1461OC on March 30, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Murphy TF, Apicella MA. Nontypeable Haemophilus influenzae: a review of clinical aspects, surface antigens, and the human immune response to infection. Rev Infect Dis 1987;9:1–15. [DOI] [PubMed] [Google Scholar]
- 2.Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med 2002;347:465–471. [DOI] [PubMed] [Google Scholar]
- 3.Sethi S, Murphy TF. Bacterial infection in chronic obstructive pulmonary disease in 2000: a state-of-the-art review. Clin Microbiol Rev 2001;14:336–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bresser P, Out TA, van Alphen L, Jansen HM, Lutter R. Airway inflammation in nonobstructive and obstructive chronic bronchitis with chronic Haemophilus influenzae airway infection: comparison with noninfected patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;162:947–952. [DOI] [PubMed] [Google Scholar]
- 5.Patel IS, Seemungal TA, Wilks M, Lloyd-Owen SJ, Donaldson GC, Wedzicha JA. Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax 2002;57:759–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill AT, Campbell EJ, Hill SL, Bayley DL, Stockley RA. Association between airway bacterial load and markers of airway inflammation in patients with stable chronic bronchitis. Am J Med 2000;109:288–295. [DOI] [PubMed] [Google Scholar]
- 7.Wilkinson TM, Patel IS, Wilks M, Donaldson GC, Wedzicha JA. Airway bacterial load and FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003;167:1090–1095. [DOI] [PubMed] [Google Scholar]
- 8.Noel GJ, Hoiseth SK, Edelson PJ. Type b capsule inhibits ingestion of Haemophilus influenzae by murine macrophages: studies with isogenic encapsulated and unencapsulated strains. J Infect Dis 1992;66:178–182. [DOI] [PubMed] [Google Scholar]
- 9.Craig JE, Cliffe A, Garnett K, High NJ. Survival of nontypeable Haemophilus influenzae in macrophages. FEMS Microbiol Lett 2001;203:5–61. [DOI] [PubMed] [Google Scholar]
- 10.Dorger M, Munzing S, Allmeling AM, Messmer K, Krombach F. Phenotypic and functional differences between rat alveolar, pleural, and peritoneal macrophages. Exp Lung Res 2001;27:65–76. [DOI] [PubMed] [Google Scholar]
- 11.Dinakar C, Malur A, Raychaudhuri B, Buhrow LT, Melton AL, Kavuru MS, Thomassen MJ. Differential regulation of human blood monocyte and alveolar macrophage inflammatory cytokine production by nitric oxide. Ann Allergy Asthma Immunol 1999;82:217–222. [DOI] [PubMed] [Google Scholar]
- 12.Hocking WG, Golde DW. The pulmonary-alveolar macrophage. N Engl J Med 1979;301:580–587. [DOI] [PubMed] [Google Scholar]
- 13.Fels AO, Cohn ZA. The alveolar macrophage. J Appl Physiol 1986;60:353–369. [DOI] [PubMed] [Google Scholar]
- 14.Jonsson S, Musher DM, Lawrence EC. Phagocytosis and killing of Haemophilus influenzae by alveolar macrophages: no difference between smokers and non-smokers. Eur J Respir Dis 1987;70:309–315. [PubMed] [Google Scholar]
- 15.Berenson CS, Murphy TF, Wrona CT, Sethi S. Outer membrane protein P6 of nontypeable Haemophilus influenzae is a potent and selective inducer of human macrophage proinflammatory cytokines. Infect Immun 2005;73:2728–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abe Y, Murphy TF, Sethi S, Faden HS, Dmochowski J, Harabuchi Y, Thanavala YM. Lymphocyte proliferative response to P6 of Haemophilus influenzae is associated with relative protection from exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002;165:967–971. [DOI] [PubMed] [Google Scholar]
- 17.Berenson CS, Wrona CT, Grove LJ, Maloney J, Sethi S. Nontypeable Haemophilus influenzae antigen regulation of human alveolar macrophage inflammation [abstract]. Am Soc Microbiol 2004;E-014.
- 18.Berenson CS, Garlipp MA, Wrona CT, Grove LJ, Maloney J, Sethi S. Impaired immune responsiveness of alveolar macrophages in COPD to antigens of nontypeable Haemophilus influenzae [abstract]. Am Soc Microbiol 2005;B-302.
- 19.Murphy TF, Dudas KC, Mylotte JM, Apicella MA. A subtyping system for nontypable Haemophilus influenzae based on outer-membrane proteins. J Infect Dis 1983;147:838–846. [DOI] [PubMed] [Google Scholar]
- 20.Fakih MF, Pattoli MA, Murphy TF, Berenson CS. Specific binding of Haemophilus influenzae to minor gangliosides of human respiratory epithelial cells. Infect Immun 1997;65:1695–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berenson CS, Gallery MA, Katari MS, Foster EW, Pattoli MA. Gangliosides of monocyte-derived macrophages of adults with advanced HIV infection show reduced surface accessibility. J Leukoc Biol 1998;64:11–321. [DOI] [PubMed] [Google Scholar]
- 22.Earley M, Vogt R, Shapiro H, Mandy FF, Keller K, Bellisario R, Pass KA, Marti GE, Stewart CC, Hannon WH. Report from a workshop on multianalyte microsphere assays. Cytometry 2002;50:239–242. [DOI] [PubMed] [Google Scholar]
- 23.Tetley TD. Macrophages and the pathogenesis of COPD. Chest 2002;121:156S–159S. [DOI] [PubMed] [Google Scholar]
- 24.Karalus RJ, Murphy TF. Purification and characterization of the outer membrane protein P6, a vaccine antigen of nontypeable Haemophilus influenzae. FEMS Immunol Med Microbiol 1999;26:159–166. [DOI] [PubMed] [Google Scholar]
- 25.Wu T, Chen J, Murphy TF, Green BA, Gu XX. Investigation of nontypeable Haemophilus influenzae outer membrane protein P6 as a new carrier for lipooligosaccharide conjugate vaccines. Vaccine 2005;23:5177–5185. [DOI] [PubMed] [Google Scholar]
- 26.Murphy TF, Bartos LC. Purification and analysis with monoclonal antibodies of P2, the major outer membrane protein of nontypable Haemophilus influenzae. Infect Immun 1988;56:1084–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hiltke TJ, Sethi S, Murphy TF. Sequence stability of the gene encoding outer membrane protein P2 of nontypeable Haemophilus influenzae in the human respiratory tract. J Infect Dis 2002;185:627–631. [DOI] [PubMed] [Google Scholar]
- 28.Campagnari AA, Gupta MR, Dudas KC, Murphy TF, Apicella MA. Antigenic diversity of lipooligosaccharides of nontypable Haemophilus influenzae. Infect Immun 1987;55:882–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sethi S. Etiology and management of infections in chronic obstructive pulmonary disease. Clin Pulm Med 1999;6:327–332. [Google Scholar]
- 30.Ito K, Ito M, Elliott WM, Cosio B, Caramori G, Kon OM, Barczyk A, Hayashi S, Adcock IM, Hogg JC, et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med 2005;352:1967–1976. [DOI] [PubMed] [Google Scholar]
- 31.Aldonyte R, Jansson L, Piitulainen E, Janciauskiene S. Circulating monocytes from healthy individuals and COPD patients. Respir Res 2003;4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Stefano A, Caramori G, Ricciardolo FL, Capelli A, Adcock IM, Donner CF. Cellular and molecular mechanisms in chronic obstructive pulmonary disease: an overview. Clin Exp Allergy 2004;34:1156–1167. [DOI] [PubMed] [Google Scholar]
- 33.Frankenberger M, Menzel M, Betz R, Kassner G, Weber N, Kohlhaufl M, Haussinger K, Ziegler-Heitbrock L. Characterization of a population of small macrophages in induced sputum of patients with chronic obstructive pulmonary disease and healthy volunteers. Clin Exp Immunol 2004;138:507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Churg A, Wang RD, Tai H, Wang X, Xie C, Wright JL. Tumor necrosis factor-alpha drives 70% of cigarette smoke-induced emphysema in the mouse. Am J Respir Crit Care Med 2004;170:492–498. [DOI] [PubMed] [Google Scholar]
- 35.Churg A, Wang RD, Tai H, Wang X, Xie C, Dai J, Shapiro SD, Wright JL. Macrophage metalloelastase mediates acute cigarette smoke-induced inflammation via tumor necrosis factor-alpha release. Am J Respir Crit Care Med 2003;167:1083–1089. [DOI] [PubMed] [Google Scholar]
- 36.Traves SL, Culpitt SV, Russell RE, Barnes PJ, Donnelly LE. Increased levels of the chemokines GROalpha and MCP-1 in sputum samples from patients with COPD. Thorax 2002;57:590–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanino M, Betsuyaku T, Takeyabu K, Tanino Y, Yamaguchi E, Miyamoto K, Nishimura M. Increased levels of interleukin-8 in BAL fluid from smokers susceptible to pulmonary emphysema. Thorax 2002;7:405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fitting C, Dhawan S, Cavaillon JM. Compartmentalization of tolerance to endotoxin. J Infect Dis 2004;189:1295–1303. [DOI] [PubMed] [Google Scholar]
- 39.Dobrovolskaia MA, Vogel SN. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect 2002;4:903–914. [DOI] [PubMed] [Google Scholar]
- 40.Wang JH, Doyle M, Manning BJ, Di Wu Q, Blankson S, Redmond HP. Induction of bacterial lipoprotein tolerance is associated with suppression of toll-like receptor 2 expression. J Biol Chem 2002;277:6068–36075. [DOI] [PubMed] [Google Scholar]
- 41.Oshikawa K, Sugiyama Y. Regulation of toll-like receptor 2 and 4 gene expression in murine alveolar macrophages. Exp Lung Res 2003;9:401–412. [DOI] [PubMed] [Google Scholar]
- 42.Lin SM, Frevert CW, Kajikawa O, Wurfel MM, Ballman K, Mongovin S, Wong VA, Selk A, Martin TR. Differential regulation of membrane CD14 expression and endotoxin-tolerance in alveolar macrophages. Am J Respir Cell Mol Biol 2004;31:162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macaubas C, DeKruyff RH, Umetsu DT. Respiratory tolerance in the protection against asthma. Curr Drug Targets Inflamm Allergy 2003;2:175–186. [DOI] [PubMed] [Google Scholar]
- 44.Redpath S, Ghazal P, Gascoigne NR. Hijacking and exploitation of IL-10 by intracellular pathogens. Trends Microbiol 2001;9:86–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.