Abstract
Rationale: Bronchopulmonary dysplasia (BPD), a chronic lung disease of newborns triggered by oxygen and barotrauma, is characterized by arrested alveolarization. Increased levels of bombesin-like peptides shortly after birth mediate lung injury: anti-bombesin antibody 2A11 protects against BPD in two baboon models. The role of adaptive immunity in BPD has not been explored previously.
Objectives: Our goal was to test the hypothesis that thymic architecture and/or T-cell function is altered with BPD, leading to autoimmunity and immunodeficiency.
Methods: Thymic structure was analyzed by histopathology of thymic architecture and immunohistochemistry for thymic maturation markers (terminal deoxynucleotidyl transferase, proliferating cell nuclear antigen, CD4, and CD8). Thymic cortical epithelial cells (nurse cells) were studied using HLA-DR and protein gene product 9.5 as markers. Functional analysis was performed with “mixed lymphocyte reaction” of thymocyte or splenocyte responder cells with autologous lung cells as the stimulators.
Measurements and Main Results: 2A11 treatment attenuates thymic cortical involution in BPD animals, sustaining terminal deoxynucleotidyl transferase–positive prothymocytes and thymocyte proliferation. BPD animals have increased CD4+ cells in thymic cortex and lung interstitium, which are reduced by 2A11. Conversely, cortical protein gene product 9.5/HLA-DR–positive thymic nurse cells are depleted in BPD animals, but are preserved by 2A11-treatment. Whereas fetal thymocytes and splenocytes respond to phythemagglutinin/ionomycin and to a lesser extent, to autologous lung, BPD thymocytes and splenocytes are phythemagglutinin/ionomycin-unresponsive, and yet react strongly to autologous lung. The 2A11 normalizes these responses.
Conclusions: These observations suggest that bombesin-like peptides mediate premature thymic maturation and thymic nurse-cell depletion, leading to autoreactive T cells that could contribute to lung injury.
Keywords: animal model, bombesin, immunohistochemistry, mixed lymphocyte reaction, thymic nurse cells
Bronchopulmonary dysplasia (BPD) is a chronic lung disease of premature newborns (1), with contributing causes including oxygen toxicity, barotrauma, infection, and immaturity (2–4). The clinical definition of BPD as oxygen requirement at 36-wk gestational age closely correlates with long-term outcomes (5, 6). Although medical advances have reduced its severity, BPD remains a major unsolved disease state, afflicting more than 10,000 children in the United States annually, leading to increased morbidity and mortality (3). A distinction exists between severe “old BPD” and milder “modern-day BPD” (7): “Old BPD,” seen primarily in the presurfactant era, is characterized by hyperinflated lung segments alternating with regions of atelectasis and fibrosis; in contrast, “modern-day BPD” is now clinically predominant, characterized by enlarged alveoli with reduced septation and impaired capillary development (8, 9).
Newborn animal models of BPD that are clinically and pathologically most similar to human BPD are two baboon models described by Escobedo and colleagues (10) and Coalson and colleagues (11, 12). First is the hyperoxic model of BPD, wherein preterm baboons delivered at 140-d gestation (term = 180 d) are treated with 100% O2 for 10 d and develop clinical and pathologic changes of severe BPD (140 d/100% O2); in contrast, 140-d animals given maintenance levels of O2 pro re nada (PRN) do not develop BPD-like changes. Recently, a more clinically relevant “interrupted gestation” model of BPD has been developed in 125-d gestational age (125 d) baboons maintained on O2 PRN for 14–21 d, resulting in mild-to-moderate BPD, characterized by arrested alveolarization similar to “modern-day BPD.” Increased numbers of pulmonary neuroendocrine (NE) cells containing bombesin-like peptides (BLPs) occur in infants with BPD (13). We observed increased BLP-positive pulmonary NE cells in hyperoxic premature baboons with BPD (14). Urine BLP levels are elevated in baboons at risk for BPD during the first three postnatal days in both the hyperoxic and interrupted gestation models, and the magnitude of the BLP levels correlates with BPD severity later developing in the same animals (14). This observation has been validated in premature human infants: elevated urine BLP levels occur in most BPD infants between Days 2–5 of life; in multiple logistic regression analyses, a single elevated urine BLP value confers a 10-fold increased risk of developing BPD (15). Finally, treatment of hyperoxic preterm baboons with 2A11, a well-characterized blocking anti-bombesin antibody, abrogates the development of BPD according to clinical and pathologic criteria (14). Similar observations have been made in the 125-d/PRN model (16).
In very-low-birth-weight infants, small thymus size at birth is a strong predictive radiographic sign of respiratory and/or inflammatory complications, including BPD (17), respiratory distress syndrome (RDS) (18), and subclinical chorioamnionitis (7, 19), the last itself causing a predisposition to BPD (20). Considering the pivotal role of the immune system in many inflammatory lung diseases, including asthma (21), cystic fibrosis (22), and chronic obstructive lung disease (23), we hypothesized that thymic architecture and/or T-cell function is altered in infants with BPD. At present, there is virtually nothing known about the adaptive immune system in BPD. We tested this first hypothesis directly by analyzing thymic structure and function from age-matched gestational control animals (GCs) and oxygen-treated animals with or without therapeutic intervention. We also tested the hypothesis that animals with BPD have autoimmunity as part of their immunodeficient state, evaluating the antilung reactivity of thymocytes (central T-cell development) and splenocytes (peripheral T-cell development).
This work was presented in part at the International Meetings of the American Thoracic Society in 2003 and 2004 (24, 25).
METHODS
Animals
Premature baboons delivered at 125 d (125-d/PRN model) were administered anti-bombesin antibody 2A11 intravenously at 5 mg/kg beginning 2 h after birth, followed by 2 mg/kg on Days 3, 6, and 9. Details of the protocol have been described previously (12). More information about animal care is provided in the online supplement.
Histopathology
Thymus and lung tissues were fixed in 4% paraformaldehyde, and paraffin-embedded (14). Hematoxylin and eosin staining was used to evaluate tissue architecture. The avidin-biotin complex technique was used for immunoperoxidase studies (14) after antigen retrieval with Ab-972 (Abcam, Cambridge, MA). Antibodies were as follows: mouse anti-proliferating cell nuclear antigen (PCNA) antibody clone PC10 at 1:20 (14); rabbit anti-terminal deoxynucleotidyl transferase (TdT) (Supertechs Inc., Bethesda, MD) (26) at 1:25; mouse anti–α–smooth muscle actin antibody clone 1A4 (Sigma, St. Louis, MO) at 1:125 (27); rabbit anti–protein gene product 9.5 (PGP 9.5) (Ultraclone, Cambridge, UK) at 1:500; mouse anti-CD4 ab846 (Abcam) at 1:50; mouse anti-CD8 N1592 (Dako) at 1:50 (28). The TSA biotin system was used for CD4 and CD8 immunostaining (29). The peroxidase substrate was diaminobenzedine, and methyl green was the counterstain.
Immunofluorescence methodologies have been described previously (30). Rabbit anti-PGP 9.5 (1:100) and HLA-DR (1:100 mouse anti-human HLA-DR; BD Biosciences, San Diego, CA) were incubated for 1h at 20°C, followed by secondary antibodies for 1h using Alex Fluor 568 goat anti-rabbit IgG (Molecular Probes, Eugene, OR) and Alexa Fluor 488 goat anti-mouse IgG (Invitrogen, Carlsbad, CA). DAPI 300 nM was used to label nuclei.
Brightfield images were captured using a Nikon Optiphot microscope and digitizer interfaced with a Dell computer (Dell, Round Rock, TX) using ACT-I software (Nikon, Tokyo, Japan). Areas were determined using density slice analysis (Scion Image 1.62; Scion Corp., Frederick, MD) for whole thymus sections in square inches, one entire cross-section analyzed per animal. Immunopositive cells were enumerated as “particles” at 20× magnification, and expressed as the number positive per square inch of tissue (31).
Mixed Lymphocyte Reaction
Single-cell suspensions were prepared from fresh thymus, spleen, and lung by passing cells through a fine stainless steel screen into Dulbecco's modified Eagle medium (DMEM)/5% fetal bovine serum (FBS), then through a 200-micropore polyester screen to remove debris. Cell pellets were suspended in DMEM/5% FBS at 107 cells/ml. As adapted from a general mixed lymphocyte reaction (MLR) protocol (32), lung stimulator cells were sublethally irradiated (2,000 rads). Responder cells (thymocytes and splenocytes) were plated into 96-well plates at 1 × 105/100 μl or 4 × 105/100 μl in media (DMEM + penicillin [2 × 104 U/ml], streptomycin [200 μg/ml], l-glutamine [∼ 0.5 mg/ml], and 5% FBS). A total of 100 μl of lung stimulator cells were added per well at 2 × 105/100 μl or 6 × 105/100 μl, each group in triplicate. For negative controls, cells were cultured in media alone. For positive controls, responder cells were incubated with 200 ng/ml ionomycin and 2 μg/ml phytohemagglutinin (PHA [termed collectively for simplicity]). Plates were incubated at 37°C in 5% CO2 for 5 d, then 1 μCi/well of 3H-thymidine was added for 24 h. Cells were collected on filter paper using a Combi-12 cell harvester (Molecular Devices, Sunnyvale, CA). The mean counts per minute of 3H-thymidine incorporated was calculated for each group (three wells). Results are expressed as percentage change over media alone.
Statistical Analyses
For group comparisons, we used one-way analysis of variance. Results are given as mean values (± SEM).
RESULTS
Histopathologic Analysis of the Thymus
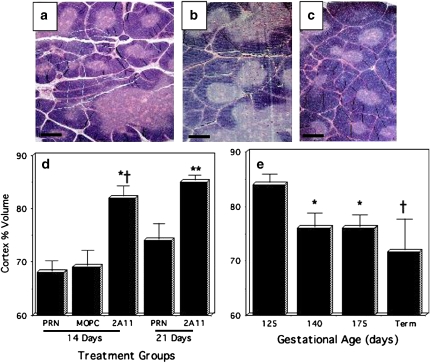
On routine slide review, we made the initial observation that the thymic cortex in 2A11-treated animals was preserved in fetal-like morphology without apparent thinning or involution. To quantify the relative amount of thymic cortex, we measured the volume of thymic cortex and expressed this as a percentage of the volume of cortex plus medulla, which is best visualized in hematoxylin and eosin–stained slides (Figures 1a–1c). The results of morphometric analyses are given in Figures 1d and 1e. After 14 d, the relative volume of thymic cortex was significantly higher in 2A11-treated 125-d/PRN animals (Figures 1c and 1d) as compared with 125-d/PRN animals treated with the negative control isotype-matched IgG, Clone MOPC21 (MOPC) (Figures 1b and 1d; p = 0.004) and also compared with untreated 125-d/PRN animals (Figures 1A and 1D; p = 0.001). Similarly, in 125-d/PRN animals maintained for 21 d, the thymic cortical volume in the 2A11-treated group was significantly higher than it was in untreated 125-d/PRN animals (p = 0.03; Figure 1d). There was no significant difference in thymic weight between the different treatment groups (data not shown). The thymic cortical volume in 125-d/PRN animals at 14 d was significantly lower than that of fetal GCs (Figure 1e) at 125 d (p < 0.0001), 140 d (p = 0.02), and 175 d (p = 0.04), but was similar to that of full-term normal control animals after a natural delivery. By comparison, the thymic cortical volume of 2A11-treated 125-d/PRN animals at 14–21 d (Figures 1c and 1d) was the same as that of 125-d GCs (Figure 1e), which was even greater than that of the age-matched 140-d GCs.
Figure 1.
Thymic histopathology from preterm baboons given O2 pro re nada (PRN) ± anti-bombesin antibody 2A11. (a–c) Hematoxylin and eosin–stained sections of thymus from 125-d/PRN animals. Scale bar in lower left corner = 500 μm. (a) Bronchopulmonary dysplasia (BPD) control 125-d/PRN × 14-d animal, no intervention (n = 10). (b) Image shows thymus from a 125-d/PRN × 14 d animal treated with MOPC21 (negative control IgG) (n = 6). (c) Image shows thymus from a 125-d/PRN × 14 d animal treated with 2A11 (n = 5). (d and e) Quantitative morphometric analysis for volume percent of thymic cortex on the entire slide expressed as a percentage of the total thymic tissue volume (cortex + medulla). (d) Bar graph shows 125-d/PRN: groups include: 125-d/PRN × 21 d with no intervention (n = 5); and 125-d/PRN × 21 d + 2A11 (n = 5). *p = 0.004 compared with 125-d/PRN + MOPC; †p = 0.001 compared with 125-d/PRN × 14 d with no intervention; **p = 0.03 compared with 125-d/PRN × 21 d with no intervention. (e) Gestational control animals (GCs): 125 d (n = 4); 140 d (n = 5); 175 d (n = 3); and full-term natural delivery (n = 3). p Values compared with 125-d GCs: *p < 0.001; †p < 0.0001.
Immunostaining for Markers of T-Cell Maturation
As a first approach to analyzing T-cell development, we studied TdT immunostaining as a marker of undifferentiated thymocytes (prothymocytes) (26). Compared with thymus from the untreated control 125-d/PRN group (Figure 2a), relative numbers of TdT+ cortical cells were significantly greater in 2A11-treated 125-d/PRN animals (Figure 2b), both at 14 d (compare Figures 2a and 2b) and at 21 d (photomicrographs not shown). Morphometric analysis of thymic TdT immunostaining in thymus of 125-d/PRN animals at 14 d (Figure 2c) demonstrates significantly more TdT+ cells/in2 in the 2A11-treated group as compared with MOPC-treated 125-d/PRN control animals (p = 0.04), and also compared with untreated 125-d/PRN animals (p = 0.0001). Similarly, after 21 d (Figure 2c), TdT+ cells were higher in the 2A11-treated group as compared with untreated 125-d/PRN animals. In normal thymic development (Figure 2d), TdT was higher in 125-d GCs than 140-d GCs (p < 0.02), and declined progressively up to full-term animals with a natural delivery (p < 0.01 comparing 125-d GCs to either 175-d GCs or term animals).
Figure 2.
Quantitative morphometric analysis of terminal deoxynucleotidyl transferase (TdT) immunostaining in thymus from preterm baboons given O2 PRN ± 2A11. (a–b) TdT-immunostained sections of thymus from 125-d/PRN animals. Scale bar in lower left corner = 50 μm. Arrowheads indicate thymic cortex (capsule). (a) BPD control 125-d/PRN × 21-d animal, no intervention (n = 3). (b) Image shows 125-d/PRN × 21 d treated with 2A11 (n = 5). (c and d) Quantitative morphometric analysis of 14-d/PRN and 21-d/PRN animals for numbers of TdT+ nuclei per total area of the thymic cortex tissue section in square inches. Similar results were obtained when numbers of TdT+ cells were normalized for the area of the cortex plus medulla. (c) Groups tested were: 125-d/PRN × 14-d animal, no intervention (n = 5); 125-d/PRN × 14 d + MOPC21 (n = 5); 125-d/PRN × 14 d + 2A11 (n = 3); and the 21-d animals listed in a and b above. *p < 0.0001 compared with 125-d/PRN × 14 d without intervention; †p < 0.04 compared with 125-d/PRN × 14 d + MOPC21; **p < 0.0002 compared with 125-d/PRN × 21 d with no intervention. (D) GCs for numbers of TdT+ nuclei/in2 of thymic cortex: 125 d (n = 4); 140 d (n = 5); 175 d (n = 3); and full-term natural delivery (n = 3). *p < 0.02 and †p < 0.01 compared with 125-d GC group. M = medulla.
To determine whether altered T-cell maturation is associated with changes in cell proliferation, we performed immunostaining for PCNA (Figure 3). Results of quantitative image analysis of 125-d/PRN animals are given in Figure 3c, and results from GCs are presented in Figure 3d. At 14 d, the prevalence of PCNA-positive thymocytes was the same in 125-d/PRN animals with or without 2A11 or MOPC treatment (Figure 3c). In contrast, at 21 d, the untreated 125-d/PRN group (Figure 3a) had fewer PCNA-positive thymocytes than the 2A11-treated group (Figure 3b). Quantitative analysis demonstrates that PCNA-positive thymocytes were approximately threefold elevated at 21 d in 2A11-treated animals as compared with the untreated 125-d/PRN control animals (Figure 3c; p < 0.005).
Figure 3.
Proliferating cell nuclear antigen (PCNA)–immunostaining of thymuses from 125-d/PRN baboons ± 2A11. (a and b) PCNA-immunostained sections of thymus from 125-d/PRN animals. Scale bar in lower left corner = 50 μm. Arrows indicate thymic cortex (capsule). (a) BPD control 125-d/PRN × 21-d animal, no intervention (n = 3). (b) Image shows 125-d/PRN × 21 d treated with 2A11 (n = 6). (c and d) Quantitative analysis for numbers of PCNA+ nuclei/in2 of thymic cortex tissue. Similar results were obtained when numbers of PCNA+ cells were normalized for the area of the cortex plus medulla. Groups tested were: (c) 125-d/PRN × 14-d animal, no intervention (n = 6); 125-d/PRN × 14 d + MOPC21 (n = 6); 125-d/PRN × 14 d + 2A11 (n = 4); *p < 0.0001 compared with 125-d/PRN × 21 d with no intervention. (d) GCs for PCNA+ nuclei/in2 of thymic cortex tissue section: 125 d (n = 4); 140 d (n = 4); 175 d (n = 3); and full-term natural delivery (n = 3). p Value compared with 125-d GCs: *p < 0.001. M = medulla.
Immunostaining of Thymus for CD4 and CD8
We performed immunohistochemical analyses of thymus from 125-d/PRN animals for CD4 and CD8. The results are given in Figure 4. At 14 d, untreated 125-d/PRN animals had CD4+ cells widely scattered throughout the thymic cortex (Figure 4a). In contrast, CD4+ cells were markedly decreased in thymuses of 2A11-treated 125-d/PRN animals (Figure 4b). Similarly, relative numbers of CD8+ cells were higher in the thymic cortex of untreated 125-d/PRN animals at 14 d (Figure 4c) as compared with 2A11-treated 125-d/PRN animals at 14 d (Figure 4d). Quantification using computerized image analysis (Figure 4e) demonstrates that, at 14 d, thymic CD4+ and CD8+ cells were significantly lower in 2A11-treated 125-d/PRN animals compared with baboons with no intervention (p = 0.015 for CD4; p = 0.0001 for CD8) or compared with MOPC-treated control animals (p = 0.0025 for CD4; p = 0.02 for CD8). However, at 21 d, there was no longer a difference in relative numbers of CD4+ or CD8+ cells between the 2A11-treated and untreated 125-d/PRN animals (Figure 4c).
Figure 4.
CD4 and CD8 immunostaining of thymuses from 125-d/PRN baboons. (a–d) Immunostained sections of thymus from 125-d/PRN animals. Scale bar in lower left corner = 50 μm. Arrowheads indicate thymic cortex (capsule). (a) CD4-immunostaining of thymus from one representative control 125-d/PRN × 14-d animal (n = 3). (b) CD4 immunostaining of thymus from one representative 125-d/PRN × 14-d animal treated with 2A11 (n = 3). (c) CD8 immunostaining of thymus from one representative control 125-d/PRN × 14-d animal (n = 5). (d) CD8 immunostaining of thymus from one representative 125-d/PRN × 14-d animal treated with 2A11 (n = 3). (e) Quantitative morphometric analysis for numbers of CD+ cells per total area of each thymic tissue field (4 fields/animal). Groups tested were: 125-d/PRN × 14-d animals, no intervention (n = 5); 125-d/PRN × 14 d + MOPC21 (n = 6); 125-d/PRN × 14 d + 2A11 (n = 3); 125-d/PRN × 21 d, no intervention (n = 3); 125-d/PRN × 21 d + 2A11 (n = 5); *p < 0.0005; †p < 0.0025 compared with 125-d/PRN × 14 d with no intervention. *p < 0.002, †p < 0.04 compared with MOPC-treated 125-d/PRN group. M = medulla.
Immunostaining and Immunofluorescence for Thymic Epithelial Cells
To determine whether BLPs might be produced by thymic NE cells, as suggested in one study of murine thymus (33), we performed immunostaining for BLPs and PGP 9.5, the neuronal/NE isoform of ubiquitin hydroxylase. Surprisingly, we observed numerous large (> 12 μm) cells with dendritic morphology and cytoplasmic PGP9.5 immunostaining throughout the cortex of 2A11-treated animals (Figure 5a). Quantitative morphometric analysis (Figure 5b) verifies the presence of abundant PGP9.5+ cells in thymus from 2A11-treated baboons at 14 d and at 21 d. In contrast, using the given experimental conditions, PGP9.5 immunostaining was only marginally detectable in untreated 125-d/PRN animals at either 14 d or 21 d. Compared with MOPC-treated or untreated 125-d/PRN animals at 14 d, there were over four times or seven times more PGP+ cells in the thymus in 125-d GCs and 140-d GCs, respectively (Figure 5b; p < 0.001 between 140-d GCs and 14-d/PRN). BLP immunostaining was consistently negative in the thymus in spite of excellent positive controls.
Figure 5.
Thymic nurse cells in 125-d/PRN baboons ± 2A11 treatment. (a) PGP9.5 immunostaining of thymus from one representative 125-d/PRN × 21-d animal that was treated with 2A11. Scale bar in lower left hand corner = 50 μm. Thymic cortex is located in the region between the two arrows. (b) Quantification of PGP9.5+ cells per in2 thymic cortex. Groups tested were: 125 GCs (n = 4); 125-d/PRN × 14-d animals, no intervention (n = 5); 125-d/PRN × 14 d + 2A11 (n = 4); 125-d/PRN × 14 d + MOPC (n = 5); 125-d/PRN × 21-d animals, no intervention (n = 4); 125-d/PRN × 21 d + 2A11 (n = 5); 140 GCs (n = 5); Term (n = 3). *p = 0.005 compared with corresponding 125-d/PRN group with no intervention. †p < 0.04 compared with MOPC-treated 125-d/PRN group. Compared with 125 d GC: 140-d GC: p < 0.001; Term: p < 0.0001. ¥p < 0.001 for 125-d/PRN × 14 d with or without MOPC compared with 140-d GCs. **p = 0.002 compared to 125-d/PRN × 21 d with no intervention. Compared with 140-d GC: 14-d/PRN: p = 0.08. (c–e) Immunofluorescence staining of a thymus section from a representative 125-d/PRN × 21 d + 2A11 animal to verify the identity of the PGP9.5-positive cells from a as thymic nurse cells. Scale bar in the lower left corner = 50 μm. (c) PGP9.5 using phycoerythrin (red). (d) HLA-DR using fluorescein isothiocyanate (green). (e) Merged images from c and d demonstrate colocalization of both PGP9.5 and HLA-DR in nearly 100% of the positive cortical thymocytes, which appear yellow. Infrequent foci of PGP9.5-positive staining that lacked HLA-DR positivity were morphologically identified as nerve fibers or intrinsic ganglia (data not shown). (f) Phase contrast image of the same field as shown in c–e demonstrates thymic cortex in the lower half of the photomicrograph, with the capsule indicated by yellow asterisks and the approximate location of the corticomedullary junction indicated by a broken red line, so that the thymic medulla is at the top of the photo. A Hassall's corpuscle is indicated by an arrow. M = medulla.
To verify the identity of the PGP9.5+ cells as thymic cortical epithelial “nurse” cells, we performed double immunofluorescence staining of thymus for PGP 9.5 and HLA-DR (34, 35). The results of this study are given in Figures 5c–5e. In thymic cortex, there was near-perfect concordance of fluorescence for PGP 9.5 (Figure 5c, red cells) and HLA-DR (Figure 5d, green cells). When Figures 5c and 5d are superimposed, it is apparent that all red cells are also green, with the merged images being yellow (Figure 5e). The orientation of the tissue section is given in a phase contrast view of the same tissue section (Figure 5f).
Mixed Lymphocyte Reaction
As a first approach to evaluating T-cell function in BPD, we performed cocultures of splenocytes or thymocytes together with a single cell suspension of sublethally irradiated lung cells, as detailed in Methods. After 5 d, 3H-thymidine was added for 24 h, and cells were harvested to quantify thymidine incorporation. The pooled results of 12 experiments are given in Figure 6.
Figure 6.
Mixed lymphocyte reaction with autologous lung cells as targets for responder splenocytes or thymocytes. (a) For each animal, the maximal responses of thymocytes to autologous lung cells and to PHA are given. (b) The ratio of maximal responsiveness to lung versus PHA was calculated and the results pooled for both thymocytes and splenocytes from each group, as shown: 125-d GCs (125GC; n = 2), 125-d/PRN × 6 d (n = 3), 125-d/PRN × 14 d (n = 3), 125-d/PRN × 14 d + 2A11 (n = 4), and 140-d GC (n = 4). Note that the 140-d GC group is an age-matched control for the 125-d/PRN × 14-d animals. *p < 0.03; †p < 0.003 compared with 140-d GCs. **p < 0.03 compared with 125-d/PRN × 14 d with no intervention.
Thymocytes from 125-d and 140-d GCs had mean responses over baseline (media alone) of 1.3- and 1.7-fold, respectively, to autologous lung cells versus 2.1- and 8.8-fold to the nonspecific stimulant PHA (Figure 6a). At 6-d postnatal age, 125-d/PRN animals had mean responses of 2.7-fold to lung and 7.7-fold to PHA. In contrast, after 14 d, the 125-d/PRN thymocytes responded threefold to lung, but only 1.4-fold to PHA. These responses were reversed by 2A11 treatment: anti-lung reactivity dropped to 2.3-fold, and PHA responses were modestly increased to twofold over baseline (Figure 6a). Similar responses to autologous lung cells and to PHA were demonstrated by splenocytes (data not shown). However, the moderate baseline variability between experiments precluded statistical significance.
To normalize the data and allow results from different animals to be pooled for interpretation, we calculated the ratio of lung responsiveness over PHA responsiveness for thymocytes and splenocytes from each animal (L/PHA; Figure 6b). The individual variability between the groups was minimized and statistical significance was attained. For thymocytes, the peak L/PHA in 125-d/PRN × 14-d animals (2.7) was significantly greater than the ratio of 0.4 in 140-d GCs (p < 0.03). For splenocytes, the peak L/PHA in 125-d/PRN × 14-d animals (2.1) was significantly greater than the ratio of 0.6 in 140-d GCs (p = 0.002), and was also significantly greater than results from the corresponding 2A11-treated group (p < 0.03).
Lung Immunostaining for T Cells and Dendritic Cells
Lung sections from all 125-d/PRN animals were immunostained for CD4, CD8, and HLA-DR. Representative results of CD4 immunostaining are given in Figure 7. At 14 d, abundant CD4+ cells were present throughout the interstitial lung parenchyma in untreated (Figure 7a) and MOPC-treated (Figure 7b) 125-d/PRN animals. In contrast, 2A11-treated 125-d/PRN animals had rare CD4+ cells in the lung interstitium, although there were CD4+ cells in the perivascular and peribronchial regions (Figure 7c). Only rare CD8+ cells were detectable in lung sections from baboons in any of these groups. HLA-DR+ cells with a dendritic morphology were present throughout the interstitial lung parenchyma in all groups of 125-d/PRN animals. Relative numbers of DR+ cells in the lung were the same in 125-d/PRN animals either untreated or 2A11-treated versus 140-d GCs (data not shown). The only significant difference we observed was between 2A11-treated and MOPC-treated 125-d/PRN animals (0.11 ± 0.03 cells/in2 vs. 0.28 ± 0.02 cells/in2, respectively; p = 0.006).
Figure 7.
Immunodetection of CD4+ cells in lung tissue from 125-d/14-d PRN baboons ± MOPC or 2A11. (a) A representative 125-d/PRN × 14-d BPD animal (out of n = 3). (b) A representative MOPC-treated 125-d/14-d PRN animal (n = 3). (c) A representative 2A11-treated 125-d/14-d PRN animal (n = 3).
DISCUSSION
This study represents the first investigation of adaptive immunity in BPD. Using anti-BLP antibody treatment of a baboon model of BPD, we demonstrate that BLP accelerates thymocyte maturation together with loss of thymic nurse cells. Thymic nurse cells are cortical epithelial cells that play an important role in negative selection (36, 37). Consequently, autoreactive T cells are released from the thymus into the peripheral circulation, including that of the lung and spleen. There are abundant CD4+ cells (but not CD8+ cells or dendritic cells) in the lung parenchyma of BPD animals with lung injury, but not in 2A11-treated animals. Similarly, in a murine model of chronic lung disease, CD4+ T cells and autoimmunity have been implicated in the pathogenesis of lung injury (38). In the baboon BPD model, the splenocytes and thymocytes are also immunodeficient, with diminished responsiveness to PHA at 14 d, consistent with impaired T-cell receptor signaling that could be attributed to inadequate thymic education due to early maturation. Thymic involution can be due to stress, glucocorticoids, or other chemicals trophic for the thymus (39). Peripheral factors could also be involved in mediating organ-specific autoimmunity and immunodeficiency, such as altered regulatory T cells (40–43), decreased circulating dendritic cells (44, 45), abnormal extrathymic lymphocyte maturation (46), or a T-cell signaling defect related to chronic lung injury (47).
Thymic involution occurs either as a normal physiologic process during postnatal development or as a pathologic event in premature infants with acute injury, infection, or stress. Reversible changes can occur in response to hyperoxia, during which the thymus gland undergoes maturation of diverse cell populations (48, 49). In one study of neonates undergoing open-heart surgery, the thymuses had severe depletion of cortical CD4+ CD8+ cells and reduced cortical volume (50) compared with those of normal children. Anti-BLP blocking antibody 2A11 has multiple effects on the developing postnatal thymus, sustaining a more fetal-like thymic phenotype. The anti-bombesin antibody 2A11 abrogates thymic involution such that the thymic cortex at 3 wk of age is similar to 140-d GCs. In addition, 2A11 treatment diminishes premature thymocyte maturation, and leads to persistence of immature thymocytes. Although 2A11 appears to mainly prevent premature cell differentiation, there are also effects on cell proliferation: (1) improved thymocyte PCNA labeling at 21 d, possibly related to lowering BLP levels to the optimal dose range for cell proliferative responses; and (2) improved PHA responsiveness of thymocytes and splenocytes. Cumulatively, 2A11 is a potent immunomodulatory agent: reducing anti-lung autoreactivity and enhancing PHA responsiveness to levels similar to 140-d GCs, supporting the concept that 2A11 is effectively normalizing development of adaptive immunity.
A candidate mechanism for increased autoimmune reactivity in animals with BPD is the reduction in thymic nurse cells that can regulate thymocyte differentiation and positive–negative thymocyte selection (36, 51). In the present study, we observe numerous thymic nurse cells in normal full-term newborn baboons in spite of physiologic cortical involution, similar to the persistence of thymic dendritic cells during normal thymic involution (52). Thus, the reduction in thymic nurse cells in 125-d/PRN animals appears to be a pathologic condition, suggesting that thymic nurse cells do not survive during involution associated with injury, infection, or stress. Reduced numbers of thymic nurse cells have been associated with a variety of autoimmune conditions with immunodeficiency, including thyroiditis and systemic lupus erythematosis (36, 53). The 2A11-induced increase in thymic nurse cells appears to be due to two factors: (1) BLP blockade, because 2A11-treated animals have significantly more nurse cells than do MOPC-treated control animals; and (2) nonspecific immunostimulation by IgG, because MOPC21 induces a small increase in numbers of these cortical epithelial cells. Thus, the data do support a specific role for a BLP-induced loss of thymic nurse cells. In other studies, BLP has been shown to be immunomodulatory by impairing the generation and function of tumor-associated dendritic cells (54).
The alterations in thymic structure and thymocyte function present at 14–21 d after birth are consistent with the occurrence of elevated urine BLP levels in preterm baboons and human infants during the first week after birth (15, 55). It appears that over 90% of this urine BLP is derived from the lung (16). GRP mRNA levels for gastrin-releasing peptide (GRP), the major pulmonary BLP in mammals, are highly elevated (4- to 16-fold) in 125-d/PRN lung tissue at 24 h of age (M. Sunday, unpublished data), consistent with a role in acute lung injury in BPD, which is further supported by the rapidly beneficial effect of 2A11 in the hyperoxic baboon model of BPD (14). Recently, BLPs have been demonstrated to have a major role in promoting acute lung injury in septic mice (56, 57). BLP overproduction in the lung could lead to high BLP levels in the bronchial and pulmonary venous blood, which directly supplies the thymus gland. The differences in thymic histology that we observed between the treatment groups could also have been secondary to cytokines released by the innate immune system as part of a systemic inflammatory response, triggered by BLP—especially considering the association between small thymus size and subclinical chorioamnionitis (7). We did not observe BLP immunoreactivity in any thymus studied, consistent with studies of human thymus (58), in spite of a report of BLP immunostaining in murine thymus (33). In pilot studies, we have detected mRNA for bombesin/GRP-preferring receptors in thymus from 125-d/PRN baboons (M. Sunday, unpublished data), suggesting that some of the BLP effects on thymus could be direct. Another possibility is that 2A11 could block BLP-induced secretion of adrenocorticotropic hormone and glucocorticoids (59), and thus secondarily reduce stress-induced thymic involution.
In summary, the present investigation demonstrates that newborn baboons with BPD have an acquired immunodeficiency and T-cell autoreactivity, both of which are improved by treatment with anti-bombesin antibodies. BPD-associated changes in T-cell function are associated with significant alterations in thymic structure, including accelerated T-cell maturation and greatly reduced numbers of thymic nurse cells. Recent studies indicate that the thymus also exports long-lived, fully committed T-cell precursors that can colonize peripheral organs (60). Inadequate negative selection in the thymus would lead to release of relatively uneducated/autoreactive thymocytes, which could contribute to lung injury in BPD. Numerous CD4+ cells are present in the lung parenchyma of animals with BPD, but not in 2A11-treated animals. These observations suggest that adaptive immunity can be modulated by the pulmonary NE system. Autoreactive T cells could cause lung injury via recognition of autoantigens such as autologous histocompatibility antigens either directly (51), or after oxidation or nitrosation.
Cumulatively, these observations suggest that blocking BLPs protects against BPD at multiple levels: both indirectly, by promoting development of normal adaptive immunity, and also directly, by reducing acute lung injury (14, 57), by reducing fibrosis, and by preserving normal alveolarization (61).
Supplementary Material
Acknowledgments
The authors thank Dr. Jacqueline Coalson for helpful discussions.
Supported by National Institutes of Health grants UO1-HL52638 (M.E.S.), UO1-HL52636 (BPD Resource Center at the University of Texas at San Antonio), and P51RR13986 for facilities support at the Primate Center, Southwest Foundation for Medical Research.
Current affiliation for Dr. Jong-Hwan Lee: Department of Biotechnology and Bioengineering, Dong-Eui University, Daegu, South Korea.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200511-1784OC on March 30, 2006
Conflict of Interest Statement: D.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.-H.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. F.C. is a co-inventor of US Government (NGI) Patent 5,109,115 (April 28, 1992), which covers the neutralizing anti-bombesin/GRP monoclonal antibody ZA11 (MoAb–ZA11), used in this study to block BLP activity. No financial support/funding has been received from the pharmaceutical industry or the private sector for this reagent. F.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.E.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Northway WH, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline membrane disease. N Engl J Med 1967;276:357–368. [DOI] [PubMed] [Google Scholar]
- 2.Jobe AH. Antenatal factors and the development of bronchopulmonary dysplasia. Semin Neonatol 2003;8:9–17. [DOI] [PubMed] [Google Scholar]
- 3.Allen J, Zwerdling R, Ehrenkranz R, Gaultier C, Geggel R, Greenough A, Kleinman R, Klijanowicz A, Martinez F, Ozdemir A, et al. Statement on the care of the child with chronic lung disease of infancy and childhood. Am J Respir Crit Care Med 2003;168:356–396. [DOI] [PubMed] [Google Scholar]
- 4.Jobe AH, Ikegami M. Mechanisms initiating lung injury in the preterm. Early Hum Dev 1998;53:81–94. [DOI] [PubMed] [Google Scholar]
- 5.Davis JM, Parad RB, Michele T, Allred E, Price A, Rosenfeld W. Pulmonary outcome at 1 year corrected age in premature infants treated at birth with recombinant human CuZn superoxide dismutase. Pediatrics 2003;111:469–476. [DOI] [PubMed] [Google Scholar]
- 6.Parad RB, Berger TM. Chronic lung disease. In: Cloherty JP, Stark AR, editors. Manual of neonatal care, 4th ed. Philadelphia–New York: Lippincott-Raven; 1998. pp. 378–388.
- 7.Toti P, Buonocore G, Tanganelli P, Catella AM, Palmeri ML, Vatti R, Seemayer TA. Bronchopulmonary dysplasia of the premature baby: an immunohistochemical study. Pediatr Pulmonol 1997;24:22–28. [DOI] [PubMed] [Google Scholar]
- 8.Thibeault DW, Mabry SM, Ekekezie II, Truog WE. Lung elastic tissue maturation and perturbations during the evolution of chronic lung disease. Pediatrics 2000;106:1452–1459. [DOI] [PubMed] [Google Scholar]
- 9.Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;164:1971–1980. [DOI] [PubMed] [Google Scholar]
- 10.Escobedo MB, Hilliard JL, Smith F, Meredith K, Walsh W, Johnson D, Coalson JJ, Kuehl TJ, Null DM, Robotham JL. A baboon model of bronchopulmonary dysplasia, I: clinical features. Exp Mol Pathol 1982;37:323–334. [DOI] [PubMed] [Google Scholar]
- 11.Coalson JJ, Kuehl TJ, Escobedo MB, Hilliard JL, Smith F, Meredith K, Null DM, Walsh W, Johnson D, Robotham JL. A baboon model of bronchopulmonary dysplasia, II: pathologic features. Exp Mol Pathol 1982;37:335–350. [DOI] [PubMed] [Google Scholar]
- 12.Coalson JJ, Winter VT, Siler-Khodr T, Yoder BA. Neonatal chronic lung disease in extremely immature baboons. Am J Respir Crit Care Med 1999;160:1333–1346. [DOI] [PubMed] [Google Scholar]
- 13.Johnson DE, Lock JE, Elde RP, Thompson TR. Pulmonary neuroendocrine cells in hyaline membrane disease and bronchopulmonary dysplasia. Pediatr Res 1982;16:446–454. [DOI] [PubMed] [Google Scholar]
- 14.Sunday ME, Yoder BA, Cuttitta F, Haley KJ, Emanuel RL. Bombesin-like peptide mediates lung injury in a baboon model of bronchopulmonary dysplasia. J Clin Invest 1998;102:584–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cullen A, Van Marter LJ, Moore M, Parad R, Sunday ME. Urine bombesin-like peptide elevation precedes clinical evidence of bronchopulmonary dysplasia. Am J Respir Crit Care Med 2002;165:1093–1097. [DOI] [PubMed] [Google Scholar]
- 16.Sunday ME, Yoder BA, Cullen A, Cuttitta F, Emanuel RL. Neuropeptides in lung development and injury. FASEB J 1999;13:A1154 (abstract 857.4). [Google Scholar]
- 17.De Felice C, Latini G, Del Vecchio A, Toti P, Bagnoli F, Petraglia F. Small thymus at birth: a predictive radiographic sign of bronchopulmonary dysplasia. Pediatrics 2002;110:386–388. [DOI] [PubMed] [Google Scholar]
- 18.Chen CM, Yu KY, Lin HC, Yeh GC, Hsu HH. Thymus size and its relationship to perinatal events. Acta Paediatr 2000;89:975–978. [DOI] [PubMed] [Google Scholar]
- 19.Toti P, De Felice C, Stumpo M, Schurfeld K, Di Leo L, Vatti R, Bianciardi G, Buonocore G, Seemayer TA, Luzi P. Acute thymic involution in fetuses and neonates with chorioamnionitis. Hum Pathol 2000;31:1121–1128. [DOI] [PubMed] [Google Scholar]
- 20.Yoon BH, Romero R, Kim KS, Park JS, Ki SH, Kim BI, Jun JK. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol 1999;181:773–779. [DOI] [PubMed] [Google Scholar]
- 21.Holt PG, Upham JW, Sly PD. Contemporaneous maturation of immunologic and respiratory functions during early childhood: implications for development of asthma prevention strategies. J Allergy Clin Immunol 2005;116:16–24; quiz 25. [DOI] [PubMed] [Google Scholar]
- 22.Hausler M, Schweizer K, Biesterfel S, Opladen T, Heimann G. Peripheral decrease and pulmonary homing of CD4+CD45RO+ helper memory T cells in cystic fibrosis. Respir Med 2002;96:87–94. [DOI] [PubMed] [Google Scholar]
- 23.Korn S, Wiewrodt R, Walz YC, Becker K, Mayer E, Krummenauer F, Buhl R. Characterization of the interstitial lung and peripheral blood T cell receptor repertoire in cigarette smokers. Am J Respir Cell Mol Biol 2005;32:142–148. Epub 2004 Nov 11. [DOI] [PubMed] [Google Scholar]
- 24.Rosen DM, Sunday ME. Anti-bombesin-like peptide (BLP) antibody 2A11 arrests thymic involution in preterm baboons with bronchopulmonary dysplasia (BPD) [abstract]. Am J Respir Crit Care Med 2003;167:A730. [Google Scholar]
- 25.Rosen DM, Sunday ME. Anti-bombesin-like peptide (BLP) antibody 2A11 arrests thymic involution and thymocyte differentiation while attenuating bronchopulmonary dysplasia (BPD) [abstract]. Am J Respir Crit Care Med 2004;169:A79. [Google Scholar]
- 26.Bollum FJ. Antibody to terminal deoxynucleotidyl transferase. Proc Natl Acad Sci USA 1975;72:4119–4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G. A monoclonal antibody against α-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol 1986;103:2787–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mason DY, Cordell JL, Gaulard P, Tse AG, Brown MH. Immunohistological detection of human cytotoxic/suppressor T cells using antibodies to a CD8 peptide sequence. J Clin Pathol 1992;45:1084–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaufmann O, Baume H, Dietel M. Detection of oestrogen receptors in non-invasive and invasive transitional cell carcinomas of the urinary bladder using both conventional immunohistochemistry and the tyramide staining amplification (TSA) technique. J Pathol 1998;186:165–168. [DOI] [PubMed] [Google Scholar]
- 30.Katakai T, Hara T, Sugai M, Gonda H, Nambu Y, Matsuda E, Agata Y, Shimizu A. Chemokine-independent preference for T-helper-1 cells in transendothelial migration. J Biol Chem 2002;277:50948–50958. Epub 2002 Oct 20. [DOI] [PubMed] [Google Scholar]
- 31.Pua ZJ, Stonestreet BS, Cullen A, Shahsafaei A, Sadowska GB, Sunday ME. Histochemical analyses of altered fetal lung development following single versus multiple courses of antenatal steroids. J Histochem Cytochem 2005;53:1459–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W, editors. Current protocols in immunology. New York: John Wiley and Sons; 1996. pp. 3.12.6–3.12.7.
- 33.Del Rio M, Hernanz A, De la Fuente M. Bombesin, gastrin-releasing peptide, and neuromedin C modulate murine lymphocyte proliferation through adherent accessory cells and activate protein kinase C. Peptides 1994;15:15–22. [DOI] [PubMed] [Google Scholar]
- 34.Brelinska R, Ostalska D, Zabel M. Subtypes of thymic epithelial cells defined by neuroendocrine markers. Histochem Cell Biol 2000;114:239–244. [DOI] [PubMed] [Google Scholar]
- 35.Brelinska R, Paczkowska A, Kowalska K, Jaroszewski J. Lympho-epithelial interactions in rat thymus during pregnancy. Folia Histochem Cytobiol 2002;40:175–176. [PubMed] [Google Scholar]
- 36.Guyden JC, Pezzano M. Thymic nurse cells: a microenvironment for thymocyte development and selection. Int Rev Cytol 2003;223:1–37. [DOI] [PubMed] [Google Scholar]
- 37.Cotta-de-Almeida V, Bertho AL, Villa-Verde DM, Savino W. Phenotypic and functional alterations of thymic nurse cells following acute Trypanosoma cruzi infection. Clin Immunol Immunopathol 1997;82:125–132. [DOI] [PubMed] [Google Scholar]
- 38.Bruder D, Westendorf AM, Geffers R, Gruber AD, Gereke M, Enelow RI, Buer J. CD4 T lymphocyte–mediated lung disease: steady state between pathological and tolerogenic immune reactions. Am J Respir Crit Care Med 2004;170:1145–1152. [DOI] [PubMed] [Google Scholar]
- 39.Bodey B, Bodey BJ, Siegel SE, Kaiser HE. Involution of the mammalian thymus, one of the leading regulators of aging. In Vivo 1997;11:421–440. [PubMed] [Google Scholar]
- 40.Shevach EM. Regulatory T cells in autoimmunity. Annu Rev Immunol 2000;18:423–449. [DOI] [PubMed] [Google Scholar]
- 41.Laurie KL, Van Driel IR, Gleeson PA. The role of CD4+CD25+ immunoregulatory T cells in the induction of autoimmune gastritis. Immunol Cell Biol 2002;80:567–573. [DOI] [PubMed] [Google Scholar]
- 42.McHugh RS, Shevach EM. Cutting edge: depletion of CD4+CD25+ regulatory T cells is necessary, but not sufficient, for induction of organ-specific autoimmune disease. J Immunol 2002;168:5979–5983. [DOI] [PubMed] [Google Scholar]
- 43.Gregori S, Mangia P, Bacchetta R, Tresoldi E, Kolbinger F, Traversari C, Carballido JM, de Vries JE, Korthauer U, Roncarolo MG. An anti-CD45RO/RB monoclonal antibody modulates T cell responses via induction of apoptosis and generation of regulatory T cells. J Exp Med 2005;201:1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brivio F, Lissoni P, Gilardi R, Ferrante R, Vigore L, Curzi L, Uggeri F, Nespoli A, Fumagalli L. Abrogation of surgery-induced decline in circulating dendritic cells by subcutaneous preoperative administration of IL-2 in operable cancer patients. J Biol Regul Homeost Agents 2000;14:200–203. [PubMed] [Google Scholar]
- 45.Hesselink DA, Betjes MG, Verkade MA, Athanassopoulos P, Baan CC, Weimar W. The effects of chronic kidney disease and renal replacement therapy on circulating dendritic cells. Nephrol Dial Transplant 2005;20:1868–1873. [DOI] [PubMed] [Google Scholar]
- 46.Bubanovic IV. Crossroads of extrathymic lymphocytes maturation pathways. Med Hypotheses 2003;61:235–239. [DOI] [PubMed] [Google Scholar]
- 47.Sayeed MM. Alterations in cell signaling and related effector functions in T lymphocytes in burn/trauma/septic injuries. Shock 1996;5:157–166. [DOI] [PubMed] [Google Scholar]
- 48.Tosi P, Kraft R, Luzi P, Cintorino M, Fankhauser G, Hess MW, Cottier H. Involution patterns of the human thymus, I: size of the cortical area as a function of age. Clin Exp Immunol 1982;47:497–504. [PMC free article] [PubMed] [Google Scholar]
- 49.Hale LP, Braun RD, Gwinn WM, Greer PK, Dewhirst MW. Hypoxia in the thymus: role of oxygen tension in thymocyte survival. Am J Physiol Heart Circ Physiol 2002;282:H1467–H1477. [DOI] [PubMed] [Google Scholar]
- 50.Varas A, Jimenez E, Sacedon R, Rodriguez-Mahou M, Maroto E, Zapata AG, Vicente A. Analysis of the human neonatal thymus: evidence for a transient thymic involution. J Immunol 2000;164:6260–6267. [DOI] [PubMed] [Google Scholar]
- 51.Penninger J, Wick G. Thymic nurse cell lymphocytes react against self major histocompatibility complex. Eur J Immunol 1992;22:79–83. [DOI] [PubMed] [Google Scholar]
- 52.Varas A, Sacedon R, Hernandez-Lopez C, Jimenez E, Garcia-Ceca J, Arias-Diaz J, Zapata AG, Vicente A. Age-dependent changes in thymic macrophages and dendritic cells. Microsc Res Tech 2003;62:501–507. [DOI] [PubMed] [Google Scholar]
- 53.Takeoka Y, Taguchi N, Shultz L, Boyd RL, Naiki M, Ansari AA, Gershwin ME. Apoptosis and the thymic microenvironment in murine lupus. J Autoimmun 1999;13:325–334. [DOI] [PubMed] [Google Scholar]
- 54.Makarenkova VP, Shurin GV, Tourkova IL, Balkir L, Pirtskhalaishvili G, Perez L, Gerein V, Siegfried JM, Shurin MR. Lung cancer-derived bombesin-like peptides down-regulate the generation and function of human dendritic cells. J Neuroimmunol 2003;145:55–67. [DOI] [PubMed] [Google Scholar]
- 55.Sunday ME. Bioactive peptides and lung development. In: Gaultier C, Bourbon JR, Post M, editors. Lung development, 1 ed. Oxford, UK: Oxford University Press; 1999. pp. 304–326.
- 56.Dal-Pizzol F, Di Leone LP, Ritter C, Martins MR, Reinke A, Pens Gelain D, Zanotto-Filho A, de Souza LF, Andrades M, Barbeiro DF, et al. Gastrin-releasing peptide receptor antagonist effects on an animal model of sepsis. Am J Respir Crit Care Med 2006;173:84–90. [DOI] [PubMed] [Google Scholar]
- 57.Ganter MT, Pittet JF. Bombesin-like peptides: modulators of inflammation in acute lung injury? Am J Respir Crit Care Med 2006;173:1–2. [DOI] [PubMed] [Google Scholar]
- 58.Piantelli M, Maggiano N, Larocca LM, Ricci R, Ranelletti FO, Lauriola L, Capelli A. Neuropeptide-immunoreactive cells in human thymus. Brain Behav Immun 1990;4:189–197. [DOI] [PubMed] [Google Scholar]
- 59.Olsen L, Knigge U, Warberg J. Gastrin-releasing peptide stimulation of corticotropin secretion in male rats. Endocrinology 1992;130:2710–2716. [DOI] [PubMed] [Google Scholar]
- 60.Lambolez F, Arcangeli ML, Joret AM, Pasqualetto V, Cordier C, Di Santo JP, Rocha B, Ezine S. The thymus exports long-lived fully committed T cell precursors that can colonize primary lymphoid organs. Nat Immunol 2006;7:76–82. [DOI] [PubMed] [Google Scholar]
- 61.Ashour K, Shan L, Lee JH, Schlicher W, Wada K, Wada E, Sunday ME. Bombesin inhibits alveolarization and promotes pulmonary fibrosis in newborn mice. Am J Respir Crit Care Med 2006;173:1377–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.