Abstract
Objective
Endometrial cancer is the most common type of gynecologic cancer in the United States. In this study, we propose that a marine sponge compound, psammaplysene A (PsA) induces apoptosis in endometrial cancer cells through forced nuclear expression of FOXO1.
Methods
Ishikawa and ECC1 cells were treated with varying doses of PsA. FOXO1 protein localization was observed using immunofluorescent staining of cells. The effects of PsA on cell viability and proliferation were assessed using a cell viability assay and a BrdU incorporation assay respectively. Cell cycle analysis was performed using flow cytometry. To assess the role of FOXO1 in PsA-induced apoptosis, FOXO1 was silenced in ECC1 cells using siRNA technique, and overexpressed in Ishikawa cells using an adenovirus containing FOXO1 cDNAs. Western blots were used to measure levels of FOXO1 and cleaved PARP proteins.
Results
Treatment of both ECC1 and Ishikawa cells with PsA caused an increase in nuclear FOXO1 protein, a dramatic decrease in cell viability of approximately 5-fold (p<0.05) and minimal effect on proliferation. Furthermore, treatment of cells with PsA doubled the percentage of cells in the G2/M phase (p<0.05). PsA induced apoptosis in endometrial cancer cells. When FOXO1 was silenced in ECC1 cells and treated with PsA, the incidence of apoptosis decreased. In addition, overexpression of FOXO1 with PsA treatment increased apoptosis.
Conclusions
Increasing nuclear FOXO1 function is important for the induction of apoptosis of endometrial cancer cells by PsA.
Introduction
In the United States, cancer of the uterine corpus is the most common of the gynecologic malignancies, with an estimated 39,080 new cases diagnosed in 2007. Despite the frequent detection of early-stage cancers and the evolving use of adjuvant chemotherapy for advanced disease, the death rate from this malignancy has increased and currently claims 7,400 lives among US women per year [1]. The majority (75-80%) of endometrial adenocarcinomas are known as estrogen-dependent, or type I carcinomas, and consist of the endometrioid histologic subtype [2]. PTEN, a tumor suppressor gene, is known to be mutated in 40-50% of type I endometrial cancers [3]. Normally, the PTEN gene plays a role in blocking cell cycle progression, inducing apoptosis, and negatively regulating the PI3-K/AKT cell survival pathway via dephosphorylation and inactivation of PIP2, PIP3 and AKT. With a loss of PTEN function, one observes the constitutive activation of the PI3K/AKT signal transduction pathway, a characteristic of many cancers [4]. This constitutive activation results in the inhibition of several downstream proapoptotic targets through phosphorylation, including the Forkhead box O (FOXO) proteins [5,6].
The transcription factor FOXO1 is a member of the FOXO subfamily of the Forkhead/winged helix family and plays a role in several crucial cellular processes such as cell survival, cell-cycle progression, and oxidative-stress resistance [reviewed in 7]. In particular, FOXO1 is known to induce apoptosis through its localization to the cell nucleus and its transcription of several genes involved in the apoptotic pathway [8]. Cell homeostasis is maintained via the constant shuttling of FOXO1 between the nucleus and cytoplasm. When FOXO1 is phosphorylated by AKT at Thr24, Ser256 and Ser319 this causes export of FOXO1 from the nucleus to the cytoplasm [5,6]. A PTEN loss-of-function mutation therefore results in the constitutive action of AKT, the phosphorylation, inactivation, and nuclear export of FOXO1, and the inability to restrain cell cycle progression is thought to contribute to carcinogenesis [9, 10]. We and others have demonstrated that levels of FOXO1 in endometrial tumors and cancer cells are significantly lower or absent [11,12]. This is in part due to ubiquitination, involving Skp2, and degradation of the FOXO1 protein [11-13]. Overexpression of the constitutively active FOXO1 in endometrial cancer cells promoted cell cycle arrest and apoptosis [11] supporting its role as a tumor suppressor.
In the emerging field of targeted biologic therapy for the treatment of cancer, identifying small molecules that can modulate loss-of-function mutations, as is the case for PTEN, has proven difficult. Therefore, attention has shifted to finding targets downstream of such loss-of-function mutations, which might be more amenable to small molecule modulation. In the case of the PTEN mutation, discovering small molecules that could restore the function of FOXO1, thus compensating for the PTEN mutation, might ultimately prove more productive. We have shown that an AKT inhibitor, API-59CJ-OMe, promoted apoptosis and sensitized endometrial cancer cells to chemotherapeutic agents and that FOXO1 played an important role in this process [14]. In this study we investigate another small molecule, psammaplysene A (PsA), that has demonstrated ability to localize FOXO1 into the nucleus of numerous cancer cells [15]. PsA is a bromotyrosine derivative from a marine sponge of the Verongida order, genus Psammaplysilla, collected in the Indian Ocean. A cell-based screen of more than 18,000 compounds from the National Cancer Institutes Structural Diversity Set, Chemridge DiverSetE, and a small collection of NCI marine extracts were tested for their ability to relocalize FOXO1 to the nucleus in PTEN null cells. From these compounds, 89 relocalized FOXO1 to the nucleus and based on availability and potency, 42 compounds were further tested [15]. One compound that was identified as B6-7-1 and further analysis of this extract, including both activity-guided fractionation and fractionation via silica gel chromatography, revealed two pure active compounds which were named psammaplysene A and psammaplysene B, with PsA being the more active inhibitor of FOXO1 nuclear export [16]. Characterized as a dimeric bromotyrosine-derived metabolite with the molecular formula C27H35Br4N3O3, PsA has since been synthesized to allow for further study of its effects on FOXO1 [17,18]. We demonstrate in this study that treatment of type I endometrial cancer cell lines with PsA promotes apoptosis and that FOXO1 plays an active role in this process.
Material and Methods
Cell lines and reagents
The Ishikawa and ECC-1 endometrial cancer cell lines were provided by B. Lessey. Ishikawa cells were maintained in MEM (Invitrogen, Carlsbad, CA) and ECC1 cells were maintained in DMEM/F12 (Invitrogen) supplemented with 10% fetal bovine serum (FBS), sodium pyruvate, and antibiotics (penicillin G 5000 U/mL, streptomycin 5000μg/mL). The PsA was obtained from J. Clardy (Harvard Medical School, Boston, MA).
Immunofluorescent staining
Cells were grown on glass cover slips and then treated with either a control vehicle (DMSO) or PsA 100nM or PsA 1μM for 24 hours. Cells were then fixed with 4% paraformaldehyde (Sigma Chemical Co., St. Louis, MO) and cover slips were washed with phosphate-buffered NaCl solution and then permeabilized with 0.1% Triton-0.1% deoxycholate (Sigma). Cells were blocked with 5% bovine serum albumin (BSA, Sigma) made in PBS. Subsequently, the FOXO1 primary antibody (1:1000, Bethyl Laboratories, Montgomery, TX) made in 5% BSA was added to each sample and incubated for 2h at room temperature. A fluorescein secondary peroxidase-conjugated goat antirabbit IgG (1:50, Vector Laboratories Inc., Burlingame, CA) was used. Finally, cells were mounted with Vectashield Hard Set mounting medium for fluorescence and visualized using a fluorescent inverted microscope, Axiovert 200 (Zeiss, Jena, Germany).
Cell proliferation and viability assays
Ishikawa and ECC1 cells were trypsinized, counted, then plated in 96-well plates at a concentration of 2 × 104 cells/well and allowed to incubate overnight. Cells were then treated with either a control vehicle or increasing concentrations of PsA, 1nM, 10nM, 100nM and 1μM. After a 24-hour treatment period, a cell viability assay was performed. Using the Quick Cell Proliferation Assay Kit (BioVision, Mountain View, CA), 10μL WST-1 Reagent/ECS (electro-coupling solution) was added per well and incubated for 2 hours at 37C. Samples were then read on the Synergy HT from Bio-Tek (Winooski, VT) with the KC4 3.4 software at 420nm to determine cell viability. Cell proliferation was studied by using the pyrimidine analogue BrdU (5-bromo-2′-deoxyuridine, a thymidine substitute) which is incorporated into cellular DNA and measured with a colorimetric assay (Roche Penzberg, Germany). When treating the cells with either vehicle or increasing concentrations of PsA, the BrdU labeling solution was added to the cells 24h before harvesting. Cells were fixed and denatured using the provided FixDenat solution. An anti-Brdu monoclonal antibody conjugated with peroxidase was then added and the immune complexes detected with a subsequent substrate reaction. The reaction product was quantified by measuring absorbance at 370nm again using the Synergy HT ELISA reader (BioTek, Winooski, VT) using the KC4 3.4 software.
Cell cycle analysis
Cells were grown to 80% confluence then treated with vehicle or 100nM, 500nM, and 1μM PsA for 24 hours. Cells were trypsinized and fixed with 75% ethanol for 2 hours. Then, cells were resuspended in 1mL propidium iodide (PI) staining solution containing 50μg/mL PI (Sigma), 2mg RNAase A (Invitrogen), and 0.1% Triton X-100 (Fisher Scientific, Pittsburgh, PA) made in PBS. Cells were analyzed for G0/G1, S, and G2/M fractions on a Coulter EPICS-XL flow cytometer (Beckman Coulter, Fullerton, CA).
Western blot analysis
Total cell lysates were obtained using RIPA buffer [50mM Tris-HCL (pH 8.0), 150mM NaCl, 0.5% sodium deoxycholate, 1% IGEPAL (Sigma), 0.1% SDS] plus protease inhibitors and sodium orthovanadate. Protein concentration was measured using the micro BCA protein assay kit (Pierce). Isolated protein samples were run on a 10% Tris-HCL acrylamide precast gel (Bio-Rad, Hercules, CA) and were transferred onto polyvinylidene difluoride membranes (Whatman Inc, Sanford, ME). Membranes were blocked in 5% nonfat milk made with TBS-T at room temperature and incubated in FOXO1 primary antibody (1:5000 from Bethyl laboratories) or cleaved PARP primary antibody (1:1000 Cell Signaling) in 1% nonfat milk overnight at 4C. After the incubation, membranes were washed three times with TBS-T and incubated in secondary peroxidase-conjugated goat anti-rabbit IgG (1:10,000 for FOXO1, 1:2000 for cleaved PARP, BioRad) in 1% nonfat milk. Membranes were then washed and developed using either the ECL Plus Western Blot Detection System kit (Amersham, Piscataway, NJ) or the Supersignal West Dura Extended Duration Substrate (Pierce). Membranes were stripped with Restore Western Blot Stripping Buffer (Pierce) and reprobed with a monoclonal antibody to β-actin (1:10,000, Sigma).
Small interfering RNA (siRNA)
ECC1 cells were grown in 60-mm plates to 50% confluence Dharmacon (Lafayette, CO). SMARTpool siRNA control, targeted to the luciferase gene, and an siRNA specific to FOXO1 were transiently transfected into the cells using Lipofectamine 2000 (Invitrogen) according to the manufacturers protocol for siRNA transfection. Cells were transfected for 4 hours followed by removal of the transfection media and replacement with DMEM F12 (Invitrogen) with 10% FBS, sodium pyruvate and penicillin/streptomycin. Cells were kept in culture overnight then treated with either a control vehicle or 1μM PsA for 24 hours, at which time they were harvested with RIPA buffer for western blot analysis of total protein. Silencing of FOXO1 was verified by immunoblot analysis using FOXO1 antibody (1:5000, Bethyl).
Adenovirus infection
Ishikawa cells were grown to 60% confluence and then infected with 100 multiplicity of infection (MOI) with adenoviral constructs of either AD-CMV (empty vector), Wild-Type FOXO1 or the triple mutant FOXO1 (mutated at Thr24, Ser256 and Ser319 and which remains constitutively active) cDNAs for 24 hours. Then, cells were treated with either vehicle or 1μM PsA and incubated for 24 hours. Finally, total cell lysates were obtained using RIPA buffer and western blot analysis performed for both FOXO1 followed by cleaved PARP as detailed above.
Statistical analysis
Statistical analysis was performed using one-way ANOVA and the Student's t test for pairwise comparisons. P <0.05 was considered significant.
Results
Effect of PsA on FOXO1 localization in endometrial cancer cells
Two immortalized human endometrial cancer cell lines, Ishikawa and ECC1, were utilized in this study. These cell lines have been derived from well-differentiated, steroid-responsive endometrial adenocarcinomas and used in numerous studies as representative of type 1 endometrial cancer. The Ishikawa cell line is one of the best-characterized endometrial cell lines [19–24]. These cells maintain functional steroid receptors to estrogen, progesterone, and androgen. The ECC1 cells, although not as well studied, originate from a well differentiated adenocarcinoma that was passed in nude mice (25). These cells are also hormonally responsive and maintain estrogen receptors alpha and beta, progesterone receptors A and B, androgen receptors, and the steroid receptor coactivators NCOA1, NCOA2, and NCOA3 [26]. It has previously been reported that Ishikawa cells carry a PTEN mutation whereas ECC1 cells do not [27,28].
With the aforementioned knowledge that PsA can promote nuclear localization of FOXO1, thus contributing to cell cycle arrest and apoptosis, ECC1 and Ishikawa cells were treated with PsA and localization of FOXO1 was determined using immunofluorescent staining. Treatment with 1uM PsA indeed increased nuclear FOXO1 protein levels in both cell lines, regardless of PTEN status (Fig 1).
Figure 1. FOXO1 protein localization in ECC1 and Ishikawa cell lines after treatment with PsA.
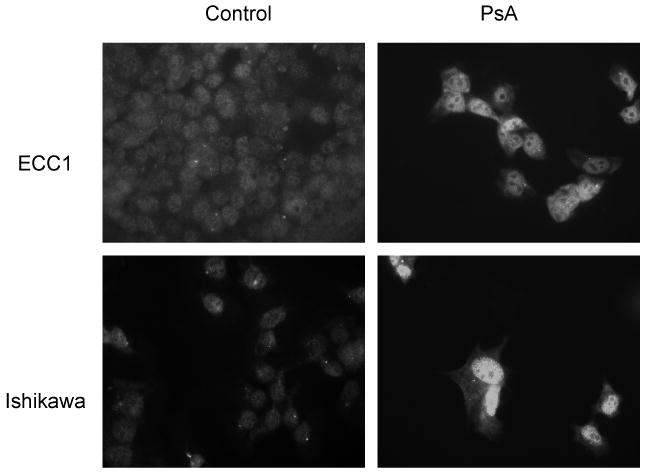
ECC1 and Ishikawa cells were treated with 1uM PsA for 24h. Immunofluorescent staining was done for FOXO1 and visualized under the fluorescent microscope. Magnification 630X.
Effects of PsA on Cell Viability, Proliferation and Apoptosis
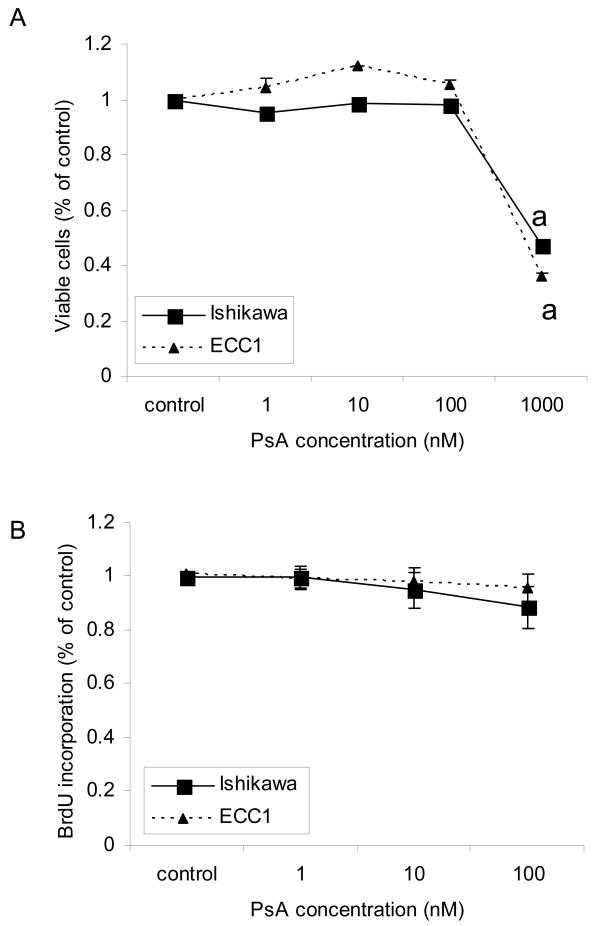
Treatment of ECC1 and Ishikawa cells with 1uM PsA for 24h caused significant cell death as evidenced by the dramatic decrease in the number of cells adherent to the polystyrene dish after approximately 16 hours of treatment (Fig 2). In order to quantify viability after treatment, ECC1 and Ishikawa cells were treated with increasing doses of PsA (10nM, 100nM, 500nM and 1uM) for 24h and a cell viability assay was done. As seen in Fig 3A, concentrations up to 100nM did not significantly affect viability of cells during the 24h treatment period whereas 1uM PsA significantly decreased the number of viable cells approximately 5-fold (p<0.05). In addition, cell proliferation was monitored by BrdU incorporation to determine whether PsA at concentrations that did not affect cell viability, affected proliferation. As shown in Fig 3B, cell proliferation was not significantly affected after treatment with 1nM, 10nM or 100nM PsA. Furthermore, cell cycle analysis confirmed that 100nM PsA did not change the distribution of cells whereas 1uM PsA significantly increased the percentage of cells by approximately 2-fold in the G2/M phase in both cell lines (Fig 4A, 4B; p<0.05). Finally, to determine whether 1uM PsA increased apoptosis in ECC1 and Ishikawa cells, western blot for cleaved PARP was done. Both ECC1 and Ishikawa cells underwent apoptosis at 1uM PsA as shown by the cleaved PARP product and not at 100nM PsA (Fig 4C).
Figure 2. Cell morphology after treatment with PsA. Effect of API-59CJ-OME on Ishikawa cells.
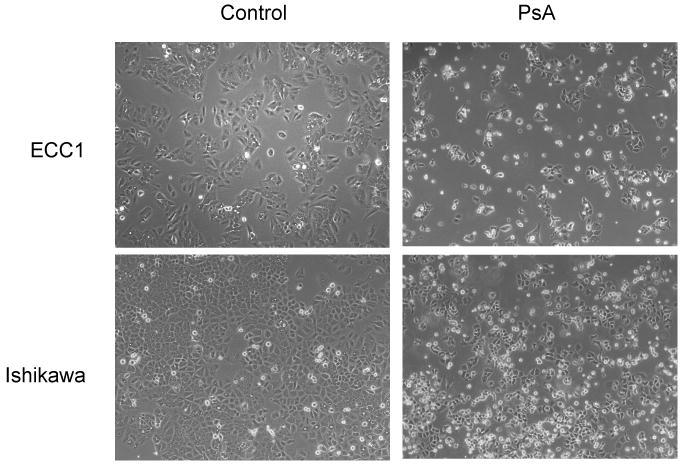
A. ECC1 and Ishikawa cells were treated with 1uM PsA for 16h. Cells were visualized using phase contrast microscopy. Magnification 100X.
Figure 3. Effect of PsA on cell viability and proliferation.
ECC1 and Ishikawa cells were treated with 0, 1, 10, 100, or 1000 nM PsA for 24h. A) Cell viability was measured using the WST assay and B) cell proliferation was measured using the BrdU incorporation assay. Data represent the mean ± SEM of six independent experiments. Statistical differences from the untreated control are noted as “a”
Figure 4. Effect of PsA on cell cycle progression and apoptosis.
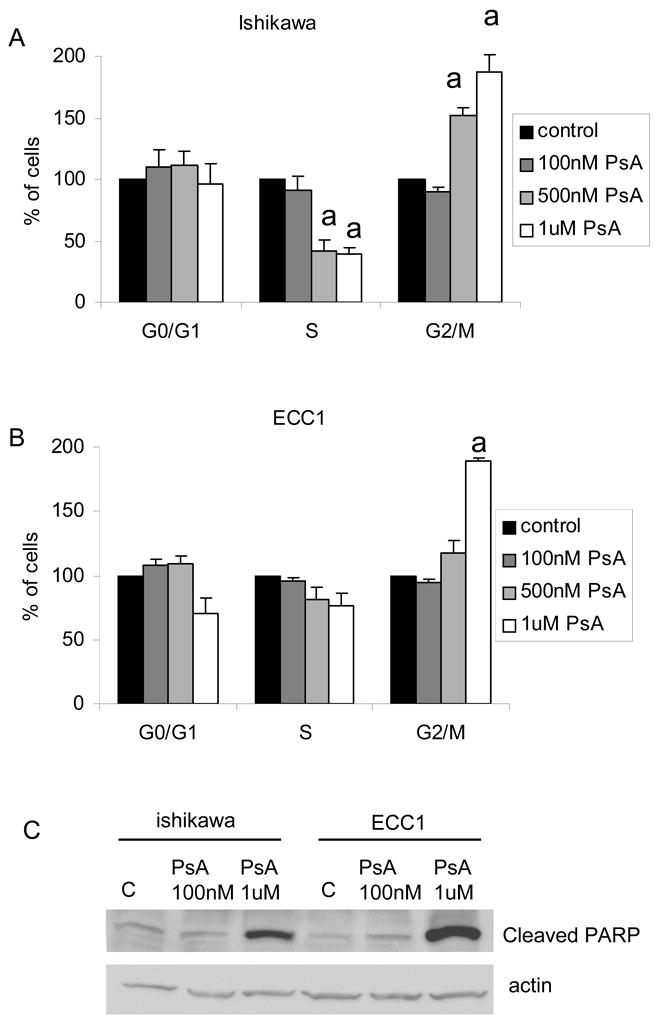
A) Ishikawa or B) ECC1 cells were treated with 100, 500 or 1000 nM PsA 24h and processed for cell cycle analysis using flow cytometry. Percentage of cells in each phase (Go/G1, S or G2/M) are presented. Data represent the mean ± SEM of three independent experiments. Statistical differences from the corresponding untreated control are noted as “a” C) Ishikawa or ECC1 cells were treated with 100nM or 1uM PsA for 24h. Cell lysates were run on Western blot for cleaved PARP and actin. Data are representative of at least three independent experiments.
The role of FOXO1 in PsA-induced apoptosis
Our data thus far demonstrate that PsA increases nuclear FOXO1 protein expression and is promoting apoptosis in both the Ishikawa and ECC1 cells. In order to determine whether FOXO1 contributes to PsA-induced apoptosis, we first knocked down FOXO1 in ECC1 cells using siRNA. ECC1 cells were chosen since levels of endogenous FOXO1 protein were higher than those of Ishikawa cells (Fig 5A). Transient transfection of either the control (siControl) siRNA or siRNA to FOXO1 (siFOXO1) was done in ECC1 cells, followed by treatment with vehicle or 1uM PsA for 24h. Western blots showed that FOXO1 was efficiently silenced in both untreated and PsA treated cells (Fig 5B). Increased levels of cleaved PARP were also found in cells treated with PsA in both siControl and siFOXO1 transfected cells. Interestingly, lower levels of FOXO1 were observed in siFOXO1 transfected cells treated with PsA compared to the siControl cells treated with PsA. These data highly implicate FOXO1 to play a significant role in PsA-induced apoptosis of ECC1 cells.
Figure 5. Role of FOXO1 in PsA-induced apoptosis.
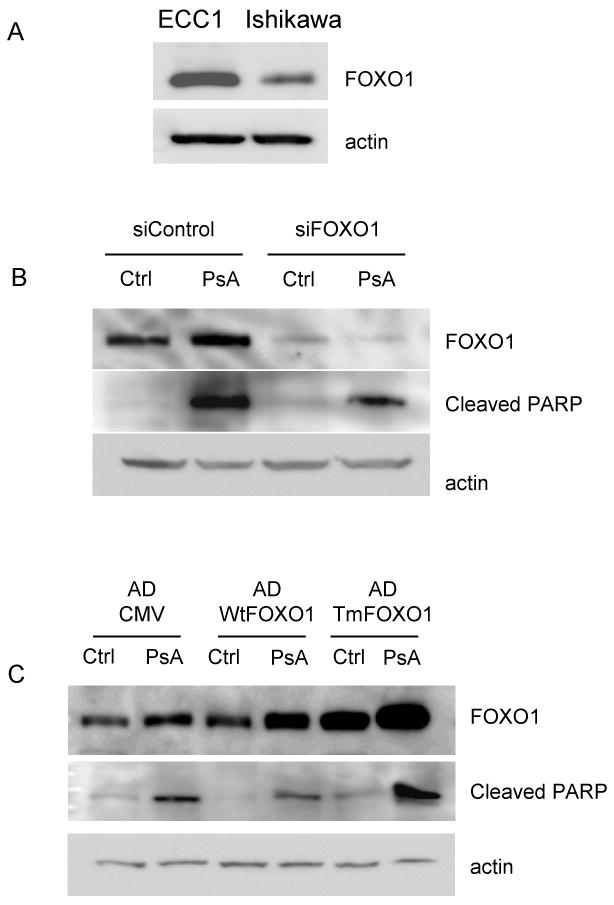
A) Ishikawa and ECC1 cells lysates were run on Western blot for FOXO1 and actin. B) ECC1 cells were transiently transfected with siRNA to FOXO1 (siFOXO1) or to a control luciferase gene (siControl). Cells were then treated with 1uM PsA for 24h. Western blots for FOXO1, cleaved PARP and actin were done. C) Ishikawa cells were infected with adenoviruses containing constructs for empty CMV (AD-CMV), wild type FOXO1 (WtFOXO1) or the triple mutant FOXO1 (TmFOXO1) cDNAs. Cells were then treated with 1uM PsA for 24h. Western blots for FOXO1, cleaved PARP, and actin were done. Data are representative of at least three independent experiments.
As another approach, recombinant FOXO1 was overexpressed in Ishikawa cells, which have low endogenous FOXO1. Ishikawa cells were infected with adenoviruses containing the empty CMV vector (ADCMV), the wildtype FOXO1 (WtFOXO1) or the triple-mutant FOXO1 (Tm-FOXO1) cDNAs. Cells were then treated with vehicle or 1uM PsA. As expected, the protein expression of FOXO1 was highest in the cells infected with TM-FOXO1 (Fig 5C). Interestingly, treatment of cells with PsA increased total FOXO1 protein in AD-CMV, WtFOXO1 and TmFOXO1 infected cells. PsA treatment also caused an increase in cleaved PARP product indicative of increased apoptosis. The highest levels of cleaved PARP was observed in AD-TmFOXO1 infected cells treated with PsA. These results suggest that higher FOXO1 levels promote apoptosis, supporting the observations in Fig 5B.
Discussion
The search for cytotoxic agents active against type 1 endometrial cancer will continue to expand into the realm targeted drug therapy. The PI3K/AKT signaling pathway is crucial to many aspects of cell growth and survival, and its activation, often via a PTEN mutation, results in a disturbance of control of cell growth and survival, contributing to competitive growth advantage, metastatic competence, and, not infrequently, resistance to therapy. Acknowledging that inhibition of the PI3K/AKT signaling pathway in PTEN null cells can control deviant cell growth, psammaplysene A shows promise as a modulator of downstream targets of the PTEN tumor suppressor gene mutation, specifically the transcription factor FOXO1. In this study, we demonstrate that in endometrial cancer cells treated with PsA, nuclear localization of FOXO1 is increased, that decreased cell viability via increased apoptosis occurs, and that FOXO1 plays a significant role in PsA-induced apoptosis. Interestingly, these findings were demonstrated in human endometrial cancer cell lines both with and without PTEN mutations.
The role of FOXO transcription factors was initially suggested by the observation that three of the four known FOXO genes were found at chromosomal breakpoints in certain types of tumor, such as rhabdomyosarcoma for FOXO1 and acute myeloid leukemia for FOXO 3 and FOXO4 [29]. FOXO factors play a pivotal role in cell fate decisions, and the loss of FOXO1 function has been identified as a critical event in tumorigenesis, compromising the cells ability to inhibit proliferation and promote death, or apoptosis. In this study, using cleaved PARP as an apoptotic marker, we observed that endometrial cancer cells treated with PsA underwent apoptosis at much higher rates than untreated cells. In the presence of PsA, by either enforcing nuclear localization (PTEN null ishikawa cells) or increasing the already existing nuclear levels of FOXO1 (PTEN + ECC1 cells), FOXO1 triggered apoptosis. Modur et al [30] found that in PTEN-deficient prostate carcinoma cell lines, FOXO1 and FOXO3 were cytoplasmically sequestered and inactive and that expression of the pro-apoptotic marker TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) was decreased. Overexpression of FOXO1 and FOXO3 in this cell line resulted in apoptosis and increased expression of TRAIL, suggesting that FOXO proteins regulate cell survival by modulating the expression of death receptor ligands such as TRAIL and FasL. In addition to these death receptor ligands, FOXO proteins have been shown to be involved in the activation of the Bcl-2 family, most notably the pro-apoptotic protein Bim. Sunters et al [31] showed that in paclitaxel-sensitive breast cancer, upregulation of FOXO3a by paclitaxel resulted in increased level of Bim mRNA and protein, leading to apoptosis in breast cancer cells. While an exact apoptotic pathway was not investigated in our study, PsA appears to be modulating FOXO1 levels and localization regardless of the PTEN mutation status. Treatment of cells with PsA also increased total FOXO1 protein levels suggesting an increase in FOXO1 mRNA expression and/or rescue of FOXO1 protein from ubiquitination and proteosomal degradation.
Our study is the first to demonstrate the effects of PsA in human endometrial cancer cells. Kau et al [15] determined that PsA acted neither through CRM1 nuclear export receptor inhibition nor through reduced AKT phosphorylation to enforce FOXO1 in the nucleus. It was deduced that PsA interacts with a still-unknown target, downstream of AKT, to inhibit FOXO1 nuclear export in the presence of a PTEN mutation [15]. Marine sponges of the order Verongida are known to produce a wide variety of structurally diverse bromotyrosine derivatives, from simple bromotyrosine monomers to the more complex bastadins, cyclic macromolecules often containing several bromotyrosine units [32]. The marine sponge Psammaplysilla is responsible for another group of bromotyrosine derivatives, the psammaplins which are distinct from psammaplysenes. Since its discovery in 1987, psammaplin A is one of the most studied of this group. Initial work attributed psammaplin A with having antibacterial activity as well as anti-enzyme mechanisms such as the inhibition of mycothiol-S-conjugate amidase, topoisomerase II, and chitinase [33-35]. A study by Park et al [32] demonstrated this compound to have cytoxicity towards several human cancer cell lines including lung, ovarian, and colon cancers, with a mechanism of action not established. Since then, further work to determine anti-tumor effects has demonstrated that Psammaplin A can suppress angiogenesis in vitro through the inhibition of aminopeptidase N (APN) [36] and can inhibit the actions of histone deacetylase (HDAC) and DNA methyltransferase (DN-MT) [37,38] in human cervical, breast, and lung cancer cell lines. In a very recent report by Ahn et al [39], Psammaplin A was found to inhibit cell proliferation in Ishikawa cells through cell cycle arrest at the G1 or G2/M phases, and to induce apoptotic cell death. This line of investigation is promising as HDAC inhibitors are believed to be promising classes of new antitumor agents in current clinical trials. Now, with the identification of Psammaplysene A and its actions in the PI3K/AKT/FOXO1 pathway, the opportunity to incorporate these various marine sponge extracts into new and existing cytotoxic regimens for the treatment of endometrial cancer seems more and more promising.
The FOXO1 transcription factors lies at the interface of several crucial cellular processes including apoptosis, cell-cycle progression, and oxidative-stress resistance. As the role of phosphorylation of FOXO proteins in tumorigenesis becomes increasingly apparent, further work is needed to elucidate the role of post-translational modifications that influence transcriptional activity. In this study we have demonstrated a relationship between the marine sponge extract PsA and its ability to enforce nuclear localization of FOXO1 in endometrial cancer cells. Understanding the mechanisms of action of PsA could pave the way towards use of PsA as an adjunct targeted therapy in the treatment of advanced or recurrent grade 1 endometrial cancer.
Acknowledgments
Funded by: Grants from NIH, CA127674 (JJK) and CA24487 (JC) and a grant from the Friends of Prentice (JJK)
Footnotes
Conflicts of Interests: The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Fanning J, Evans MC, Peters AJ, Samuel M, Harmon ER, Bates JS. Endometrial adenocarcinoma histologic subtypes: clinical and pathologic profile. Gynecol Oncol. 1989;32:288–291. doi: 10.1016/0090-8258(89)90626-4. [DOI] [PubMed] [Google Scholar]
- 3.Tashiro H, Blazes MS, Wu R, Cho KR, Bose S, Wang SI, Li J, Parsons R, Ellenson LH. Mutations in PTEN are frequent in endometrial carcinoma but rare in other common gynecological malignancies. Cancer Res. 1997;57:3935–3940. [PubMed] [Google Scholar]
- 4.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 5.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 6.Biggs WH, 3rd, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci U S A. 1999;96:7421–7426. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang H, Tindall DJ. FOXO factors: a matter of life and death. Future Oncol. 2006;2:83–89. doi: 10.2217/14796694.2.1.83. [DOI] [PubMed] [Google Scholar]
- 8.Alikhani M, Alikhani Z, Graves DT. FOXO1 functions as a master switch that regulates gene expression necessary for tumor necrosis factor-induced fibroblast apoptosis. J Biol Chem. 2005;280:12096–12102. doi: 10.1074/jbc.M412171200. [DOI] [PubMed] [Google Scholar]
- 9.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 10.Burgering BM, Kops GJ. Cell cycle and death control: long live Forkheads. Trends Biochem Sci. 2002;27:352–360. doi: 10.1016/s0968-0004(02)02113-8. [DOI] [PubMed] [Google Scholar]
- 11.Ward EC, Hoekstra AV, Blok LJ, Hanifi-Moghaddam P, Lurain JR, Singh DK, Buttin BM, Schink JC, Kim JJ. The regulation and function of the forkhead transcription factor, Forkhead box O1, is dependent on the progesterone receptor in endometrial carcinoma. Endocrinology. 2008;149:1942–1950. doi: 10.1210/en.2007-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goto T, Takano M, Albergaria A, Briese J, Pomeranz KM, Cloke B, Fusi L, Feroze-Zaidi F, Maywald N, Sajin M, Dina RE, Ishihara O, Takeda S, Lam EW, Bamberger AM, Ghaem-Maghami S, Brosens JJ. Mechanism and functional consequences of loss of FOXO1 expression in endometrioid endometrial cancer cells. Oncogene. 2008;27:9–19. doi: 10.1038/sj.onc.1210626. [DOI] [PubMed] [Google Scholar]
- 13.Huang H, Regan KM, Wang F, Wang D, Smith DI, van Deursen JM, Tindall DJ. Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc Natl Acad Sci U S A. 2005;102:1649–1654. doi: 10.1073/pnas.0406789102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoekstra AV, Ward EC, Hardt JL, Lurain JR, Singh DK, Buttin BM, Schink JC, Kim JJ. Chemosensitization of endometrial cancer cells through AKT inhibition involves FOXO1. Gynecol Oncol. 2008;108:609–618. doi: 10.1016/j.ygyno.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Kau TR, Schroeder F, Ramaswamy S, Wojciechowski CL, Zhao JJ, Roberts TM, Clardy J, Sellers WR, Silver PA. A chemical genetic screen identifies inhibitors of regulated nuclear export of a Forkhead transcription factor in PTEN-deficient tumor cells. Cancer Cell. 2003;4:463–476. doi: 10.1016/s1535-6108(03)00303-9. [DOI] [PubMed] [Google Scholar]
- 16.Schroeder FC, Kau TR, Silver PA, Clardy J. The psammaplysenes, specific inhibitors of FOXO1a nuclear export. J Nat Prod. 2005;68:574–576. doi: 10.1021/np049624z. [DOI] [PubMed] [Google Scholar]
- 17.Georgiades SN, Clardy J. Total synthesis of psammaplysenes A and B, naturally occurring inhibitors of FOXO1a nuclear export. Org Lett. 2005;7:4091–4094. doi: 10.1021/ol0513286. [DOI] [PubMed] [Google Scholar]
- 18.Georgiades SN, Clardy J. Preparation of a psammaplysene-based library. Org Lett. 2006;8:4251–4254. doi: 10.1021/ol061599w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishida M, Kasahara K, Kaneko M, Iwasaki H, Hayashi K. Establishment of a new human endometrial adenocarcinoma cell line, Ishikawa cells, containing estrogen and progesterone receptors. Nippon Sanka Fujinka Gakkai Zasshi. 1985;37:1103–1111. [PubMed] [Google Scholar]
- 20.Hata H, Holinka CF, Pahuja SL, Hochberg RB, Kuramoto H, Gurpide E. Estradiol metabolism in Ishikawa endometrial cancer cells. J Steroid Biochem. 1987;26:699–704. doi: 10.1016/0022-4731(87)91042-9. [DOI] [PubMed] [Google Scholar]
- 21.Croxtall JD, Elder MG, White JO. Hormonal control of proliferation in the Ishikawa endometrial adenocarcinoma cell line. J Steroid Biochem. 1990;35:665–669. doi: 10.1016/0022-4731(90)90306-d. [DOI] [PubMed] [Google Scholar]
- 22.Lessey BA, Ilesanmi AO, Castelbaum AJ, Yuan L, Somkuti SG, Chwalisz K, Satyaswaroop PG. Characterization of the functional progesterone receptor in an endometrial adenocarcinoma cell line (Ishikawa): progesterone-induced expression of the alpha1 integrin. J Steroid Biochem Mol Biol. 1996;59:31–39. doi: 10.1016/s0960-0760(96)00103-3. [DOI] [PubMed] [Google Scholar]
- 23.Castelbaum AJ, Ying L, Somkuti SG, Sun J, Ilesanmi AO, Lessey BA. Characterization of integrin expression in a well differentiated endometrial adenocarcinoma cell line (Ishikawa) J Clin Endocrinol Metab. 1997;82:136–142. doi: 10.1210/jcem.82.1.3658. [DOI] [PubMed] [Google Scholar]
- 24.Lovely LP, Appa Rao KB, Gui Y, Lessey BA. Characterization of androgen receptors in a well-differentiated endometrial adenocarcinoma cell line (Ishikawa) J Steroid Biochem Mol Biol. 2000;74:235–241. doi: 10.1016/s0960-0760(00)00127-8. [DOI] [PubMed] [Google Scholar]
- 25.Satyaswaroop PG, Zaino RJ, Mortel R. Human endometrial adenocarcinoma transplanted into nude mice: growth regulation by estradiol. Science. 1983;219:58–60. doi: 10.1126/science.6849115. [DOI] [PubMed] [Google Scholar]
- 26.Mo B, Vendrov AE, Palomino WA, DuPont BR, Apparao KB, Lessey BA. ECC-1 cells: a well-differentiated steroid-responsive endometrial cell line with characteristics of luminal epithelium. Biol Reprod. 2006;75:387–394. doi: 10.1095/biolreprod.106.051870. [DOI] [PubMed] [Google Scholar]
- 27.Jin X, Gossett DR, Wang S, Yang D, Cao Y, Chen J, Guo R, Reynolds RK, Lin J. Inhibition of AKT survival pathway by a small molecule inhibitor in human endometrial cancer cells. Br J Cancer. 2004;91:1808–1812. doi: 10.1038/sj.bjc.6602214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou C, Gehrig PA, Whang YE, Boggess JF. Rapamycin inhibits telomerase activity by decreasing the hTERT mRNA level in endometrial cancer cells. Mol Cancer Ther. 2003;2:789–795. [PubMed] [Google Scholar]
- 29.Barr FG. Gene fusions involving PAX and FOX family members in alveolar rhabdomyosarcoma. Oncogene. 2001;20:5736–5746. doi: 10.1038/sj.onc.1204599. [DOI] [PubMed] [Google Scholar]
- 30.Modur V, Nagarajan R, Evers BM, Milbrandt J. FOXO proteins regulate tumor necrosis factor-related apoptosis inducing ligand expression. Implications for PTEN mutation in prostate cancer. J Biol Chem. 2002;277:47928–47937. doi: 10.1074/jbc.M207509200. [DOI] [PubMed] [Google Scholar]
- 31.Sunters A, Fernandez de Mattos S, Stahl M, Brosens JJ, Zoumpoulidou G, Saunders CA, Coffer PJ, Medema RH, Coombes RC, Lam EW. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J Biol Chem. 2003;278:49795–49805. doi: 10.1074/jbc.M309523200. [DOI] [PubMed] [Google Scholar]
- 32.Park Y, Liu Y, Hong J, Lee CO, Cho H, Kim DK, Im KS, Jung JH. New bromotyrosine derivatives from an association of two sponges, Jaspis wondoensis and Poecillastra wondoensis. J Nat Prod. 2003;66:1495–1498. doi: 10.1021/np030162j. [DOI] [PubMed] [Google Scholar]
- 33.Kim D, Lee IS, Jung JH, Lee CO, Choi SU. Psammaplin A, a natural phenolic compound, has inhibitory effect on human topoisomerase II and is cytotoxic to cancer cells. Anticancer Res. 1999;19:4085–4090. [PubMed] [Google Scholar]
- 34.Nicholas GM, Eckman LL, Ray S, Hughes RO, Pfefferkorn JA, Barluenga S, Nicolaou KC, Bewley CA. Bromotyrosine-derived natural and synthetic products as inhibitors of mycothiol-S-conjugate amidase. Bioorg Med Chem Lett. 2002;12:2487–2490. doi: 10.1016/s0960-894x(02)00385-2. [DOI] [PubMed] [Google Scholar]
- 35.Tabudravu JN, Eijsink VG, Gooday GW, Jaspars M, Komander D, Legg M, Synstad B, van Aalten DM. Psammaplin A, a chitinase inhibitor isolated from the Fijian marine sponge Aplysinella rhax. Bioorg Med Chem. 2002;10:1123–1128. doi: 10.1016/s0968-0896(01)00372-8. [DOI] [PubMed] [Google Scholar]
- 36.Shim JS, Lee HS, Shin J, Kwon HJ. Psammaplin A, a marine natural product, inhibits aminopeptidase N and suppresses angiogenesis in vitro. Cancer Lett. 2004;203:163–169. doi: 10.1016/j.canlet.2003.08.036. [DOI] [PubMed] [Google Scholar]
- 37.Kim DH, Shin J, Kwon HJ. Psammaplin A is a natural prodrug that inhibits class I histone deacetylase. Exp Mol Med. 2007;39:47–55. doi: 10.1038/emm.2007.6. [DOI] [PubMed] [Google Scholar]
- 38.Pina IC, Gautschi JT, Wang GY, Sanders ML, Schmitz FJ, France D, Cornell-Kennon S, Sambucetti LC, Remiszewski SW, Perez LB, Bair KW, Crews P. Psammaplins from the sponge Pseudoceratina purpurea: inhibition of both histone deacetylase and DNA methyltransferase. J Org Chem. 2003;68:3866–3873. doi: 10.1021/jo034248t. [DOI] [PubMed] [Google Scholar]
- 39.Ahn MY, Jung JH, Na YJ, Kim HS. A natural histone deacetylase inhibitor, Psammaplin A, induces cell cycle arrest and apoptosis in human endometrial cancer cells. Gynecol Oncol. 2008;108:27–33. doi: 10.1016/j.ygyno.2007.08.098. [DOI] [PubMed] [Google Scholar]