Abstract
Rationale: Cystic fibrosis is caused by defects in the cystic fibrosis transmembrane conductance regulator gene, which codes for a chloride channel, but the role of this chloride channel in inflammation induced by lung infection with Pseudomonas aeruginosa remains to be defined.
Objectives: We tested the hypothesis that loss of this chloride channel alone is sufficient to cause excessive inflammation in response to inflammatory stimuli.
Methods: We investigated the response of cystic fibrosis and wild-type mice to mucoid P. aeruginosa administered by insufflation.
Measurements: The host responses measured included survival, weight change, lung morphometry, bacterial clearance, and inflammatory mediators, and cell counts were assessed in bronchoalveolar lavage fluid.
Main Results: Depending on the dose administered and frequency of dosing, cystic fibrosis mice experienced significantly higher mortality rates, greater weight loss, higher lung pathology scores, and higher inflammatory mediator and neutrophil levels compared with wild-type mice, even after the bacteria had been cleared. Surprisingly, bacteria were cleared just as rapidly in cystic fibrosis mice as in wild-type mice, and sepsis was not observed. Chronic lung infections could not be established with mucoid P. aeruginosa in either cystic fibrosis or wild-type mice.
Conclusions: Absence of this chloride channel alone appears sufficient for exaggerated inflammation and excess mortality compared with wild-type controls in the face of mucoid P. aeruginosa lung infection. To establish chronic infection, additional factors such as bacterial trapping or poor clearance may be required.
Keywords: cystic fibrosis, cystic fibrosis transmembrane conductance regulator, inflammation, pathogenesis
Cystic fibrosis (CF) is caused by mutations in the CF transmembrane conductance regulator gene (CFTR). Patients with CF are not able to effectively eliminate opportunistic pathogens such as Pseudomonas aeruginosa, which is the leading cause of morbidity and mortality in CF. Whether defects in CFTR necessarily lead to lung infection is not clear, because mice with absent murine CFTR homolog (Cftr) function do not develop spontaneous lung infection. However, Cftr mutant mice reportedly lack the marked up-regulation of activity of the epithelial sodium channel (ENaC) in their lower airways that is observed in humans with CF (1–3). A recently developed mouse model overexpressing the β subunit of ENaC expresses normal Cftr, yet exhibits mucus plugging, bacterial trapping, and inflammatory changes characteristic of the human CF lung (4–6). It is a short step to infer from these data that the dysregulation of ENaC, and not defects in Cftr per se, predisposes to the lung disease characteristic of CF. However, it is not clear, in the ENaC-overexpressing mouse, whether the inflammatory response is either (1) appropriate to the bacterial or other stimulus or (2) excessive, as it seems to be in patients with CF.
Several lines of evidence suggest that mice with defective Cftr have exaggerated inflammation compared with wild-type control animals in response to several organisms, including aerosolized Staphylococcus aureus and Burkholderia cepacia (7, 8), and repeated intranasal applications of B. cepacia have led to chronic lung infection in Cftr-deficient mice, much like that observed in patients with CF (9). It has been difficult to study responses to P. aeruginosa in mice because, at lower inoculums, the organism is rapidly cleared and at higher doses there is considerable mortality (10). We and others have approached this problem by embedding the P. aeruginosa in a matrix to retard their clearance (11–14), and have demonstrated that infected mice with defective Cftr have an exaggerated inflammatory response and greater mortality compared with wild-type control mice. In some of these studies, the exaggerated inflammatory response is associated with increased numbers of bacteria cultured from the lung, but in our own studies, it is not. However, one study demonstrated that a mucoid clinical strain of P. aeruginosa (FRD1) delivered transtracheally without matrix led to significantly greater tumor necrosis factor (TNF)-α levels in lung homogenates of CF mice compared with wild-type mice, although surprisingly, bacterial clearance rates were similar (15). Their findings led us to hypothesize that deficiency of Cftr function is sufficient to produce excessive inflammation in response to bacterial challenge; therefore, we further examined the host inflammatory response of CF and wild-type mice to free-living mucoid P. aeruginosa. Some of the results of these studies have been previously reported in the form of abstracts (16, 17).
METHODS
Mice
Congenic B6.129P2-Cftrtm1Unc heterozygotes (stock no. 2196; Cftr+/− or wild-type mice) and stock Cftrtm1Unc-TgN(FABPCFTR)#Jaw mice (stock no. 2364; CF mice) were originally obtained from the Jackson Laboratory (Bar Harbor, ME). Details and rationale of the mice used can be found in the online supplement, as well as information about animal husbandry. Only female mice were used in these studies. Wild-type mice used in lung infection studies were Cftr+/− mice. All procedures were approved by Case Western Reserve University's Animal Care and Use Committee.
P. aeruginosa Infection of Mice
Mice were 7.1 ± 1.1 wk old and weighed 18.0 ± 1.9 g at the time of inoculation. Mice were anesthetized with isoflurane using a vaporizer (VetEquip, Inc., Pleasanton, CA). Mice were inoculated with 0.02 ml of bacterial broth (105–109 cfu/mouse) by insufflation using a sterile pipette placed just above the nostril. Details are provided in the online supplement.
Bronchoalveolar Lavage
Mice were killed using greater than 70% carbon dioxide in air followed by exsanguination by direct cardiac puncture, in accordance with 2000 American Veterinary Medical Association Panel on Euthanasia Guidelines. After the mice were killed, bronchoalveolar lavage (BAL) was performed, and cell counts were performed on resuspended cell pellets; the sterile filtered supernatant was processed for evaluation of mediators, as described elsewhere (18). Murine cytokines measured were TNF-α, interleukin (IL)-1β, IL-6, IL-4, IL-5, IL-10, IL-12, and IFN-γ read by a Luminex 100 using murine Lincoplex Multiplex immunoassay kits (Linco Research, Inc., St. Charles, MO) and reagents from R&D Systems (Minneapolis, MN) to generate standard curves. The murine neutrophil chemokines, macrophage inflammatory protein (MIP)-2, and keratinocyte chemoattractant (KC) were measured by ELISA, according to the manufacturer's recommendations (R&D Systems) or as with the aforementioned mediators. Values that fell below the limits of detection for the assay were assigned a value equal to the lowest limit of detection for each assay. Values were corrected for urea dilution (19) and expressed as ng/ml epithelial lining fluid (ELF).
Lung Histopathology
After BAL, lungs were prepared for histologic examination. Five-micron sections were stained with hematoxylin and eosin (H&E), and adjacent sections were stained with alcian blue and periodic acid–Schiff (PAS). The area of the lung that was inflamed was assessed from H&E- stained sections by quantitative morphometric techniques. Details have been published previously (20). Qualitative production of mucin was assessed in airways and airway epithelial cells from alcian blue/PAS–stained slides.
Bacterial Clearance
The right and left lung were placed in 9 ml of cold, sterile phosphate-buffered saline and homogenized. Tenfold serial dilutions of lung homogenates were plated on tryptic soy agar, in duplicate, and the number of colonies counted 20 to 24 h after incubation at 37°C.
Statistics
The statistical software packages SigmaPlot version 2.03 or SAS version 9.1 (SAS Institute, Cary, NC) were used. Data are represented as the means ± SEM of the raw data. Data from untreated control animals are shown at time-point zero. Details of the statistical analyses are provided in the online supplement.
RESULTS
Effect of Isoflurane
Inhalation anesthetics can induce an inflammatory response in the lung after prolonged exposure (21, 22) and may therefore confound the inflammatory response to experimental P. aeruginosa infection in mice using isoflurane anesthesia. Thus, we determined the effect of 5 min of exposure to isoflurane anesthesia on the inflammatory response of CF and wild-type mice. These data are provided in Table E1 of the online supplement. Briefly, we detected no change in total or differential cell counts in BAL fluid, no increase in TNF-α and IL-1β levels above the limits of detection of the assays, and no significant change in IL-6 levels in ELF compared with untreated control animals. Thus, isoflurane is unlikely to confound our results.
Response of Mice to a Single Dose of Mucoid P. aeruginosa
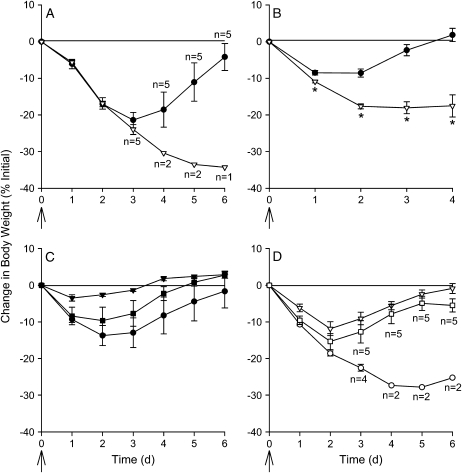
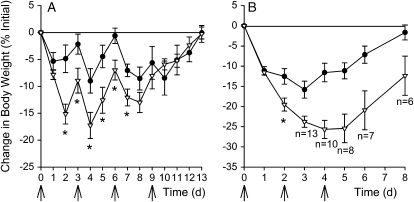
CF and wild-type mice were inoculated with a single dose of P. aeruginosa (n = 6/group). All mice receiving 109 cfu died 2 d after infection. Mice that received 108 cfu started to die 3 d after infection; CF mice had a greater mortality rate than wild-type mice 6 d after infection (83.3 and 16.7%, respectively; p = 0.08; Fisher's exact test). The change in body weight over time is shown in Figure 1A, with changes in sample size through death indicated in the figure. A single inoculation with 5 × 105 or 5 × 106 cfu administered per CF mouse (n = 10/group) was not associated with mortality or weight loss; however, a dose of 5 × 107 cfu administered per CF mouse was associated with significant weight loss yet no mortality where cultures of whole blood and spleen homogenates were negative, and BAL cultures showed only the organism inoculated. Combining data from six experiments, CF mice lost significantly (p ⩽ 0.006) more weight than wild-type mice 1, 2, 3, and 4 d after a single intranasal administration of P. aeruginosa (Figure 1B). Mortality was not observed in either CF or wild-type mice if the bacteria were fixed in gluteraldehyde. The changes in body weight over time are shown in Figures 1C and 1D, with changes in sample size through death indicated in Figure 1D. One CF mouse that received heat-killed bacteria died 3 d after inoculation; necropsy revealed a consolidated right cranial lung lobe.
Figure 1.
Weight change after a single inoculation with P. aeruginosa. Cystic fibrosis (CF; open symbols) and wild-type (closed symbols) mice were inoculated on Day 0 with P. aeruginosa by insufflation (indicated by the arrow). (A) Data are from a single experiment. Mice received approximately 108 cfu (6 mice/group), were weighed daily, and survivors were killed on Day 6. (B) Data were combined from six experiments. Mice were killed at each time point, leading to the decline in the number of mice over time. Mice received approximately 5 × 107 cfu, and weighed 1 (71 mice/group), 2 (53 mice/group), 3 (23–26 mice/group), and 4 d (12–13/group) after infection. Three CF mice died in the group to be killed on Day 3, and one died in the group to be killed on Day 4. *Significantly different (p ⩽ 0.006) from wild-type mice using a two-way analysis of variance (ANOVA) model to test for differences between the groups while controlling for the experiment. The Bonferroni method was used to adjust for multiple testing; a p value < 0.01 was considered significant. (C) Wild-type and (D) CF mice (n = 6/group) were inoculated with either live bacteria (circles), gluteraldehyde-fixed bacteria (triangles), or heat-killed bacteria (squares). Reductions in sample sizes indicated in A and D are due to spontaneous death due to overwhelming pulmonary infection. As a result of censorship by death, statistical analysis was not performed.
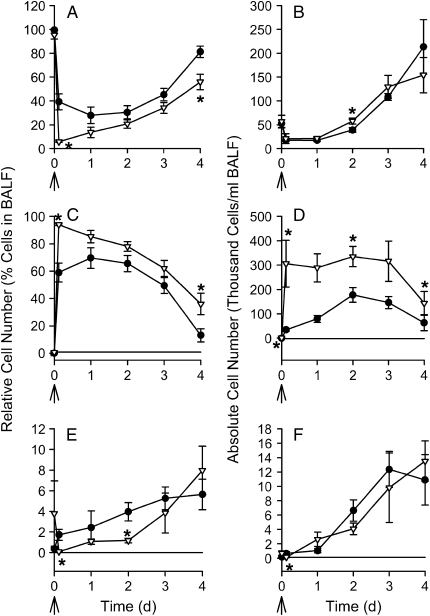
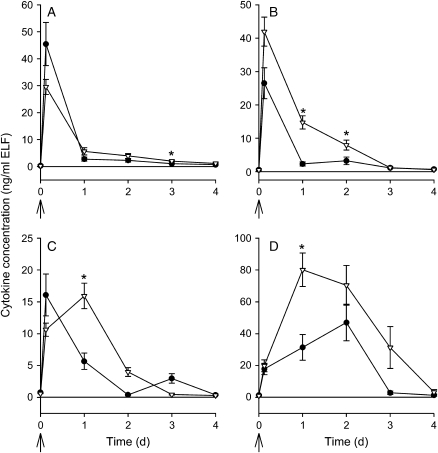
The host inflammatory response, as measured by BAL cell counts and ELF cytokines, was assessed in mice represented in Figure 1B. Relative and absolute cell counts (i.e., differential cell counts and total leukocyte counts) were determined from the BAL fluid (Figure 2); there were significant differences (p ⩽ 0.009) in the cell counts between CF mice and wild-type mice at several time points as indicated in the figure. At earlier time points, differences in relative cell counts were observed starting 2 h after infection (Figure E1). Results from TNF-α, IL-6, MIP-2, and KC are shown in Figure 3, and significant differences between CF and wild-type mice are indicated in the figure. Other cytokine levels were either at or below detectable limits (IL-12, IL-13, IFN-γ, and granulocyte-macrophage colony–stimulating factor), or were not significantly different between CF and wild-type mice (IL-1β and IL-10) and therefore the data are not shown.
Figure 2.
Leukocytes in bronchoalveolar lavage fluid (BALF) after a single inoculation with P. aeruginosa. Wild-type (closed circles) and CF mice (open triangles) were inoculated with a single dose of P. aeruginosa. Mice were killed 3 h, (n = 18/group), 1 d (18 mice/group), 2 d (27 mice/group), 3 d (11–13 mice/group), and 4 d (12–13/group) after infection (B), and represent mice from Figure 1B. BAL was performed and relative or differential (A, C, and E) and absolute or total numbers (B, D, and F) of alveolar macrophages (A and B), neutrophils (C and D), and lymphocytes (E and F) were enumerated, as described. *Significantly different (p ⩽ 0.009) from wild-type control mice at the same time point (due to data that were not normally distributed, the Van Elteren's test was used adjusting for differences between experiments and the Bonferroni method was used to adjust for multiple testing; p < 0.01 was considered significant). Arrows indicate the timing of infection. Data points at t = 0 represent untreated mice.
Figure 3.
Cytokine response after a single inoculation with P. aeruginosa. Wild-type (closed circles) and CF mice (open triangles) were inoculated with a single dose of P. aeruginosa. Mice were killed 3 h (n = 18/group), 1 d (18 mice/group), 2 d (27 mice/group), 3 d (11–13 mice/group), and 4 d (12–13/group) after infection, and represent mice from Figure 1B. BAL was performed and inflammatory mediators (A) macrophage inflammatory protein-2, (B) keratinocyte chemoattractant, (C) tumor necrosis factor-α, and (D) interleukin-6 were measured, as described. Data are normalized for urea dilution (ng/ml epithelial lining fluid [ELF]). Arrows indicate the timing of infection. Data points at t = 0 represent untreated mice. *Significantly different from wild-type control mice at the same time point (Van Elteren's test was used adjusting for differences between experiments and the Bonferroni method was used to adjust for multiple testing; p < 0.01 was considered significant).
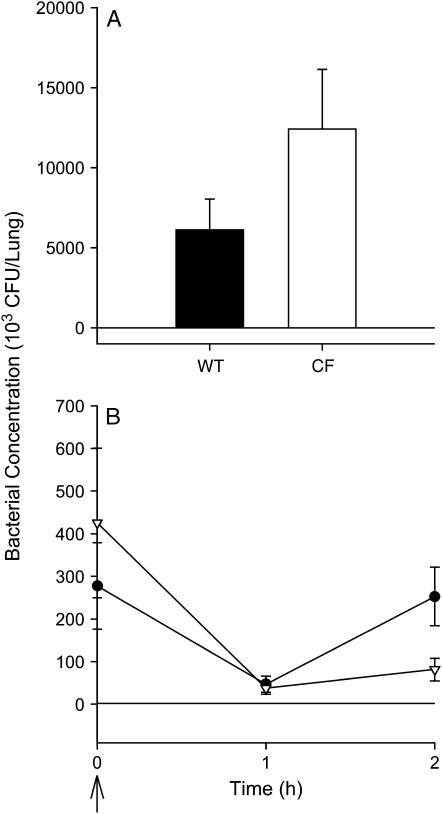
The numbers of bacteria were quantitated in the lungs of mice after a single intranasal inoculation with P. aeruginosa in three experiments (Figure 4). The number of bacteria recovered in lung homogenates was significantly less than the inoculum (∼ 5 × 107 cfu/mouse) immediately after inoculation, and 1, 2, and 3 h later in both CF and wild-type mice. By 1 to 2 d after infection, bacteria were no longer detectable in most mice from either strain. There were no significant differences in the number of bacteria cultured from CF and wild-type mice at any time point studied.
Figure 4.
Bacterial numbers in lung homogenates after a single inoculation with P. aeruginosa. CF (open bar, open triangles) and wild-type (WT) mice (solid bar, closed circles) were inoculated with a single dose of P. aeruginosa. (A) WT and CF mice (n = 26 and 27, respectively; data combined from four experiments) were killed 3 h after infection (average, 6 × 107 cfu/mouse). (B) Mice were killed immediately, 1 h, or 2 h after infection (average, 4 × 107 cfu/mouse, n = 12/group). Data are combined from two experiments. There were no significant differences between the CF and WT mice at any time point studied (Van Elteren's test was used adjusting for differences between experiments).
Lung sections were made from mice killed 2 d after a single inoculation with P. aeruginosa; sections illustrating lung inflammation are shown in Figure 5. There was mild to moderate pneumonia present in some areas of the lungs of both CF and wild-type mice, though in a few mice endobronchial inflammation could be detected. H&E-stained sections were assessed for the presence or absence of inflammation using morphometric techniques 3 h, 1 d, and 2 d after infection. There were no significant differences in the degree of inflammation between CF and wild-type mice at any of the time points studied when evaluating the left and right lung separately, though the degree of inflammation tended to increase with time (Figures E2A and E2B). Due to the similarity of the results in the right lung and the left lung, the data were pooled for the entire lung (Figure E2C). Again, there were no significant differences at any time point studied.
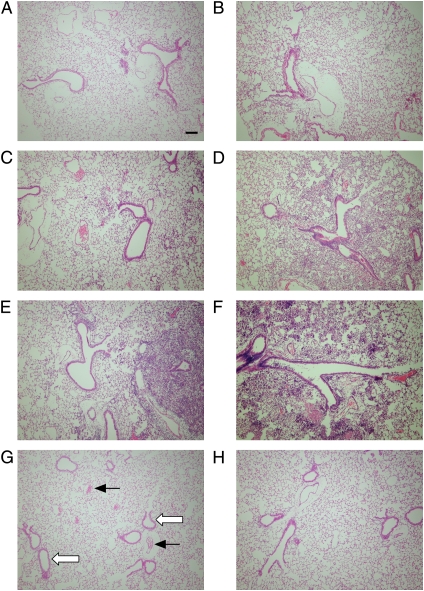
Figure 5.
Histology sections representing areas of inflammation in distal airways with adjacent alveoli after a single inoculation with P. aeruginosa. Wild-type (A, C, and E) and CF (B, D, and F) mice were inoculated with PA M57–15 by insufflation (∼ 5 × 107 cfu/mouse), and killed 3 h (A and B), 1 d (C and D), or 2 d later (E and F; n = 6/group). Control wild-type and CF mice (G and H, respectively) were untreated. After BAL, the lungs were prepared for histologic examination, and stained with hematoxylin and eosin. Examples of blood vessels (solid arrows) and airway epithelia (open arrows) are indicated in an untreated wild-type mouse (G). Original magnification, 10×; bar = 100 μm.
Response of Mice to Multiple Doses of Mucoid P. aeruginosa
Experiments were performed to evaluate whether repeated administrations of P. aeruginosa (∼ 5 × 107 cfu/mouse/administration) could develop into a chronic lung infection in mice. P. aeruginosa was administered by insufflation to CF and wild-type mice every 3 d for a total of four doses, similar to the protocol used to create chronic B. cepacia lung infection (9). After a single administration, CF mice lost weight the first 2 d, and then regained some of the weight lost by the third day (Figure 6A). After the second dose, mice lost weight the first day; however, the mice started to gain weight the second and third day. By the third dose, although mice lost weight, the changes in body weight of the CF and wild-type mice became more similar, and by the fourth dose, the change in weights of the two groups mice were nearly indistinguishable. Mice were killed 13 d after the initial inoculum and BAL performed. TNF-α, IL-1β, IL-6, MIP-2, and KC in BAL fluid were at or near the limits of detection for the assays in almost all mice.
Figure 6.
Change in body weight after repeated administrations of P. aeruginosa. CF and wild-type mice were inoculated with P. aeruginosa by insufflation (arrows), and weighed daily. (A) CF (open triangles) and wild-type mice (closed circles) were inoculated on Days 0, 3, 6, and 9 (∼ 5 × 107 cfu/mouse; n = 10–11/group), and killed on Day 13. All but one CF mouse survived to the termination of the study. *Significantly different differences in change in body weight from wild-type mice (p ⩽ 0.038; unpaired Student's t test, the Bonferroni method was used to adjust for multiple testing). (B) CF (open triangles) and wild-type mice (closed circles) were inoculated on Days 0 (5 × 107 cfu/mouse), 2 (108 cfu/mouse), and 4 (3 × 107 cfu/mouse; n = 15/group), and killed on Day 8. Changes in sample size indicated in the figure are due to death. *Significant differences in change in body weight from wild type mice comparing groups where data are not censored by death (p = 0.009, unpaired Student t test; the Bonferroni method was used to adjust for multiple testing on Days 1 and 2).
Because we observed that mice recovered some of their body weight 3 d after infection, mice were inoculated every 2 d in subsequent experiments (Figure 6B). After the first day, CF and wild-type control mice lost a similar amount of weight. However, CF mice continued to lose more weight than wild-type mice thereafter. Some mice died after the second and third dose; a fourth dose was not administered, although survival and body weight were monitoried until 8 d after infection. CF mice had significantly greater mortality compared with wild-type control animals (60 vs. 0%; p < 0.001). Survivors were killed 8 d after the first bacterial administration, and BAL performed. Lung sections stained with H&E were evaluated for inflammation. TNF-α, IL-1β, IL-6, MIP-2, and KC in BAL fluid were at or near the limits of detection for the assays in almost all mice, and there was no significant difference in the area of inflammation between the two groups of mice.
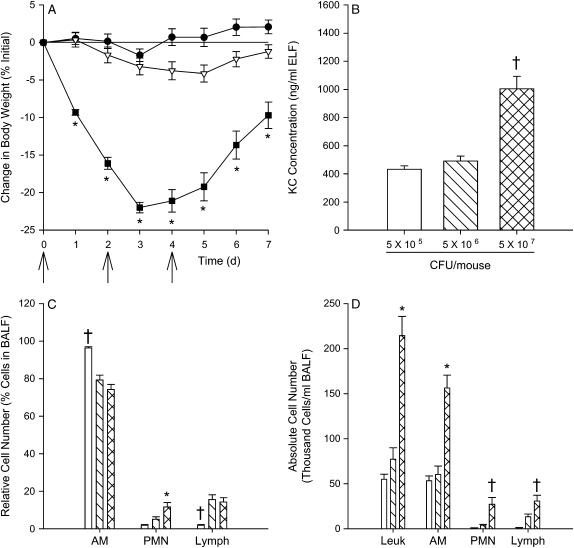
Because doses of approximately 108 cfu/mouse or greater led to mortality of the CF mice, a dose–response curve was generated. Three different doses were administered every 2 d for a total of three doses, and the mice were weighed daily (Figure 7A). Mice in the highest dosing group lost significantly more weight than those in the other two dosing groups. Mice were killed 7 d after the first dose, and BAL was performed. Mice receiving approximately 5 × 107 cfu/mouse lost significantly more weight than the other two doses at all time points studied. All cytokine concentrations but KC were still at or near the limits of detection for most mice. KC concentration was significantly greater in mice receiving the highest bacterial dose compared with the other dosing groups (Figure 7B). Relative alveolar macrophage and lymphocyte numbers were greatest and fewest, respectively, in mice receiving the lowest bacterial dose compared with the other two dosing groups, and relative neutrophil numbers were greatest in mice receiving the highest bacterial dose compared with the other two groups (Figure 7C). Absolute cell counts were significantly greater in mice receiving the highest bacterial dose compared with the other two dosing groups (Figure 7D).
Figure 7.
Dose response study. CF mice were inoculated on Days 0, 2, and 4 with a low (∼ 5 × 105 cfu/mouse; closed circles or open bars), medium (∼ 5 × 106 cfu/mouse; open triangles or hatched bars), or high (∼ 5 × 107 cfu/mouse; closed squares or cross-hatched bars) dose of P. aeruginosa, weighed daily and killed on Day 7 (n = 10/group). All mice survived to the termination of the study. After the mice were killed, BAL was performed for cytokine analysis and cell counts. (A) Change in body weight after infection. (B) KC concentration in ELF. (C) Relative cell numbers of alveolar macrophages (AM), neutrophils (PMN), and lymphocytes (Lymph) in BALF. (D) Absolute cell counts of leukocytes (Leuk), AM, PMN, and Lymph in BALF. *Significantly different (p < 0.001 using a one way ANOVA, and p < 0.001 using a Tukey test to make pairwise comparisons) from the other two groups. †Significantly different from mice receiving the other two dosing groups (p < 0.001 using the Kruskal-Wallis test and p < 0.05 using Dunn's method to make pairwise comparisons). The Bonferroni method was used to adjust for multiple comparisons.
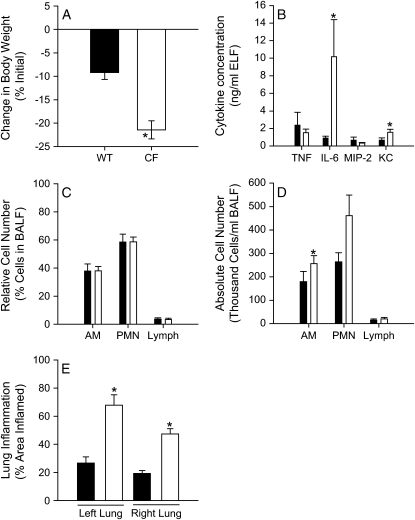
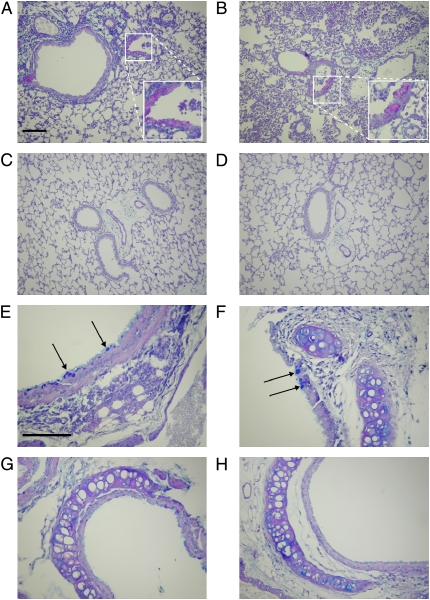
Because cytokines could not be readily detected in mice after repeated doses 7 d after the initial infection, earlier time points were considered. CF and wild-type mice were inoculated every 2 d and killed 4 d after the first dose. One CF mouse died on the last day due to pulmonary obstruction as a result of the infection. After the surviving mice were killed, BAL was performed and lung sections were stained with H&E. Again, CF mice lost significantly more weight than wild-type mice (Figure 8A). There were significantly more IL-6 and KC in the ELF of CF mice compared with wild-type control animals (Figure 8B). There were no significant differences in the relative cell counts (Figure 8C), which is different than after a single inoculation shown in Figure 2C, although there were significantly greater absolute numbers of alveolar macrophages in the BAL fluid of CF mice compared with wild-type control mice (Figure 8D). In addition, there was a significantly greater area of the lung that was inflamed in the CF mice compared with the wild-type mice (Figure 8E). Figure 9 shows sections of lungs from these mice stained with alcian blue and PAS. There appeared to be mucus plugs in the distal airways of CF mice (Figure 9B), more so than wild-type mice (Figure 9A), and that alcian blue–stained airway epithelial cells were primarily found in the proximal airways (Figures 9E and 9F), whereas PAS-stained cells were found in the distal airways (Figures 9A and 9B). Similar parts of the airways are shown for untreated mice for comparison (Figures 9C, 9D, 9G, and 9H).
Figure 8.
Inflammation after repeated administrations of P. aeruginosa. CF (open bars) and wild-type (WT) mice (closed bars) were inoculated with P. aeruginosa by insufflation (∼ 5 × 107 cfu/mouse) on Days 0 and 2, and killed on Day 4 (n = 15/group). All but one CF mouse survived the duration of the experiment, and BALF was not processed for cytokines from one CF mouse due to technical difficulties. (A) Change from initial body weight. (B) Inflammatory mediator concentration in ELF (n = 13–15/group). (C) Relative and (D) absolute cell counts in BALF (n = 14–15/group). (E) The area of inflamed lung was evaluated in seven mice from each group. *Significantly different from CF mice (p < 0.05 was considered significant; unpaired Student's t test or Mann-Whitney rank sum test).
Figure 9.
Lung histology sections after repeated administrations of P. aeruginosa. Wild-type (A and E) and CF (B and F) mice were inoculated intranasally with P. aeruginosa on Day 0 (2.6 × 107 cfu) and Day 2 (6.9 × 108 cfu), and killed on Day 4. Lung sections from untreated wild-type (C and G) and CF (D and H) mice in similar areas of the lung are shown for comparison. BAL was performed and the lungs were prepared for sectioning and stained with alcian blue and periodic acid–Schiff (PAS). A magenta color indicates the presence of neutral polysaccharides (PAS positive), whereas acid mucopolysaccharides and 1,2-glycol acid substrates stain a dark blue (alcian blue positive; arrows). Endobronchial inflammation can be seen in the lumen of airway from a wild-type and CF mouse (A and B). Original magnification, 20× (A–D) and 40× (E–H); bar = 100 μm.
DISCUSSION
Studying the pathophysiology of CF lung disease is critical for developing novel treatments; however, because there are no adequate controls in the human population, other models must be considered. Animal models of CF lung disease must be used because inflammatory processes are complex and almost surely involve interactions among multiple cell types. However, understanding the strengths and limitations of the animal models available is essential for critically evaluating individual pieces of the disease process and putting those pieces together to make a more complete story.
The data reported here support the hypothesis that CFTR deficiency is sufficient to produce increased inflammatory responses to free-living P. aeruginosa insufflated into the lung. At higher doses, CF mice suffer higher mortality than wild-type mice. At slightly lower doses, they survive, but suffer greater weight loss with slower recovery than their wild-type counterparts. This is, however, not due to excessive or protracted bacterial retention in the airways, for the CF mice cleared the bacteria just as rapidly as wild-type mice. Rather, it is the result of increased and prolonged inflammatory responses.
CF mice had an exaggerated inflammatory response to P. aeruginosa compared with wild-type control mice both after a single dose and multiple doses, as assessed by the area of the lung that was inflamed and the levels of inflammatory cytokine mediators and neutrophils in BAL fluid. In addition, bacterial clearance was just as rapid in CF mice as in wild-type mice. Control studies showed that these results could not be attributed to the inhalational anesthesia used. The use of the CF mouse strain selected eliminated dietary or nutritional differences as a variable for these experiments (as these mice, although maintaining the CF phenotype in the lung, grow normally, unlike Cftr−/− mice, which require a liquid diet to survive and remain small compared with wild-type littermates [18]). In the agarose bead model, the CF mice had pulmonary responses similar to Cftr−/− mice (13, 23). Therefore, we attribute the differences between groups observed in this study to lack of Cftr activity. Rapid clearance of the bacteria by the CF mice in this study was likely due to the robust, early neutrophil response. Despite the fact that bacteria are cleared within 2 d of administration, CF mice have a prolonged inflammatory response as indicated by excessive levels in the proinflammatory mediator TNF-α and the murine neutrophil chemokine KC compared with wild-type mice.
In another publication using free-living bacteria, mice with Cftr mutations were reported to have reduced ability to kill P. aeruginosa compared with wild-type mice (24). The discrepancy in our results and theirs probably results from differences in experimental conditions, the most important of which is likely to be the mice themselves. In the earlier study, mice from that colony were reportedly spontaneously infected, so the colony was maintained routinely on antibiotics, which were stopped 24 h before challenge. In contrast, our mice were not treated with antibiotics at any time and sentinel animals (both wild-type mice and Cftr mutants) were not infected with any of the agents reported in the other investigators' colony. Moreover, in our experiments, qualitative bacteriology cultures on BAL fluid in challenged mice showed only the bacteria inoculated; this was not the case in the prior study. In addition, the outcome measures were performed differently. The earlier investigators used a technique designed to detect the difference between bacteria that were ingested by CF and wild-type lung cells, as well as the multiple of infectious inoculum, whereas the method described here simply measured the number of bacteria in the entire lung and these are reported as the number of cfu/lung.
CF mice maintained under specific pathogen–free conditions do not spontaneously acquire lung infections, and they are capable of rapid clearance and killing of even substantial and repeated inocula of pathogenic organisms. In contrast, patients with CF do spontaneously acquire bacterial colonization of the airways early in life and eventually become unable to clear the infecting organisms. Whether bacterial retention is due to (1) changes in the CF lung and how it responds to the bacterium, (2) changes in how the bacterium responds to the CF lung, or (3) a combination of the two is not clear. Recent observations in mouse models, including that presented here, may shed some insight to this conundrum.
First, Cftr−/− mice do not have the same up-regulation of ENaC activity that is observed in the lower airways of patients with CF (25). Mall and colleagues demonstrated that overexpression of the β subunit of ENaC directed to the airways by a Clara cell–specific promoter in mice led to mucus plugging of the lower airways, mimicking the natural pathophysiology of CF lung disease (6). In addition, they found that bacterial clearance of a nonmucoid clinical strain of P. aeruginosa (108 cfu/mouse) was slower than in wild-type control mice, although it did not produce mortality. The differences between Cftr−/− mice and the ENaC-overexpressing mice probably do not reside entirely in the increase in goblet cells and mucins, however, because induction of increased mucin production and increased goblet cell number in Cftr−/− mice still did not permit chronic infection with free-living P. aeruginosa challenge (26). Second, neutrophil necrosis and the release of DNA and actin likely enhance the conversion of nonmucoid P. aeruginosa to the mucoid form (27). Also, mucoid P. aeruginosa in the presence of alginate is retained more readily in the lungs of CF mice than nonmucoid P. aeruginosa, and mortality rates were significantly higher in CF mice than in Balb/c wild-type control mice (28). Last, we report that planktonic mucoid P. aeruginosa can be cleared readily from the lungs of CF and wild-type mice without mortality.
Therefore, it is likely that trapping and retention of bacteria in the CF lung is a combination of two factors: that of the CF lung and that of the bacterium. First, it requires thick, sticky mucus, which may be attributable to abnormalities in ENaC. The lungs of CF mice appear to be well hydrated, which may be due to normal activity of ENaC and increased activity of a calcium-dependent chloride transporter (25). Although mucus production is stimulated in both wild-type and CF mice after challenge with P. aeruginosa, this does not appear to occur to the extent that it does in mice overexpressing the β subunit of ENaC. Second, retention is facilitated by conversion of P. aeruginosa from a nonmucoid form into a mucoid form, and the alginate coating protects the bacterium from clearance in CF mice (28). Therefore, it is not surprising that chronic lung infections could not be established in CF mice under these experimental conditions. However, our data clearly show that defective Cftr, in the absence of both increased function of ENaC in the lower airways and an overabundance of alginate in the challenge bacteria, is sufficient to induce an increased and protracted inflammatory response despite efficient clearance of the bacteria.
There are several possible mechanisms responsible for the inflammatory response after repeated administration of P. aeruginosa, some of them under active investigation in our lab. For instance, if the host already has its defenses recruited to defend against the first inoculum (e.g., neutrophils are already in the airway, macrophages already activated, serum already leaked into the airway), the host may control the second inoculum better, without additional recruitment of cells or increase in cytokines. It is also possible that due to the repeated administration and continual clearance of the bacteria, providing a continual stimulus, a different cell population is now recruited. Indeed, lymphocyte numbers are increasing, as indicated by BAL cell counts and in observing histologic sections over time. The lymphocytic response may be more important later on in the infection. In addition, due to absent Cftr, the CF mice have a more prolonged neutrophilia with the continued production of KC.
In conclusion, the interpretation of our results combined with the work of others suggest that the impaired mucociliary clearance, mucus plugging, and trapping of inhaled bacteria in CF may be more closely associated with the up-regulation of ENaC initially than with deficiency of CFTR activity per se, but that once an infectious or inflammatory stimulus is applied, Cftr deficiency permits the inflammatory response to be exaggerated. This exaggerated response probably allows increased airway damage from neutrophil products and provides a favorable setting for further bacterial invasion. Also, overproduction of alginate by P. aeruginosa protects the organism from clearance by the CF lung, further exacerbating the cycle of chronic infection and exaggerated inflammation. Therefore, the vicious cycle of infection and inflammation in the CF lung probably arises from at least two of the functions of CFTR, one of which has yet to be fully characterized.
Supplementary Material
Acknowledgments
The authors thank the Case Pediatric Animal Core members, particularly Alma Genta Wilson, Veronica Peck and Ebony Boyd for breeding and maintaining the mice, and Christiaan van Heeckeren and James Poleman for performing the experiments; Thomas E. Shaw, Jr., for point counting the lung histology slides; Case Western Reserve University (Case) Cystic Fibrosis Center's Inflammatory Mediator Core personnel, particularly Christopher Statt and Eureka Bryant, for their expert technical assistance in performing cytokine analyses; and Case Cystic Fibrosis Center's Morphology Core technician, Claudia Garner, for expert technical assistance in tissue sectioning and staining.
Supported by National Institutes of Health grants P30 DK27651 and HL60293, and grants from the Cystic Fibrosis Foundation.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200506-917OC on November 4, 2005
Conflict of Interest Statement: A.M.v.H. received $1,000 in 2002 for consulting with GelTex Pharmaceuticals, Inc., regarding mouse models of P. aeruginosa lung infection. M.D.S. does not have a financial relationship with a commercial entity that has an interest in the subject matter of the manuscript. W.X. does not have a financial relationship with a commercial entity that has an interest in the subject matter of the manuscript. P.B.D. received $1,000 in 2002 for consulting for Centacor on inflammation in CF.
References
- 1.Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DW. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med 1995;151:1075–1082. [DOI] [PubMed] [Google Scholar]
- 2.Balough K, McCubbin M, Weinberger M, Smits W, Ahrens R, Fick R. The relationship between infection and inflammation in the early stages of lung disease from cystic fibrosis. Pediatr Pulmonol 1995;20: 63–70. [DOI] [PubMed] [Google Scholar]
- 3.Grubb BR, Vick RN, Boucher RC. Hyperabsorption of Na+ and raised Ca(2+)-mediated Cl- secretion in nasal epithelia of CF mice. Am J Physiol 1994;266:C1478–C1483. [DOI] [PubMed] [Google Scholar]
- 4.Noah TL, Black HR, Cheng PW, Wood RE, Leigh MW. Nasal and bronchoalveolar lavage fluid cytokines in early cystic fibrosis. J Infect Dis 1997;175:638–647. [DOI] [PubMed] [Google Scholar]
- 5.Muhlebach MS, Stewart PW, Leigh MW, Noah TL. Quantitation of inflammatory responses to bacteria in young cystic fibrosis and control patients. Am J Respir Crit Care Med 1999;160:186–191. [DOI] [PubMed] [Google Scholar]
- 6.Mall M, Grubb BR, Harkema JR, O'Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med 2004;10:487–493. [DOI] [PubMed] [Google Scholar]
- 7.Davidson DJ, Dorin JR, McLachlan G, Ranaldi V, Lamb D, Doherty C, Govan J, Porteous DJ. Lung disease in the cystic fibrosis mouse exposed to bacterial pathogens. Nat Genet 1995;9:351–357. [DOI] [PubMed] [Google Scholar]
- 8.Koehler DR, Sajjan U, Chow YH, Martin B, Kent G, Tanswell AK, McKerlie C, Forstner JF, Hu J. Protection of Cftr knockout mice from acute lung infection by a helper-dependent adenoviral vector expressing Cftr in airway epithelia. Proc Natl Acad Sci USA 2003;100: 15364–15369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sajjan U, Thanassoulis G, Cherapanov V, Lu A, Sjolin C, Steer B, Wu YJ, Rotstein OD, Kent G, McKerlie C, et al. Enhanced susceptibility to pulmonary infection with Burkholderia cepacia in Cftr(−/−) mice. Infect Immun 2001;69:5138–5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George SE, Kohan MJ, Gilmour MI, Taylor MS, Brooks HG, Creason JP, Claxton LD. Pulmonary clearance and inflammatory response in C3H/HeJ mice after intranasal exposure to Pseudomonas spp. Appl Environ Microbiol 1993;59:3585–3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gosselin D, Stevenson MM, Cowley EA, Griesenbach U, Eidelman DH, Boule M, Tam MF, Kent G, Skamene E, Tsui LC, et al. Impaired ability of Cftr knockout mice to control lung infection with Pseudomonas aeruginosa. Am J Respir Crit Care Med 1998;157:1253–1262. [DOI] [PubMed] [Google Scholar]
- 12.Heeckeren A, Walenga R, Konstan MW, Bonfield T, Davis PB, Ferkol T. Excessive inflammatory response of cystic fibrosis mice to bronchopulmonary infection with Pseudomonas aeruginosa. J Clin Invest 1997;100:2810–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Heeckeren AM, Scaria A, Schluchter MD, Ferkol TW, Wadsworth S, Davis PB. Delivery of CFTR by adenoviral vector to cystic fibrosis mouse lung in a model of chronic Pseudomonas aeruginosa lung infection. Am J Physiol Lung Cell Mol Physiol 2004;286:L717–L726. [DOI] [PubMed] [Google Scholar]
- 14.McMorran BJ, Palmer JS, Lunn DP, Oceandy D, Costelloe EO, Thomas GR, Hume DA, Wainwright BJ. G551D CF mice display an abnormal host response and have impaired clearance of Pseudomonas lung disease. Am J Physiol Lung Cell Mol Physiol 2001;281:L740–L747. [DOI] [PubMed] [Google Scholar]
- 15.Chroneos ZC, Wert SE, Livingston JL, Hassett DJ, Whitsett JA. Role of cystic fibrosis transmembrane conductance regulator in pulmonary clearance of Pseudomonas aeruginosa in vivo. J Immunol 2000;165: 3941–3950. [DOI] [PubMed] [Google Scholar]
- 16.van Heeckeren AM, Downey G, Davis PB. Towards a novel model of chronic P. aeruginosa lung infection in CF mice [abstract]. Pediatr Pulmonol Suppl 2003;25:227. [Google Scholar]
- 17.van Heeckeren AM, Downey G, Davis PB. Modeling the natural pathophysiology of P. aeruginosa lung infection in CF mice [abstract]. Pediatr Pulmonol Suppl 2003;25:287. [Google Scholar]
- 18.van Heeckeren AM, Schluchter MD, Drumm ML, Davis PB. Role of Cftr genotype in the response to chronic Pseudomonas aeruginosa lung infection in mice. Am J Physiol Lung Cell Mol Physiol 2004;287: L944–L952. [DOI] [PubMed] [Google Scholar]
- 19.Rennard SI, Basset G, Lecossier D, O'Donnell KM, Pinkston P, Martin PG, Crystal RG. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol 1986;60: 532–538. [DOI] [PubMed] [Google Scholar]
- 20.van Heeckeren A, Ferkol T, Tosi M. Effects of bronchopulmonary inflammation induced by pseudomonas aeruginosa on adenovirus- mediated gene transfer to airway epithelial cells in mice. Gene Ther 1998;5:345–351. [DOI] [PubMed] [Google Scholar]
- 21.Nader-Djalal N, Knight PR, Bacon MF, Tait AR, Kennedy TP, Johnson KJ. Alterations in the course of acid-induced lung injury in rats after general anesthesia: volatile anesthetics versus ketamine. Anesth Analg 1998;86:141–146. [DOI] [PubMed] [Google Scholar]
- 22.Shayevitz JR, Rodriguez JL, Gilligan L, Johnson KJ, Tait AR. Volatile anesthetic modulation of lung injury and outcome in a murine model of multiple organ dysfunction syndrome. Shock 1995;4:61–67. [DOI] [PubMed] [Google Scholar]
- 23.van Heeckeren AM, Schluchter M, Xue L, Alvarez J, Freedman S, St.George J, Davis PB. Nutritional effects on host response to lung infections with mucoid Pseudomonas aeruginosa in mice. Infect Immun 2004;72:1479–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schroeder TH, Reiniger N, Meluleni G, Grout M, Coleman FT, Pier GB. Transgenic cystic fibrosis mice exhibit reduced early clearance of Pseudomonas aeruginosa from the respiratory tract. J Immunol 2001;166: 7410–7418. [DOI] [PubMed] [Google Scholar]
- 25.Grubb BR, Paradiso AM, Boucher RC. Anomalies in ion transport in CF mouse tracheal epithelium. Am J Physiol 1994;267:C293–C300. [DOI] [PubMed] [Google Scholar]
- 26.Cressman VL, Hicks EM, Funkhouser WK, Backlund DC, Koller BH. The relationship of chronic mucin secretion to airway disease in normal and CFTR-deficient mice. Am J Respir Cell Mol Biol 1998;19:853–866. [DOI] [PubMed] [Google Scholar]
- 27.Walker TS, Tomlin KL, Worthen GS, Poch KR, Lieber LG, Saavedra MT, Fessler MB, Malcolm KC, Vasil ML, Nick JA. Enhanced Pseudomonas aeruginosa biofilm development mediated by human neutrophils. Infect Immun 2005;73:3693–3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffmann N, Rasmussen TB, Jensen PO, Stub C, Hentzer M, Molin S, Ciofu O, Givskov M, Johansen HK, Hoiby N. Novel mouse model of chronic Pseudomonas aeruginosa lung infection mimicking cystic fibrosis. Infect Immun 2005;73:2504–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.