Abstract
Rationale: Chronic exposure to indoor nitrogen dioxide (NO2) is a public health concern. Over half of U.S. households have a source of NO2, and experimental data suggest potential for adverse respiratory effects.
Objective: To examine associations of indoor NO2 exposure with respiratory symptoms among children with asthma.
Methods: NO2 was measured using Palmes tubes, and respiratory symptoms in the month before sampling were collected during home interviews of mothers of 728 children with active asthma. All were younger than 12 yr, lived at the sampled home for at least 2 mo, and had asthma symptoms or used maintenance medication within the previous year.
Measurements: Respiratory symptoms (wheeze, persistent cough, shortness of breath, chest tightness).
Results: Mean (SD) NO2 was 8.6 (9.1) ppb in homes with electric ranges and 25.9 (18.1) ppb in homes with gas stoves. In models stratified by housing type (a factor associated with socioeconomic status), gas stove presence and elevated NO2 were each significantly associated with respiratory symptoms, controlling for age, ethnicity, medication, mold/mildew, water leaks, and season of sampling. Among children in multifamily housing, exposure to gas stoves increased likelihood of wheeze (odds ratio [OR], 2.27; 95% confidence interval [95% CI], 1.15, 4.47), shortness of breath (OR, 2.33; 95% CI, 1.12, 5.06), and chest tightness (OR, 4.34; 95% CI, 1.76, 10.69), whereas each 20-ppb increase in NO2 increased both likelihood of any wheeze (OR, 1.52; 95% CI, 1.04, 2.21) or chest tightness (OR, 1.61; 95% CI, 1.04, 2.49), and days of wheeze (rate ratio (RR), 1.33; 95% CI, 1.05, 1.68) or chest tightness (RR, 1.51; 95% CI, 1.18, 1.91).
Conclusion: Exposure to indoor NO2 at levels well below the Environmental Protection Agency outdoor standard (53 ppb) is associated with respiratory symptoms among children with asthma in multifamily housing.
Keywords: asthma, children, gas stoves, indoor environment, nitrogen dioxide, respiratory symptoms
The effect on respiratory health of chronic exposure to low levels of indoor nitrogen dioxide (NO2) continues to be a public health concern. One reason for concern is the large number of people exposed. The 2000 U.S. Census (1) indicates that more than half of all households in the United States use gas, and the primary source of residential NO2 is a gas-fueled cooking appliance.
Although the toxicology of NO2 exposure suggests the potential for respiratory symptoms and loss of lung function (2, 3), evidence from three decades of epidemiologic studies linking NO2 exposure to adverse health effects has been inconsistent. Some inconsistency may be explained by differences in measures of exposure (acute [4, 5] vs. chronic [4–11]). Populations studied have also varied and include healthy children (4, 5, 12–15) and infants (16–19), as well as children with asthma (4, 5, 20–23) and infants at risk for developing asthma (24, 25).
Studies of acute, controlled exposure to NO2 have been largely negative (26); however, some effects on lung function (27) and airway reactivity (28) have been observed in adults with asthma. None of the acute, controlled exposure studies included children.
Despite an extensive literature on the effects of chronic NO2 exposure, of two reviews published in 1999, one (5) recommended “a revision of the NO2 guidelines to protect asthmatics and the general population, especially children,” whereas the other (4) concluded there was “inconsistent evidence of adverse effects.” Since this time, a randomized trial has demonstrated a reduction in symptoms in children with asthma when exposure to high levels of NO2 in schools was reduced (23).
The Yale Childhood Asthma Study (24, 25, 29) enrolled 1,002 families who had a newborn infant and an older child with physician-diagnosed asthma. The infants are at high risk of developing asthma, because in addition to their diagnosed sibling, 49% have a parent with asthma, and 77% a parent with allergies. In this cohort, infants living in homes with NO2 concentrations exceeding 17.4 ppb (highest quartile) had increased frequency of days of wheeze and persistent cough and twice the frequency of shortness of breath in the first year of life (25). These data support the hypothesis that children with atopy or asthma may be more sensitive to NO2 exposure. In the present analysis, we examine the association of exposure to household NO2 on the older sibling with asthma in the Yale cohort. A previous report related to NO2 exposure in these children was presented in abstract form (30).
METHODS
Participants
From 1997 through 1999, 1,002 families living in Connecticut and southwestern Massachusetts with an infant and at least one older child with physician-diagnosed asthma were enrolled in a cohort to study the early development of asthma (24, 25, 29). Subjects for the present study were 728 older children with asthma (one per cohort family). To be eligible for analysis, the child with asthma was younger than 12 yr old at the time the family enrolled, had active asthma (exhibited respiratory symptoms or used asthma medication within the year before enrollment), and had lived at the enrollment address for at least 2 mo before NO2 sampling. If two children in a family met the eligibility criteria, the child with more severe asthma was selected. Of 1,002 children, two had questionable asthma diagnoses, four were ineligible due to age, 37 had not lived in the home for at least 2 mo, 34 were missing data on symptoms and medication use, and 85 did not have active asthma at enrollment. An additional 112 were missing NO2 measurements, leaving a total of 728 for the current analysis. The Human Investigation Committee of Yale University, New Haven, Connecticut, approved this study, and all respondents (mothers of study subjects) gave informed consent, and children 7 yr or older gave assent before participation.
Data Collection
At enrollment, a trained research assistant visited the home and collected extensive information from the study participant's mother on the family's ethnicity, education, smoking in the home, housing characteristics, and use of household appliances fueled by natural gas. Mothers were also asked about the number of days of respiratory symptoms (wheeze, persistent cough, shortness of breath, chest tightness) experienced by the child and medications used, including β2-agonists and maintenance medication (inhaled or systemic steroids, cromolyn sodium, long-acting β2-agonists, leukotriene inhibitors), for each month of the previous year.
NO2 was measured in each home using a Palmes tube (31) placed in the main living area for 10 to 14 d after the enrollment visit.
Data Analysis
Health outcomes of interest were respiratory symptoms such as wheeze, persistent cough, shortness of breath, and chest tightness during the month before home interview. Each symptom was quantified in two ways: (1) as a binary variable, never (0) or ever (1) experienced that symptom during the previous month, and (2) the number of days that symptom was present (0–31 d).
Exposure variables included sources of NO2 in the home (gas stoves, gas dryers, presence of tobacco smokers) and measured levels of NO2. For the purpose of illustration (Figure 1), and in some preliminary analyses, NO2 levels were dichotomized as less than 20 ppb versus 20 ppb or more. A concentration of 20 ppb was the median concentration of indoor NO2 reported for an inner-city population (32). In all multivariate models, measured NO2 was entered as a continuous variable. Other variables included season of sampling (classified as warmer months [April–October] or cooler months [November–March]), housing characteristics (multi- vs. single-family, number of rooms, water leaks, visible mold), and ethnicity. Use of maintenance medication (inhaled steroid, cromolyn, long-acting β2-agonist, or leukotriene inhibitor during the year before enrollment) was examined as a proxy for asthma severity and provided a reasonable alternative to using respiratory symptoms to classify asthma severity. Because smoking (environmental tobacco smoke) is a source of NO2 exposure, it was included in models examining NO2 sources, but not included in models examining measured NO2 concentration.
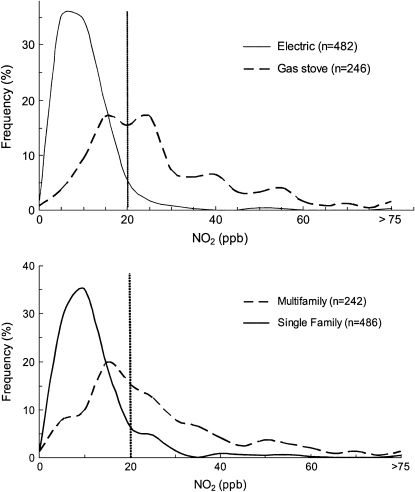
Figure 1.
Frequency distribution of NO2 measured in homes where the cooking is done using electric ranges (solid line) or gas stoves (dashed line; upper panel) and in multifamily (dashed line) or single-family (solid line) homes (lower panel). Vertical line at 20 ppb indicates median concentration of indoor NO2 reported for an inner-city population (32).
In Connecticut, residence in apartments is confined to larger cities (e.g., Bridgeport, New Haven, Hartford) and is strongly associated with lower socioeconomic status. More affluent families live in single-family homes in suburban towns. Exposure to NO2 sources was anticipated to be associated with multifamily housing and lower socioeconomic status since many of the suburban towns do not have gas lines. Logistic regression analyses were used to investigate interactions among exposure to NO2 (gas stove use and NO2 of ⩾ 20 ppb), respiratory symptoms, and multi- versus single-family housing . Significant interactions were detected for housing status and NO2 with wheeze, shortness of breath, and chest tightness. Final analyses were stratified by housing type. (For unstratified analyses, see the online supplement).
Unadjusted associations were examined with χ2 analyses. Logistic regression analyses examined adjusted associations between each symptom as a binary variable (present or absent during the previous month) and either reported use of gas sources (gas stove, gas dryer, smoker in the home) or measured NO2 as a continuous variable. Poisson regression analyses examined the association of NO2 as a continuous variable with increased number of symptom days. Final models included age and ethnicity of the child, maintenance medication use, season of sampling, and housing characteristics (mold/mildew, water leaks) as potential confounding factors.
RESULTS
The mean (SD) concentration of indoor NO2 was 8.6 (9.1) ppb (median, 7.0; interquartile range [IQR], 6.8) in homes with electric ranges and 25.9 (18.1) ppb (median, 21.6; IQR, 18.6) in homes with gas stoves. The overlapping distributions of measured NO2 in these homes is illustrated in Figure 1, top panel. The lower panel illustrates the distribution of NO2 levels by housing type. Mean (SD) NO2 levels measured in multifamily homes were 22.9 (17.0) ppb with a median of 18.9 and an IQR of 19.6. Measurements in single-family homes were much lower: mean, 10.2 (12.3); median, 7.6; and IQR, 7.6.
Children using maintenance medication were more likely to have respiratory symptoms than children who did not (Table 1), indicating more severe asthma in the former children. Children also had more reported symptoms during colder months, corresponding to the time of year when respiratory viruses are present. Among children living in multifamily housing, those 6 yr and older were more likely to experience chest tightness (29.6%) than younger children (11.2%).
TABLE 1.
UNADJUSTED ASSOCIATIONS BETWEEN RESPIRATORY SYMPTOMS IN THE MONTH BEFORE SAMPLING AND PERSONAL FACTORS, MAINTENANCE MEDICATION USE, AND SEASON OF NO2 SAMPLING STRATIFIED BY HOUSING TYPE (SOUTHERN NEW ENGLAND, 1998–2000)
| Multifamily Housing
|
Single-Family Housing
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Factors | n (%) | Wheeze (%) | Persistent Cough (%) | Shortness of Breath (%) | Chest Tightness (%) | n (%) | Wheeze (%) | Persistent Cough (%) | Shortness of Breath (%) | Chest Tightness (%) |
| Housing group | 242 | 27.0 | 32.6 | 21.6 | 17.4 | 486 | 29.2 | 34.8 | 20.2 | 16.5 |
| Personal factors | ||||||||||
| Sex | ||||||||||
| Male | 151 (62.4) | 28.5 | 34.4 | 22.5 | 17.9 | 307 (63.2) | 31.6 | 35.6 | 21.2 | 16.9 |
| Female | 91 (37.6) | 24.4 | 29.7 | 20.0 | 16.5 | 178 (36.8) | 25.3 | 33.5 | 18.4 | 15.6 |
| Age, yr | ||||||||||
| < 6 | 161 (66.5) | 25.0 | 31.7 | 18.8 | 11.2 | 310 (63.8) | 28.2 | 35.6 | 19.7 | 14.8 |
| ⩾ 6 | 81 (33.5) | 30.9 | 34.6 | 27.2 | 29.6 | 176 (36.2) | 31.2 | 33.5 | 21.0 | 19.3 |
| Ethnicity | ||||||||||
| White, Asian, other | 68 (28.1) | 28.4 | 32.4 | 22.4 | 20.6 | 422 (86.8) | 28.7 | 35.2 | 19.4 | 14.9 |
| Black | 44 (18.2) | 25.0 | 40.9 | 27.3 | 18.2 | 30 (6.2) | 36.7 | 33.3 | 23.3 | 30.0 |
| Hispanic | 130 (53.7) | 26.9 | 30.0 | 19.2 | 15.4 | 34 (7.0) | 29.4 | 32.4 | 26.5 | 23.5 |
| Housing characteristics | ||||||||||
| No. rooms in home | ||||||||||
| < 6 | 203 (83.9) | 27.7 | 33.0 | 20.3 | 17.2 | 85 (17.6) | 32.9 | 36.5 | 22.4 | 22.4 |
| ⩾ 6 | 39 (16.1) | 23.1 | 30.8 | 28.2 | 18.0 | 398 (82.4) | 28.4 | 34.4 | 19.8 | 17.3 |
| Mold/mildew | ||||||||||
| Yes | 95 (39.4) | 30.5 | 39.0 | 25.3 | 21.0 | 215 (44.3) | 27.4 | 30.2 | 22.8 | 15.8 |
| No | 146 (60.6) | 24.8 | 28.8 | 19.3 | 15.1 | 270 (55.7) | 30.9 | 38.3 | 18.2 | 17.0 |
| Water leaks | ||||||||||
| Yes | 72 (29.8) | 34.7 | 38.9 | 26.6 | 20.8 | 167 (34.4) | 31.7 | 41.3 | 25.2 | 19.2 |
| No | 170 (70.2) | 23.7 | 30.0 | 20.7 | 15.9 | 318 (65.6) | 28.1 | 31.2 | 17.6 | 15.1 |
| Maintenance medication use | ||||||||||
| Yes | 94 (38.8) | 44.7 | 44.7 | 38.3 | 28.7 | 276 (56.8) | 34.4 | 41.1 | 23.6 | 20.6 |
| No | 148 (61.2) | 15.6 | 25.0 | 10.9 | 10.1 | 210 (43.2) | 22.5 | 26.7 | 15.7 | 11.0 |
| Season of NO2 sampling | ||||||||||
| Nov–Mar | 97 (40.1) | 35.0 | 42.3 | 28.9 | 25.6 | 177 (36.4) | 35.8 | 45.2 | 26.6 | 22.0 |
| Apr–Oct | 145 (59.9) | 21.5 | 26.2 | 16.7 | 11.7 | 309 (63.6) | 25.6 | 28.9 | 16.5 | 13.3 |
Significant (p < 0.05, χ2 test) differences between children with and without symptoms are shown in boldface type.
Exposure to NO2 (Table 2) was not associated with most personal or housing characteristics: specifically, not with maintenance medication use or season of sampling. However, measured NO2 (⩾ 20 ppb) was associated with ethnicity: whites were least likely to have high exposure and Hispanics most likely.
TABLE 2.
UNADJUSTED ASSOCIATIONS BETWEEN HOUSEHOLD SOURCE OF NO2 (GAS STOVES) AND MEASURED NO2 ⩾ 20 ppb, PERSONAL FACTORS, MAINTENANCE MEDICATION USE, AND SEASON OF NO2 SAMPLING STRATIFIED BY HOUSING TYPE (SOUTHERN NEW ENGLAND, 1998–2000)
| Multifamily Housing
|
Single-Family Housing
|
|||||
|---|---|---|---|---|---|---|
| Factors | n (%) | Gas stove (%) | NO2 ⩾ 20 ppb (%) | n (%) | Gas stove (%) | NO2 ⩾ 20 ppb (%) |
| Housing group | 242 | 54.6 | 45.9 | 486 | 23.5 | 9.3 |
| Personal factors | ||||||
| Sex | ||||||
| Male | 151 (62.4) | 57.0 | 44.4 | 307 (63.2) | 24.4 | 8.8 |
| Female | 91 (37.6) | 50.6 | 48.4 | 178 (36.8) | 21.8 | 10.1 |
| Age, yr | ||||||
| < 6 | 161 (66.5) | 53.4 | 46.6 | 310 (63.8) | 24.2 | 9.0 |
| ⩾ 6 | 81 (33.5) | 56.8 | 44.4 | 176 (36.2) | 22.2 | 9.7 |
| Ethnicity | ||||||
| White, Asian, other | 68 (28.1) | 45.6 | 35.3 | 422 (86.8) | 22.8 | 8.1 |
| Black | 44 (18.2) | 47.7 | 40.9 | 30 (6.2) | 26.7 | 13.3 |
| Hispanic | 130 (53.7) | 61.5 | 53.1 | 34 (7.0) | 29.4 | 20.6 |
| Housing characteristics | ||||||
| No. rooms in home | ||||||
| < 6 | 203 (83.9) | 55.2 | 47.8 | 85 (17.6) | 28.2 | 14.1 |
| ⩾ 6 | 39 (16.1) | 51.3 | 35.9 | 399 (82.4) | 22.6 | 8.3 |
| Mold/mildew | ||||||
| Yes | 95 (39.4) | 55.8 | 47.4 | 215 (44.3) | 19.1 | 7.4 |
| No | 146 (60.6) | 54.1 | 45.2 | 270 (55.7) | 26.7 | 10.7 |
| Water leaks | ||||||
| Yes | 72 (29.8) | 43.1 | 41.7 | 167 (34.4) | 24.6 | 12.0 |
| No | 170 (70.2) | 59.4 | 47.6 | 318 (65.6) | 22.6 | 7.9 |
| Maintenance medication use | ||||||
| Yes | 94 (38.8) | 53.2 | 41.5 | 276 (56.8) | 25.7 | 7.6 |
| No | 148 (61.2) | 55.4 | 48.6 | 210 (43.2) | 20.5 | 11.4 |
| Season of NO2 sampling | ||||||
| Nov–Mar | 97 (40.1) | 54.6 | 47.4 | 177 (36.4) | 22.6 | 9.6 |
| Apr–Oct | 145 (59.9) | 54.5 | 44.8 | 309 (63.6) | 24.0 | 9.1 |
Significant (p < 0.05, χ2 tests) differences between factor categories for gas stove use and NO2 ⩾ 20 ppb are shown in boldface type.
Among children living in multifamily housing, exposure to gas stoves was associated with wheeze, shortness of breath, and chest tightness (Table 3). Increases in measured NO2 were associated with increases in each respiratory symptom; however, only the association with chest tightness was statistically significant. For children in single-family homes, neither exposure to gas stoves nor to measured NO2 was associated with any respiratory symptom.
TABLE 3.
UNADJUSTED ASSOCIATIONS BETWEEN RESPIRATORY SYMPTOMS IN THE MONTH BEFORE SAMPLING AND NO2 EXPOSURE (HOUSEHOLD SOURCES OR MEASURED CONCENTRATIONS) STRATIFIED BY HOUSING TYPE (SOUTHERN NEW ENGLAND, 1998–2000)
| Multifamily Housing
|
Single-Family Housing
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Factors | n (%) | Wheeze (%) | Persistent Cough (%) | Shortness of Breath (%) | Chest Tightness (%) | n (%) | Wheeze (%) | Persistent Cough (%) | Shortness of Breath (%) | Chest Tightness (%) |
| Housing group | 242 | 27.0 | 32.6 | 21.6 | 17.4 | 486 | 29.3 | 34.8 | 20.2 | 16.5 |
| Household sources of NO2 | ||||||||||
| Gas stove | ||||||||||
| Yes | 132 (54.6) | 32.6 | 34.1 | 27.3 | 24.2 | 114 (23.5) | 23.9 | 35.1 | 19.3 | 14.0 |
| No | 110 (45.4) | 20.2 | 30.9 | 14.7 | 9.1 | 372 (76.5) | 30.9 | 34.8 | 20.4 | 17.2 |
| Gas dryer | ||||||||||
| Yes | 18 (7.4) | 27.8 | 38.9 | 44.4 | 27.8 | 51 (10.5) | 27.4 | 35.3 | 19.6 | 19.6 |
| No | 224 (92.6) | 26.9 | 32.1 | 19.7 | 16.5 | 435 (89.5) | 29.5 | 34.8 | 20.2 | 16.1 |
| Smoker in the home | ||||||||||
| Yes | 47 (19.4) | 30.4 | 38.3 | 23.9 | 23.4 | 35 (7.2) | 40.0 | 31.4 | 22.9 | 11.4 |
| No | 195 (80.6) | 26.2 | 31.3 | 21.0 | 15.9 | 451 (92.8) | 28.4 | 35.1 | 20.0 | 16.8 |
| Indoor NO2, ppb | ||||||||||
| < 10 | 46 (19.0) | 20.0 | 26.1 | 17.8 | 13.0 | 317 (65.4) | 28.7 | 34.5 | 20.8 | 16.4 |
| 10–19 | 85 (35.1) | 23.5 | 34.1 | 17.6 | 10.6 | 124 (25.5) | 33.3 | 34.7 | 21.8 | 17.7 |
| ⩾ 20 | 111 (45.9) | 32.4 | 34.2 | 26.1 | 24.3 | 45 (9.3) | 22.2 | 37.8 | 11.1 | 13.3 |
Significant (p < 0.05, χ2 test) differences between children with and without symptoms are shown in boldface type.
Adjusted logistic regression models confirmed these relationships (Table 4). Children in multifamily housing exposed to gas stoves were more likely to experience wheeze, shortness of breath, and chest tightness. Gas source was not associated with respiratory symptoms for children living in single-family homes. Measured NO2 (using concentration as a continuous variable) replaced gas sources in the logistic and Poisson regression models, as shown in Table 5. For each 20-ppb increase in NO2, children in multifamily housing were more likely to wheeze (odds ratio [OR], 1.52; 95% confidence interval [95% CI], 1.04, 2.21) and to have chest tightness (OR, 1.61; 95% CI, 1.04, 2.49). In Poisson models with days of symptoms as the outcome variables, for each 20-ppb increase in NO2, children in multifamily housing experienced more days of wheeze in the month before the interview (relative risk [RR], 1.33; 95% CI, 1.05, 1.68) and more days of chest tightness (RR, 1.51; 95% CI, 1.18, 1.91). No significant relationships between symptoms and measured NO2 were evident for children living in single-family homes.
TABLE 4.
ESTIMATES OF ODDS RATIOS AND 95% CONFIDENCE INTERVALS FROM LOGISTIC REGRESSION MODELS FOR HOUSEHOLD SOURCES OF NO2 RELATED TO RESPIRATORY SYMPTOMS IN THE MONTH BEFORE SAMPLING (SOUTHERN NEW ENGLAND, 1998–2000)
| Wheeze | Persistent Cough | Shortness of Breath | Chest Tightness | |
|---|---|---|---|---|
| Factors | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Multifamily housing | ||||
| Gas stove | 2.27 (1.15, 4.47) | 1.19 (0.66, 2.16) | 2.38 (1.12, 5.06) | 4.34 (1.76, 10.69) |
| Gas dryer | 0.78 (0.23, 2.57) | 1.19 (0.40, 3.53) | 2.39 (0.77, 7.43) | 1.09 (0.31, 3.90) |
| Smoker in the home | 1.08 (0.48, 2.39) | 1.30 (0.64, 2.62) | 0.98 (0.41, 2.33) | 1.60 (0.62, 4.12) |
| Single-family Housing | ||||
| Gas stove | 0.61 (0.35, 1.05) | 0.92 (0.55, 1.51) | 0.91 (0.50, 1.64) | 0.68 (0.34, 1.32) |
| Gas dryer | 1.02 (0.50, 2.12) | 0.98 (0.49, 1.94) | 0.93 (0.42, 2.07) | 1.41 (0.61, 3.26) |
| Smoker in the home | 1.92 (0.92, 4.04) | 0.90 (0.41, 1.96) | 1.26 (0.53, 2.97) | 0.62 (0.20, 1.90) |
Definition of abbreviations: CI = confidence interval; OR = odds ratio.
Significant (p < 0.05) results shown in boldface type. Separate models were run for each symptom, and all models were adjusted for age, ethnicity, mold/mildew, water leaks, maintenance medication use, and season of sampling. Analyses were stratified by housing type.
TABLE 5.
RESULTS OF MODELS RELATING SYMPTOMS IN THE MONTH BEFORE SAMPLING TO LEVELS OF NO2 MEASURED INDOORS
| Model | Wheeze | Persistent Cough | Shortness of Breath | Chest Tightness |
|---|---|---|---|---|
| Multifamily housing | ||||
| Logistic regression predicting any symptom, OR (95% CI) | 1.52 (1.04, 2.21) | 1.06 (0.75, 1.49) | 1.28 (0.85, 1.91) | 1.61 (1.04, 2.49) |
| Poisson regression predicting days of symptom, RR (95% CI) | 1.33 (1.05, 1.68) | 1.07 (0.84, 1.35) | 1.23 (0.95, 1.59) | 1.51 (1.18, 1.91) |
| Single-family housing | ||||
| Logistic regression predicting any symptom, OR (95% CI) | 0.99 (0.71, 1.38) | 1.07 (0.78, 1.47) | 0.83 (0.52, 1.31) | 1.10 (0.78, 1.57) |
| Poisson regression predicting days of symptom, RR (95% CI) | 0.98 (0.78, 1.22) | 0.91 (0.69, 1.20) | 0.86 (0.63, 1.18) | 0.92 (0.68, 1.25) |
Definition of abbreviations: CI = confidence interval; OR = odds ratio; RR = rate ratio.
Significant (p < 0.05) results shown in boldface type. Separate models were run for each symptom, and all models were adjusted for age, ethnicity, mold/mildew, water leaks, maintenance medication use, and season of sampling. Analyses were stratified by housing type. Estimates of OR and 95% CI are from logistic regression models predicting any symptom and rate ratios (RRs) from Poisson models predicting number of days of symptom (southern New England, 1998–2000). ORs and RRs are given for each 20-ppb increase in NO2.
DISCUSSION
This study of children with physician-diagnosed asthma observed associations between exposure to NO2 and respiratory symptoms (wheeze and chest tightness). The association was consistent whether exposure was estimated from use of a gas stove in the home or by measured NO2 concentration. In addition, exposure to NO2 increased both risk of experiencing symptoms (any wheeze or any chest tightness) and number of days of symptoms (wheeze or chest tightness) in the month before interview. The association between NO2 exposure and respiratory symptoms was limited to children in multifamily housing. To date, this is the largest study to examine the effects of NO2 on children with asthma. The study population was quite diverse and included both white and nonwhite children, children living in single-family and multifamily homes, and children living in urban and suburban environments.
A strength of the study is the short period of recall for respondents and the close proximity of reported symptoms to NO2 measurement. Mothers reported their child's symptoms for the month immediately before NO2 measurement. This ensured that respiratory symptoms were compared with NO2 measurements in the same season. Ideally, we would have measured symptoms at exactly the same time as NO2 was being monitored, although this would not necessarily have reduced recall bias by the mother. NO2 in homes is unlikely to vary over a single month (33, 34), particularly within the same season, and the NO2 we measured is expected to reasonably predict NO2 in the prior month when symptoms were assessed.
A potential limitation of the study is that the same research assistant who collected symptom information from the mother also observed and recorded the presence of a gas stove. This is unlikely to have introduced bias, because the research assistant could not know the measured NO2 levels. All associations between gas stove use and respiratory symptoms were confirmed by associations using measured NO2.
A further limitation may be assessing personal exposure by passive monitors placed in one room rather than using personal badges worn by study subjects. However, outdoor levels can be quite variable depending on proximity to traffic and other combustion sources (35), and the dominant influence on personal exposure to NO2 has been shown to be indoor concentration (36–39). Indoor concentration is largely influenced by use of a gas stove (36, 38). Measurements were not made in other environments where the child spent considerable time, such as school, but we would not expect children to be exposed to NO2 sources (e.g., cooking stoves, unvented gas heaters) inside schools in our study area. Unmeasured exposure to NO2 in other environments would contribute to random misclassification and would bias the finding toward the null. Despite this potential misclassification, we were able to detect associations between respiratory symptoms and NO2 in the home.
Use of personal monitors would not have overcome another study limitation: the strong association of NO2 exposure with housing characteristics, lower socioeconomic status, and ethnicity. To reduce any confounding, analyses were stratified by housing status and children were compared within groups that were similar in socioeconomic status and housing type. Stratification by housing type did not eliminate the association of ethnicity with NO2 exposure. Furthermore, ethnicity was not significantly associated with asthma symptoms in either the stratified or unstratified analyses and confounding was controlled in multivariate analyses. However, it was difficult to control for potential confounding variables (e.g., housing characteristics, ethnicity) without reducing the effect of NO2. It is possible that residual confounding remains or, alternatively, that the results underestimate the effect of NO2.
No effect of NO2 was detected among children living in single-family homes. NO2 in single-family homes (mean [SD] = 10.2 [12.3] ppb) was much lower than in multifamily housing (22.9 [17.0] ppb), which may explain this observation. In addition, single-family homes were much larger (82% had six or more rooms). Exposure was measured in one location (the room where the family spent the most time), and not in the child's bedroom. In a single-family house, the child's bedroom is often on a different floor. Thus, personal exposure of a child in a single-family home could have been considerably less than the measured concentration of NO2 in this study.
It could also be argued that exposure to NO2 was simply a marker for poor housing conditions and that any association between NO2 and respiratory symptoms indicates a risk from poor housing. This appears unlikely because analyses were stratified so that children were compared with other children in similar housing. In addition, housing characteristics that might contribute to respiratory symptoms (water leaks and visible mold) were not associated with NO2 exposure in stratified analyses and were controlled in multivariate analyses.
The significant association we observed between gas stove use and respiratory symptoms is consistent with other studies of children with asthma. In a meta-analysis (5) of three cross-sectional studies that included children with asthma (40–42), the combined OR was 1.20 (95% CI, 1.11, 1.30) for an asthma diagnosis and 1.12 (95% CI, 1.04, 1.20) for any wheeze in the presence of gas stoves. Garrett and colleagues (43) prospectively investigated 148 Australian children, including 53 children with asthma and 88 children with atopy. Among all children, respiratory symptoms were more common in those exposed to a gas stove (OR, 2.32; 95% CI, 1.04, 5.18) and a significant association was found between an asthma diagnosis and presence of a gas stove (OR, 2.23; 95% CI, 1.06, 4.72).
Associations between measured NO2 and respiratory symptoms were also consistent with the few studies that specifically investigated effects of NO2 exposure on children with asthma. A community-based study of 125 self-reported individuals with asthma included 48 children younger than 15 yr (20). Mean daily exposure to household NO2 was 32 ppb (range, 24–44 ppb) in homes with gas stoves and 12 ppb (range, 11–15 ppb) in homes with electric stoves. For each 20-ppb increase in NO2, the OR for chest tightness was 1.12 (95% CI, 1.07, 1.18). In a study of 114 children with asthma in England, the authors investigated whether NO2 exposure before viral infections could potentiate an asthmatic response (21, 22). Exposures to NO2 were generally low (66% were < 7.5 ppb), but compared with exposures of 4 ppb or less, exposures greater than 15 ppb were associated with an RR of 1.9 (95% CI, 1.1, 3.4) for subsequent asthma episode (21). NO2 exposure greater than 7.5 ppb in the week before infection also resulted in increased severity of lower respiratory symptoms (21). Although our study did not include data to directly address this issue, our results are consistent with their findings.
A recent randomized trial provides strong evidence that reducing NO2 exposure results in a reduction of asthma symptoms (23). Australian schools (n = 18) using unflued gas heaters were randomly assigned to have replacement flued gas or electric heaters installed (23). Passive NO2 monitors were placed in the 10 control and 8 replacement schools, and a total of 199 children with asthma were monitored for 12 wk after heater replacement. Mean NO2 levels were 15.5 (SD, 6.6) ppb and 47.0 (26.8) ppb in the electric heated and unflued gas-heated classrooms, respectively. The RR of symptoms in the gas-heated classroom was 2.44 (95% CI, 1.02, 14.29) for difficulty breathing, 2.22 (95% CI, 1.23, 4.00) for chest tightness, and 2.56 (95% CI, 1.08, 5.88) for asthma attacks (23).
In conclusion, this study has demonstrated an association between indoor NO2 and increased respiratory symptoms among children with asthma. The NO2 levels to which these children responded are common in homes using gas stoves (32). There are currently no U.S. standards for indoor levels of NO2, but the levels associated with significant health effects among the children in multifamily housing are similar to the outdoor annual average exposure of 21 ppb (40 μg/m3) recommended by the World Health Organization (44), and well below the outdoor hourly peak exposure of 106 ppb (200 μg/m3) set by the World Health Organization (44) and outdoor annual average exposure of 53 ppb (100 μg/m3) set by the U.S. Environmental Protection Agency (45).
Supplementary Material
Supported by grants ES07456, ES05410, and ES11013 from the National Institute of Environmental Health Sciences.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200408-1123OC on October 27, 2005
Conflict of Interest Statement: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.U.S. Census Bureau. 2000 census of population and housing. Summary File 3; Table H40: House Heating Fuel. Available from: http://factfinder.census.gov.
- 2.Utell M, Frampton MW. Oxides of nitrogen. In: Roth RA, editor. Comprehensive toxicology: oxides of nitrogen. Cambridge, UK: Cambridge University Press; 1997. pp. 303–312.
- 3.Samet JM, Utell MJ. The risk of nitrogen dioxide: what have we learned from epidemiological and clinical studies? Toxicol Ind Health 1990;6: 247–262. [PubMed] [Google Scholar]
- 4.Basu R, Samet JM. A review of the epidemiological evidence on health effects of nitrogen dioxide exposure from gas stoves. J Environ Med 1999;1:173–187. [Google Scholar]
- 5.Nitschke M, Smith BJ, Pilotto LS, Pisaniello DL, Abramson MJ, Ruffin RE. Respiratory health effects of nitrogen dioxide exposure and current guidelines. Int J Environ Health Res 1999;9:39–53. [Google Scholar]
- 6.Fuhlbrigge A, Weiss S. Domestic gas appliances and lung disease. Thorax 1997;52:S58–S62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chauhan AJ. Gas cooking appliances and indoor pollution. Clin Exp Allergy 1999;29:1009–1013. [DOI] [PubMed] [Google Scholar]
- 8.Jarvis D. Gas cooking and respiratory disease. Thorax 1999;54:1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strachan DP. The role of environmental factors in asthma. BMJ 2000;56: 865–882. [DOI] [PubMed] [Google Scholar]
- 10.Brunekreef B. NO2: the gas that won't go away. Clin Exp Allergy 2001;31: 1170–1172. [DOI] [PubMed] [Google Scholar]
- 11.Samet JM, Bell ML. Commentary: nitrogen dioxide and asthma redux. Int J Epidemiol 2004;33:215–216. [DOI] [PubMed] [Google Scholar]
- 12.Hasselblad V, Eddy D, Kotchmar D. Synthesis of environmental evidence: nitrogen dioxide epidemiology studies. J Air Waste Manag Assoc 1992;42:662–671. [DOI] [PubMed] [Google Scholar]
- 13.Mukala K, Alm S, Tiittanen P, Salonen RO, Jantunen M, Pekkanen J. Nitrogen dioxide exposure assessment and cough among preschool children. Arch Env Health 2000;55:431–438. [DOI] [PubMed] [Google Scholar]
- 14.Shima M, Adachi M. Effect of outdoor and indoor nitrogen dioxide on respiratory symptoms in schoolchildren. Int J Epidemiol 2000;29:862–870. [DOI] [PubMed] [Google Scholar]
- 15.Ponsonby A-L, Glasgow N, Gatenby P, Mullins R, McDonald AD, Hurwitz M, Pradith B, Attewell R. The relationship between low level nitrogen dioxide exposure and child lung function after cold air challenge. Clin Exp Allergy 2001;31:1205–1212. [DOI] [PubMed] [Google Scholar]
- 16.Samet JM, Lambert WE, Skipper BJ, Cushing AH, Hunt WC, Young SA, McLaren LC, Schwab M, Spengler JD. Nitrogen dioxide and respiratory illness in infants. Am Rev Respir Dis 1993;148:1258–1265. [DOI] [PubMed] [Google Scholar]
- 17.Magnus P, Nafstad P, Oie L, Carlsen KCL, Becher G, Kongerud J, Carlsen K-H, Samuelson SO, Botten G, Bakketeig L. Exposure to nitrogen dioxide and the occurrence of bronchial obstruction in children below 2 years. Int J Epidemiol 1998;27:995–999. [DOI] [PubMed] [Google Scholar]
- 18.Sunyer J, Puig C, Torrent M, Garcia-Algar O, Calico I, Munoz-Ortiz L, Barnes M, Cullinan P. Nitrogen dioxide is not associated with respiratory infection during the first year of life. Int J Epidemiol 2004;33:116–120. [DOI] [PubMed] [Google Scholar]
- 19.Emenius G, Svartengren M, Korsgaard J, Nordvall L, Pershagen G, Wickman M. Building characteristics, indoor air quality and recurrent wheezing in very young children (BAMSE). Indoor Air 2004;14:34–42. [DOI] [PubMed] [Google Scholar]
- 20.Smith BJ, Nitschke M, Pilotto LS, Ruffin RE, Pisaniello DL, Wilson KJ. Health effects of daily indoor nitrogen dioxide exposure in people with asthma. Eur Respir J 2000;16:879–885. [DOI] [PubMed] [Google Scholar]
- 21.Linaker CH, Coggon D, Holgate ST, Clough J, Josephs L, Chauhan AJ, Inskip HM. Personal exposure to nitrogen dioxide and risk of airflow obstruction in asthmatic children with upper respiratory infection. Thorax 2000;55:930–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chauhan AJ, Inskip HM, Linaker CH, Smith S, Schreiber J, Johnston SL, Holgate ST. Personal exposure to nitrogen dioxide (NO2) and the severity of virus-induced asthma in children. Lancet 2003;361:1939–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pilotto LS, Nitschke M, Smith BJ, Pisaniello D, Ruffin RE, McElroy HJ, Martin J, Hiller JE. Randomized controlled trial of unflued gas heater replacement on respiratory health of asthmatic schoolchildren. Int J Epidemiol 2003;33:208–214. [DOI] [PubMed] [Google Scholar]
- 24.Belanger K, Beckett W, Triche E, Bracken M, Holford T, Ren P, McSharry J-E, Gold D, Platts-Mills T, Leaderer B. Symptoms of wheeze and persistent cough in the first year of life: associations with indoor allergens, air contaminants and maternal history of asthma. Am J Epidemiol 2003;158:195–202. [DOI] [PubMed] [Google Scholar]
- 25.van Strien RT, Gent JF, Belanger K, Triche E, Bracken MB, Leaderer BP. Exposure to NO2 and nitrous acid and respiratory symptoms in the first year of life. Epidemiology 2004;15:471–478. [DOI] [PubMed] [Google Scholar]
- 26.Berglund M. Health risk evaluation of nitrogen oxides: exposure. Scand J Work Environ Health 1993;19:37–43. [PubMed] [Google Scholar]
- 27.Bauer MA, Utell MJ, Morrow PE, Speers DM, Gibb FR. Inhalation of 0.30 ppm nitrogen dioxide potentiates exercise-induced bronchospasm in asthmatics. Am Rev Respir Dis 1986;134:1203–1208. [DOI] [PubMed] [Google Scholar]
- 28.Folinsbee LJ. Does nitrogen dioxide exposure increase airways responsiveness? Toxicol Ind Health 1992;8:273–283. [PubMed] [Google Scholar]
- 29.Gent JF, Ren P, Belanger K, Triche E, Bracken MB, Holford TR, Leaderer BP. Levels of household mold associated with respiratory symptoms in the first year of life in a cohort at risk for asthma. Environ Health Perspect 2002;110:A781–A786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gent JF, Belanger K, Triche EW, Holford TR, Beckett WS, Bracken MB, Leaderer BP. Respiratory symptoms and indoor nitrogen dioxide: a retrospective study of 682 asthmatic children [abstract]. Am J Respir Crit Care Med 2004;170:A162. [Google Scholar]
- 31.Palmes ED, Gunnison AF, DiMattio J, Tomczyk C. Personal sampler for nitrogen dioxide. Am Ind Hyg Assoc J 1976;37:570–577. [DOI] [PubMed] [Google Scholar]
- 32.Breysse PN, Buckley TJ, Williams D, Beck CM, Jo S-J, Merriman B, Kanchanaraksa S, Swartz LJ, Callahan KA, Butz AM, et al. Indoor exposures to air pollutants and allergens in the homes of asthmatic children in inner-city Baltimore. Environ Res 2005;95:167–176. [DOI] [PubMed] [Google Scholar]
- 33.Brunekreef B, Noy D, Clausing P. Variability of exposure measurements in environmental epidemiology. Am J Epidemiol 1987;125:892–898. [DOI] [PubMed] [Google Scholar]
- 34.Spengler JD, Schwab M, McDermott A, Lambert WE, Samet JM. Research report: nitrogen dioxide and respiratory illness in children. Part IV: effects of housing and meteorologic factors on indoor nitrogen dioxide concentrations. Res Rep Health Eff Inst 1996; Dec (58):1–29; discussion 31–36. [PubMed]
- 35.Liard R, Zureik M, Le Moullec Y, Soussan D. M, Grimfeld A, Neukirch F. Use of personal passive samplers for measurement of NO2, NO, and O3 levels in panel studies. Environ Res 1999;81:339–348. [DOI] [PubMed] [Google Scholar]
- 36.Leaderer B, Zagraniski R, Berwick M, Stolwijk J. Assessment of exposure to indoor air contaminants from combustion sources: methodology and application. Am J Epidemiol 1986;124:275–289. [DOI] [PubMed] [Google Scholar]
- 37.Hoek G, Brunekreef B, Meijer R, Scholten A, Boleij J. Indoor nitrogen dioxide pollution and respiratory symptoms of schoolchildren. Int Arch Occup Environ Health 1984;55:79–86. [DOI] [PubMed] [Google Scholar]
- 38.Levy JI, Lee K, Spengler JD, Yanagisawa Y. Impact of residential nitrogen dioxide exposure on personal exposure: an international study. J Air Waste Manag Assoc 1998;48:553–560. [DOI] [PubMed] [Google Scholar]
- 39.Linaker CH, Chauhan AJ, Inskip HM, Holgate ST, Coggon D. Personal exposures of children to nitrogen dioxide relative to concentrations in outdoor air. Occup Environ Med 2000;57:472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dekker C, Dales R, Bartlett S, Brunekreef B, Zwanenburgy H. Childhood asthma and the indoor environment. Chest 1991;100:922–926. [DOI] [PubMed] [Google Scholar]
- 41.Kuhr J, Hendel Kramer A, Karmaus W, Moseler M, Weiss K, Stephan V, Urbanek R. Air pollutant burden and bronchial asthma in school children. Soz Prav Med 1991;36:67–73. [DOI] [PubMed] [Google Scholar]
- 42.Volkmer RE, Ruffin RE, Wigg NR, Davies N. The prevalence of respiratory symptoms in South Australian preschool children. II Factors associated with indoor air quality. J Paediatr Child Health 1995;31:116–120. [DOI] [PubMed] [Google Scholar]
- 43.Garrett MH, Hooper MA, Hooper BM, Abramson MJ. Respiratory symptoms in children and indoor exposure to nitrogen dioxide and gas stoves. Am J Respir Crit Care Med 1998;158:891–895. [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization. Air quality guidelines for Europe, 2nd ed. Copenhagen: World Health Organization; 2000.
- 45.U.S. Environmental Protection Agency. National ambient air quality standards (naaqs). Updated July 29, 2005. Available online at http://epa.gov/air/criteria.html [accessed December 12, 2005.]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.