Abstract
Objective: Our goal is to determine short-term intraindividual biologic and measurement variability in spirometry of patients with a wide range of stable chronic obstructive pulmonary disease severity, using datasets from the National Emphysema Treatment Trial (NETT) and the Lung Health Study (LHS). This may be applied to determine criteria that can be used to assess a clinically meaningful change in spirometry.
Methods: A total of 5,886 participants from the LHS and 1,215 participants from the NETT performed prebronchodilator spirometry during two baseline sessions. We analyzed varying criteria for absolute and percent change of FEV1 and FVC to determine which criterion was met by 90% of the participants.
Results: The mean ± SD FEV1 for the initial session was 2.64 ± 0.60 L (75.1 ± 8.8% predicted) for the LHS and 0.68 ± 0.22 L (23.7 ± 6.5% predicted) for the NETT. The mean ± SD number of days between test sessions was 24.9 ± 17.1 for the LHS and 85.7 ± 21.7 for the NETT. As the degree of obstruction increased, the intersession percent difference of FEV1 increased. However, the absolute difference between tests remained relatively constant despite the severity of obstruction (0.106 ± 0.10 L). Over 90% of participants had an intersession FEV1 difference of less than 225 ml irrespective of the severity of obstruction.
Conclusions: Absolute changes in FEV1 rather than percent change should be used to determine whether patients with chronic obstructive pulmonary disease have improved or worsened between test sessions.
Keywords: forced expiratory volume, obstructive lung diseases, reproducibility of measurements, spirometry, vital capacity
In patients with chronic obstructive pulmonary disease (COPD), spirometry, including FEV1 and FVC, is a widely used measure of progression of disease and response to treatment. According to past American Thoracic Society (ATS) standards for spirometry, within-session reproducibility was considered to be 200 ml or a 5% change in FEV1, whichever is larger (1), based on data from the Third National Health and Nutrition Examination Survey (NHANES III) (2). The current proposed ATS/European Respiratory Society guidelines for spirometry standardization recommend within-session reproducibility within 150 ml for FEV1 and FVC (3). Small previous studies suggested that patients with COPD may have greater variability within and between spirometry test sessions than do individuals with normal lung function (4). Previous articles have reported the clinical predictors associated with variability in FEV1 in the Lung Health Study (LHS) (5). The current study expands the results by adding data from the National Emphysema Treatment Trial (NETT) cohort and specifically examining the effect of baseline lung function on intersession variability. There are, however, no generally accepted standards for short-term reproducibility between test sessions that can be clinically used to define improvement or worsening.
Two studies of patients with COPD, the NETT and the LHS, provide large datasets in which spirometry was performed on two separate occasions during a baseline evaluation. Taken together, these data are useful for evaluating intersession variability in COPD spirometry results because they include a wide range of COPD severity and represent a variety of testing sites.
Using these datasets, we addressed the following questions: What is the short-term variability of FEV1 and FVC between test sessions in patients with COPD? Does intersession variability depend on the severity of COPD? Should suitable criteria for minimal significant change in FEV1 or FVC be based on percent change, absolute change, or both? The data in the current article were presented as an abstract and poster at the May 2005 International Conference of the ATS (6).
METHODS
Patient Selection
We selected participants for this study from the pool of participants of two randomized controlled trials: 5,886 participants from the LHS and 1,215 patients from the NETT. All participants had airflow obstruction by pulmonary function testing (PFT) and smoking history. The LHS participants were all active smokers at the time of enrollment, and the NETT participants were nonsmokers for at least 4 mo at the time of enrollment. All participants had at least two separate tests of spirometry during the baseline evaluation. Both the LHS and NETT were approved by the institutional review board/ethics committees of all participating institutions.
The LHS (7) was a multicenter, randomized clinical trial funded by the National Heart, Lung, and Blood Institute with the purpose of determining whether a smoking intervention program and inhaled bronchodilators slowed the decline of FEV1. Participants were aged 34 to 67 yr, with a mean age of 48 yr, and mild to moderate airway obstruction (FEV1 from 90 to 50% predicted) (8).
The NETT (9) was a multicenter, randomized clinical trial that compared lung volume reduction surgery with medical management with medical management alone to determine the efficacy of lung volume reduction surgery. Participants were aged 39 to 84 yr, with a mean age of 66 yr, with severe emphysema (FEV1 ⩽ 45% predicted) (10).
Pulmonary Function Testing
Spirometry methods met or exceeded ATS standards (1). In both studies, participants had prior experience with spirometry. There were differences, however, between the PFT performed in the LHS and the NETT trials. All participants in the LHS were tested using standardized equipment by technicians who were centrally trained and certified. Participants of the NETT study were tested in clinical pulmonary function laboratories using a variety of spirometers in centers that were site-visited and certified by the trial investigators. In both trials, spirometry used for comparison was obtained before bronchodilator treatment, with a mean of 25 d (LHS) and 86 d (NETT) between sessions (7, 9). The NETT subjects had undergone pulmonary rehabilitation training between sessions, an intervention that would not be expected to alter FEV1 (9, 11). In both studies, spirometry was performed when patients were stable and not suffering from an exacerbation.
Statistical Analysis
Descriptive analyses were performed assessing the age, sex, and race of participants of each group studied. Box plots of intersession differences of pulmonary function were created by deciles of percent-predicted lung function (8, 10). Comparisons were made between the two study sessions for FEV1 and FVC, calculating the mean difference and standard deviation between sessions as the second test minus the first. We also calculated the mean absolute difference and standard deviation as the absolute difference between the two test sessions. The absolute difference is the difference between two measurements irrespective of sign (i.e., it is always positive). Percent difference was calculated as the second session value minus the first session value divided by the first session value. The absolute percent difference was calculated as the absolute difference between the second and first session values divided by the first session value. Stata version 8.0 (Stata Corp, College Station, TX) was used for statistical calculations.
To evaluate alternative sets of criteria for intersession reproducibility, we calculated the proportion of study participants meeting candidate criteria that covered a range of absolute differences for each level of percent change. We plotted the proportion of participants meeting each of the criteria as isopleths of percent difference for each absolute difference. For the purpose of comparison with previous studies of pulmonary function testing reproducibility, we used a criterion value that would be met by 90% of the population (12–14).
RESULTS
Demographic and Pulmonary Function Characteristics
A total of 7,101 participants had two separate PFT sessions (5,886 subjects from the LHS and 1,215 from the NETT). Participant characteristics and pulmonary function tests are summarized in Table 1. The average age ± SD was 48.5 ± 6.8 yr for LHS participants and 66.4 ± 6.1 yr for NETT. A majority of the participants were white in both groups. Because the ethnic groups were categorized differently for the LHS and NETT, the groups were divided into white and nonwhite for the purposes of the present study. Males comprised about 62% of both populations.
TABLE 1.
PARTICIPANT CHARACTERISTICS
| Variable | NETT (n = 1,215) | LHS (n = 5,886) | Total (n = 7,101) |
|---|---|---|---|
| Age | 66.4 ± 6.1 | 48.5 ± 6.8 | 51.6 ± 9.6 |
| Race, % white | 94.9 | 95.8 | 95.6 |
| Sex, % male | 61.4 | 62.9 | 62.6 |
| FEV1, L | 0.68 ± 0.22 | 2.64 ± 0.60 | 2.30 ± 0.92 |
| FVC, L | 2.14 ± 0.72 | 4.20 ± 0.94 | 3.84 ± 1.19 |
| FEV1, % predicted | 23.7 ± 6.5 | 75.1 ± 8.8 | 66.3 ± 21.2 |
| FVC, % predicted | 57.1 ± 14.7 | 96.4 ± 10.7 | 89.7 ± 18.7 |
| Interval between tests, d | 85.7 ± 21.7 | 24.9 ± 17.1 | 35.3 ± 29.1 |
| Difference FEV1, L | 0.007 ± 0.12 | −0.011 ± 0.15 | −0.008 ± 0.14 |
| Absolute difference FEV1, L | 0.089 ± 0.08 | 0.110 ± 0.10 | 0.106 ± 0.10 |
| Difference FEV1, % | 2.54 ± 17.4 | −0.35 ± 5.8 | 0.14 ± 9.0 |
| Absolute difference FEV1, % | 13.33 ± 11.4 | 4.29 ± 3.9 | 5.84 ± 6.8 |
| Difference FVC, L | 0.072 ± 0.42 | −0.023 ± 0.20 | −0.007 ± 0.25 |
| Absolute difference FVC, L | 0.317 ± 0.28 | 0.150 ± 0.13 | 0.178 ± 0.18 |
| Difference FVC, % | 5.48 ± 20.9 | −0.47 ± 4.9 | 0.55 ± 10.0 |
| Absolute difference FVC, % | 15.81 ± 14.8 | 3.65 ± 3.3 | 5.73 ± 8.2 |
Definition of abbreviations: LHS = Lung Health Study; NETT = National Emphysema Treatment Trial.
Results are shown as mean ± SD. Data were not available on one participant from the LHS (n = 5,886).
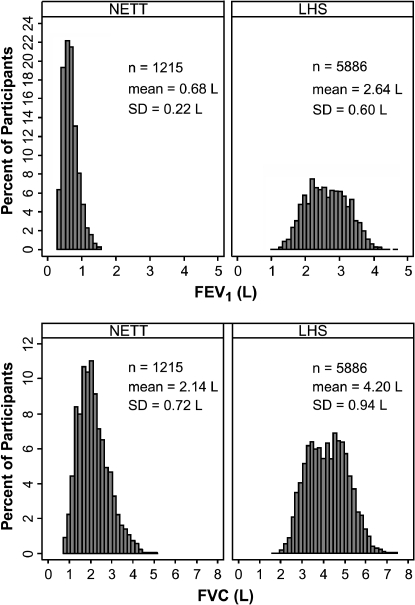
The two pulmonary function tests were administered a mean ± SD of 24.8 ± 17.1 and 85.7 ± 21.7 d apart, respectively, in the LHS and NETT studies. In accordance with the study designs, the baseline mean ± SD FEV1 (Table 1) was higher for the LHS participants than for the NETT participants (2.64 ± 0.60 vs. 0.68 ± 0.22 L), and the FEV1 percent predicted for the LHS group was higher than the NETT group: mean ± SD percent-predicted FEV1 of 75.1 ± 8.8 versus 23.7 ± 6.5%. With a range of FEV1 from 1.07 to 4.70 L, and a range of percent-predicted values from 48.2 to 95.7%, the LHS participants generally had mild to moderate COPD. In contrast, NETT participants with a lower range of FEV1 values at baseline (0.29 to 1.58 L) and a range of percent-predicted values from 9.1 to 45.7% would be categorized as having severe or very severe COPD (15). Therefore, the two study populations taken together represented a wide range of COPD severity. The histograms of distribution of FEV1 and FVC for each of the studies are given in Figure 1.
Figure 1.
Distributional plots for FEV1 and FVC in the two study populations. LHS = Lung Health Study; NETT = National Emphysema Treatment Trial.
The interval between test sessions was longer for the NETT than the LHS. However, the mean absolute difference in FEV1 between the first and second test sessions was similar (0.089 ml in the NETT vs. 0.110 ml in the LHS; Table 1). Moreover, there was no significant correlation between the testing interval and the difference between tests within each of the studies. Therefore, we combined both study groups in a single analysis.
Reproducibility of FEV1
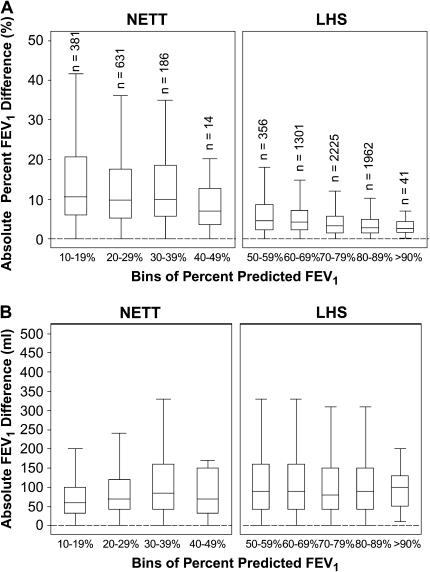
To determine the reproducibility of the FEV1, we compared the intersession absolute difference and absolute percent difference of FEV1 stratified by deciles of percent predicted. As the percent-predicted FEV1 increased, the percent difference in FEV1 became smaller (Figure 2A). However, the absolute difference remained relatively stable as the percent-predicted FEV1 increased (Figure 2B), and there was no notable difference between the two study populations.
Figure 2.
Box and whisker plots for the percent difference (A) and absolute difference (B) for repeat FEV1 measurements as a function of FEV1 percent predicted. Boxes represent the interquartile range (IQR) bounded by the 25th and 75th percentiles with the bar as the median. The whiskers represent the distribution of values between the upper fence (75th percentile + 1.5 × IQR) and the lower fence (25th percentile – 1.5 × IQR). Outliers are not displayed in the graph. Each bin corresponds to a decile range of percent-predicted FEV1. Example: Bin 1 = 10 to 19.9% predicted FEV1. There are identical numbers of participants (n) in corresponding bins of percent-predicted FEV1 for A and B. Bins for the range of 0 to 9% predicted FEV1 for NETT and 40 to 49% predicted FEV1 for LHS are not displayed as there are fewer than five participants per bin (n = 3 and n = 1, respectively).
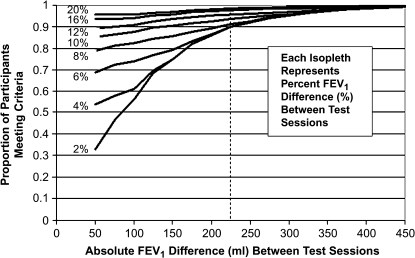
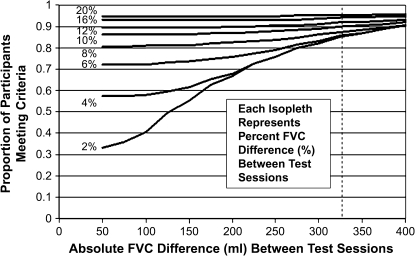
To compare different potential criteria for FEV1 reproducibility in terms of percent change or absolute change, we plotted the percent of patients meeting varying sets of criteria (Figure 3). Each isopleth represents the percent of patients within a calculated percent difference category from 2 to 20%. At absolute differences above 225 ml, more than 90% of participants met the criteria regardless of the percent difference. If two criteria were used, then a percent change of 10% or 150 ml was met by at least 90% of participants.
Figure 3.
Proportion of patients as a function of absolute difference or percent difference between two measurements of FEV1. Each isopleth indicates the proportion of patients meeting the candidate criteria for the labeled percent difference as the absolute FEV1 difference varies. The percent difference is the absolute percent difference of the FEV1 between the two test sessions. For example, to determine the proportion of participants who met criteria for less than 6% difference in FEV1 between visits or less than 150 ml absolute difference between visits, follow the 6% isopleth to 150-ml value on the x-axis and read the proportion meeting the criteria on the y-axis. (Slightly less than 80% of participants met this criteria.)
Reproducibility of FVC
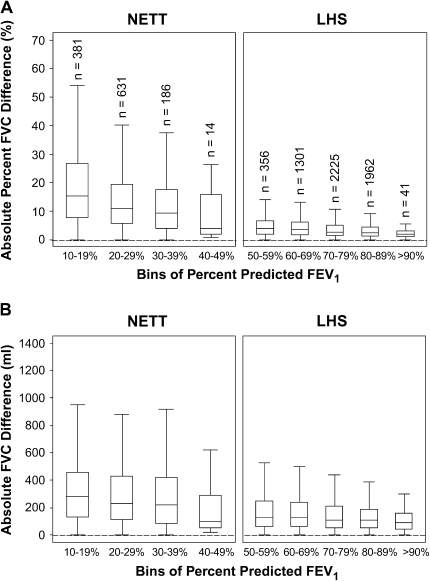
The FVC intersession variability in the NETT was considerably greater than in the LHS. In the NETT, the SD of the absolute FVC difference was 0.28 L compared with 0.13 L in the LHS. This is a larger SD than the SD of the absolute FEV1 difference (0.08 L in the NETT compared with 0.10 L in the LHS; Table 1). To determine the FVC variability, we calculated the intersession absolute difference and absolute percent difference stratified by deciles of percent-predicted FEV1. As the degree of airflow obstruction increased (i.e., declining percent-predicted FEV1), the absolute and percent difference in FVC increased (Figures 4A and 4B).
Figure 4.
Box and whisker plots for the percent difference (A) and absolute difference (B) for repeat FVC measurements as a function of FEV1 percent predicted. Boxes represent the IQR bounded by the 25th and 75th percentiles with the bar as the median. The whiskers represent the distribution of values between the upper fence (75th percentile + 1.5 × IQR) and the lower fence (25th percentile – 1.5 × IQR). Outliers are not displayed in the graph. Each bin corresponds to a decile range of percent-predicted FEV1. Example: Bin 1 = 10 to 19.9% predicted FEV1. There are identical numbers of participants (n) in corresponding bins of percent-predicted FEV1 for A and B. Bins for the range of 0 to 9% predicted FEV1 for NETT and 40 to 49% predicted FEV1 for LHS are not displayed as there are fewer than five participants per bin (n = 3 and n = 1, respectively).
Within each study, the variability of absolute FVC difference was relatively constant for each decile of FVC percent predicted, but was higher for the NETT than for the LHS (Figure E1 of the online supplement). To include 90% of the test session for both studies, the reproducibility criteria would have to exceed a 10% change or 325 ml absolute difference. If only an absolute criterion for FVC is used, then 90% of the test sessions are reproducible within 400 ml (Figure 5).
Figure 5.
Proportion of patients as a function of absolute difference or percent difference between two measurements of FVC. Each isopleth indicates the proportion of patients meeting the candidate criteria for the labeled percent difference as the absolute FVC difference varies. The percent difference is the absolute percent difference of the FVC between the two test sessions.
DISCUSSION
There are no formal recommendations to determine what percent change or absolute value change is clinically or statistically meaningful between measurements of FEV1 taken during two test sessions. The major finding of this analysis is that the absolute difference of FEV1 between two spirometry sessions did not vary with the baseline level of FEV1. As a corollary, the percent change was larger as the baseline FEV1 declined. Thus, the absolute change in FEV1, rather than the percent change, is a more stable criterion for determining whether a measured change in FEV1 exceeds the expected day-to-day variability in patients with COPD.
Based on the present study, criteria for intersession FEV1 reproducibility can be defined on the basis of the values represented in Figure 3. At least 90% of all patients studied met a criterion of reproducibility at 225 ml absolute difference in FEV1. Above the absolute cutoff of 225 ml, the percent difference in FEV1 had little effect on the fraction of subjects meeting the reproducibility threshold. Below the 225-ml cutoff, the effect of percent difference of FEV1 increased. This result suggests that a clinically useful rule for detecting change in FEV1 in an individual patient is an absolute change greater than 225 ml, irrespective of the baseline level of FEV1. An alternative approach is to accept a percent difference of greater than 10% with an absolute change of at least 150 ml. If we were to use a value of percent difference alone as a criterion for reproducibility, we would overestimate the number of patients with more severe COPD who were considered to have improved or worsened. In turn, we would underestimate the percent of patients with true changes in function in those with less severe COPD.
The ATS published criteria for standardization and reproducibility of spirometry in 1995 with a recommendation for intrasession reproducibility as a change of 5% or 200 ml (1). This was based on Hankinson and Moon Bang's study using the NHANES III population (2). However, a recent publication citing 18,000 patients who had undergone spirometry suggests that the 200-ml cutoff may be too lenient, with 90% of patients able to reproduce two FEV1 measurements within 120 ml, or 6.1% (12). The current guidelines for intrasession reproducibility are now based only on an absolute change of 150 ml (3). We would expect variability between testing sessions to be larger than within a test session because of both biological and technical variation.
Although there are no predetermined guidelines for intersession changes in patients with COPD, many people have adopted the ATS definition of a bronchodilator effect, which is 200 ml or 12% change (1, 16). Intersession variability of spirometry has been studied in normal adults and patients with COPD. In a meta-analysis including 73 patients, Pennock and colleagues found that week-to-week variability, expressed as percent change, was greater in patients with asthma or COPD than in normal patients, and recommended that a significant change was 12% in normal subjects and 23% in patients with obstructive disease (4). Another study showed that day-to-day FEV1 must change by 17% in patients with chronic bronchitis to be significant, as compared with the 5% change needed in normal patients (17). On the basis of these data, experts have suggested that changes in FEV1 and FVC that exceed the 95% confidence limits of short-term variability occur when the spirometric values change 10 to 15% from the baseline value (1, 18–20). Brand and colleagues examined the short-term changes in FEV1 in response to bronchodilators in a population of adults with obstructive airway disease and found that the percent change in FEV1 was more dependent on the baseline FEV1 than the absolute change or change in percent-predicted FEV1 (21). In another evaluation of patients with COPD and bronchodilator responsiveness, Calverly and coworkers showed that the post-bronchodilator absolute change in FEV1 was not influenced by the prebronchodilator FEV1, whereas the percent change resulted in a curvilinear relationship (22). The implication of this is that bronchodilator testing might also use an absolute criterion for reproducibility rather than a percent change or combination of absolute and percent change. The intersession variation of FEV1 in COPD is similar to that seen in studies of diurnal variation in FEV1 in general populations. Borsboom and colleagues found an average variation in FEV1 of 2.8% (86 ml) compared with the current results in COPD populations of 2.54% (89 ml; Table 1), suggesting that factors other than changes in airway caliber may contribute to the observed variability (23).
If a patient with COPD shows improvement or worsening of FEV1 that exceeds the limits defined by our study results, then it is reasonable to conclude that the individual has had an improvement or worsening of lung function in the interval. Whether this change is clinically meaningful depends on the clinical situation. However, some experts have suggested that the minimal clinically important difference between two clinical measurements can be estimated by the standard error of the measurement, which is approximated at 0.1 L for FEV1 (24–27). It remains to be seen how these changes are related to minimal clinically important differences in symptoms.
The FVC was more variable in the NETT than in the LHS, and more variable than FEV1. The NETT study, conducted in clinical pulmonary function labs with varying equipment, likely represents general community conditions more than LHS, which had very rigorous spirometry quality control. In addition, because the patients in the NETT had more severe obstruction, the measurement of FVC may have been more dependent on the duration of expiratory effort. If so, the inspiratory capacity might be a more accurate measure than FVC. This effect of severity of airflow obstruction and duration of expiratory effort may have varied between test sessions, and the effects of that variability would have been greater in the NETT subjects who had more severe obstruction. Overall, however, it seems reasonable to suggest that a change in FVC would have to be greater than 10% or 325 ml to reflect a change in FVC.
There are several limitations to the present study. First, our study included participants with mild to moderate COPD and severe COPD by using information from two prior published studies, the LHS and the NETT. By using data from two different studies, we have selected a heterogeneous group of participants. However, the large numbers of participants, the wide range of COPD severity, and the multisite recruitment and testing improve the generalizability of the study sample to the general COPD population. Second, the PFTs were performed with different procedures in the NETT group and LHS group, confounding disease severity and testing procedures. The LHS studies were done in research clinics with similar equipment; the NETT used differing equipment in clinical pulmonary function laboratories. Both LHS and NETT procedures dictated that spirometric testing should be done at the same time of day; however, data were not excluded if this could not be accomplished. All laboratories used equipment and techniques that met or exceeded the ATS recommendations. The LHS technicians received feedback on quality control on a case by case basis from a central reading facility, whereas this was not the case in the NETT. Because all participants were enrolled in a clinical trial, there is the possibility of a bias toward greater motivation of participants or clinical staff and more stable health, such that variability could be even greater in community settings. Third, there was a difference in the interval between the two measurements in the two studies (25 d for the LHS vs. 85 d in the NETT); however, the observed mean differences in FEV1 between sessions between the two studies were similar. Fourth, there was no intervention or treatment between sessions in the LHS group, whereas the NETT participants did receive pulmonary rehabilitation between sessions. We believe this is not a concern because the NETT study participants showed no statistically significant difference in FEV1 between pre- and postrehabilitation. Finally, the NETT participants were all diagnosed with emphysema, whereas the LHS patients were diagnosed with COPD that was not specified as emphysema only, a difference that might have influenced test variability between the two study cohorts. Nevertheless, the wide range of severity in COPD and the large numbers of participants receiving two separate lung function tests should allow us to use these data to represent the COPD population more accurately than in previous studies. Review of LHS and NETT results separately would permit us to draw similar conclusions about FEV1 reproducibility. We are less certain about FVC reproducibility, which depends more on local laboratory testing procedures in COPD.
In summary, the current study provides data validating the use of absolute difference over percent difference in FEV1 to assess intersession reproducibility and a clinically significant intersession change in FEV1 in patients with COPD. Percent changes alone in FEV1 should not be used to assess alterations in spirometry in patients with COPD. On the basis of these results, we suggest that a change of 225 ml absolute difference in FEV1 could be used as a threshold to evaluate changes in lung function in patients with COPD. That is, a change of more than 225 ml has a high likelihood of representing a true change in lung function. Smaller changes may still represent biological and important changes in lung function, but also have greater likelihood of representing measurement noise. We are less confident about what criterion would constitute a significant change in FVC; however, the use of 10% change or 325 ml would be met by 90% of our participants.
Supplementary Material
APPENDIX
The Lung Health Study
The principal investigators and senior staff of the clinical and coordinating centers, the NHLBI, and members of the Safety and Data Monitoring Board of the Lung Health Study are as follows:
Case Western Reserve University, Cleveland, OH: M.D. Altose, M.D. (Principal Investigator), C.D. Deitz, Ph.D. (Project Coordinator); Henry Ford Hospital, Detroit, MI: M.S. Eichenhorn, M.D. (Principal Investigator), K.J. Braden, A.A.S. (Project Coordinator), R.L. Jentons, M.A.L.L.P. (Project Coordinator); Johns Hopkins University School of Medicine, Baltimore, MD: R.A. Wise, M.D. (Principal Investigator), C.S. Rand, Ph.D. (Co-Principal Investigator), K.A. Schiller (Project Coordinator); Mayo Clinic, Rochester, MN: P.D. Scanlon, M.D. (Principal Investigator), G.M. Caron (Project Coordinator), K.S. Mieras, L.C. Walters; Oregon Health Sciences University, Portland: A.S. Buist, M.D. (Principal Investigator), L.R. Johnson, Ph.D. (LHS Pulmonary Function Coordinator), V.J. Bortz (Project Coordinator); University of Alabama at Birmingham: W.C. Bailey, M.D. (Principal Investigator), L.B. Gerald, Ph.D., M.S.P.H. (Project Coordinator); University of California, Los Angeles: D.P. Tashkin, M.D. (Principal Investigator), I.P. Zuniga (Project Coordinator); University of Manitoba, Winnipeg: N.R. Anthonisen, M.D. (Principal Investigator, Steering Committee Chair), J. Manfreda, M.D. (Co-Principal Investigator), R.P. Murray, Ph.D. (Co-Principal Investigator), S.C. Rempel-Rossum (Project Coordinator); University of Minnesota Coordinating Center, Minneapolis: J.E. Connett, Ph.D. (Principal Investigator), P.L. Enright, M.D., P.G. Lindgren, M.S., P. O'Hara, Ph.D., (LHS Intervention Coordinator), M.A. Skeans, M.S., H.T. Voelker; University of Pittsburgh, Pittsburgh, PA: R.M. Rogers, M.D. (Principal Investigator), M.E. Pusateri (Project Coordinator); University of Utah, Salt Lake City: R.E. Kanner, M.D. (Principal Investigator), G.M. Villegas (Project Coordinator); Safety and Data Monitoring Board: M. Becklake, M.D., B. Burrows, M.D. (deceased), P. Cleary, Ph.D., P. Kimbel, M.D. (Chairperson; deceased), L. Nett, R.N., R.R.T. (former member), J.K. Ockene, Ph.D., R.M. Senior, M.D. (Chairperson), G.L. Snider, M.D., W. Spitzer, M.D. (former member), O.D. Williams, Ph.D.; Morbidity and Mortality Review Board: T.E. Cuddy, M.D., R.S. Fontana, M.D., R.E. Hyatt, M.D., C.T. Lambrew, M.D., B.A. Mason, M.D., D.M. Mintzer, M.D., R.B. Wray, M.D.; National Heart, Lung, and Blood Institute staff, Bethesda, MD: S.S. Hurd, Ph.D. (Former Director, Division of Lung Diseases), J.P. Kiley, Ph.D. (Former Project Officer and Director, Division of Lung Diseases), G. Weinmann, M.D. (Former Project Officer and Director, Airway Biology and Disease Program, DLD), M.C. Wu, Ph.D. (Division of Epidemiology and Clinical Applications).
The NETT Research Group
Members of the NETT Research Group are as follows:
Office of the Chair of the Steering Committee, University of Pennsylvania, Philadelphia, PA: Alfred P. Fishman, M.D. (Chair); Betsy Ann Bozzarello; Ameena Al-Amin.
Clinical Centers
Baylor College of Medicine, Houston, TX: Marcia Katz, M.D. (Principal Investigator); Carolyn Wheeler, R.N., B.S.N. (Principal Clinic Coordinator); Elaine Baker, R.R.T., R.P.F.T.; Peter Barnard, Ph.D., R.P.F.T.; Phil Cagle, M.D.; James Carter, M.D.; Sophia Chatziioannou, M.D.; Karla Conejo-Gonzales; Kimberly Dubose, R.R.T.; John Haddad, M.D.; David Hicks, R.R.T., R.P.F.T.; Neal Kleiman, M.D.; Mary Milburn-Barnes, C.R.T.T.; Chinh Nguyen, R.P.F.T.; Michael Reardon, M.D.; Joseph Reeves-Viets, M.D.; Steven Sax, M.D.; Amir Sharafkhaneh, M.D.; Owen Wilson, Ph.D.; Christine Young, P.T.; Rafael Espada, M.D. (Principal Investigator 1996–2002); Rose Butanda (1999–2001); Minnie Ellisor (2002); Pamela Fox, M.D. (1999–2001); Katherine Hale, M.D. (1998–2000); Everett Hood, R.P.F.T. (1998–2000); Amy Jahn (1998–2000); Satish Jhingran, M.D. (1998–2001); Karen King, R.P.F.T. (1998–1999); Charles Miller III, Ph.D. (1996–1999); Imran Nizami, M.D. (Co-Principal Investigator, 2000–2001); Todd Officer (1998–2000); Jeannie Ricketts (1998–2000); Joe Rodarte, M.D. (Co-Principal Investigator, 1996–2000); Robert Teague, M.D. (Co-Principal Investigator, 1999–2000); Kedren Williams (1998–1999).
Brigham and Women's Hospital, Boston, MA: John Reilly, M.D. (Principal Investigator); David Sugarbaker, M.D. (Co-Principal Investigator); Carol Fanning, R.R.T. (Principal Clinic Coordinator); Simon Body, M.D.; Sabine Duffy, M.D.; Vladmir Formanek, M.D.; Anne Fuhlbrigge, M.D.; Philip Hartigan, M.D.; Sarah Hooper, E.P.; Andetta Hunsaker, M.D.; Francine Jacobson, M.D.; Marilyn Moy, M.D.; Susan Peterson, R.R.T.; Roger Russell, M.D.; Diane Saunders; Scott Swanson, M.D. (Co-Principal Investigator, 1996–2001).
Cedars-Sinai Medical Center, Los Angeles, CA: Rob McKenna, M.D. (Principal Investigator); Zab Mohsenifar, M.D. (Co-Principal Investigator); Carol Geaga, R.N. (Principal Clinic Coordinator); Manmohan Biring, M.D.; Susan Clark, R.N., M.N.; Jennifer Cutler, M.D.; Robert Frantz, M.D.; Peter Julien, M.D.; Michael Lewis, M.D.; Jennifer Minkoff-Rau, M.S.W.; Valentina Yegyan, B.S., C.P.F.T.; Milton Joyner, B.A. (1996–2002).
Cleveland Clinic Foundation, Cleveland, OH: Malcolm DeCamp, M.D. (Principal Investigator); James Stoller, M.D. (Co-Principal Investigator); Yvonne Meli, R.N.C. (Principal Clinic Coordinator); John Apostolakis, M.D.; Darryl Atwell, M.D.; Jeffrey Chapman, M.D.; Pierre DeVilliers, M.D.; Raed Dweik, M.D.; Erik Kraenzler, M.D.; Rosemary Lann, L.I.S.W.; Nancy Kurokawa, R.R.T., C.P.F.T.; Scott Marlow, R.R.T.; Kevin McCarthy, R.C.P.T.; Pricilla McCreight, R.R.T., C.P.F.T.; Atul Mehta, M.D.; Moulay Meziane, M.D.; Omar Minai, M.D.; Mindi Steiger, R.R.T.; Kenneth White, R.P.F.T.; Janet Maurer, M.D. (Principal Investigator, 1996–2001); Terri Durr, R.N. (2000–2001); Charles Hearn, D.O. (1998–2001); Susan Lubell, P.A.-C. (1999–2000); Peter O'Donovan, M.D. (1998–2003); Robert Schilz, D.O. (1998–2002).
Columbia University, New York, NY, in consortium with Long Island Jewish Medical Center, New Hyde Park, NY: Mark Ginsburg, M.D. (Principal Investigator); Byron Thomashow, M.D. (Co-Principal Investigator); Patricia Jellen, M.S.N., R.N. (Principal Clinic Coordinator); John Austin, M.D.; Matthew Bartels, M.D.; Yahya Berkmen, M.D.; Patricia Berkoski, M.S., R.R.T. (Site Coordinator, LIJ); Frances Brogan, M.S.N., R.N.; Amy Chong, B.S., C.R.T.; Glenda DeMercado, B.S.N.; Angela DiMango, M.D.; Sandy Do, M.S., P.T.; Bessie Kachulis, M.D.; Arfa Khan, M.D.; Berend Mets, M.D.; Mitchell O'Shea, B.S., R.T., C.P.F.T.; Gregory Pearson, M.D.; Leonard Rossoff, M.D.; Steven Scharf, M.D., Ph.D. (Co-Principal Investigator, 1998–2002); Maria Shiau, M.D.; Paul Simonelli, M.D.; Kim Stavrolakes, M.S., P.T.; Donna Tsang, B.S.; Denise Vilotijevic, M.S., P.T.; Chun Yip, M.D.; Mike Mantinaos, M.D. (1998–2001); Kerri McKeon, B.S., R.R.T., R.N. (1998–1999); Jacqueline Pfeffer, M.P.H., P.T. (1997–2002).
Duke University Medical Center, Durham, NC: Neil MacIntyre, M.D. (Principal Investigator); R. Duane Davis, M.D. (Co-Principal Investigator); John Howe, R.N. (Principal Clinic Coordinator); R. Edward Coleman, M.D.; Rebecca Crouch, R.P.T.; Dora Greene; Katherine Grichnik, M.D.; David Harpole, Jr., M.D.; Abby Krichman, R.R.T.; Brian Lawlor, R.R.T.; Holman McAdams, M.D.; John Plankeel, M.D.; Susan Rinaldo-Gallo, M.E.D.; Sheila Shearer, R.R.T.; Jeanne Smith, A.C.S.W.; Mark Stafford-Smith, M.D.; Victor Tapson, M.D.; Mark Steele, M.D. (1998–1999); Jennifer Norten, M.D. (1998–1999).
Mayo Foundation, Rochester, MN: James Utz, M.D. (Principal Investigator); Claude Deschamps, M.D. (Co-Principal Investigator); Kathy Mieras, C.C.R.P. (Principal Clinic Coordinator); Martin Abel, M.D.; Mark Allen, M.D.; Deb Andrist, R.N.; Gregory Aughenbaugh, M.D.; Sharon Bendel, R.N.; Eric Edell, M.D.; Marlene Edgar; Bonnie Edwards; Beth Elliot, M.D.; James Garrett, R.R.T.; Delmar Gillespie, M.D.; Judd Gurney, M.D.; Boleyn Hammel; Karen Hanson, R.R.T.; Lori Hanson, R.R.T.; Gordon Harms, M.D.; June Hart; Thomas Hartman, M.D.; Robert Hyatt, M.D.; Eric Jensen, M.D.; Nicole Jenson, R.R.T.; Sanjay Kalra, M.D.; Philip Karsell, M.D.; Jennifer Lamb; David Midthun, M.D.; Carl Mottram, R.R.T.; Stephen Swensen, M.D.; Anne-Marie Sykes, M.D.; Karen Taylor; Norman Torres, M.D.; Rolf Hubmayr, M.D. (1998–2000); Daniel Miller, M.D. (1999–2002); Sara Bartling, R.N. (1998–2000); Kris Bradt (1998–2002).
National Jewish Medical and Research Center, Denver, CO: Barry Make, M.D. (Principal Investigator); Marvin Pomerantz, M.D. (Co-Principal Investigator); Mary Gilmartin, R.N., R.R.T. (Principal Clinic Coordinator); Joyce Canterbury; Martin Carlos; Phyllis Dibbern, P.T.; Enrique Fernandez, M.D.; Lisa Geyman, M.S.P.T.; Connie Hudson; David Lynch, M.D.; John Newell, M.D.; Robert Quaife, M.D.; Jennifer Propst, R.N.; Cynthia Raymond, M.S.; Jane Whalen-Price, P.T.; Kathy Winner, O.T.R.; Martin Zamora, M.D.; Reuben Cherniack, M.D. (Principal Investigator, 1997–2000).
Ohio State University, Columbus, OH: Philip Diaz, M.D. (Principal Investigator); Patrick Ross, M.D. (Co-Principal Investigator); Tina Bees (Principal Clinic Coordinator); Jan Drake; Charles Emery, Ph.D.; Mark Gerhardt, M.D., Ph.D.; Mark King, M.D.; David Rittinger; Mahasti Rittinger.
St. Louis University, St. Louis, MO: Keith Naunheim, M.D. (Principal Investigator); Robert Gerber, M.D. (Co-Principal Investigator); Joan Osterloh, R.N., M.S.N. (Principal Clinic Coordinator); Susan Borosh; Willard Chamberlain, D.O.; Sally Frese; Alan Hibbit; Mary Ellen Kleinhenz, M.D.; Gregg Ruppel; Cary Stolar, M.D.; Janice Willey; Francisco Alvarez, M.D. (Co-Principal Investigator, 1999–2002); Cesar Keller, M.D. (Co-Principal Investigator, 1996–2000).
Temple University, Philadelphia, PA: Gerard Criner, M.D. (Principal Investigator); Satoshi Furukawa, M.D. (Co-Principal Investigator); Anne Marie Kuzma, R.N., M.S.N. (Principal Clinic Coordinator); Roger Barnette, M.D.; Neil Brister, M.D.; Kevin Carney, R.N., C.C.T.C.; Wissam Chatila, M.D.; Francis Cordova, M.D.; Gilbert D'Alonzo, D.O.; Michael Keresztury, M.D.; Karen Kirsch; Chul Kwak, M.D.; Kathy Lautensack, R.N., B.S.N.; Madelina Lorenzon, C.P.F.T.; Ubaldo Martin, M.D.; Peter Rising, M.S.; Scott Schartel, M.D.; John Travaline, M.D.; Gwendolyn Vance, R.N., C.C.T.C.; Phillip Boiselle, M.D. (1997–2000); Gerald O'Brien, M.D. (1997–2000).
University of California, San Diego, San Diego, CA: Andrew Ries, M.D., M.P.H. (Principal Investigator); Robert Kaplan, Ph.D. (Co-Principal Investigator); Catherine Ramirez, B.S., R.C.P. (Principal Clinic Coordinator); David Frankville, M.D.; Paul Friedman, M.D.; James Harrell, M.D.; Jeffery Johnson; David Kapelanski, M.D.; David Kupferberg, M.D., M.P.H.; Catherine Larsen, M.P.H.; Trina Limberg, R.R.T.; Michael Magliocca, R.N., C.N.P.; Frank J. Papatheofanis, M.D., Ph.D.; Dawn Sassi-Dambron, R.N.; Melissa Weeks.
University of Maryland at Baltimore, Baltimore, MD, in consortium with Johns Hopkins Hospital, Baltimore, MD: Mark Krasna, M.D. (Principal Investigator); Henry Fessler, M.D. (Co-Principal Investigator); Iris Moskowitz (Principal Clinic Coordinator); Timothy Gilbert, M.D.; Jonathan Orens, M.D.; Steven Scharf, M.D., Ph.D.; David Shade; Stanley Siegelman, M.D.; Kenneth Silver, M.D.; Clarence Weir; Charles White, M.D.
University of Michigan, Ann Arbor, MI: Fernando Martinez, M.D. (Principal Investigator); Mark Iannettoni, M.D. (Co-Principal Investigator); Catherine Meldrum, B.S.N., R.N., C.C.R.N. (Principal Clinic Coordinator); William Bria, M.D.; Kelly Campbell; Paul Christensen, M.D.; Kevin Flaherty, M.D.; Steven Gay, M.D.; Paramjit Gill, R.N.; Paul Kazanjian, M.D.; Ella Kazerooni, M.D.; Vivian Knieper; Tammy Ojo, M.D.; Lewis Poole; Leslie Quint, M.D.; Paul Rysso; Thomas Sisson, M.D.; Mercedes True; Brian Woodcock, M.D.; Lori Zaremba, R.N.
University of Pennsylvania, Philadelphia, PA: Larry Kaiser, M.D. (Principal Investigator); John Hansen-Flaschen, M.D. (Co-Principal Investigator); Mary Louise Dempsey, B.S.N., R.N. (Principal Clinic Coordinator); Abass Alavi, M.D.; Theresa Alcorn; Selim Arcasoy, M.D.; Judith Aronchick, M.D.; Stanley Aukberg, M.D.; Bryan Benedict, R.R.T.; Susan Craemer, B.S., R.R.T., C.P.F.T.; Ron Daniele, M.D.; Jeffrey Edelman, M.D.; Warren Gefter, M.D.; Laura Kotler-Klein, M.S.S.; Robert Kotloff, M.D.; David Lipson, M.D.; Wallace Miller, Jr., M.D.; Richard O'Connell, R.P.F.T.; Staci Opelman, M.S.W.; Harold Palevsky, M.D.; William Russell, R.P.F.T.; Heather Sheaffer, M.S.W.; Rodney Simcox, B.S.R.T., R.R.T.; Susanne Snedeker, R.R.T., C.P.F.T.; Jennifer Stone-Wynne, M.S.W.; Gregory Tino, M.D.; Peter Wahl; James Walter, R.P.F.T.; Patricia Ward; David Zisman, M.D.; James Mendez, M.S.N., C.R.N.P. (1997–2001); Angela Wurster, M.S.N., C.R.N.P. (1997–1999).
University of Pittsburgh, Pittsburgh, PA: Frank Sciurba, M.D. (Principal Investigator); James Luketich, M.D. (Co-Principal Investigator); Colleen Witt, M.S. (Principal Clinic Coordinator); Gerald Ayres; Michael Donahoe, M.D.; Carl Fuhrman, M.D.; Robert Hoffman, M.D.; Joan Lacomis, M.D.; Joan Sexton; William Slivka; Diane Strollo, M.D.; Erin Sullivan, M.D.; Tomeka Simon; Catherine Wrona, R.N., B.S.N.; Gerene Bauldoff, R.N., M.S.N. (1997–2000); Manuel Brown, M.D. (1997–2002); Elisabeth George, R.N., M.S.N. (Principal Clinic Coordinator, 1997–2001); Robert Keenan, M.D. (Co-Principal Investigator, 1997–2000); Theodore Kopp, M.S. (1997–1999); Laurie Silfies (1997–2001).
University of Washington, Seattle, WA: Joshua Benditt, M.D. (Principal Investigator), Douglas Wood, M.D. (Co-Principal Investigator); Margaret Snyder, M.N. (Principal Clinic Coordinator); Kymberley Anable; Nancy Battaglia; Louie Boitano; Andrew Bowdle, M.D.; Leighton Chan, M.D.; Cindy Chwalik; Bruce Culver, M.D.; Thurman Gillespy, M.D.; David Godwin, M.D.; Jeanne Hoffman; Andra Ibrahim, M.D.; Diane Lockhart; Stephen Marglin, M.D.; Kenneth Martay, M.D.; Patricia McDowell; Donald Oxorn, M.D.; Liz Roessler; Michelle Toshima; Susan Golden (1998–2000).
Other Participants
Agency for Healthcare Research and Quality, Rockville, MD: Lynn Bosco, M.D., M.P.H.; Yen-Pin Chiang, Ph.D.; Carolyn Clancy, M.D.; Harry Handelsman, D.O.
Centers for Medicare and Medicaid Services, Baltimore, MD: Steven M Berkowitz, Ph.D.; Tanisha Carino, Ph.D.; Joe Chin, M.D.; JoAnna Baldwin; Karen McVearry; Anthony Norris; Sarah Shirey; Claudette Sikora; Steven Sheingold, Ph.D. (1997–2004).
Coordinating Center, The Johns Hopkins University, Baltimore, MD: Steven Piantadosi, M.D., Ph.D. (Principal Investigator); James Tonascia, Ph.D. (Co-Principal Investigator); Patricia Belt; Amanda Blackford, Sc.M.; Karen Collins; Betty Collison; Ryan Colvin, M.P.H.; John Dodge; Michele Donithan, M.H.S.; Vera Edmonds; Gregory L. Foster, M.A.; Julie Fuller; Judith Harle; Rosetta Jackson; Shing Lee, Sc.M.; Charlene Levine; Hope Livingston; Jill Meinert; Jennifer Meyers; Deborah Nowakowski; Kapreena Owens; Shangqian Qi, M.D.; Michael Smith; Brett Simon, M.D.; Paul Smith; Alice Sternberg, Sc.M.; Mark Van Natta, M.H.S.; Laura Wilson, Sc.M.; Robert Wise, M.D.
Cost-Effectiveness Subcommittee: Robert M. Kaplan, Ph.D. (Chair); J. Sanford Schwartz, M.D. (Co-Chair); Yen-Pin Chiang, Ph.D.; Marianne C. Fahs, Ph.D.; A. Mark Fendrick, M.D.; Alan J. Moskowitz, M.D.; Dev Pathak, Ph.D.; Scott Ramsey, M.D., Ph.D.; Steven Sheingold, Ph.D.; A. Laurie Shroyer, Ph.D.; Judith Wagner, Ph.D.; Roger Yusen, M.D.
Cost-Effectiveness Data Center, Fred Hutchinson Cancer Research Center, Seattle, WA: Scott Ramsey, M.D., Ph.D. (Principal Investigator); Ruth Etzioni, Ph.D.; Sean Sullivan, Ph.D.; Douglas Wood, M.D.; Thomas Schroeder, M.A.; Karma Kreizenbeck; Kristin Berry, M.S.; Nadia Howlader, M.S.
CT Scan Image Storage and Analysis Center, University of Iowa, Iowa City, IA: Eric Hoffman, Ph.D. (Principal Investigator); Janice Cook-Granroth, B.S.; Angela Delsing, R.T.; Junfeng Guo, Ph.D.; Geoffrey McLennan, M.D.; Brian Mullan, M.D.; Chris Piker, B.S.; Joseph Reinhardt, Ph.D.; Blake Robinswood; Jered Sieren, R.T.R.; William Stanford, M.D.
Data and Safety Monitoring Board: John A. Waldhausen, M.D. (Chair); Gordon Bernard, M.D.; David DeMets, Ph.D.; Mark Ferguson, M.D.; Eddie Hoover, M.D.; Robert Levine, M.D.; Donald Mahler, M.D.; A. John McSweeny, Ph.D.; Jeanine Wiener-Kronish, M.D.; O. Dale Williams, Ph.D.; Magdy Younes, M.D.
Marketing Center, Temple University, Philadelphia, PA: Gerard Criner, M.D. (Principal Investigator); Charles Soltoff, M.B.A.
Project Office, National Heart, Lung, and Blood Institute, Bethesda, MD: Gail Weinmann, M.D. (Project Officer); Joanne Deshler (Contracting Officer); Dean Follmann, Ph.D.; James Kiley, Ph.D.; Margaret Wu, Ph.D. (1996–2001).
Other Acknowledgments
Arthur Gelb, M.D., Lakewood Regional Medical Center, Lakewood, CA.
Supported by contract N01-HR-46002 from the Division of Lung Diseases, National Heart, Lung, and Blood Institute, and National Institutes of Health (The Lung Health Study). The National Emphysema Treatment Trial is supported by contracts with the National Heart, Lung, and Blood Institute (N01HR76101, N01HR76102, N01HR76103, N01HR76104, N01HR76105, N01HR76106, N01HR76107, N01HR76108, N01HR76109, N01HR76110, N01HR76111, N01HR76112, N01HR76113, N01HR76114, N01HR76115, N01HR76116, N01HR76118, and N01HR76119); the Centers for Medicare and Medicaid Services (formerly the Health Care Financing Administration); and the Agency for Healthcare Research and Quality. Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT, supplied Atrovent and placebo inhalers.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200506-975OC on February 23, 2006
Conflict of Interest Statement: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med 1995;152:1107–1136. [DOI] [PubMed] [Google Scholar]
- 2.Hankinson JL, Moon Bang K. Acceptability and reproducibility criteria of the American Thoracic Society as observed in a sample of the general population. Am Rev Respir Dis 1991;143:516–521. [DOI] [PubMed] [Google Scholar]
- 3.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinton CPM, Gustafsson P, et al. Standardisation of spirometry. Eur Respir J 2005;26:319–338. [DOI] [PubMed] [Google Scholar]
- 4.Pennock BE, Rogers RM, McCaffree DR. Changes in measured spirometric indices: what is significant? Chest 1981;80:97. [DOI] [PubMed] [Google Scholar]
- 5.Enright PL, Connett JE, Kanner RE, Johnson LR, Lee WW. Spirometry in the Lung Health Study: II. Determinants of short-term intraindividual variability. Am J Respir Crit Care Med 1995;151:406–411. [DOI] [PubMed] [Google Scholar]
- 6.Herpel LB, Kanner R, Lee SM, Fessler H, Sciurba F, Connett J, Wise RA. Variability of spirometry in chronic obstructive pulmonary disease: results from two clinical trials [abstract]. Proc Am Thorac Soc 2005;2:A642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connett JE, Kusek JW, Bailey WC, O'Hara P, Wu M. Design of the Lung Health Study: a randomized trial of early intervention for chronic obstructive pulmonary disease. Control Clin Trials 1993;14:3S–19S. [DOI] [PubMed] [Google Scholar]
- 8.Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis 1981;123:659–664. [DOI] [PubMed] [Google Scholar]
- 9.The National Emphysema Treatment Trial Research Group. Rationale and design of the National Emphysema Treatment Trial: a prospective randomized trial of lung volume reduction surgery. Chest 1999;116:1750–1761. [DOI] [PubMed] [Google Scholar]
- 10.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999;159:179–187. [DOI] [PubMed] [Google Scholar]
- 11.Ries AL, Kaplan RM, Limberg TM, Prewitt LM. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Ann Intern Med 1995;122:823–832. [DOI] [PubMed] [Google Scholar]
- 12.Enright PL, Beck KC, Sherrill DL. Repeatability of spirometry in 18,000 adult patients. Am J Respir Crit Care Med 2004;169:235–238. [DOI] [PubMed] [Google Scholar]
- 13.Enright PL, Adams AB, Boyle PJ, Sherrill DL. Spirometry and maximal respiratory pressure references from healthy Minnesota 65- to 85-year-old women and men. Chest 1995;108:663–669. [DOI] [PubMed] [Google Scholar]
- 14.Punjabi NM, Shade D, Patel AM, Wise RA. Measurement variability in single-breath diffusing capacity of the lung. Chest 2003;123:1082–1089. [DOI] [PubMed] [Google Scholar]
- 15.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) workshop summary. Am J Respir Crit Care Med 2001;163:1256–1276. [DOI] [PubMed] [Google Scholar]
- 16.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948–968. [DOI] [PubMed] [Google Scholar]
- 17.Rozas CJ, Goldman AL. Daily spirometric variability: normal subjects and subjects with chronic bronchitis with and without airflow obstruction. Arch Intern Med 1982;142:1287–1291. [DOI] [PubMed] [Google Scholar]
- 18.Lorber DB, Kaltenborn W, Burrows B. Responses to isoproterenol in a general population sample. Am Rev Respir Dis 1963;118:855–861. [DOI] [PubMed] [Google Scholar]
- 19.Reis AL. Response to bronchodilators. In: Clausen JL, editor. Pulmonary function testing: guidelines and controversies. New York: Academic Press; 1982. pp. 215–221.
- 20.Sourk RL, Nugent KM. Bronchodilator testing: confidence intervals derived from placebo inhalations. Am Rev Respir Dis 1983;128:153–157. [DOI] [PubMed] [Google Scholar]
- 21.Brand PL, Quanjer PH, Postma DS, Kerstjen HA, Koeter GH, Dikhuijzen PN, Sluiter HJ. Interpretation of bronchodilator response in patients with obstructive airways disease. The Dutch Chronic Non-Specific Lung Disease (CNSLD) Study Group. Thorax 1992;47:429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calverly PMA, Burge PS, Spencer S, Anderson JA, Jones PW; ISOLDE Study Investigators. Bronchodilator reversibility testing in chronic obstructive pulmonary disease. Thorax 2003;58:659–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borsboom GJJM, van Pelt W, van Houwelingen HC, van Vianen BG, Schouten JP, Quanjer PH. Diurnal variation in lung function in subgroups from two dutch populations. Am J Respir Crit Care Med 1999;159:1163–1171. [DOI] [PubMed] [Google Scholar]
- 24.Wyrwich KW, Tierney WM, Wolinsky FD. Further evidence supporting an SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J Clin Epidemiol 1999;52:861–873. [DOI] [PubMed] [Google Scholar]
- 25.Redelmeier DA, Goldstein RS, Min ST, Hyland RH. Spirometry and dyspnea in patients with COPD: when small differences mean little. Chest 1996;109:1163–1168. [DOI] [PubMed] [Google Scholar]
- 26.Sloan JA. Assessing the minimally clinically significant difference: scientific considerations, challenges, and solutions. J Chronic Obstructive Pulmonary Dis 2005;2:57–62. [DOI] [PubMed] [Google Scholar]
- 27.Donohue JF. Minimally clinically important differences in COPD lung function. J Chronic Obstructive Pulmonary Dis 2005;2:111–124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.