Abstract
Rationale: Acute inflammation and vascular leak are cardinal features of acute lung injury and the acute respiratory distress syndrome. Nonspecific tissue inflammation and injury in response to infectious and noninfectious insults lead to oxidative stress and the generation of lipid oxidation products, which may inhibit the acute inflammatory response to bacterial components.
Objective: To test the hypothesis that oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (OxPAPC) may attenuate the acute lung inflammatory response to lipopolysaccharide (LPS) and enhance lung vascular barrier recovery, we used in vivo and in vitro models of LPS-induced lung injury.
Methods: Rats received intratracheal aerosolized LPS (5 mg/kg) or sterile water with concurrent intravenous injection of OxPAPC (0.5–6.0 mg/kg) or saline alone. Nonoxidized PAPC was used as a control. At 18 h, bronchoalveolar lavage was performed and the lungs were removed for histologic analysis. Measurements of endothelial transmonolayer electrical resistance and immunofluorescent analysis of monolayer integrity were used in an in vitro model of LPS-induced lung vascular barrier dysfunction.
Measurements and Main Results: In vivo, aerosolized intratracheal LPS induced lung injury with profound increases in bronchoalveolar lavage neutrophils, protein content, and the inflammatory cytokines interleukin 6 and interleukin 1β, as well as tissue neutrophils. OxPAPC, but not nonoxidized PAPC, markedly attenuated the LPS-induced tissue inflammation, barrier disruption, and cytokine production over a range of doses. In vitro, oxidized phospholipids attenuated LPS-induced endothelial barrier disruption and reversed LPS-induced cytoskeletal remodeling and disruption of monolayer integrity.
Conclusions: These studies demonstrate in vivo and in vitro protective effects of oxidized phospholipids on LPS-induced lung dysfunction.
Keywords: bacterial wall lipopolysaccharide, bronchoalveolar lavage, interleukin 6, interleukin 1β, lung endothelial permeability
Tissue inflammation and increased vascular leak are cardinal features of acute lung injury (ALI), a severe illness associated with a mortality of 30 to 50% (1). Despite recent advances in low tidal volume mechanical ventilation and a better understanding of the underlying inflammatory pathophysiology of ALI, there remain few effective treatments for this devastating illness. ALI and the more severe acute respiratory distress syndrome (ARDS) represent a spectrum of common syndromes in response to a variety of infectious and noninfectious insults. The immune response to insults, such as infection or shock, includes recruitment of neutrophils and other inflammatory cells, induction of proinflammatory cytokines, and subsequent generation of reactive oxygen intermediates, which cause tissue damage and contribute to the induction and perpetuation of ALI (2, 3). One consequence of enhanced oxidative stress is the peroxidation of phospholipids, abundantly present in the surfactant layer, into active metabolites, which have been observed in a variety of acute and chronic inflammatory diseases of the lung, including ARDS and asthma (3, 4). In addition, reactive nitrogen species generated during acute inflammation may also modify blood-borne and cellular lipids, representing a novel source of bioactive oxidized or nitrated phospholipids (5, 6). Increased production of exhaled isoprostanes serves as an index of oxidant stress and lipid oxidation in a number of lung pathologies (see Reference 7 for review) and is elevated in ALI (8). After tissue insult, oxidized phospholipids released from membrane vesicles may serve as stress signals, triggering both pro- and antiinflammatory cascades. One of the major plasma membrane phospholipids is 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (PAPC), which on oxidative modification forms a heterogeneous group of compounds that contain various modified residues at the sn-2 position of the phospholipid. Products of PAPC oxidation (OxPAPC) have been previously suggested as possible culprits in the chronic vascular inflammation involved in atherogenesis (9–12). However, previous studies have also demonstrated that OxPAPC attenuates LPS-triggered inflammatory cascade via antagonistic interaction with the LPS coreceptors, LPS-binding protein and CD14, which competitively blocks the ability of LPS to bind its major receptor, TLR-4 (13), thus blunting the nuclear factor (NF)-κB–mediated expression of inflammatory genes. Furthermore, it was shown that OxPAPC also attenuates LPS-induced tumor necrosis factor (TNF)-α production and inhibits the CpG-triggered inflammatory cascade by interfering with Toll-like receptor 9 (TLR9)–mediated NF-κB activation (14). Administration of a mixture of LPS and OxPAPC decreased inflammatory cell recruitment and even protected against LPS-mediated lethal shock in various models of acute inflammation (13, 14). In addition, previous studies from our laboratories have shown that OxPAPC is a potent regulator of endothelial barrier function, enhancing basal barrier function and attenuating thrombin-induced endothelial barrier dysfunction (15).
This study used an in vivo model to demonstrate for the first time that a single intravenous injection of OxPAPC significantly attenuates lung barrier dysfunction and acute inflammation induced by intratracheal LPS administration. Moreover, OxPAPC is also effective in inhibiting the LPS-induced endothelial barrier disruption in cultured human pulmonary artery endothelial cells. Further studies may lead to development of a new type of a dual-action drug combining antiinflammatory and barrier-protective properties for the treatment of a plethora of lung pathologies, including ALI and ARDS. Selected parts of this study were presented at the American Thoracic Society International Conference in San Diego, California, May 20–25, 2005 (16).
METHODS
Reagents and Cell Culture
All biochemical reagents, including vinculin antibody, LPS, and PAPC, were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise indicated. PAPC was oxidized by exposure of dry lipid to air for 72 h. The extent of oxidation was monitored by positive-ion electrospray mass spectrometry as described previously (17). Each batch of oxidized phospholipids was standardized by thin-layer chromatography. In addition, quality control of the OxPAPC composition in each batch was also performed by positive-ion electrospray mass spectrometry and judged by a standard pattern of characteristic peaks with mass-to-change ratio (m/z) of 616.4, 632.4, 810.5, 828.5, corresponding to major oxygenated and fragmented products of PAPC oxidation. PAPC and OxPAPC preparations were shown to be negative for endotoxin by the limulus amebocyte assay performed after PAPC oxidation before in vivo and in vitro experiments (BioWhittaker, Frederick, MD). All reagents used for immunofluorescent staining were purchased from Molecular Probes (Eugene, OR); β-catenin antibody was purchased from BD Transduction Laboratories (San Diego, CA). Human pulmonary artery endothelial cells were obtained from Clonetics, BioWhittaker, Inc., maintained according to the vendor's protocol, and used at Passages 6–10.
In Vivo Model of ALI
Adult male Sprague-Dawley rats, 250 to 350 g (Charles River Laboratories, Wilmington, MA), were anesthetized with an intraperitoneal injection of ketamine (75 mg/kg) and acepromazine (1.5 mg/kg). Animals were intratracheally intubated with a 19-G liquid microsprayer (model IA-1B; Penn-Century, Inc., Philadelphia, PA) and given an aerosolized spray of sterile water (200 μl) or bacterial LPS (5 mg/kg, Escherichia coli O127:B8). Rats were randomized to concurrently receive sterile saline solution or OxPAPC (0.5–6.0 mg/kg), or nonoxidized PAPC (1.5 mg/kg) by intravenous injection in the external jugular vein to yield the following experimental groups: control, LPS only, OxPAPC only, LPS + OxPAPC (0.5–6.0 mg/kg), and LPS + PAPC. At 18 h, animals were killed by exsanguination under anesthesia. A 3-ml blood sample was drawn from the hepatic vein for serum analysis. Tracheotomy was performed and the trachea was cannulated with a 14-G intravenous catheter, which was tied into place. Bronchoalveolar lavage (BAL) was performed on the left lung using 3 ml of sterile phosphate-buffered saline. BAL cell counting was performed using a standard hemacytometer technique. Differential cell counts were performed on Diff-Quick–stained (Baxter Diagnostics, McGaw Park, IL) slides. The BAL protein concentration was determined by a modified Lowrey colorimetric assay using a Bio-Rad DC protein assay kit (Bio-Rad Laboratories, Hercules, CA). Interleukin (IL)-6 and IL-1β concentrations were measured using ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. The left lung was fixed in buffered formalin and processed for hematoxylin and eosin staining and histologic analysis. Additional details on the experimental methods used are provided in the online supplement.
Measurement of Transendothelial Electrical Resistance
The cellular barrier properties were evaluated by measuring transendothelial electrical resistance (TER) across confluent human pulmonary artery endothelial monolayers using an electrical cell-substrate impedance sensing system (Applied Biophysics, Troy, NY) as described previously (18, 19). Briefly, cells were grown on small gold electrodes (10−4 cm2), in complete culture medium containing 10% fetal bovine serum and growth factor supplement. Two hours before TER measurements, the culture medium was changed to a medium containing 2% fetal calf serum. The total electrical resistance was measured dynamically across the monolayer, and the effects of stimulation with LPS (200 ng/ml), OxPAPC (5 μg/ml, 10 μg/ml) were monitored over 24 h. Increased TER was noted as cells adhered and spread over the microelectrode and was maximal at full confluence, whereas cell retraction, paracellular gap formation, rounding, or loss of adhesion were reflected by a decrease in TER. Resistance was normalized to the initial voltage and expressed as a fraction of the normalized resistance value. TER values from at least six microelectrodes corresponding to each experimental condition were pooled at discrete time points using custom-designed Epool software and plotted versus time as the mean ± SE as previously described (20–22).
Immunofluorescent Staining and Endothelial Cell Imaging
After agonist treatment, endothelial cells grown on glass coverslips were subjected to double immunofluorescent staining as previously described (18, 23). β-Catenin and vinculin were detected using specific antibodies, and F-actin filaments were visualized by staining with Texas red–conjugated phalloidin. After immunostaining, the glass slides were analyzed using a Nikon video-imaging system (Nikon Instech Co., Tokyo, Japan).
Statistical Methods
All data are presented as mean ± SEM. Group comparisons were evaluated by the analysis of variance test with post hoc Newman-Keuls multiple comparison test. p values less than 0.05 were considered statistically significant.
RESULTS
Effect of OxPAPC on LPS-induced Lung Inflammation and Barrier Dysfunction
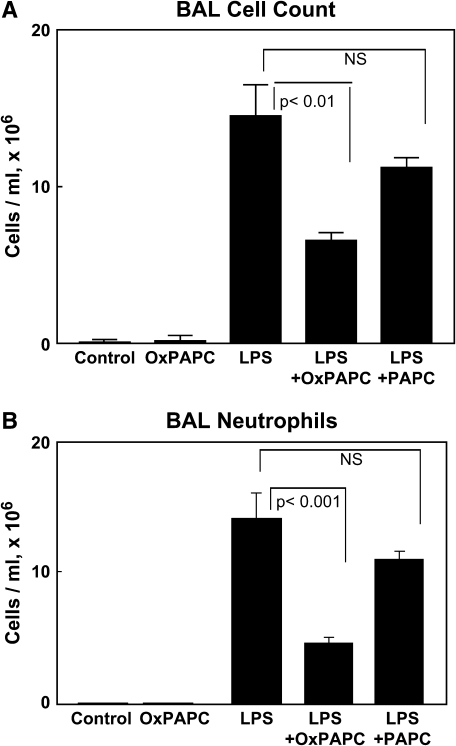
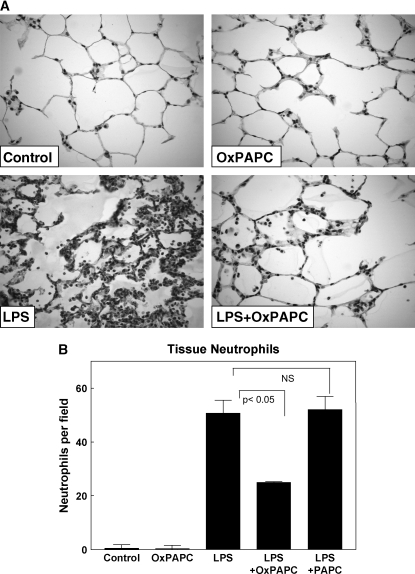
An in vivo model of ALI was used to evaluate the effects of intravenous OxPAPC on LPS-induced lung injury. Rats received a single dose of aerosolized LPS (5 mg/kg) reconstituted in water or sterile water alone followed immediately by intravenous injection of OxPAPC (1.5 mg/kg) or sterile saline via the jugular vein. Injection of nonoxidized PAPC (1.5 mg/kg) was performed as a control. After 18 h, blood sampling, BAL, and tissue harvesting were performed. Aerosolized LPS induced a prominent acute inflammatory response in the lung (Figure 1A) with a dramatic increase in BAL total cell counts at 18 h (1.45 × 107 ± 0.20 vs. 7.30 × 104 ± 1.7 cells/ml in controls, p < 0.001). The LPS-induced increase in BAL cell count was significantly attenuated by OxPAPC (6.56 × 106 ± 0.53 vs. 1.45 × 107 ± 0.20 cells/ml for LPS alone, p < 0.05), whereas treatment with nonoxidized PAPC did not significantly reduce BAL cell counts. Figure 1B demonstrates that the LPS-induced increase in BAL cell count was almost entirely attributable to the recruitment and influx of polymorphonuclear cells (neutrophils). Histologic analysis of lung tissue using paraffin-embedded lung sections stained for hematoxylin and eosin yielded similar results and showed dramatic attenuation of LPS-induced tissue neutrophil accumulation by intravenously administered OxPAPC (Figure 2A, lower panels). LPS treatment induced dramatic increase in tissue polymorphonuclear cells (50.6 ± 4.9 vs. 0.44 ± 0.11 cells/field for control, p < 0.001), which was significantly attenuated by the intravenous injection of OxPAPC (24.9 ± 0.31 vs. 50.6 ± 4.9 cells/field for LPS alone, p < 0.05), but not by nonoxidized PAPC (Figure 2B). Intravenously administered OxPAPC did not exhibit noticeable effects on BAL total cell count, BAL protein content, or tissue neutrophils in the absence of LPS. Taken together, these results demonstrate a protective effect of intravenous OxPAPC against LPS-induced lung inflammation induced by LPS.
Figure 1.
Intravenous oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (OxPAPC) attenuates LPS-induced neutrophil accumulation in bronchoalveolar lavage (BAL). Aerosolized intratracheal LPS (5 mg/kg) and intravenous OxPAPC were administered 18 h before cytologic analysis of BAL fluid. LPS induced a marked increase in BAL total cell count (A), an effect that was almost entirely due to influx of neutrophils (B). Intravenous OxPAPC (1.5 mg/kg) markedly attenuated this response, whereas injection of nonoxidized PAPC (1.5 mg/kg) was without effect. There were no significant differences in cell counts between animals treated with vehicle or OxPAPC alone. (n = 6–9/group).
Figure 2.
Histologic assessment of the effect of OxPAPC on LPS-induced lung injury. Whole lungs (4–6 animals from each experimental group) were agarose inflated in situ, fixed in 10% formalin, and used for histologic evaluation by hematoxylin and eosin staining as described in Methods. Histologic analysis of lung tissue (40× magnification; A) and quantitative analysis of lung tissue neutrophil count (B) obtained from rats exposed to aerosolized LPS demonstrate prominent neutrophilic inflammation, which was dramatically attenuated by cotreatment with intravenous OxPAPC. Nonoxidized PAPC had no effect on LPS-induced tissue inflammation.
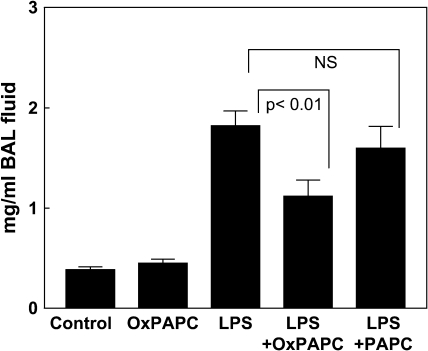
Next, using BAL protein content as a marker of barrier disruption and lung injury, we examined effects of OxPAPC on LPS-induced lung vascular leak. Results depicted in Figure 3 show that intratracheal challenge with aerosolized LPS significantly increased the total protein concentration in BAL fluid, as compared with control animals (1.82 ± 0.15 vs. 0.383 ± 0.029 mg/ml, p < 0.001). Concurrent treatment with intratracheal LPS and intravenous OxPAPC significantly attenuated BAL protein concentrations compared with LPS alone (1.15 ± 0.13 vs. 1.82 ± 0.15 mg/ml, p < 0.001), whereas treatment with nonoxidized PAPC had no significant effect on the LPS-induced changes. There was no significant difference in BAL protein between OxPAPC-treated animals and control animals in the absence of LPS. These results strongly suggest a protective effect of OxPAPC against LPS-induced lung vascular barrier dysfunction in vivo.
Figure 3.
Intravenous OxPAPC attenuates LPS-induced lung barrier dysfunction. BAL protein concentration was assessed at 18 h after aerosolized intratracheal LPS challenge or sham spray. Intravenous OxPAPC (1.5 mg/kg) significantly reduced the pronounced increase in BAL protein induced by LPS administration, whereas injection of nonoxidized PAPC (1.5 mg/kg) was without effect. (n = 6–9/group).
Effect of OxPAPC on LPS-induced Production of Proinflammatory Cytokines
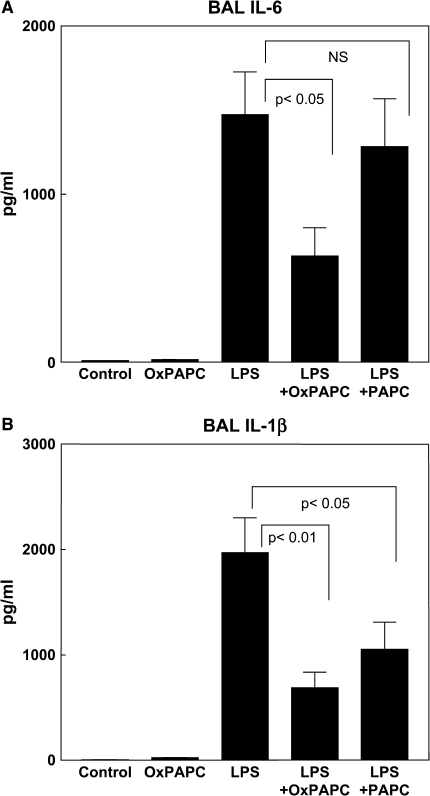
To further investigate the role of OxPAPC in a model of LPS-induced ALI, we analyzed levels of proinflammatory cytokine production in serum and BAL fluid after agonist stimulation. IL-6 and IL-1β were detected and quantified in BAL and serum samples using commercially available rat-specific IL-6 and IL-1β ELISA assays (Figure 4). The levels of IL-6 and IL-1β in both serum and BAL fluid from control animals were minimal, although still within the detection limit of the ELISA, and there were no significant changes in either IL-1β or IL-6 concentrations in serum or BAL from the rats treated with OxPAPC alone. Aerosolized intratracheal LPS induced a dramatic increase in the concentration of IL-6 (1,472 ± 256 vs. 8.44 ± 1.62 pg/ml in controls; p < 0.001) in the BAL fluid. Cotreatment with OxPAPC markedly attenuated this response, resulting in a 57% decrease in BAL IL-6 levels compared with LPS alone (631 ± 168 vs. 1,472 ± 256 pg/ml, p < 0.05), whereas nonoxidized PAPC did not significantly attenuate the LPS effect (Figure 4A). Similarly, LPS induced a robust increase in the concentration of IL-1β in the BAL fluid (1,973 ± 333 vs. 4.1 ± 2.11 pg/ml in control, p < 0.001). This effect was significantly (65%) attenuated with a single intravenous dose of OxPAPC (687 ± 150 vs. 1,973 ± 333 pg/ml for LPS alone, p < 0.05; Figure 4B). In contrast to IL-6 results, intravenous injection of nonoxidized PAPC caused statistically significant reduction of LPS-induced IL-1β production in the BAL fluid at 18 h. Notably, the LPS-induced cytokine response was confined to the alveolar compartment. No significant differences in the serum levels of IL-6 or IL-1β were observed between control rats and rats treated with OxPAPC, LPS, or the combination of LPS and OxPAPC (data not shown). These results reveal inhibitory effects of OxPAPC on LPS-induced production of proinflammatory cytokines and suggest an important role for OxPAPC in lung protection against LPS-induced inflammation and lung injury.
Figure 4.
Intravenous OxPAPC attenuates LPS-induced proinflammatory cytokine production. Interleukin (IL)-6 (A) and IL-1β (B) were measured in the BAL fluid of rats exposed to aerosolized LPS or sham spray with or without concurrent intravenous OxPAPC or PAPC (1.5 mg/kg). OxPAPC inhibited the LPS-induced increase in proinflammatory cytokines IL-6 and IL-1β (n = 6–9/group). PAPC attenuated LPS-induced rise in IL-1β, but had no effect on LPS-induced increase in IL-6.
Dose Dependence of OxPAPC Effects on Pulmonary Inflammation and Barrier Disruption
We next analyzed OxPAPC-induced effects on the parameters of lung injury over a range of OxPAPC doses (0.5, 1.5, 3.0, and 6.0 mg/kg). OxPAPC caused a significant decrease of total cell count in BAL fluid in LPS-stimulated animals over a broad range of doses (Table 1) with a maximal inhibitory dose of 1.5 mg/kg, which induced a 55% decrease in total cell count compared with animals treated with LPS alone. Likewise, measurements of BAL protein concentrations showed that intravenous injection of OxPAPC attenuated the LPS-induced increase in BAL fluid protein in the range of OxPAPC doses from 0.5 to 6.0 mg/kg, with maximal reduction at 1.5 mg/kg (39% decrease vs. LPS alone) and 3 mg/kg (37% decrease vs. LPS alone). OxPAPC significantly suppressed the LPS-induced rise in BAL IL-6 in the 0.5 to 3.0 mg/kg range with maximal inhibition (54–57%) at 1.5 to 3.0 mg/kg, but lost this inhibitory effect at the highest OxPAPC dose (6.0 mg/kg). Likewise, OxPAPC significantly attenuated the LPS-induced increase in BAL IL-1β over the entire range tested (0.5–6.0 mg/kg). Maximal inhibition of LPS-induced IL-1β production (65% inhibition) was observed with the OxPAPC dose of 1.5 mg/kg. Intravenous injection of nonoxidized PAPC (1.5 mg/kg) did not affect LPS-induced changes in BAL protein, cell count, or IL-6 levels, but did decrease LPS-induced production of IL-1β.
TABLE 1.
EFFECT OF INTRAVENOUS OxPAPC ON BRONCHOALVEOLAR LAVAGE FLUID CONTENTS AFTER INTRATRACHEAL LPS ADMINISTRATION
| Protein (mg/ml) | Cell Count (cells/ml × 106) | IL-6 (pg/ml) | IL-1β (pg/ml) | |
|---|---|---|---|---|
| Control | 0.38 ± 0.03* | 0.07 ± 0.02* | 8.44 ± 1.62* | 4.10 ± 2.11* |
| LPS | 1.82 ± 0.15 | 14.48 ± 2.02 | 1472 ± 256 | 1973 ± 333 |
| LPS + OxPAPC, 0.5 mg/kg | 1.24 ± 0.19† | 7.59 ± 1.51† | 700 ± 156† | 1111 ± 144‡ |
| LPS + OxPAPC, 1.5 mg/kg | 1.12 ± 0.17‡ | 6.56 ± 0.53‡ | 631 ± 168† | 687 ± 150‡ |
| LPS + OxPAPC, 3.0 mg/kg | 1.15 ± 0.13‡ | 8.79 ± 2.05† | 674 ± 49† | 1091 ± 223† |
| LPS + OxPAPC, 6.0 mg/kg | 1.36 ± 0.10† | 10.04 ± 1.23† | 1029 ± 266§ | 1028 ± 181† |
| LPS + PAPC, 1.5 mg/kg | 1.60 ± 0.22§ | 11.24 ± 0.64§ | 1280 ± 286§ | 1053 ± 263† |
Definition of abbreviations: IL = interleukin; OxPAPC = oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine.
p < 0.001 compared with LPS.
p < 0.05 compared with LPS.
p < 0.01 compared with LPS.
Not significant compared with LPS.
Effect of OxPAPC on LPS-induced Pulmonary Endothelium Barrier Disruption
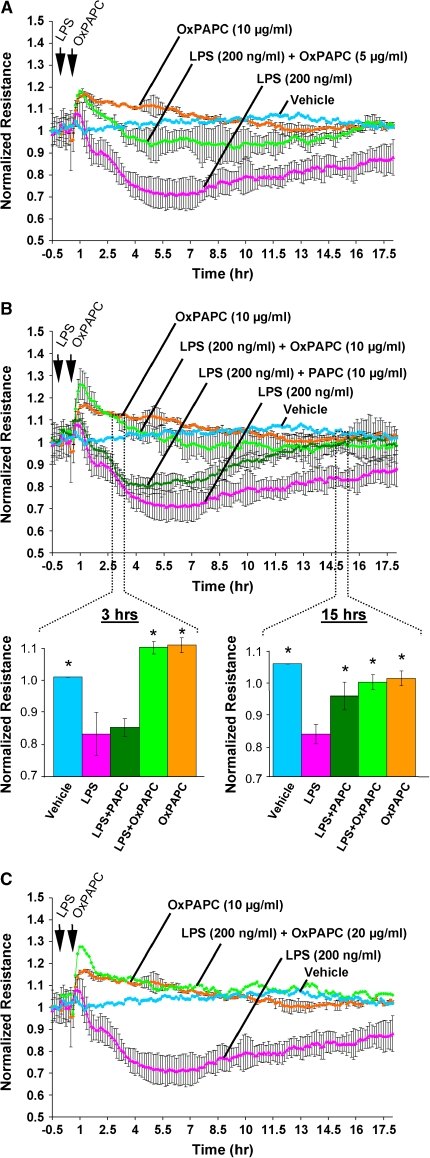
To examine the specific role of the pulmonary endothelium in LPS-induced lung dysfunction and to test the potential protective effects of OxPAPC on endothelial barrier, we measured changes in TER in human pulmonary endothelial cell cultures challenged with LPS and OxPAPC. As shown in Figure 5, LPS (200 ng/ml) stimulation caused significant increase in permeability, which reached maximum (60% of original resistance levels) by 5 h of LPS stimulation. These results reflect dramatic endothelial cell barrier dysfunction induced by LPS treatment. As we have shown previously, treatment of human pulmonary endothelial cell cultures with OxPAPC alone caused significant and sustained increase in TER, reflecting OxPAPC's barrier-protective effects (15). Our current results demonstrate a dose-dependent protective effect of OxPAPC against LPS-induced barrier dysfunction. Even low OxPAPC concentrations (5 μg/ml) introduced 20 min after LPS treatment significantly attenuated LPS-induced dysfunction of the pulmonary endothelial monolayers (Figure 5A). Importantly, addition of OxPAPC (10 μg/ml) 20 min after LPS challenge completely abolished LPS-induced endothelial cell barrier dysfunction and maintained basal resistance levels (Figure 5B) at the basal level, whereas nonoxidized PAPC had no effect on the LPS-induced TER decline observed during the first 5 h of LPS challenge. However, this treatment promoted restoration of endothelial cell–barrier properties at later time points (10–16 h post-stimulation) in contrast to cells incubated with LPS alone. The OxPAPC at the concentration of 20 μg/ml elevated resistance levels above the baseline registered in nonstimulated controls (Figure 5C). In the next series of experiments, LPS-induced decreases in TER were linked to actomyosin rearrangement.
Figure 5.
Effects of oxidized phospholipids and LPS on transendothelial electrical resistance (TER) changes in human pulmonary endothelial cells. Human pulmonary endothelial cells were grown on gold microelectrodes and used for TER measurements, as described in Methods. Cells were challenged with LPS (200 ng/ml) followed by OxPAPC addition (5 μg/ml [A], 10 μg/ml [B], 20 μg/ml [C]) or PAPC addition (10 μg/ml, B) at the times indicated by arrows. Control cells were stimulated with either LPS or OxPAPC alone. Inset shows statistical analysis of TER changes at 3 h and 15 h of LPS and OxPAPC stimulation depicted in B. *p < 0.01 compared with LPS alone. OxPAPC abolished endothelial barrier disruption induced by LPS. Shown are cumulative data of five independent experiments. Results are represented as mean + SE.
Effect of OxPAPC on LPS-induced Pulmonary Endothelium Cytoskeletal and Cell Contact Remodeling
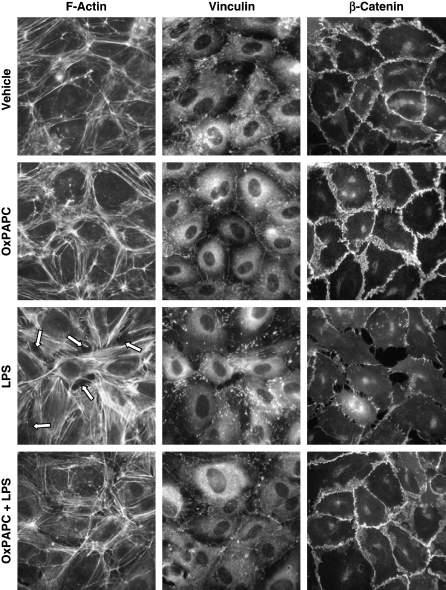
To assess effects of OxPAPC on cytoskeletal rearrangements and cell contact remodeling associated with LPS-induced barrier dysfunction, we performed immunofluorescent staining of human pulmonary endothelial cells treated with LPS alone or with a combination of LPS and OxPAPC. In unstimulated cells, F-actin was primarily organized into peripheral actin bundles and more diffuse cortical actin structures, which were even more pronounced in cells stimulated with OxPAPC (20 μg/ml, 6 h; Figure 6, left panels). After 6-h LPS (200 ng/ml) stimulation, F-actin reorganized into thicker stress fibers in the center of the cells, whereas the peripheral actin band was significantly weakened. These changes were associated with appearance of paracellular gaps (shown by arrows) indicating endothelial cell barrier compromise. OxPAPC treatment 20 min after LPS stimulation dramatically attenuated LPS-induced stress fibers and gap formation observed after 6 h of stimulation (Figure 6, bottom left panel). Because hyperpermeability induced by edemagenic agonists such as thrombin has been suggested to be mediated by changes in cell–cell adhesion (24–26) and focal adhesions (27, 28), we next examined LPS effects on remodeling of focal adhesions and integrity of adherence junctions. Immunofluorescent analysis of vinculin redistribution, which reflects focal adhesion remodeling in LPS-stimulated pulmonary endothelial cells, showed formation of discrete enlarged focal adhesions (Figure 6, middle column), which serve as anchoring sites for contracting actomyosin filaments and have been previously linked to thrombin-induced endothelial barrier dysfunction (18, 27, 29). OxPAPC treatment alone induced peripheral vinculin distribution observed as a continuous line of focal adhesions at the cell–cell interface. Furthermore, OxPAPC treatment of LPS-challenged endothelial monolayers abolished the “thrombinlike” pattern of focal adhesion remodeling (Figure 6, lower middle panel), which was also associated with preservation of endothelial monolayer integrity (Figure 5). OxPAPC stimulation significantly increased accumulation within cell–cell interface areas of a key adherence junction protein β-catenin, which links adherence junctions to actin cytoskeleton (30, 31). In contrast, LPS stimulation caused partial β-catenin dissociation from the areas of cell–cell interface, thus indicating disruption of adherence junctions (Figure 6, right panels). Importantly, OxPAPC prevented LPS-induced β-catenin dissociation from cell–cell junctional complexes and disruption of endothelial monolayer integrity (Figure 6, lower right panel).
Figure 6.
Effects of OxPAPC on LPS-induced actin cytoskeletal and focal adhesion rearrangement and adherens junction disassembly in human pulmonary endothelial cells. Human pulmonary endothelial cells grown on coverslips were preincubated with vehicle (0.1% DMSO), OxPAPC (20 μg/ml), or LPS (200 ng/ml), or cells were challenged with LPS (200 ng/ml) and OxPAPC (20 μg/ml) for 6 h. F-actin, vinculin, and β-catenin were visualized using Texas red–conjugated phalloidin (left column), anti-vinculin antibody (middle column) and anti–β-catenin antibody (right column), respectively, as described in Methods. OxPAPC exhibited protective effects against LPS-induced stress fiber and paracellular gap formation (shown by arrows), focal adhesion remodeling, and disruption of cell–cell contacts. Shown are representative results of four independent experiments.
Taken together, these data demonstrate that OxPAPC prevents LPS-induced barrier dysfunction in pulmonary endothelium via attenuation of contractile response, activation of focal adhesions, and partial disassembly of adherence junction complexes induced by LPS.
DISCUSSION
Acute inflammation and disruption of vascular integrity are cardinal features of acute lung injury, contributing to the high morbidity and mortality associated with this condition. Using an in vivo model, we show in this study that a single intravenous injection of OxPAPC significantly attenuates pulmonary inflammation and vascular barrier dysfunction after intratracheal LPS administration. Intravenous OxPAPC significantly reduced tissue and BAL neutrophil counts, BAL levels of IL-6 and IL-1β, and BAL protein concentration after intratracheal administration of aerosolized LPS, with maximal protection at the 1.5- to 3.0-mg/kg dose range. Previous studies showed that administration of a mixture of LPS or a virus-related proinflammatory agent, CpG, and OxPAPC decreased inflammatory cell recruitment and even protected against LPS-mediated lethal shock in various models of acute inflammation (13, 14). The novel aspect of this study is the use of separate routes of administration for LPS and OxPAPC. To avoid direct competitive inhibition of LPS-mediated proinflammatory signaling by OxPAPC and mimic a more clinical scenario, we used intratracheal administration of aerosolized LPS to induce lung injury and evaluated the ability of concurrent intravenous administration of OxPAPC to attenuate the inflammatory and barrier-disruptive responses.
Oxidative modification of PAPC forms a heterogeneous group of compounds, which can be separated into two major classes: those generated by oxidative fragmentation of sn-2 fatty acid residues, and those generated by addition of oxygen atoms to the sn-2 fatty acid. Examples of biologically active “fragmented oxidized phospholipids” are 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-phosphocholine (POVPC), 1-palmitoyl- 2-glutaroyl-sn-glycero-phosphocholine (PGPC) (17), and 5-keto- 6-octendioic acid ester of 2-lyso-phosphocholine (KOdiA-PC) (32). Examples of “oxygenated phospholipids” include 1-palmitoyl- 2-(5,6-epoxyisoprostane E2)-sn-glycero-3-phosphocholine (5,6-PEIPC) (33), and esterified epoxycyclopentenones, such as 1-palmitoyl-2-(5,6-epoxycyclopentenone)-sn-glycero-3-phosphorylcholine (5,6-PECPC) (34). In addition to these products, rearrangement of endoperoxide intermediates of phospholipid-esterified arachidonic acid during PAPC oxidation results in formation of a third class of esterified γ-ketoaldehydes called isolevuglandins or isoketals (isoketal-PC) (35), which also exhibit biological activities (36). The biological activities of oxidized phospholipids belonging to either the fragmented or oxygenated classes sometimes overlap, but are clearly defined by functional groups present in the oxidatively modified fatty acid moiety (see Reference 37 for review). Examples of the biological effects of oxidized phospholipids include inhibition of LPS-induced E-selectin and vascular cell adhesion molecule expression, blunting the LPS-induced neutrophil adhesion to vascular endothelium, differential regulation of endothelial permeability by oxidized and fragmented PAPC adducts, “platelet activating factor–like” proinflammatory effects, and induction of monocyte adhesion (37).
Although OxPAPC was protective in our model over a range of doses, treatment of LPS-challenged animals with high OxPAPC doses revealed a trend to increased levels of protein, cell elements, and cytokines in BAL as compared with LPS-challenged mice treated with lower OxPAPC concentrations. This waning of the protective effect at high OxPAPC doses is consistent with our previous findings (15), which showed that high OxPAPC concentrations alone caused pulmonary endothelial barrier dysfunction. Although fragmented phospholipids still exhibit potent inhibitory effects on LPS/NF-κB–mediated inflammatory signaling cascade (38), our previous studies delineated barrier-disruptive effects of specific fragmented phospholipids (POVPC, PGPC, lyso-PC) present in OxPAPC preparation, which appear to dominate over the barrier-protective effects of oxygenated products (PECPC and PEIPC) toward pulmonary vascular endothelium, when OxPAPC was administered at high concentration. Our current study shows that the optimal effects of OxPAPC against LPS-induced lung dysfunction have been achieved in the lower range of concentrations (1.5–3.0 mg/kg).
In our studies, nonoxidized PAPC failed to attenuate an early phase of LPS-induced barrier dysfunction in vitro and LPS-induced increases in BAL protein, cell counts, and IL-6 production in vivo. However, PAPC reduced IL-1β production in vivo and promoted EC barrier recovery at late time points in the in vitro model of LPS-induced endothelial dysfunction. Because PAPC may undergo oxidation in the course of both in vivo and in vitro experiments, we speculate that partial attenuation of LPS-induced effects on some parameters of lung dysfunction is attributed to the partial PAPC oxidation in the course of experiment. Further studies in our lab are underway to monitor oxidation of phospholipids in the biological fluids.
Results from cell culture experiments (Figures 5 and 6) showed that the addition of OxPAPC after LPS treatment abolished LPS-induced barrier disruption in cultured human pulmonary endothelial cells, restoring TER to basal or even supranormal levels. These results complement our published data showing direct barrier enhancement by OxPAPC alone and accelerated barrier restoration by OxPAPC in a model of thrombin-induced endothelial hyperpermeability (15). That study demonstrated the direct vascular endothelial barrier–enhancing effects of OxPAPC in the absence of inflammatory stimuli, and showed that these effects were mediated by changes in the actin cytoskeleton, focal adhesions, and adherens junctions in a Rac- and Cdc42-dependent fashion (Reference 15 and K. Birukov, unpublished data). Furthermore, the results of our current study strongly suggest a dual nature of OxPAPC protective effects toward lung barrier function: first, via blunting of LPS/TLR4/NF-κB proinflammatory signaling cascade, and second, via direct effects on cytoskeletal remodeling and enhancement of cell–cell interactions (Figure 6), which are most likely driven by Rac/Cdc42-mediated mechanisms described previously (15). Taken together, these results suggest that endogenous oxidized phospholipids may act as a negative feedback mechanism, which not only attenuates the acute inflammatory response to endotoxin and gram-negative sepsis associated with activation of TLR4 but also directly reduces the lung vascular leak and neutrophil extravasation. In addition, oxidized phospholipids likely exhibit an even broader range of antiinflammatory effects. Recent studies have demonstrated that OxPAPC may also inhibit inflammatory cytokine response induced by TLR2 and TLR9 ligands (14, 39).
The endothelial barrier plays a key role in regulating lung function, both in health and in states of injury, governing vascular permeability and inflammatory cell recruitment to the lung. OxPAPC inhibits LPS-induced E-selectin expression and neutrophil adhesion to endothelial cells in vitro and induces endothelial cytoskeletal remodeling (11, 15). However, the proinflammatory effects of oxidized phospholipids in atherogenesis are also well recognized (40). How do these findings concur with antiinflammatory effects described in this study? OxPAPC alone had no significant effect on neutrophilic inflammation, as measured by tissue and BAL neutrophils, or barrier disruption, as measured by BAL protein, in our in vivo model. Likewise, OxPAPC alone showed a very modest increase in the IL-6 (14.7 ± 1.93 vs. 8.44 ± 1.62 pg/ml in controls) and IL-1β levels (21.8 ± 5 vs. 4.1 ± 2.1 pg/ml in controls), which was statistically significant by t test. In comparison, the magnitude of the LPS-induced inflammatory response reflected by cytokine production and neutrophil infiltration was more than two orders of magnitude greater than for OxPAPC alone (Figures 1, 2, and 4). These results suggest that the minimal acute proinflammatory effects of OxPAPC are negligible when compared with the magnitude of LPS-induced inflammation. Second, OxPAPC-induced chronic vascular inflammation is associated with sustained increases in the local OxPAPC levels associated with accumulation of oxidized lipids in the atherosclerotic plaque. In contrast, intravenous OxPAPC injection in the treatment of LPS-induced lung inflammation is generalized and time-limited, when given concurrent to the inflammatory stimulus. Thus, chronic monocytic inflammation is unlikely to be induced by brief OxPAPC exposure and does not necessarily preclude the potential short-term therapeutic application of oxidized phospholipid compounds in the treatment of acute inflammatory syndromes.
It remains to be elucidated whether OxPAPC is able to traverse the endothelial layer to exert effects directly on alveolar epithelial and inflammatory cells; however, we speculate that intravenously administered OxPAPC exerts its protective effects in our in vivo model by directly enhancing endothelial barrier function, preventing neutrophil recruitment and propagation of the inflammatory cytokine cascades in the lung parenchyma, as well as by directly inhibiting the LPS-induced inflammatory cascades. Although further studies need to be done to identify the specific OxPAPC receptors that mediate its actions on the lung vasculature and potentially the alveolar epithelium, its broad beneficial effects in preventing acute lung injury may have far-reaching clinical implications.
Do oxidized phospholipids have a therapeutic potential? Inflammation appears to be the major problem in a variety of conditions from sepsis and ALI to pancreatitis and burns, even after resolution of the initial insult. In sepsis and ALI, in particular, there has been much interest in the development of therapeutics aimed at attenuating the acute inflammatory response. The recent identification of activated protein C, which exhibits antiinflammatory, antithrombotic, and fibrinolytic activities, represents the first effective antiinflammatory therapy for the treatment of severe sepsis, significantly decreasing morbidity and mortality. However, studies of corticosteroids, well known for their antiinflammatory properties, in the treatment of sepsis and ARDS have shown only modest benefit in human trials despite promising preliminary data from animal studies (41–43). Thus, newer treatments aimed specifically at blunting the excessive inflammatory response are being studied. For example, recent studies by Peng and colleagues (44) showed that, consistent with effects on systemic barrier enhancement, sphingosine 1-phosphate and its pharmacologic analog FTY720 significantly reduced pulmonary/renal vascular leak associated with LPS-induced ALI and inflammation. The efficacy of intravenous OxPAPC in attenuating ALI caused by intratracheal aerosolized LPS suggests a possible therapeutic role for this group of compounds, at least in the acute setting. Moreover, identification of specific oxidized phospholipid species exhibiting potent barrier-protective properties (15) together with antiinflammatory effects strongly supports clinical significance of oxidized phospholipids as a potential new group of therapeutic agents. Further studies will be needed to determine the specific mechanisms of action of OxPAPC in vivo, but our current study, coupled with prior work, suggests the possibility of acute administration of exogenous oxidized phospholipids as a novel therapeutic strategy for various conditions leading to ALI and/or sepsis.
Supplementary Material
Supported by National Institutes of Health NHLBI grants HL076259 and HL075349 for K.G.B., and HL58064 and HL73994 for J.G.N.G.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200511-1737OC on March 2, 2006
Conflict of Interest Statement: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334–1349. [DOI] [PubMed] [Google Scholar]
- 2.Lang JD, McArdle PJ, O'Reilly PJ, Matalon S. Oxidant-antioxidant balance in acute lung injury. Chest 2002;122:314S–320S. [DOI] [PubMed] [Google Scholar]
- 3.Chabot F, Mitchell JA, Gutteridge JM, Evans TW. Reactive oxygen species in acute lung injury. Eur Respir J 1998;11:745–757. [PubMed] [Google Scholar]
- 4.Wood LG, Gibson PG, Garg ML. Biomarkers of lipid peroxidation, airway inflammation and asthma. Eur Respir J 2003;21:177–186. [DOI] [PubMed] [Google Scholar]
- 5.Pennathur S, Bergt C, Shao B, Byun J, Kassim SY, Singh P, Green PS, McDonald TO, Brunzell J, Chait A, et al. Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species. J Biol Chem 2004;279:42977–42983. [DOI] [PubMed] [Google Scholar]
- 6.Kalyanaraman B. Nitrated lipids: a class of cell-signaling molecules. Proc Natl Acad Sci USA 2004;101:11527–11528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrow JD, Roberts LJ. The isoprostanes: their role as an index of oxidant stress status in human pulmonary disease. Am J Respir Crit Care Med 2002;166:S25–S30. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter CT, Price PV, Christman BW. Exhaled breath condensate isoprostanes are elevated in patients with acute lung injury or ARDS. Chest 1998;114:1653–1659. [DOI] [PubMed] [Google Scholar]
- 9.Bochkov VN, Mechtcheriakova D, Lucerna M, Huber J, Malli R, Graier WF, Hofer E, Binder BR, Leitinger N. Oxidized phospholipids stimulate tissue factor expression in human endothelial cells via activation of ERK/EGR-1 and Ca(++)/NFAT. Blood 2002;99:199–206. [DOI] [PubMed] [Google Scholar]
- 10.Lusis AJ. Atherosclerosis. Nature 2000;407:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leitinger N, Tyner TR, Oslund L, Rizza C, Subbanagounder G, Lee H, Shih PT, Mackman N, Tigyi G, Territo MC, et al. Structurally similar oxidized phospholipids differentially regulate endothelial binding of monocytes and neutrophils. Proc Natl Acad Sci USA 1999;96:12010–12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole AL, Subbanagounder G, Mukhopadhyay S, Berliner JA, Vora DK. Oxidized phospholipid-induced endothelial cell/monocyte interaction is mediated by a cAMP-dependent R-Ras/PI3-kinase pathway. Arterioscler Thromb Vasc Biol 2003;23:1384–1390. [DOI] [PubMed] [Google Scholar]
- 13.Bochkov VN, Kadl A, Huber J, Gruber F, Binder BR, Leitinger N. Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nature 2002;419:77–81. [DOI] [PubMed] [Google Scholar]
- 14.Ma Z, Li J, Yang L, Mu Y, Xie W, Pitt B, Li S. Inhibition of LPS- and CpG DNA-induced TNF-α response by oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol 2004;286:L808–L816. [DOI] [PubMed] [Google Scholar]
- 15.Birukov KG, Bochkov VN, Birukova AA, Kawkitinarong K, Rios A, Leitner A, Verin AD, Bokoch GM, Leitinger N, Garcia JG. Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circ Res 2004;95:892–901. [DOI] [PubMed] [Google Scholar]
- 16.Nonas SA, Miller IL, Kawkitinarong K, Garcia JG, Birukov KG. Protective effects of oxidized phospholipids on endotoxin-induced lung injury [abstract]. Proc Am Thorac Soc 2005;2:A837. [Google Scholar]
- 17.Watson AD, Leitinger N, Navab M, Faull KF, Horkko S, Witztum JL, Palinski W, Schwenke D, Salomon RG, Sha W, et al. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J Biol Chem 1997;272:13597–13607. [DOI] [PubMed] [Google Scholar]
- 18.Birukova AA, Smurova K, Birukov KG, Kaibuchi K, Garcia JGN, Verin AD. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res 2004;67:64–77. [DOI] [PubMed] [Google Scholar]
- 19.Garcia JG, Schaphorst KL, Shi S, Verin AD, Hart CM, Callahan KS, Patterson CE. Mechanisms of ionomycin-induced endothelial cell barrier dysfunction. Am J Physiol 1997;273:L172–L184. [DOI] [PubMed] [Google Scholar]
- 20.Birukova AA, Adyshev D, Gorshkov B, Bokoch GM, Birukov KG, Verin AA. GEF-H1 is involved in agonist-induced human pulmonary endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 2006;290:L540–L548. [DOI] [PubMed] [Google Scholar]
- 21.Birukova AA, Birukov KG, Gorshkov B, Liu F, Garcia JG, Verin AD. MAP kinases in lung endothelial permeability induced by microtubule disassembly. Am J Physiol Lung Cell Mol Physiol 2005;289:L75–L84. [DOI] [PubMed] [Google Scholar]
- 22.Singleton PA, Dudek SM, Chiang ET, Garcia JG. Regulation of sphingosine 1-phosphate-induced endothelial cytoskeletal rearrangement and barrier enhancement by S1P1 receptor, PI3 kinase, Tiam1/Rac1, and alpha-actinin. FASEB J 2005;19:1646–1656. [DOI] [PubMed] [Google Scholar]
- 23.Birukova AA, Birukov KG, Smurova K, Adyshev DM, Kaibuchi K, Alieva I, Garcia JG, Verin AD. Novel role of microtubules in thrombin-induced endothelial barrier dysfunction. FASEB J 2004;18:1879–1890. [DOI] [PubMed] [Google Scholar]
- 24.Dejana E. Endothelial adherens junctions: implications in the control of vascular permeability and angiogenesis. J Clin Invest 1997;100:S7–10. [PubMed] [Google Scholar]
- 25.Sandoval R, Malik AB, Minshall RD, Kouklis P, Ellis CA, Tiruppathi C. Ca(2+) signalling and PKCalpha activate increased endothelial permeability by disassembly of VE-cadherin junctions. J Physiol 2001;533:433–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winter MC, Kamath AM, Ries DR, Shasby SS, Chen YT, Shasby DM. Histamine alters cadherin-mediated sites of endothelial adhesion. Am J Physiol 1999;277:L988–L995. [DOI] [PubMed] [Google Scholar]
- 27.Shikata Y, Birukov KG, Birukova AA, Verin AD, Garcia JG. Involvement of site-specific FAK phosphorylation in sphingosine-1 phosphate- and thrombin-induced focal adhesion remodeling: role of Src and GIT. FASEB J 2003;17:2240–2249. [DOI] [PubMed] [Google Scholar]
- 28.Schaphorst KL, Pavalko FM, Patterson CE, Garcia JG. Thrombin-mediated focal adhesion plaque reorganization in endothelium: role of protein phosphorylation. Am J Respir Cell Mol Biol 1997;17:443–455. [DOI] [PubMed] [Google Scholar]
- 29.van Nieuw Amerongen GP, Natarajan K, Yin G, Hoefen RJ, Osawa M, Haendeler J, Ridley AJ, Fujiwara K, van Hinsbergh VW, Berk BC. GIT1 mediates thrombin signaling in endothelial cells: role in turnover of RhoA-type focal adhesions. Circ Res 2004;94:1041–1049. [DOI] [PubMed] [Google Scholar]
- 30.Aberle H, Schwartz H, Kemler R. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem 1996;61:514–523. [DOI] [PubMed] [Google Scholar]
- 31.Kaibuchi K, Kuroda S, Fukata M, Nakagawa M. Regulation of cadherin-mediated cell-cell adhesion by the Rho family GTPases. Curr Opin Cell Biol 1999;11:591–596. [DOI] [PubMed] [Google Scholar]
- 32.Podrez EA, Poliakov E, Shen Z, Zhang R, Deng Y, Sun M, Finton PJ, Shan L, Gugiu B, Fox PL, et al. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. J Biol Chem 2002;277:38503–38516. [DOI] [PubMed] [Google Scholar]
- 33.Watson AD, Subbanagounder G, Welsbie DS, Faull KF, Navab M, Jung ME, Fogelman AM, Berliner JA. Structural identification of a novel pro-inflammatory epoxyisoprostane phospholipid in mildly oxidized low density lipoprotein. J Biol Chem 1999;274:24787–24798. [DOI] [PubMed] [Google Scholar]
- 34.Subbanagounder G, Wong JW, Lee H, Faull KF, Miller E, Witztum JL, Berliner JA. Epoxyisoprostane and epoxycyclopentenone phospholipids regulate monocyte chemotactic protein-1 and interleukin-8 synthesis: formation of these oxidized phospholipids in response to interleukin-1beta. J Biol Chem 2002;277:7271–7281. [DOI] [PubMed] [Google Scholar]
- 35.Brame CJ, Boutaud O, Davies SS, Yang T, Oates JA, Roden D, Roberts LJ II. Modification of proteins by isoketal-containing oxidized phospholipids. J Biol Chem 2004;279:13447–13451. [DOI] [PubMed] [Google Scholar]
- 36.Zhang W, Salomon RG. Oxidized phospholipids, isolevuglandins, and atherosclerosis. Mol Nutr Food Res 2005;49:1050–1062. [DOI] [PubMed] [Google Scholar]
- 37.Bochkov VN, Leitinger N, Birukov KG. Role of oxidized phospholipids in acute lung injury. Curr Respir Med Rev 2006;2:27–37. [Google Scholar]
- 38.Bochkov VN, Leitinger N. Anti-inflammatory properties of lipid oxidation products. J Mol Med 2003;81:613–626. [DOI] [PubMed] [Google Scholar]
- 39.Walton KA, Cole AL, Yeh M, Subbanagounder G, Krutzik SR, Modlin RL, Lucas RM, Nakai J, Smart EJ, Vora DK, et al. Specific phospholipid oxidation products inhibit ligand activation of toll-like receptors 4 and 2. Arterioscler Thromb Vasc Biol 2003;23:1197–1203. [DOI] [PubMed] [Google Scholar]
- 40.Leitinger N. Oxidized phospholipids as triggers of inflammation in atherosclerosis. Mol Nutr Food Res 2005;49:1063–1071. [DOI] [PubMed] [Google Scholar]
- 41.Meduri GU, Carratu P, Freire AX. Evidence of biological efficacy for prolonged glucocorticoid treatment in patients with unresolving ARDS. Eur Respir J Suppl 2003;42:57s–64s. [DOI] [PubMed] [Google Scholar]
- 42.Minneci PC, Deans KJ, Banks SM, Eichacker PQ, Natanson C. Meta-analysis: the effect of steroids on survival and shock during sepsis depends on the dose. Ann Intern Med 2004;141:47–56. [DOI] [PubMed] [Google Scholar]
- 43.Thompson BT. Glucocorticoids and acute lung injury. Crit Care Med 2003;31:S253–S257. [DOI] [PubMed] [Google Scholar]
- 44.Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JG. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med 2004;169:1245–1251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.