Abstract
Rationale: Recently, respiratory syncytial virus (RSV) RNA has been identified by reverse transcriptase–polymerase chain reaction (RT-PCR) from a high percentage of patients with stable chronic obstructive pulmonary disease (COPD). These data raise the possibility of persistent low-grade infection in this population, which could have implications in COPD pathogenesis.
Objectives: RSV persistence was investigated by testing respiratory secretions from subjects with COPD during illness and at regular intervals over 1 yr.
Methods: Nasal and sputum samples from subjects with COPD were tested by one-tube nested RT-PCR for RSV every 2 mo and during respiratory illnesses for 1 yr. Subjects positive for RSV were evaluated weekly until negative in two consecutive samples. Nasal secretions and serum were tested for RSV antibody. A rise of fourfold or greater was defined as evidence of RSV infection.
Results: A total of 112 patients were enrolled and the illnesses of 92 patients were evaluated. RSV was detected by RT-PCR in 6/92 (6.5%) illness nasal samples versus 0/685 routine nasal samples and in 5/69 (7.2%) illness sputum samples versus 3 /315 (0.9%) routine. Four additional RSV infections were identified by serum antibody responses. Of the RSV infections 86% were associated with serum or nasal antibody responses and 73% had symptoms of acute respiratory illness.
Conclusions: Most RSV infections in patients with COPD are associated with symptomatic respiratory illnesses and measurable immune responses. Our data do not support the concept of RSV persistence in this population.
Keywords: chronic obstructive pulmonary disease exacerbation, persistent infection, viral infection
Chronic obstructive pulmonary disease (COPD) is a group of disorders characterized by airflow obstruction that can be associated with breathing-related symptoms such as chronic cough, dyspnea, and wheezing (1). It is estimated that approximately 24 million adults in the United States have impaired lung function due to COPD. During 2000, COPD was responsible for 8 million physician office visits, 1.5 million emergency room visits, 726,000 hospitalizations, and 119,000 deaths. Recurrent acute exacerbations of COPD contribute substantially to the morbidity and mortality of this condition and may be due to infections with bacteria, viruses, or both (2–4). In studies of COPD exacerbation, respiratory syncytial virus (RSV) has been identified with variable frequency ranging from 0.8 to 22% depending on the diagnostic methods used (5–16). Recently investigators from the United Kingdom and Germany identified RSV RNA by reverse transcriptase–polymerase chain reaction (RT-PCR) from a high percentage (24–28%) of patients with stable COPD as well as from ill patients experiencing acute exacerbations (17, 18). RSV detection was associated with elevated markers of inflammation and typically was at low copy number. These data have called into question the utility of RT-PCR for the diagnosis of acute RSV infection in patients with COPD and raised the possibility of persistent low-grade RSV infection in this population. The latter possibility, if confirmed, could have important implications in the pathogenesis of COPD.
In our laboratory, we have developed a sensitive and specific real-time, one-tube nested RT-PCR for the diagnosis of RSV in adults (19, 20). Because the assay is performed without opening the reaction tube, PCR contamination has been markedly reduced while the sensitivity of a nested assay is retained. Therefore, we sought to explore the question of RSV persistence in patients with COPD by testing upper- and lower-respiratory-tract secretions at regular intervals over 1 yr in a cohort of subjects with COPD.
METHODS
Subjects
Volunteers were recruited from pulmonary practices and rehabilitation programs and were eligible if they were 40 yr or older, had a diagnosis of COPD by a physician, had no history of asthma, and were past or active smokers. Informational letters inviting participation were mailed to potential subjects with a diagnosis of COPD by their pulmonary physicians or the director of the pulmonary rehabilitation center. All subjects provided written informed consent before enrollment. The study was approved by the Rochester General Hospital and the University of Rochester Research Subjects Review Board.
Study Design
Subjects were enrolled between July and October of 2004 and were followed for 12 mo. Participants underwent an enrollment visit and routine visits every 2 mo (a total of seven visits). In addition, subjects were evaluated for respiratory illnesses that were defined as the presence of any of the following symptoms: nasal congestion, sore throat, hoarseness, new or increased-from-baseline cough, sputum production, dyspnea, and wheezing. Respiratory samples were collected weekly from subjects testing positive for RSV during an illness until the subjects were RSV negative during 2 consecutive weeks. RSV-positive subjects also collected daily sputum samples at home between weekly visits if possible.
Enrollment
Medical history, medication use, and functional data were collected and results of pulmonary function tests were recorded if available. Subjects underwent a directed respiratory physical exam. A nasal-swab sample was collected from a subject by gently rubbing the nasal turbinates for 5 s with a moistened cotton-tip swab and placed in 2 ml of sterile water. Expectorated sputum was collected from those who could provide it, however, sputum samples were not induced. A baseline serum was collected from all subjects.
Routine Visits
Subjects were asked to collect their first morning expectoration and return the sample with each routine visit. Nasal swabs and blood samples were obtained at the time of each routine visit. Participants were questioned about any active respiratory symptoms at the time of the visit or in the preceding week.
Illness Visits
During a clinic or home visit, nasal and serum samples were collected from ill subjects, as was expectorated sputum if possible. Data on symptoms, functional impact, medication changes, and health care utilization were collected. Convalescent blood was obtained 4 to 6 wk after illness.
Laboratory Testing
Samples from illness visits were tested weekly with samples from routine visits interspersed in a random fashion. RT-PCR assays were performed blinded with regard to specimen type (illness vs. routine). All specimens were tested by nested RT-PCR and those positive for RSV underwent quantitative RT-PCR analysis. Sputum Gram stains were performed to assess the adequacy of samples using standard criteria. All nasal and sputum specimens were aliquoted and frozen and stored at −80°C within 24 h after arrival in the laboratory.
RSV Single-Tube Nested RT-PCR
Group-specific real-time assay was performed by a one-tube nested RT-PCR method as previously described (19, 21). The lower limit of detection is 0.01–0.05 pfu/ml for RSV group A or B or for one copy of RNA.
RSV Quantitative RT-PCR
The quantitative assay for group A and B RSV utilizes the inner primers and probes for the above nested RT-PCR (21). The lower limit of detection for the assay is 1 to 10 pfu/ml.
RSV Serology
Serum IgG.
The titer of IgG in serum to the RSV fusion protein (F) and attachment proteins (Ga, Gb) was determined using established methods (22). A greater-than-fourfold rise in titer to any of the RSV antigens was considered diagnostic of recent infection.
Nasal IgA.
The titer of nasal IgA to F, Ga, and Gb was determined using published methods (23). Total protein in each nasal sample was determined (Micro BCA, Rockford, IL) and nasal IgA was corrected to a total protein of 100 μg/ml.
Genetic Analysis
The PCR products from the F gene RT-PCR amplification were purified by agarose gel electrophoresis and extraction (QIA Quick Gel Extraction Kit; Qiagen, Valencia, CA). Nucleotide sequencing was done on an automated sequencing machine (ABI Model 3100) using the negative-sense amplification primer (5′ CTCTGTCAGTTCTTG 3′). Nucleotides corresponding to F gene bases 742 to 931 were compared by alignment. The sequence of the RSV 18,537 F gene (accession number D00334) was used as a base number reference (24).
Statistical Analysis
The χ2 and Fisher's exact tests were used to compare proportions. Means from normally distributed data were compared with the Student's t test. A Mann-Whitney test was used to compare nonparametric paired samples.
RESULTS
Enrollment included 112 subjects and 95 completed the study. During the year 9 subjects dropped out of the study due to ill health and 8 died. Of the subjects, 86 (77%) made all seven routine visits and 102 (91%) made four or more visits. Most subjects (97%) were under the care of a pulmonary specialist. Subjects had a mean of 54 pack-years of smoking history and 48% complained of chronic sputum production (Table 1). Many subjects used inhaled steroids (67%), chronic oral steroids (20%), or home oxygen (47%). For 65 subjects (58%) pulmonary function test results were available; in this subset the mean FEV1 was 44 ± 19% of predicted.
TABLE 1.
SUBJECT CHARACTERISTICS
| Total Enrolled (n = 112) | RSV Positive (n = 14) | |
|---|---|---|
| Age, mean ± SD | 72 ± 10 | 75 ± 11 |
| Female, % | 51 | 57 |
| White, % | 97 | 97 |
| Exposed to children, % | 62 | 73 |
| Active smokers | 13 | 0 |
| Pack-years, mean ± SD | 54 ± 30 | 42 ± 23 |
| Influenza vaccine, % | 97 | 100 |
| Pneumococcal vaccine % | 97 | 100 |
| FEV1,% predicted | 44 ± 19 | 39 ± 12 |
| Chronic sputum, % | 47 | 43 |
| Oral steroids, % | 20 | 36 |
| Inhaled steroids, % | 67 | 86 |
| Home oxygen, % | 47 | 57 |
Definition of abbreviation: RSV = respiratory syncytial virus.
Overall, 685 routine evaluations were performed, yielding 685 nasal samples and 315 sputum samples for RT-PCR analysis. RSV RNA was detected in 0 of the 685 nasal and in 3 (0.9%) of the 315 sputum samples (Table 2). During the 12-mo follow-up period, 134 respiratory illnesses were reported, of which 92 (69%) were evaluated during the acute phase with the collection of 92 nasal and 69 sputum samples. Ill subjects were evaluated an average of 2.9 ± 1.6 d after onset of symptoms, and six nasal (6.5%) and five sputum (7.2%) samples were RT-PCR positive. The RSV detection rate in acute illness samples was significant greater than in routinely obtained nasal (p < 0.0001) and sputum samples (p = 0.006). Overall, 30% of the 384 sputum samples were judged to be adequate, 44% adequate but contaminated, and 26% inadequate.
TABLE 2.
RESPIRATORY SYNCYTIAL VIRUS REVERSE TRANSCRIPTASE–POLYMERASE CHAIN REACTION RESULTS
| +/No. Tested (%)
|
|||
|---|---|---|---|
| Samples | Illness | Routine | p Value |
| Nasal | 6/92 (6.5) | 0/685 (0) | < 0.0001 |
| Sputum | 5/69 (7.2) | 3/315 (0.9) | 0.006 |
From RT-PCR and serology, 14 subjects showed evidence of RSV infections with 1 subject showing evidence of two infections (Table 3) Seven infections were RT-PCR positive and associated with a greater-than-fourfold rise in serum or nasal antibody, three were RT-PCR positive only, and four were identified by serologic response only. Of the 15 RSV infections, 11 were identified during illness visits (subjects 1–10), including both infections in subject 1. The latter subject had two RT-PCR positive illnesses approximately 2 mo apart, the second one associated with seroconversion. Of the four RSV-infected subjects with clinically unrecognized infection, three were identified by a positive sputum RT-PCR collected on routine visits (subjects 12–14). Of these three, one had no symptoms, one complained of increased cough at the time of the visit, and the other complained of feeling unwell with increased dyspnea for 1 wk preceding the visit. However, neither of the latter two subjects perceived a “new respiratory illness” warranting an illness evaluation at the time of the positive RT-PCR sample collection. One of these subjects (subject 12) demonstrated a serologic response at the time of the positive RT-PCR.
TABLE 3.
SUMMARY OF RESPIRATORY SYNCYTIAL VIRUS–POSITIVE SUBJECTS
| Subject No. | Illness | RT-PCR | Serum IgG Rise | Nasal IgA Rise |
|---|---|---|---|---|
| 1 | 12/30/04 | + | 0 | 0 |
| 1 | 3/7/05 | + | 8-fold | 4-fold |
| 2 | 12/31/04 | + | 16-fold | 32-fold |
| 3 | 1/5/05 | + | 0 | 4-fold |
| 4 | 1/12/05 | + | 16-fold | 16-fold |
| 5 | 10/13/04 | + | 16-fold | 16-fold |
| 6 | 1/21/05 | + | 32-fold | 8-fold |
| 7 | 4/23/05 | + | 4-fold | 16-fold |
| 8 | 2/4/05 | 0 | 16-fold | 4-fold |
| 9 | 1/14/05 | 0 | 8-fold | 32-fold |
| 10 | 6/13/05 | 0 | 8-fold | 4-fold |
| 11 | Routine visit, fall 2004 | 0 | 16-fold | 8-fold |
| 12 | Routine visit, 12/7/04 | + | 32-fold | 4-fold |
| 13 | Routine visit, 1/31/05 | + | 0 | 0 |
| 14 | Routine visit, 5/6/05 | + | 0 | 0 |
Definition of abbreviation: RT-PCR = reverse transcriptase–polymerase chain reaction.
Quantitative RT-PCR was performed on all positive nasal and sputum samples and expressed as pfu/ml of sample (Table 4). The mean peak viral load by quantitative RT-PCR was 38,620 ± 64,750 pfu (range, 2–156,000) in illness samples and 82 ± 140 (range, 0.01–250) in routine samples. Due to the small numbers and the wide range of values, this difference was not statistically significant. The viral load of RSV RNA in nasal samples was lower than in sputum in almost all instances. In 9 of 11 nasal and sputum pairs collected simultaneously, the sputum viral load was higher than the nasal (p = 0.008, Mann-Whitney test). In addition, sputum samples remained positive longer than nasal samples (13.8 ± 7.6 vs. 10.1 ± 7.3 d) although this difference was not significant.
TABLE 4.
RESPIRATORY SYNCYTIAL VIRUS TITERS IN SYMPTOMATIC SUBJECTS
| Subject | Day after Illness Onset | Nasal Viral Load | Sputum Viral Load |
|---|---|---|---|
| 1 (illness 1) | 6 | 281 | NA* |
| 15 | 0 | NA | |
| 22 | 0 | NA | |
| 1 (illness 2) | 1 | 0 | 23,700 |
| 7 | 0 | 0.01 | |
| 15 | 0 | 0 | |
| 22 | 0 | 0 | |
| 2 | 5 | 0 | 128,000 |
| 15 | 0.01 | 153 | |
| 17 | NA | 142 | |
| 18 | NA | 15.5 | |
| 21 | NA | 0.01 | |
| 22 | 0 | NA | |
| 29 | 0 | 0 | |
| 31 | NA | 0 | |
| 33 | 0 | NA | |
| 3 | 1 | 747 | NA |
| 9 | 0 | NA | |
| 16 | 0 | NA | |
| 4 | 7 | 106 | 156,000 |
| 14 | 0.16 | 9,090 | |
| 19 | NA | 0.01 | |
| 21 | 10.1 | 0.01 | |
| 22 | NA | 0.01 | |
| 26 | NA | 0 | |
| 28 | 0 | 0 | |
| 35 | 0 | 0 | |
| 5 | 5 | 216 | NA |
| 15 | 0.10 | 32.9 | |
| 26 | 0 | NA | |
| 34 | 0 | NA | |
| 6 | 3 | 3.4 | 27.4 |
| 10 | 0 | 9.92 | |
| 11 | NA | 0.01 | |
| 13 | NA | 0.01 | |
| 15 | NA | 0.01 | |
| 17 | 0 | 0 | |
| 18 | NA | 0 | |
| 20 | NA | 0 | |
| 24 | 0 | 0 | |
| 31 | 0 | 0 | |
| 7 | 3 | 1.83 | 0.01 |
| 56 | 0 | NA | |
| 62 | NA | 0 |
Definition of abbreviation: NA = not available.
Because some subjects were unable to produce sputum at the time of the evaluations and because some subjects collected sputum at home between visits, not all nasal and sputum samples are part of simultaneously matched pairs.
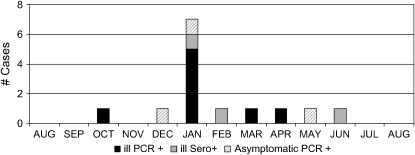
All RT-PCR positive samples were collected between October and May, with clear clustering in January (Figure 1), and all were group B by strain-specific RT-PCR, including both illness samples from subject 1. Sequences of the 190 nucleotide amplified F gene segment from these two samples were both consistent with the B1 genotype, although they differed by 2 nucleotides at positions 713 A/G and 916 A/G.
Figure 1.
Distribution of respiratory synctial virus (RSV) cases by month of identification. Cases associated with illness and reverse transcriptase–polymerase chain reaction (RT-PCR) positive nasal or sputum specimens are shown in black. Cases associated with illness and serologic response but RT-PCR negative nasal or sputum specimens are shown in gray. Asymptomatic RSV infections identified by RT-PCR are shown by diagonally lined bars. One asymptomatic infection identified by serology during the fall of 2004 is not displayed because it could not be assigned to a specific month.
The subjects who were identified as RSV positive by RT-PCR or serology did not differ significantly from subjects who did not have RSV in age, sex, smoking status, pulmonary function, or steroid use (Table 1). The clinical characteristics of the 11 RSV illnesses are outlined in Table 5. Most subjects were moderately ill with complaints of increased cough (100%), sputum (45%), and dyspnea (73%). Only 1 of 11 had symptoms limited to the upper respiratory tract. At least one office visit was made by 73 % of the subjects and one subject was hospitalized for 3 d. On average, subjects were unable to perform their activities of daily living for 9 d. Evaluated by standards set forth by Anthonsien and colleagues for acute exacerbation of COPD, 91% of those with an identified illness met exacerbation criteria (25).
TABLE 5.
RESPIRATORY SYNCYTIAL VIRUS ILLNESS CHARACTERISTICS
| Characteristic | No. (%) (n = 11) |
|---|---|
| Symptom | |
| Nasal congestion | 7 (64) |
| Sore throat | 3 (27) |
| Cough | 11 (100) |
| Sputum | 5 (45) |
| Dyspnea | 8 (73) |
| Wheeze | 9 (82) |
| Impact | |
| Office visit | 8 (73) |
| Hospitalization | 1 (9) |
| Days unable to do ADLs, mean ± SD) | 9 ± 13 |
| Days of illness, mean ± SD | 24 ± 12 |
Definition of abbreviation: ADLs = activities of daily living.
The subject who experienced two symptomatic RSV B infections was moderately ill with nasal congestion, cough, dyspnea, and wheezing lasting 3 to 4 wk during both episodes. The first illness occurred on December 31, 2004, with an evaluation 4 d later. Nasal RT-PCR was positive with a viral load of 247 pfu and sputum was unavailable. This episode was not associated with a serologic response. The second illness occurred on March 7, 2005, and she had repeatedly negative nasal specimens with positive sputum over a 2-wk period. The initial viral load in the sputum was 23,700, and this episode was associated with a eightfold rise in serum IgG and fourfold rise in nasal IgA RSV titers. Routine evaluations of both nasal and sputum specimens in April, June, and August 2005 were all negative. Throughout this period, she used nasal and inhaled corticosteroids as well as oral prednisone at 2 mg/d.
DISCUSSION
The concept that latent viral infections play a role in the pathogenesis of COPD has potentially significant implications for the management of this disease. Although it is generally accepted that certain DNA viruses, such as adenovirus, can integrate into the host cell and produce latent infection, the possibility of chronic infection with the common RNA respiratory viruses is less certain (26). Several animal studies suggest that latent infection with RSV is possible. RSV proteins and genomic RNA have been detected in the lungs of experimentally infected guinea pigs 2 mo after infection and persistent infection of B lymphocytes by bovine RSV has also been demonstrated (27, 28). More recently, Schwarze and colleagues have recovered low levels of infectious virus from the lungs of T-cell–depleted mice 150 d after the initial intranasal infection (29). Evidence for RSV latency in humans is limited. RSV RNA has been detected in archived lung tissue from infants who died in summer months, raising the question of viral persistence (30). More compelling, two recent studies of patients with COPD reported detectable RSV RNA in 24 to 28% of respiratory samples, regardless of whether samples were collected during illness or stable periods (17, 18). In addition, RSV RNA has also been detected by real-time RT-PCR in 70% of a small group of symptom-free smokers (31). In some studies, RSV RNA was identified on multiple occasions in the same subjects. Interestingly, both RSV RNA detection and elevated inflammatory markers were associated with a faster rate of decline in FEV1, suggesting a link between persistent low-grade infection and progression of COPD (32).
In our study, the identification of RSV RNA in respiratory samples from patients with COPD was most often associated an acute exacerbation and a measurable serum and mucosal immune response. However, clearly there is also a spectrum of disease ranging from asymptomatic infection or mild coryza to severe illness requiring hospitalization. In only three cases did we detect RSV RNA in respiratory secretions collected during routine visits.
The subjects with no symptoms and a robust immune response undoubtedly had asymptomatic infection. For the three subjects in whom viral RNA was identified at low copy number from a single specimen without a demonstrable immune response (subject 1, illness 1, and subjects 13 and 14), it must be questioned whether true “infection” has occurred. Possible explanations for these episodes include laboratory contamination of the RT-PCR assay, “aborted infection,” or low-grade persistence of RSV. Although all samples with low copy number or those found during routine visits were confirmed by repeat testing, contamination of the original sample cannot be excluded with absolute certainty. “Aborted infection” seems the most plausible explanation since the organism was cleared without resulting in symptoms or an immune response. It is conceivable that some pathogen encounters are successfully eliminated in the early phases by the host innate immune response or preexisting mucosal antibody. The fact that RSV RNA was only identified in sputum and not nasal samples in two of these three cases argues somewhat against this possibility. Low-grade persistence seems an unlikely alternative explanation given the clear winter temporal clustering of the RSV positive cases and that no subject had the same RSV RNA identified on more than one occasion.
One subject in our study had the same genotype of RSV identified 2 mo apart. Prolonged shedding of RSV has been described in a number of immunosuppressive conditions such as corticosteroid use and HIV infection (33). Because our subject used inhaled and oral corticosteroids on a chronic basis, prolonged infection was possible. However, the identification of two nucleotide differences in a conserved portion of the F gene in the two specimens most likely represents two different strains of RSV B1. Thus, reinfection, which has been well documented in children, seems the most likely explanation for this finding although chronic infection with the development of quasi-species cannot be ruled out without more complete sequencing data (34).
Our results are clearly different from those of previous reports that describe a high percentage of patients with stable COPD with detectable RSV RNA at low copy number. Possible explanations for this discrepancy include: different study populations, variable sensitivities of RT-PCR methodology, and assay contamination. Our study has several limitations that could have potentially affected the results. Because spirometry was not available for all patients, it is possible that the study population contained some subjects who did not have COPD and thus were not at risk for persistent RSV. Because 97% of the subjects were under the care of a pulmonary specialist we feel that this was unlikely. Another limitation of our study is that we did not have freshly induced sputum specimens from all subjects, raising concerns that viral RNA may have degraded during specimen transport. Although this is a valid concern, we have found that sputum samples may be frozen and thawed multiple times with no significant diminution in viral load. In addition, all nasal specimens were collected during clinic visits and processed immediately. The failure to identify RSV in significant numbers of subjects with stable COPD seems not to be due to an insensitive RT-PCR assay because the limit of detection of our nested assay is one RNA copy. The use of highly sensitive RT-PCR methodology requires rigorous procedures to avoid contamination and false-positive results. Thus, an alternative explanation for the disparate results of this study compared with other reports is that high rates of RSV identification in subjects with stable COPD are due to RT-PCR contamination.
The possibility of chronic RSV infection as a mechanism of disease progression could have enormous impact on the management of COPD. Our study indicates that most RSV infections in patients with COPD are associated with acute symptomatic respiratory illnesses, and we find no evidence that low-grade RSV infection occurs in a significant proportion of patients with COPD. To address this controversial problem definitively, sequencing data to rule out RT-PCR contamination and testing of lower airway samples obtained by bronchial alveolar lavage or lung biopsy are needed.
Supported by National Institutes of Health grant R21-AI-45969.
Originally Published in Press as DOI: 10.1164/rccm.200510-1681OC on December 30, 2005
Conflict of Interest Statement: A.R.F. has served as a consultant for Novartis and received $750. She served on the advisory board for Aventis for $1,500, and received a research grant of $150,000 as the primary investigator in a vaccine trial sponsored by Aventis. M.A.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. P.A.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.M.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. W.M.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. E.E.W. has served as a consultant for Novartis, Arrow and Alnylam and received $2,750. He served on the advisory board for Aventis for $1,500.
References
- 1.Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease surveillance—United States: 1971–2000. MMWR Morb Mortal Wkly Rep 2002;51:S1–S16. [Google Scholar]
- 2.Seemungal TAR, Wedzicha JA. Viral infections in obstructive airway diseases. Curr Opin Pulm Med 2003;9:111–116. [DOI] [PubMed] [Google Scholar]
- 3.Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:1418–1422. [DOI] [PubMed] [Google Scholar]
- 4.Sethi S. New developments in the pathogenesis of acute exacerbations of chronic obstructive pulmonary disease. Curr Opin Infect Dis 2004;17:113–119. [DOI] [PubMed] [Google Scholar]
- 5.Beckham JD, Cadena A, Lin J, Piedra PA, Glezen WP, Greenberg SB, Atmar RL. Respiratory viral infections in patients with chronic obstructive pulmonary disease. J Infect 2005;50:322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buscho RO, Saxtan D, Shultz PS, Finch E, Mufson MA. Infections with viruses and mycoplasma pneumoniae during exacerbations of chronic bronchitis. J Infect Dis 1978;137:377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carilli AP, Gohd R, Gordon W. A virologic study of chronic bronchitis. N Engl J Med 1964;170:123–127. [DOI] [PubMed] [Google Scholar]
- 8.Fagon J-Y, Chastre J. Severe exacerbations of COPD patients: the role of pulmonary infections. Semin Respir Infect 1996;11:109–118. [PubMed] [Google Scholar]
- 9.Glezen WP, Greenberg SB, Atmar RL, Piedra PA, Couch RB. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA 2000;283:499–505. [DOI] [PubMed] [Google Scholar]
- 10.Gump DW, Phillips CA, Forsyth BR. Role of infection in chronic bronchitis. Am Rev Respir Dis 1976;113:465–474. [DOI] [PubMed] [Google Scholar]
- 11.Lambert HP, Stern H. Infective factors in exacerbations of bronchitis and asthma. BMJ 1972;3:323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamy ME, Pouthier-Simon F, Debacker-Willame E. Respiratory viral infections in hospital patients with chronic bronchitis. Chest 1973;63:336–341. [DOI] [PubMed] [Google Scholar]
- 13.Rohde G, Wiethege A, Borg I, Kauth M, Bauser TT, Gillissen A, Bufe A, Schultze-Werninghaus G. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax 2003;58:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiselka MJ, Kent J, Cookson JB, Nicholson KG. Impact of respiratory infection in patients with chronic chest disease. Epidemiol Infect 1993;111:337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh EE, Falsey AR, Hennessey PA. Respiratory syncytial virus and other infections in persons with chronic cardiopulmonary disease. Am J Respir Crit Care Med 1999;160:791–795. [DOI] [PubMed] [Google Scholar]
- 16.Sommerville RG. Respiratory syncytial virus in acute exacerbations of chronic bronchitis. Lancet 1963;II:1247–1248. [DOI] [PubMed] [Google Scholar]
- 17.Borg I, Rohde G, Loseke S, Bittscheidt J, Schultze-Werninghaus G, Stephan V, Bufe A. Evaluation of quantitative real-time PCR for the detection of respiratory syncytial virus in pulmonary diseases. Eur Respir J 2003;21:944–951. [DOI] [PubMed] [Google Scholar]
- 18.Seemungal T, Harper-Owen R, Bhowmik A, Moric I, Sanderson G, Message S, MacCallum P, Meade TW, Jeffries DJ, Johnston SL, Wedzichia JA. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;164:1618–1623. [DOI] [PubMed] [Google Scholar]
- 19.Falsey AR, Formica MA, Walsh EE. Diagnosis of respiratory syncytial virus infection: comparison of reverse transcription-PCR to viral culture and serology in adults with respiratory illness. J Clin Microbiol 2002;40:817–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walsh EE, Falsey AR, Swinburne IA, Formica MA. Reverse transcription polymerase chain reaction (RT-PCR) for diagnosis of respiratory syncytial virus infection in adults: use of a single-tube “hanging droplet” nested PCR. J Med Virol 2001;63:259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falsey AR, Formica MA, Treanor JJ, Walsh EE. Comparison of quantitative reverse transcription-PCR to viral culture for assessment of respiratory syncytial virus shedding. J Clin Microbiol 2003;41:4160–4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falsey AR, Treanor JJ, Betts RF, Walsh EE. Viral respiratory infections in the institutionalized elderly: clinical and epidemiologic findings. J Am Geriatr Soc 1992;40:115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walsh EE, Falsey AR. A simple and reproducible method for collecting nasal secretions from frail elderly adults for measurement of virus specific IgA. J Infect Dis 1999;179:1268–1273. [DOI] [PubMed] [Google Scholar]
- 24.Johnson PR, Collins PL. The fusion glycoproteins of human respiratory syncytial virus of subgroups A and B: sequence conservation provides a structural basis for antigenic relatedness. J Gen Virol 1988;69:2623–2628. [DOI] [PubMed] [Google Scholar]
- 25.Anthonisen NR, Manfreda J, Warren CPW, Hershfield ES, Harding GKM, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987;106:196–204. [DOI] [PubMed] [Google Scholar]
- 26.Hogg JC. Role of latent viral infections in chronic obstructive pulmonary disease and asthma. Am J Respir Crit Care Med 2001;164:S71–S75. [DOI] [PubMed] [Google Scholar]
- 27.Valarcher JF, Bourhy H, Lavenu A, Bourges-Abella N, Roth M, Andreoletti O, Ave P, Schelcher F. Persistent infection of B lymphocytes by bovine respiratory syncytial virus. Virology 2001;291:55–67. [DOI] [PubMed] [Google Scholar]
- 28.Hegele RG, Hayashi S, Bramley AM, Hogg JC. Persistence of respiratory syncytial virus genome and protein after acute bronchiolitis in guinea pigs. Chest 1994;105:1848–1854. [DOI] [PubMed] [Google Scholar]
- 29.Schwarze J, O'Donnell DR, Rohwedder A, Openshaw PJM. Latency and persistence of respiratory syncytial virus despite T cell immunity. Am J Respir Crit Care Med 2004;169:801–805. [DOI] [PubMed] [Google Scholar]
- 30.Cubie HA, Duncan LA, Marshall LA, Smith NM. Detection of respiratory syncytial virus nucleic acid in archival postmortem tissue from infants. Pediatr Pathol Lab Med 1997;17:927–938. [PubMed] [Google Scholar]
- 31.Rohde G, Borg I, Arinir U, Wiethege A, Rausse R, Bufe A, Schultze-Werninghaus G. The role of RSV in respiratory infections in adults [abstract]. Proc Am Thorac Soc 2005; Available from: http://www.abstracts2view.com/ats05/view.php?nu=ATS5L-2570
- 32.Seemungal TAR, Donaldson GC, Wilkinson TW, Patel IS, Hurst JA, Wedzicha JA. High inflammatory marker indices and chronic infection hasten decline in FEV1 in COPD patients [abstract]. Proc Am Thorac Soc 2005; Available from: http://www.abstracts2view.com/ats/view.php?nu=ATS4L
- 33.Hall CB, Powell KR, MacDonald NE, Gala CL, Menegus ME, Suffin SC, Cohen HJ. Respiratory syncytial viral infection in children with compromised immune function. N Engl J Med 1986;315:77–81. [DOI] [PubMed] [Google Scholar]
- 34.Hall CB, McCarthy CA. Respiratory syncytial virus. In: Mandell G, Bennett J, Dolin R, editors. Mandell, Douglas and Bennett's principles and practice of infectious disease. New York: Churchill Livingstone; 1996. pp. 1501–1519.