Abstract
Rationale: Although it has been postulated that liver injury results in impaired clearance of bacteria from the blood, no prior study has evaluated hepatic bacterial clearance during sepsis.
Objectives: We hypothesized that liver injury during the evolution of sepsis would result in impaired hepatic bacterial clearance.
Methods: Mild and severe bacteremia were generated in C57BL/6 mice by low- and high-dose intratracheal inoculation with Pseudomonas aeruginosa.
Measurements and Main Results: The mortality rates with mild and severe bacteremia were 20% and 60%, respectively. Hepatic bacterial clearance was preserved throughout the evolution of mild bacteremia but was lost late with severe bacteremia. The loss of hepatic bacterial clearance resulted in increased systemic bacteremia and mortality. Pretreatment with a caspase inhibitor resulted in preservation of hepatic bacterial clearance with severe bacteremia and eventual control of the bacteremia. When Kupffer cells were ablated before the onset of bacteremia, there was a loss of hepatic bacterial clearance. This converted an initially mild bacteremia into severe bacteremia with increased organ injury and mortality.
Conclusions: These observations suggest that hepatic bacterial clearance may be lost during the evolution of sepsis, resulting in a failure to control bacteremia. Thus, the capacity of the liver to clear bacteria is an important determinant of the outcome in sepsis.
Keywords: apoptosis, bacteremia, infection
In the United States, there are approximately 250,000 cases of sepsis with bacteremia annually (1). Approximately 50% of cases of bacteremic sepsis develop evidence of organ injury, including liver dysfunction, and this is an important determinant of survival (1). During the course of infection, the liver clears the blood of bacteria and produces cytokines in response to the infection (2). It clears bacteria and other particulates, like endotoxin, from the blood via the hepatic reticuloendothelial system. Kupffer cells, the resident macrophages of the hepatic reticuloendothelial system, are strategically situated to perform this function because they are located in the periportal region where blood enters the liver.
Acute and chronic liver diseases are associated with an increased risk of bacteremia (3–5). In addition, it has been shown that impaired clearance of injected particulates from the blood by the liver is associated with subsequent increased severity of infection (6). A recent study found that liver disease is an independent risk factor for the development of bacteremia in patients with community-acquired pneumonia (7). Although it is clearly documented that preexisting liver disease is associated with increased severity of infection, no study has directly investigated the evolution of hepatic bacterial clearance during the evolution of sepsis. Therefore, we evaluated hepatic bacterial clearance during the evolution of bacteremia in the setting of normal preexisting liver function. We used a murine model of pneumonia and bacteremia with Pseudomonas aeruginosa strain PA103. This strain of Pseudomonas causes acute epithelial injury and bacterial dissemination via the production of type III secreted toxins (8). The type III secretion system is a major determinant of virulence and allows the bacteria to inject toxins into the host cells (9).
We evaluated the evolution of hepatic bacterial clearance using models of mild and severe bacteremia. Mild and severe bacteremias were triggered by intratracheal inoculation of 5 × 103 and 104 colony-forming units (cfu) of PA103, respectively. We found that hepatic injury increased over time only with the severe bacteremia model. This was associated with a prolonged hepatic proinflammatory response and loss of bacterial clearance by the liver. Furthermore, we found that inhibition of caspase activity with the caspase inhibitor N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (z-VAD-fmk) resulted in preservation of hepatic bacterial clearance with severe bacteremia. When Kupffer cells were ablated with gadolinium chloride (GdCl3) before the onset of bacteremia, the mild form of bacteremia evolved into a more severe form of bacteremia. In all settings, when bacterial clearance by the liver was lost, it was associated with increased injury to other organs and mortality. Some of the results of these studies have been previously reported in the form of abstracts (10, 11).
METHODS
Pneumonia and Bacteremia Models
After anesthesia with ketamine/xylazine, C57BL/6 mice (female, age 6–8 wk) (Harlan Laboratories, Indianapolis, IN) underwent intratracheal intubation with a 20-gauge Angiocath as previously described (12, 13). A 50-μl volume of P. aeruginosa, strain PA103, was instilled into the trachea. Mild and severe bacteremias were induced with 5 × 103 CFU and 5 × 104 CFU, respectively. To inhibit apoptosis, separate mice were pretreated with z-VAD-fmk (Sigma-Aldrich, St. Louis, MO) at a concentration of 10 mg/kg dissolved in dimethyl sulfoxide 1 h before generation of bacteremia. To ablate Kupffer cells, separate mice were treated with 7.5 mg/kg GdCl3 (Sigma-Aldrich) daily for 3 d before generation of bacteremia as previously described (14). Survival studies were performed on separate animals and used temperature as a surrogate endpoint for death. The criteria used were based upon prior studies showing severe hypothermia as a marker of death (15, 16). Briefly, temperature was monitored every 4 h for 24 h and then every 6 h. Animals were considered deceased if they had an absolute temperature below 27oC or a temperature below 30oC that failed to improve to above 30oC over the next 12 h. Animal studies were approved by the University of Iowa Institutional Animal Care and Use Committee.
Tissue Harvest and Homogenization
At predetermined time points, animals were killed according to Animal Care Guidelines. Blood was obtained from the portal vein and right ventricle. Organs were harvested and perfused free of intravascular cells. Tissues were homogenized, and supernatants were collected. In separate animals, livers were harvested and fixed in 4% paraformaldehyde for histologic and immunohistochemical analysis.
Serum Measurements
Interleukin 1β (IL-1β) and tumor necrosis factor α (TNF-α) were measured by ELISA (R&D Systems, Minneapolis, MN). Serum alanine aminotransferase (ALT) was determined by a kinetic assay (ThermoTrace, Melbourne, Australia). Serum creatine kinase mb fraction (CK-mb) was determined by an immunoinhibition assay (ThermoTrace).
Quantitative Real-Time Polymerase Chain Reaction
For real-time polymerase chain reaction (PCR) analysis, 2 ng of experimental sample DNA was added to 48 μl of reaction mixture containing 1× iQ SYBR Green Supermix (BioRad, Hercules, CA) and 0.2 μM each of sense and antisense primers (IDT, Coralville, IA). Primers used to amplify the outer membrane lipoprotein gene, oprL, are as follows (5′ to 3′): forward, ATGGAAATGCTGAAATTCGGC; reverse, CTTCTTCAGCTCGACGCGACG. These primers are specific for Pseudomonas spp. (17). Amplification, specificity, and quantification were determined as previously described (18). Sensitivity of the assay was determined to be 50 copies per 2-ng sample.
Caspase-3 Activity Assay
A master mix of 45-μl caspase buffer and 5-μl caspase-3 substrate (Calbiochem, San Diego, CA) per sample was added to a black 96-well plate. Baseline fluorescence was read (excitation 355 nm, emission 460 nm). After incubation for 1 h at 37oC in the dark, fluorescence was read. Baseline values were subtracted to determine relative fluorescence intensity.
Statistical Analyses
Statistical analyses were performed using Prism GraphPad software (San Diego, CA). Data are presented as mean ± SEM. Comparisons were made using analysis of variance followed by Tukey's test for multiple comparisons unless otherwise stated.
RESULTS
Mortality after Infection with PA103
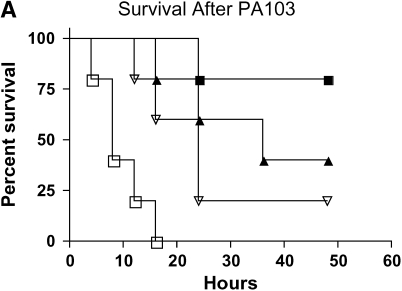
To establish appropriate models of mild and severe bacteremia, we performed survival studies using increasing intratracheal doses of PA103. Using body temperature as a surrogate endpoint for death, we found 20% and 60% mortality at 36 h with doses of 5 × 103 and 5 × 104 cfu, respectively (Figure 1A). On the basis of the survival studies, the remainder of this article uses 5 × 103 cfu as a model of mild bacteremia and 5 × 104 cfu as a model of severe bacteremia.
Figure 1.
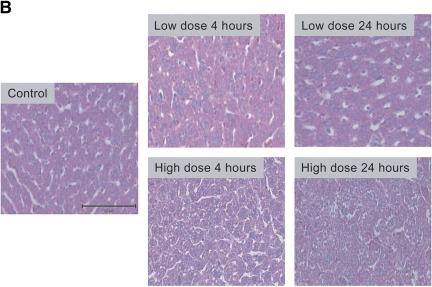
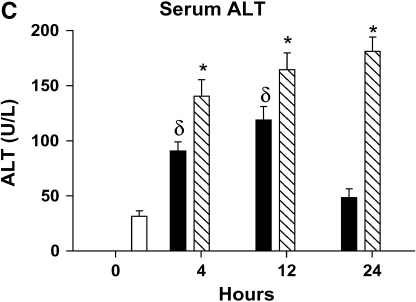
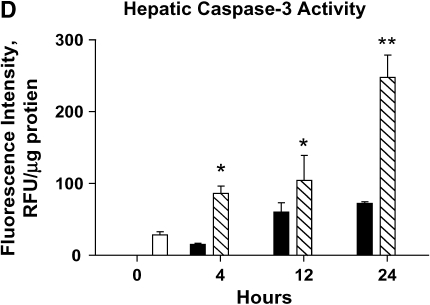
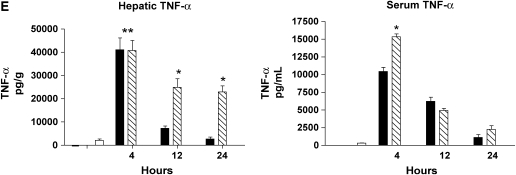
Severity of liver injury differs in mild and severe bacteremia. (A) C57BL/6 mice (n = 40) underwent endotracheal intubation and instillation of increasing doses of P. aeruginosa (strain PA103). Animals were monitored as described in Methods. Solid squares, 5 × 103; solid triangles, 5 × 104; inverted open triangles, 5 × 105; open squares 5 × 106 cfu. (B) At 4 and 24 h after generation of mild and severe bacteremia, livers were fixed in 4% paraformaldehyde. All images are hematoxylin and eosin stained. (Original magnification: ×20.) (C) Serum alanine aminotransferase (ALT) was measured after generation of mild and severe bacteremia. Each group represents seven mice. Severe bacteremia results in increased ALT compared with control and mild infection at all time points (*p < 0.001). In mild bacteremia, there was an increase in ALT compared with control at 4 and 12 h (δp < 0.001), but this returned to baseline by 24 h. (D) The caspase-3 assay shows an increase in caspase-3 activity in liver lysates at 12 and 24 h in the severe bacteremia model (*p < 0.01 and **p < 0.001, respectively). (E) Tumor necrosis factor α (TNF-α) was measured in liver lysates by ELISA. There was a significant increase in TNF-α levels at 4 h in mild and severe bacteremia compared with control (**p < 0.001). There was also an increase in hepatic TNF-α in severe bacteremia compared with mild bacteremia at 12 and 24 h (*p < 0.001). Serum TNF-α was increased in severe bacteremia at 4 h (*p < 0.05), but there was no difference at the later time points. C–E: solid bars, 5 × 103 organisms; hatched bars, 5 × 104 organisms; open bars, control.
Hepatic Inflammation Is Prolonged and Hepatic Cell Death Is Increased with Severe Bacteremia
Using these models of mild and severe sepsis, we evaluated liver injury by histology and serum ALT levels during the evolution of bacteremia. Histologic analysis shows mild hepatic injury at 4 h in the mild bacteremia model, with return of normal hepatic architecture by 24 h (Figure 1B). There is increased liver injury after 4 h of severe bacteremia and extensive hepatic cell death after 24 h as compared with control and mild bacteremia livers (Figure 1B). After 24 h of severe bacteremia, the liver shows evidence of microthrombi formation. The sections shown in Figure 1B are representative of the entire liver and were reviewed by a pathologist and a hepatologist.
Paralleling the histology, we found that serum ALT increased progressively in the severe bacteremia model (Figure 1C). In contrast, mild bacteremia was associated with an early increase in serum ALT that resolved by 24 h. The transient increase in serum ALT in mild bacteremia corresponds to the histologic evidence of mild hepatic injury that subsequently resolved. To evaluate for evidence of hepatic apoptosis during bacteremia, we measured caspase-3 activity in liver lysates. We found that hepatic caspase-3 activity increased during the course of severe bacteremia, concomitant with the increase in serum ALT (Figure 1D). This suggests that hepatic apoptosis accounts for at least some of the hepatic cell death seen during severe bacteremia.
We next evaluated hepatic levels of TNF-α during the course of sepsis. We found that mild and severe bacteremia are associated with an early and equal hepatic TNF-α response (Figure 1E). In mild bacteremia, TNF-α rapidly decreases and returns toward baseline by 12 h. In contrast, severe bacteremia is associated with a sustained elevation of TNF-α. We also found that there was increased serum TNF-α in the severe bacteremia group compared with the mild bacteremia group at 4 h, but the levels were no different at the later time points (Figure 1E). Amounts of IL-1β in liver and serum mirrored the levels of TNF-α (data not shown). These observations suggest that the degree and duration of the hepatic inflammatory response is associated with the severity of hepatic parenchymal injury.
Severe Bacteremia Is Associated with Loss of Hepatic Bacterial Clearance
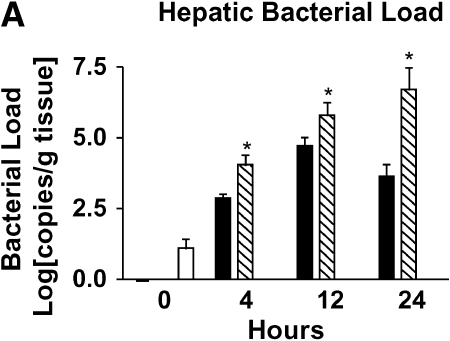
Hepatic bacterial load was evaluated using quantitative real-time PCR with primers specific for P. aeruginosa (17). We found that hepatic bacterial load increases in mild bacteremia at 12 h and trends down by 24 h (Figure 2A). In contrast, severe bacteremia is associated with increasing hepatic bacterial load over time.
Figure 2.
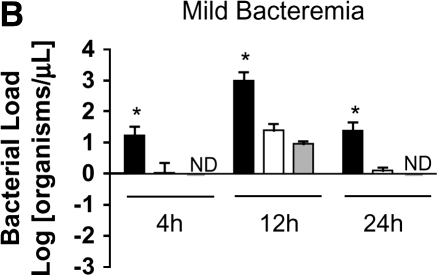
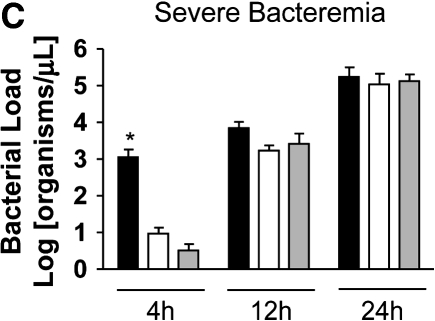
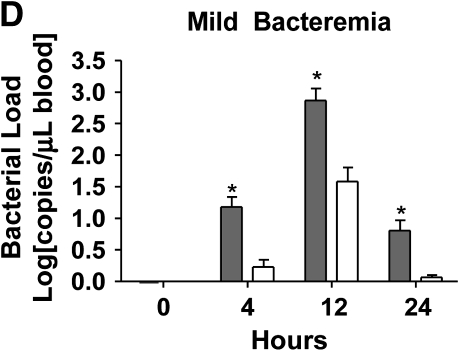
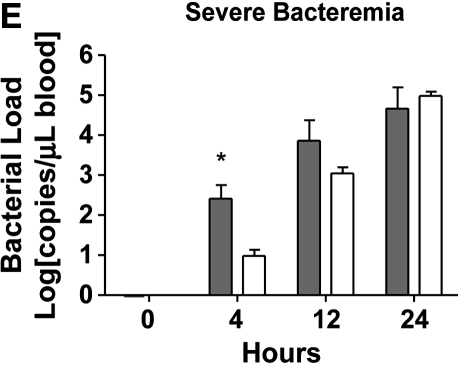
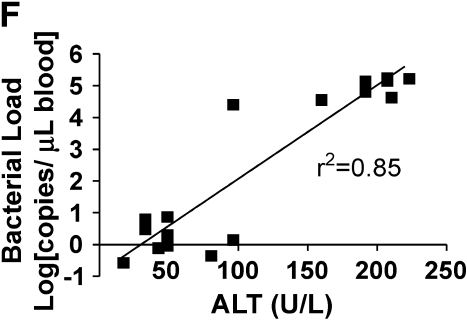
Loss of bacterial clearance occurs in severe bacteremia. (A) Bacterial load was measured in liver lysates after generation of mild or severe bacteremia by quantitative real-time PCR with primers specific for P. aeruginosa. Each group represents seven mice. A log transformation was performed to correct for unequal variances. There was increased bacterial load in severe bacteremia compared with mild bacteremia at all time points (*p < 0.001). Solid bars, 5 × 103 organisms; hatched bars, 5 × 104 organisms; open bars, control. (B) Bacterial load was measured in the portal vein (PV), right ventricle (RV), and hepatic vein (HV). In mild bacteremia, the use of the RV as a measure of hepatic bacterial clearance slightly underestimates the degree of bacterial clearance by the liver (*p < 0.05 comparing PV with RV and HV at all time points). ND = none detected. (C) In severe bacteremia, use of the RV slightly underestimates bacterial clearance at 4 h; however, bacterial clearance by the liver is lost at 12 h using HV and RV bacterial load. B, C: solid bars, PV; open bars, RV; shaded bars, HV. (D) In mild bacteremia, PV bacterial load is greater than RV bacterial load at all time points (*p < 0.05) Shaded bars, PV; open bars, RV. (E) In severe bacteremia, PV bacterial load is greater than that in the RV at 4 h (*p < 0.05). However, there is no difference at 12 or 24 h, suggesting ineffective bacterial clearance. (F) Serum ALT was compared with RV bacterial load at 24 h after infection. Linear regression analysis shows a significant correlation between degree of liver injury and the amount of bacteria in the RV (r2 = 0.85).
To evaluate bacterial clearance by the liver, we measured the bacterial concentrations in portal vein and right ventricular blood. Because the right ventricle also receives blood from the superior vena cava (SVC), we compared SVC and portal vein bacterial loads at all time points and found no significant difference (data not shown). This suggests that bacterial load in the right ventricle primarily represents bacterial clearance by the liver rather than dilution by SVC blood. To further investigate whether right ventricular bacterial load would underestimate bacterial clearance, we measured simultaneous bacterial loads in the portal vein, hepatic vein, and right ventricle in separate mice with mild and severe bacteremia. We found that in the setting of effective bacterial clearance during mild bacteremia, the use of the right ventricle slightly underestimates bacterial clearance by the liver (Figure 2B). However, loss of bacterial clearance by the liver during severe bacteremia occurs at the same time point in hepatic vein and right ventricular measurements (Figure 2C). Therefore, right ventricular bacterial load was used as a measurement of hepatic bacterial clearance in subsequent studies.
We found that in mild bacteremia there is a gradient of bacteria between the portal vein and right ventricle, suggesting effective bacterial clearance by the liver (Figure 2D). In contrast, severe bacteremia is associated with equalization of bacterial loads in the portal vein and right ventricle by 24 h, suggesting a loss of hepatic bacterial clearance late during severe bacteremia (Figure 2E). At 24 h, serum ALT correlates with right ventricular bacterial load (Figure 2F).
Caspase Inhibition Preserves Hepatic Bacterial Clearance in Severe Bacteremia
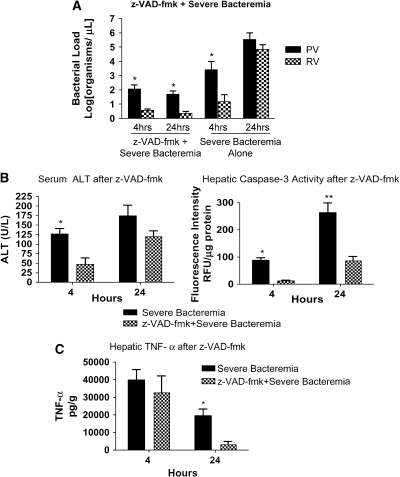
We postulated that a preservation of Kupffer cells would prevent the loss of hepatic bacterial clearance during severe bacteremia. We pretreated animals with z-VAD-fmk 1 h before generation of severe bacteremia. Pretreatment with z-VAD-fmk resulted in preservation of hepatic bacterial clearance (Figure 3A) and a decrease in bacterial load at 24 h after infection. This suggests that the loss of bacterial clearance seen during hepatic injury may be related to apoptosis of liver cells, including Kupffer cells.
Figure 3.
Caspase inhibition results in preserved hepatic bacterial clearance. (A) After pretreatment with z-VAD-fmk, mice were infected with PA103 104 cfu (severe bacteremia) and killed at 4 and 24 h. Control animals received dimethyl sulfoxide followed by PA103 104 cfu. Pretreatment with z-VAD-fmk resulted in preserved bacterial clearance at 4 and 24 h compared with severe bacteremia alone (*p < 0.01). (B) At 4 h, the severe bacteremia–alone animals had increased serum ALT compared with the animals pretreated with z-VAD-fmk (*p < 0.01). Caspase-3 activity in the liver was increased in the severe bacteremia alone mice at 4 (*p < 0.01) and 24 (**p < 0.001) h. (C) TNF-α was measured in liver lysates. At 4 h, there was no difference in hepatic TNF-α in the mice treated with z-VAD-fmk and the mice treated with severe bacteremia alone. At 24 h, there was increased TNF-α in the mice treated with severe bacteremia alone (*p < 0.01).
We found that serum ALT was decreased at 4 h in mice that received z-VAD-fmk compared with mice that were not exposed to the caspase inhibitor (Figure 3B). This provides further evidence that liver injury in sepsis is at least in part due to apoptosis. To confirm that the effect of z-VAD-fmk on the liver was associated with decreased caspase activity, we measured caspase-3 activity in liver lysates. We found that there was significantly less caspase-3 activity in the liver in animals pretreated with z-VAD-fmk (Figure 3B).
There was no difference in levels of hepatic TNF-α at 4 h between animals that were untreated and those that were pretreated with z-VAD-fmk (Figure 3C). However, over time the z-VAD-fmk mice released less hepatic TNF-α, whereas the untreated mice had a more prolonged hepatic TNF-α response. Because z-VAD-fmk preserves Kupffer cell numbers and function, this observation suggests that the increased bacterial load drives the prolonged hepatic TNF-α response in severe bacteremia.
Kupffer Cell Ablation with Gadolinium Results in a Loss of Hepatic Bacterial Clearance and Converts Mild Bacteremia to Severe Bacteremia
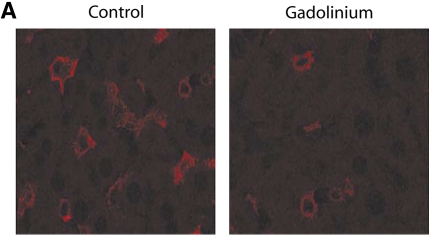
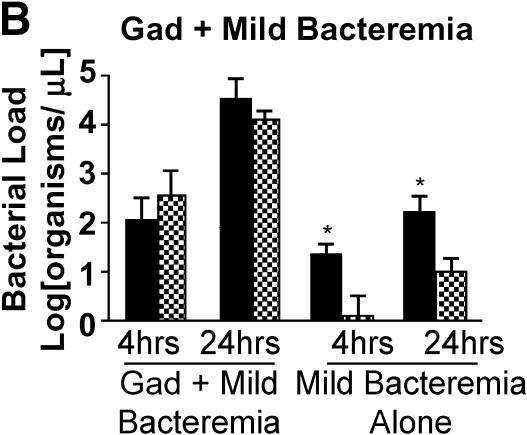
To directly evaluate the importance of Kupffer cells in bacterial clearance during bacteremia, we ablated Kupffer cells by treating animals with GdCl3. Treating animals with GdCl3 resulted in a 70% reduction in the number of Kupffer cells seen with immunohistochemistry (Figure 4A). Ablation of Kupffer cells before the onset of infection resulted in a loss of hepatic bacterial clearance even in the setting of mild bacteremia (Figure 4B). In the absence of Kupffer cells, the mild bacteremia model was converted to one of severe bacteremia. These observations show that Kupffer cells are important for the clearance of bacteria in this model.
Figure 4.
Kupffer cell ablation results in loss of hepatic bacterial clearance. (A) After pretreatment with GdCl3, livers were harvested and stained with F4/80 macrophage antibody. Compared with control, there were decreased macrophages in the GdCl3-treated liver. (B) After pretreatment with GdCl3, mice were infected with PA103 103 cfu (mild bacteremia) and killed at 4 and 16 h. Control animals received PBS followed by PA103 103 cfu. Pretreatment with GdCl3 resulted in impaired bacterial clearance at 4 and 16 h compared with mild bacteremia (*p < 0.01). Solid bars, PV; cross-hatched bars, RV. (C) At 4 h, serum ALT was increased in mice treated with mild bacteremia alone compared with mice treated with GdCl3 mice (*p < 0.001). In mice treated with GdCl3, there was increased ALT at 16 h compared with 4 h (**p < 0.001). Hepatic caspase-3 activity increased in mice treated with GdCl3 at 16 h compared with mice treated with mild bacteremia alone (*p < 0.001). (D) TNF-α was measured in liver lysates. There was significantly less TNF-α in the liver in mice treated with GdCl3at 4 h compared with mice treated with mild bacteremia alone (*p < 0.001). C, D: solid bars, mild bacteremia; open bars, gadolinium + mild bacteremia.
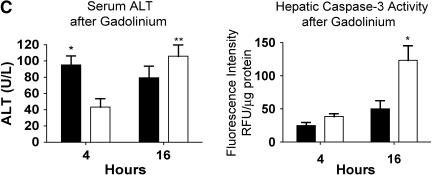
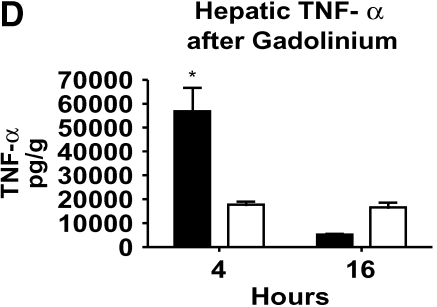
Serum ALT levels were not elevated in the animals pretreated with GdCl3 at 4 h (Figure 4C). However, there was evidence of liver injury 16 h after generation of bacteremia in the animals pretreated with GdCl3. We also found that caspase-3 activity increased at 16 h in the GdCl3-treated animals compared with the mild bacteremia alone model (Figure 4C). This suggests that a portion of the hepatic cell death seen in this model of bacteremia is due to apoptosis. The decrease in Kupffer cell number resulted in decreased hepatic TNF-α at 4 h after infection, and it did not increase at 16 h (Figure 4D). With GdCl3, liver injury occurred in the setting of low hepatic TNF-α, suggesting that liver injury in this setting is not mediated solely by the production of TNF-α. Serum TNF-α levels were also decreased in the GdCl3- treated mice (data not shown), suggesting that the Kupffer cells are an important source of systemic TNF-α with bacteremia.
Loss of Bacterial Clearance Is Associated with Increased End-Organ Damage
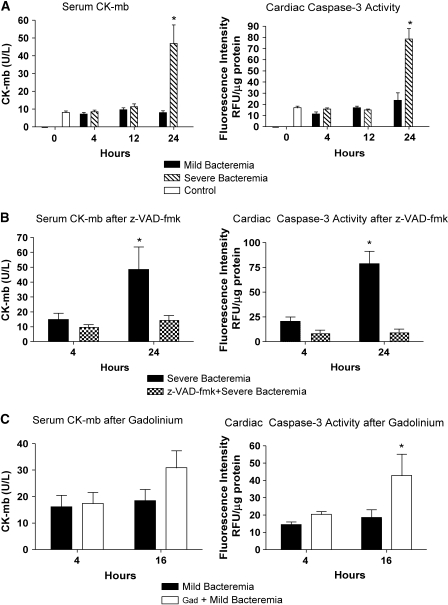
To assess whether loss of hepatic bacterial clearance predisposed to the development of end-organ damage, we measured serum CK-mb and myocardial caspase-3 activity of the left ventricle to evaluate myocardial injury. Serum CK-mb did not increase in the mild sepsis model. In contrast, serum CK-mb increased after 24 h in the severe bacteremia model (Figure 5A). This was similar to the caspase-3 activity in the severe bacteremia model.
Figure 5.
Cardiac injury in bacteremia is abated by caspase inhibition and exacerbated by Kupffer cell ablation. (A) Serum CK-mb levels were measured during mild and severe bacteremia. Serum CK-mb increased only at 24 h in the severe bacteremia model (*p < 0.001). Similarly, there was an increase in caspase-3 activity in myocardial lysates at 24 h in the severe bacteremia model (*p < 0.001). (B) Pretreatment with z-VAD-fmk followed by infection with the severe bacteremia model resulted in no evidence of increased CK-mb or cardiac caspase-3 activity. (C) Pretreatment with GdCl3 followed by infection with the mild bacteremia model resulted in a trend toward increased CK-mb levels at 16 h and evidence of increased cardiac caspase-3 activity at 16 h (*p < 0.01) compared with mice infected with mild bacteremia alone.
Inhibition of caspase activity with z-VAD-fmk before infection with the severe bacteremia model resulted in no detectable cardiac injury or apoptosis at 24 h compared with severe bacteremia alone (Figure 5B). This could be secondary to improved hepatic bacterial clearance driving the bacterial burden down and to local inhibition of apoptosis by z-VAD-fmk (19, 20). We found that there was a trend toward increased CK-mb and an earlier increase in cardiac caspase-3 activity at 16 h in the GdCl3-treated animals, whereas the animals in the mild bacteremia alone group had no increase in CK-mb or cardiac caspase activity (Figure 5C).
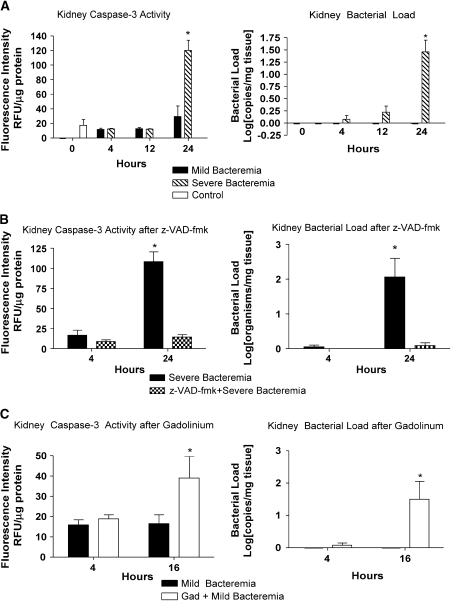
Because serum creatinine could be representative of volume status rather than kidney injury, we chose to evaluate kidney lysates for evidence of bacteria and evidence of apoptosis. We were unable to detect P. aeruginosa in the kidney until 24 h in the severe bacteremia model (Figure 6A). This time point correlates with the loss of hepatic bacterial clearance. There was no evidence of P. aeruginosa in kidney lysates in the mild bacteremia model. The amounts of bacteria in the kidney paralleled the amounts of caspase-3 activity.
Figure 6.
Renal injury in bacteremia is prevented by caspase inhibition and worsened by Kupffer cell ablation. (A) Kidney injury was evaluated by bacterial load and caspase-3 activity in kidney lysates. There was an increase in kidney caspase-3 activity in the severe bacteremia model at 24 h (*p < 0.001). Bacterial load was determined by quantitative real-time PCR with primers specific for P. aeruginosa and is reported as a log transformation. There was no P. aeruginosa detected in kidney lysates in the control animals or in the mild bacteremia model. There was a significant increase in bacterial load in the kidney in the severe bacteremia model at 24 h (*p < 0.001). (B) Pretreatment with z-VAD-fmk followed by infection with the severe bacteremia model resulted in no increase in kidney caspase-3 activity or kidney bacterial load at 24 h. (C) Pretreatment with GdCl3 followed by infection with the mild bacteremia resulted in increased kidney caspase-3 activity (*p < 0.01) and kidney bacterial load (*p < 0.01) at 16 h.
We examined renal injury in animals pretreated with z-VAD-fmk or GdCl3 followed by infection with severe or mild bacteremia, respectively. Animals with severe bacteremia that were pretreated with z-VAD-fmk had no increase in kidney bacterial load (Figure 6B). This observation is consistent with the preservation of hepatic bacterial clearance in animals pretreated with z-VAD-fmk. Animals pretreated with z-VAD-fmk had no increase in kidney caspase-3 activity. In contrast, animals with mild bacteremia pretreated with GdCl3 had an increase in kidney bacterial load at 16 h compared with the mild bacteremia alone animals, in which no bacteria was detected in the kidney (Figure 6C). The increase in kidney bacterial load in the mice treated with GdCl3 occurred earlier than the increase seen in the severe bacteremia model. These animals had an increase in kidney caspase-3 activity at the same time point. These data suggest that as hepatic bacterial clearance worsens, more bacteria are allowed systemic access, resulting in increased organ injury.
Caspase Inhibition Improves Survival and Kupffer Cell Ablation Decreases Survival in Bacteremia
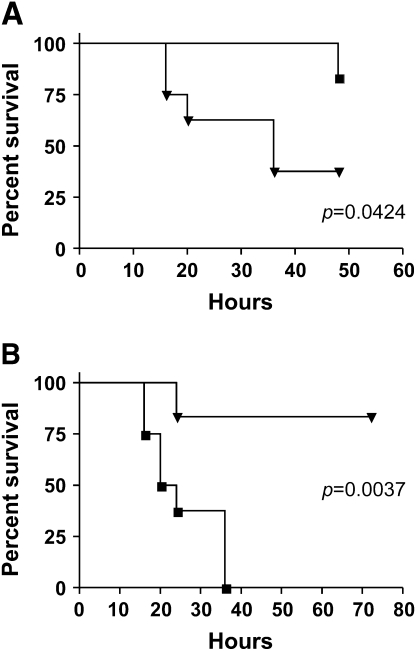
To evaluate the effect of z-VAD-fmk on mortality from sepsis, we pretreated animals with z-VAD-fmk 1 h before generation of severe bacteremia. We found that inhibition of caspase activity significantly improved survival up to 48 h compared with severe bacteremia alone (Figure 7A). Although our data suggest that inhibiting apoptosis improves hepatic bacterial clearance by decreasing apoptosis of Kupffer cells, this only in part contributes to the increased survival. The decrease in organ injury seen with z-VAD-fmk is likely due to inhibition of apoptosis at the level of the individual tissues, and this likely contributes to the increase in survival.
Figure 7.
Caspase inhibition improves survival and Kupffer cell ablation decreases survival after bacteremia. (A) Pretreatment with z-VAD-fmk followed by infection with severe bacteremia resulted in decreased mortality compared with severe bacteremia alone out to 48 h by log-rank test (p < 0.0424). Squares, z-VAD-fmk + severe bacteremia; inverted triangles, severe bacteremia. (B) Pretreatment with GdCl3 followed by infection with mild bacteremia resulted in 100% mortality at 36 h. This is significantly increased compared with mild bacteremia alone by log-rank test (p < 0.0037). Squares, gadolinium + mild bacteremia; inverted triangles, mild bacteremia.
To further evaluate the role of hepatic bacterial clearance by Kupffer cells in survival from bacteremia, we pretreated animals with GdCl3 before the generation of mild bacteremia. We found that ablation of Kupffer cells followed by infection with the mild bacteremia model resulted in decreased survival compared with mild bacteremia alone (Figure 7B). This model resulted in decreased survival compared with the severe bacteremia model as well. These data provide evidence that hepatic bacterial clearance by Kupffer cells is a determinant of the outcome of bacteremia.
DISCUSSION
In this study, we directly evaluated in vivo hepatic bacterial clearance during bacteremia in mice with normal baseline liver function. We also determined whether severe bacteremia results in a loss of hepatic bacterial clearance. We found that hepatic bacterial clearance, manifested as a gradient of bacteria between the portal vein and right ventricle, is effective in mild bacteremia. These animals experience less organ injury and mortality. In contrast, late in severe bacteremia, there is a loss of hepatic bacterial clearance, manifested as equalization of bacterial loads between the portal vein and right ventricle. These animals have a prolonged hepatic inflammatory response, experience more liver and other organ injury, and have increased mortality. We validated the use of right ventricular blood to measure bacterial clearance by comparing bacterial loads in right ventricular blood with hepatic vein blood.
A potential mechanism of decreased hepatic bacterial clearance is apoptosis of Kupffer cells. To further test this, we pretreated animals with a nonspecific caspase inhibitor and found that inhibition of apoptosis resulted in a preservation of hepatic bacterial clearance and better control of the bacteremia in severe bacteremia. In contrast, ablation of Kupffer cells resulted in a loss of hepatic bacterial clearance, increased severity of infection, and increased mortality in the setting of an initially mild bacteremia. These findings strongly suggest that hepatic bacterial clearance is an important determinant of the outcome of bacteremia. To our knowledge, these are the first studies to evaluate bacterial clearance during the evolution of bacteremia and to relate these findings to the outcome of this infection.
We used a murine model of P. aeruginosa pneumonia with bacteremia. Animals treated with PA103 developed evidence of systemic inflammation manifested by cytokine production and evidence of hypothermia. Although this does not meet the strict criteria for sepsis (21, 22), it is similar to sepsis seen in other murine models (15). This model has limitations in that it is not an exact reflection of human sepsis. The liver injury seen in this model was more severe that than normally seen in humans. The inflammatory response in this model was greater than that seen in studies of human sepsis (23), although it was similar to other animal studies (24). A potential explanation for this difference is the early timing of the TNF-α peak, which may be missed in human sepsis. Nonetheless, this model is an effective way to study hepatic clearance of circulating bacteria and its effect on end-organ injury.
We used quantitative real-time PCR with primers specific for P. aeruginosa to determine bacterial load. PCR is more sensitive than standard plating techniques in that it can quantify exact numbers of bacteria and is able to detect low-level bacteremia (18, 25). In addition, sepsis is associated with an active cellular and humoral response, resulting in bacterial killing. Standard culture techniques, which rely on bacterial viability, may not represent the true bacterial load in the setting of a brisk bactericidal response (26). PCR also allowed us to detect and quantify differences in bacterial load between the portal vein and hepatic vein or right ventricle. Using primers specific for the bacteria responsible for the primary infection allowed us to evaluate hepatic bacterial clearance of the primary infection.
Our data indicate that there is loss of hepatic bacterial clearance during severe bacteremia. We also found that this could be prevented by the inhibition of caspase activity. This suggests that apoptosis of Kupffer cells may be an important mediator of decreased bacterial clearance in sepsis. This is consistent with a prior study showing that inhibition of caspase activity improved Kupffer cell function, as determined by phagocytosis of fluorescent latex particles (27). We recognize that z-VAD-fmk has effects that are not specific to the liver. Treatment with z-VAD-fmk has been shown to prevent cardiac injury (19, 20) and improve lymphocyte survival (28) during sepsis. Caspase inhibition has also been shown to improve overall survival in murine sepsis (29). It is likely that pretreatment with z-VAD-fmk in our study resulted in decreased apoptosis of other organs and that this likely played a role in improving survival.
To further investigate the importance of Kupffer cells in sepsis, we ablated Kupffer cells with GdCl3 before mild infection. Several studies have established the efficacy of GdCl3 in ablating Kupffer cells in mice (14, 30, 31). We found a loss of hepatic bacterial clearance at all time points. Furthermore, we found that, in the absence of Kupffer cells, infection with the mild bacteremia model resulted in the generation of severe bacteremia with earlier organ injury and increased mortality. Ablation of Kupffer cells with GdCl3 also resulted in decreased hepatic and serum TNF-α. This is similar to other studies showing decreased TNF-α with Kupffer cell ablation (32–34) and suggests that Kupffer cells are an important source of TNF-α during sepsis. We found significantly increased mortality when Kupffer cells were ablated. This is consistent with a prior study showing increased mortality after cecal ligation and puncture in the setting of Kupffer cell ablation (30). Ablation of Kupffer cells before infection resulted in increased organ injury and mortality despite lower TNF-α levels. This is consistent with a prior study showing that ablation of alveolar macrophages before P. aeruginosa pneumonia resulted in increased lung injury 48 h after infection (35).
Our data suggest that apoptosis of Kupffer cells may lead to loss of bacterial clearance during the evolution of sepsis. Several studies have evaluated the importance of apoptosis of immune cells in sepsis (36–38). Hotchkiss and colleagues have shown an increase in splenic lymphocyte apoptosis in a murine model of sepsis (39). They found that this was associated with increased mortality. Furthermore, they found that this could be prevented by caspase inhibition (28). Studies have also shown evidence of macrophage apoptosis during sepsis (37). Inhibition of apoptosis has been associated with improved outcomes in animal models of sepsis (37, 40–43).
There are several potential mechanisms that could lead to Kupffer cell apoptosis in sepsis. First, TNF-α has been implicated in apoptosis during sepsis (36, 38). It is well known that sustained elevations of TNF-α can induce apoptosis via caspase activation (38). Although we have not shown that sustained TNF-α caused impaired hepatic bacterial clearance, a notable difference between our two models is the prolonged hepatic TNF-α response seen in severe bacteremia. Second, it is possible that the circulating bacteria have a direct toxic effect on Kupffer cells. Pathogen-induced macrophage apoptosis has been extensively studied (44). The YopP exotoxin of Yersinia spp. has been shown to induce macrophage apoptosis via inhibition of nuclear factor-κB (45). P. aeruginosa has been shown to induce apoptosis in a macrophage cell line via the elaboration of type III secretions (46). It is possible that the loss of hepatic bacterial clearance in our model was due to a combination of bacterial toxins and prolonged inflammation. Whatever the mechanism, hepatic bacterial clearance is an important determinant of outcome in bacteremia.
Acknowledgments
Images courtesy of The University of Iowa, Central Microscopy Facilities.
Supported by a Veterans' Administration Merit Review grant and by National Institutes of Health grants HL-073967-02 and HL-077431-01 (G.W.H.).
Originally Published in Press as DOI: 10.1164/rccm.200509-1470OC on January 6, 2006
Conflict of Interest Statement: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Pittet D, Thievent B, Wenzel RP, Li N, Auckenthaler R, Suter PM. Bedside prediction of mortality from bacteremic sepsis: aA dynamic analysis of ICU patients. Am J Respir Crit Care Med 1996;153:684–693. [DOI] [PubMed] [Google Scholar]
- 2.Arii S, Imamura M. Physiological role of sinusoidal endothelial cells and Kupffer cells and their implication in the pathogenesis of liver injury. J Hepatobiliary Pancreat Surg 2000;7:40–48. [DOI] [PubMed] [Google Scholar]
- 3.Graudal N, Milman N, Kirkegaard E, Korner B, Thomsen AC. Bacteremia in cirrhosis of the liver. Liver 1986;6:297–301. [DOI] [PubMed] [Google Scholar]
- 4.Graudal N, Hubeck B, Bonde J, Thomsen AC. The prognostic significance of bacteremia in hepatic cirrhosis. Liver 1987;7:138–141. [DOI] [PubMed] [Google Scholar]
- 5.Rolando N, Harvey F, Brahm J, Philpott-Howard J, Alexander G, Gimson A, Casewell M, Fagan E, Williams R. Prospective study of bacterial infection in acute liver failure: an analysis of fifty patients. Hepatology 1990;11:49–53. [DOI] [PubMed] [Google Scholar]
- 6.Rimola A, Soto R, Bory F, Arroyo V, Piera C, Rodes J. Reticuloendothelial system phagocytic activity in cirrhosis and its relation to bacterial infections and prognosis. Hepatology 1984;4:53–58. [DOI] [PubMed] [Google Scholar]
- 7.Metersky ML, Ma A, Bratzler DW, Houck PM. Predicting bacteremia in patients with community-acquired pneumonia. Am J Respir Crit Care Med 2004;169:342–347. [DOI] [PubMed] [Google Scholar]
- 8.Kurahashi K, Kajikawa O, Sawa T, Ohara M, Gropper MA, Frank DW, Martin TR, Wiener-Kronish JP. Pathogenesis of septic shock in Pseudomonas aeruginosa pneumonia. J Clin Invest 1999;104:743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sadikot RT, Blackwell TS, Christman JW, Prince AS. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med 2005;171:1209–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashare A, Doerschug KC, Powers LS, Monick MM, Hunninghake GW. Anti-inflammatory activity in the liver is associated with decreased bacterial clearance and increased mortality. Proc Am Thorac Soc 2005;2:A40. [Google Scholar]
- 11.Ashare A, Yarovinsky T, Monick MM, Hunninghake GW. Severe sepsis is associated with an apoptosis-mediated decrease in hepatic bacterial clearance. Chest 2005;184:379S. [Google Scholar]
- 12.Frick AG, Joseph TD, Pang L, Rabe AM, St Geme JW III, Look DC. Haemophilus influenzae stimulates ICAM-1 expression on respiratory epithelial cells. J Immunol 2000;164:4185–4196. [DOI] [PubMed] [Google Scholar]
- 13.Humlicek AL, Pang L, Look DC. Modulation of airway inflammation and bacterial clearance by epithelial cell ICAM-1. Am J Physiol Lung Cell Mol Physiol 2004;287:L598–L607. [DOI] [PubMed] [Google Scholar]
- 14.Zhou W, Zhang Y, Hosch MS, Lang A, Zwacka RM, Engelhardt JF. Subcellular site of superoxide dismutase expression differentially controls AP-1 activity and injury in mouse liver following ischemia/reperfusion. Hepatology 2001;33:902–914. [DOI] [PubMed] [Google Scholar]
- 15.Ebong S, Call D, Nemzek J, Bolgos G, Newcomb D, Remick D. Immunopathologic alterations in murine models of sepsis of increasing severity. Infect Immun 1999;67:6603–6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bast DJ, Yue M, Chen X, Bell D, Dresser L, Saskin R, Mandell LA, Low DE, de Azavedo JC. Novel murine model of pneumococcal pneumonia: use of temperature as a measure of disease severity to compare the efficacies of moxifloxacin and levofloxacin. Antimicrob Agents Chemother 2004;48:3343–3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pirnay JP, De Vos D, Duinslaeger L, Reper P, Vandenvelde C, Cornelis P, Vanderkelen A. Quantitation of Pseudomonas aeruginosa in wound biopsy samples: from bacterial culture to rapid 'real-time' polymerase chain reaction. Crit Care 2000;4:255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashare A, Powers LS, Butler NS, Doerschug KC, Monick MM, Hunninghake GW. Anti-inflammatory response is associated with mortality and severity of infection in sepsis. Am J Physiol Lung Cell Mol Physiol 2005;288:L633–L640. [DOI] [PubMed] [Google Scholar]
- 19.Neviere R, Fauvel H, Chopin C, Formstecher P, Marchetti P. Caspase inhibition prevents cardiac dysfunction and heart apoptosis in a rat model of sepsis. Am J Respir Crit Care Med 2001;163:218–225. [DOI] [PubMed] [Google Scholar]
- 20.Fauvel H, Marchetti P, Chopin C, Formstecher P, Neviere R. Differential effects of caspase inhibitors on endotoxin-induced myocardial dysfunction and heart apoptosis. Am J Physiol Heart Circ Physiol 2001;280:H1608–H1614. [DOI] [PubMed] [Google Scholar]
- 21.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992;101:1644–1655. [DOI] [PubMed] [Google Scholar]
- 22.Alberti C, Brun-Buisson C, Chevret S, Antonelli M, Goodman SV, Martin C, Moreno R, Ochagavia AR, Palazzo M, Werdan K, et al. Systemic inflammatory response and progression to severe sepsis in critically ill infected patients. Am J Respir Crit Care Med 2005;171:461–468. [DOI] [PubMed] [Google Scholar]
- 23.De Freitas I, Fernandez-Somoza M, Essenfeld-Sekler E, Cardier JE. Serum levels of the apoptosis-associated molecules, tumor necrosis factor-alpha/tumor necrosis factor type-I receptor and Fas/FasL, in sepsis. Chest 2004;125:2238–2246. [DOI] [PubMed] [Google Scholar]
- 24.Yao L, Berman JW, Factor SM, Lowy FD. Correlation of histopathologic and bacteriologic changes with cytokine expression in an experimental murine model of bacteremic Staphylococcus aureus infection. Infect Immun 1997;65:3889–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cursons RT, Jeyerajah E, Sleigh JW. The use of polymerase chain reaction to detect septicemia in critically ill patients. Crit Care Med 1999;27:937–940. [DOI] [PubMed] [Google Scholar]
- 26.Aronson MD, Bor DH. Blood cultures. Ann Intern Med 1987;106:246–253. [DOI] [PubMed] [Google Scholar]
- 27.Wanner GA, Mica L, Wanner-Schmid E, Kolb SA, Hentze H, Trentz O, Ertel W. Inhibition of caspase activity prevents CD95-mediated hepatic microvascular perfusion failure and restores Kupffer cell clearance capacity. FASEB J 1999;13:1239–1248. [DOI] [PubMed] [Google Scholar]
- 28.Hotchkiss RS, Tinsley KW, Swanson PE, Chang KC, Cobb JP, Buchman TG, Korsmeyer SJ, Karl IE. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc Natl Acad Sci USA 1999;96:14541–14546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hotchkiss RS, Chang KC, Swanson PE, Tinsley KW, Hui JJ, Klender P, Xanthoudakis S, Roy S, Black C, Grimm E, et al. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat Immunol 2000;1:496–501. [DOI] [PubMed] [Google Scholar]
- 30.Callery MP, Kamei T, Flye MW. Kupffer cell blockade increases mortality during intra-abdominal sepsis despite improving systemic immunity. Arch Surg 1990;125:36–40 (discussion: 40–41). [DOI] [PubMed] [Google Scholar]
- 31.Kinoshita M, Uchida T, Nakashima H, Ono S, Seki S, Hiraide H. Opposite effects of enhanced tumor necrosis factor-alpha production from Kupffer cells by gadolinium chloride on liver injury/mortality in endotoxemia of normal and partially hepatectomized mice. Shock 2005;23:65–72. [DOI] [PubMed] [Google Scholar]
- 32.Fukuda M, Yokoyama H, Mizukami T, Ohgo H, Okamura Y, Kamegaya Y, Horie Y, Kato S, Ishii H. Kupffer cell depletion attenuates superoxide anion release into the hepatic sinusoids after lipopolysaccharide treatment. J Gastroenterol Hepatol 2004;19:1155–1162. [DOI] [PubMed] [Google Scholar]
- 33.Lee CM, Yeoh GC, Olynyk JK. Differential effects of gadolinium chloride on Kupffer cells in vivo and in vitro. Int J Biochem Cell Biol 2004;36:481–488. [DOI] [PubMed] [Google Scholar]
- 34.Keller SA, Paxian M, Lee SM, Clemens MG, Huynh T. Kupffer cell ablation attenuates cyclooxygenase-2 expression after trauma and sepsis. J Surg Res 2005;124:126–133. [DOI] [PubMed] [Google Scholar]
- 35.Kooguchi K, Hashimoto S, Kobayashi A, Kitamura Y, Kudoh I, Wiener-Kronish J, Sawa T. Role of alveolar macrophages in initiation and regulation of inflammation in Pseudomonas aeruginosa pneumonia. Infect Immun 1998;66:3164–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Power C, Fanning N, Redmond HP. Cellular apoptosis and organ injury in sepsis: a review. Shock 2002;18:197–211. [DOI] [PubMed] [Google Scholar]
- 37.Wesche DE, Lomas-Neira JL, Perl M, Chung CS, Ayala A. Leukocyte apoptosis and its significance in sepsis and shock. J Leukoc Biol 2005;78:325–337. [DOI] [PubMed] [Google Scholar]
- 38.Oberholzer C, Oberholzer A, Clare-Salzler M, Moldawer LL. Apoptosis in sepsis: a new target for therapeutic exploration. FASEB J 2001;15:879–892. [DOI] [PubMed] [Google Scholar]
- 39.Hotchkiss RS, Swanson PE, Cobb JP, Jacobson A, Buchman TG, Karl IE. Apoptosis in lymphoid and parenchymal cells during sepsis: findings in normal and T- and B-cell-deficient mice. Crit Care Med 1997;25:1298–1307. [DOI] [PubMed] [Google Scholar]
- 40.Hotchkiss RS, Coopersmith CM, Karl IE. Prevention of lymphocyte apoptosis: a potential treatment of sepsis? Clin Infect Dis 2005;41:S465–S469. [DOI] [PubMed] [Google Scholar]
- 41.Wesche-Soldato DE, Chung CS, Lomas-Neira J, Doughty LA, Gregory SH, Ayala A. In vivo delivery of caspase-8 or Fas siRNA improves the survival of septic mice. Blood 2005;106:2295–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bommhardt U, Chang KC, Swanson PE, Wagner TH, Tinsley KW, Karl IE, Hotchkiss RS. Akt decreases lymphocyte apoptosis and improves survival in sepsis. J Immunol 2004;172:7583–7591. [DOI] [PubMed] [Google Scholar]
- 43.Weaver JG, Rouse MS, Steckelberg JM, Badley AD. Improved survival in experimental sepsis with an orally administered inhibitor of apoptosis. FASEB J 2004;18:1185–1191. [DOI] [PubMed] [Google Scholar]
- 44.Navarre WW, Zychlinsky A. Pathogen-induced apoptosis of macrophages: a common end for different pathogenic strategies. Cell Microbiol 2000;2:265–273. [DOI] [PubMed] [Google Scholar]
- 45.Ruckdeschel K, Mannel O, Schrottner P. Divergence of apoptosis-inducing and preventing signals in bacteria-faced macrophages through myeloid differentiation factor 88 and IL-1 receptor-associated kinase members. J Immunol 2002;168:4601–4611. [DOI] [PubMed] [Google Scholar]
- 46.Hauser AR, Engel JN. Pseudomonas aeruginosa induces type-III-secretion-mediated apoptosis of macrophages and epithelial cells. Infect Immun 1999;67:5530–5537. [DOI] [PMC free article] [PubMed] [Google Scholar]