Abstract
Rationale: A large body of epidemiologic literature has found an association of increased fine particulate air pollution (PM2.5) with acute and chronic mortality. The effect of improvements in particle exposure is less clear.
Objectives: Earlier analysis of the Harvard Six Cities adult cohort study showed an association between long-term ambient PM2.5 and mortality between enrollment in the mid-1970s and follow-up until 1990. We extended mortality follow-up for 8 yr in a period of reduced air pollution concentrations.
Methods: Annual city-specific PM2.5 concentrations were measured between 1979 and 1988, and estimated for later years from publicly available data. Exposure was defined as (1) city-specific mean PM2.5 during the two follow-up periods, (2) mean PM2.5 in the first period and change between these periods, (3) overall mean PM2.5 across the entire follow-up, and (4) year-specific mean PM2.5. Mortality rate ratios were estimated with Cox proportional hazards regression controlling for individual risk factors.
Measurements and Main Results: We found an increase in overall mortality associated with each 10 μg/m3 increase in PM2.5 modeled either as the overall mean (rate ratio [RR], 1.16; 95% confidence interval [CI], 1.07–1.26) or as exposure in the year of death (RR, 1.14; 95% CI, 1.06–1.22). PM2.5 exposure was associated with lung cancer (RR, 1.27; 95% CI, 0.96–1.69) and cardiovascular deaths (RR, 1.28; 95% CI, 1.13–1.44). Improved overall mortality was associated with decreased mean PM2.5 (10 μg/m3) between periods (RR, 0.73; 95% CI, 0.57–0.95).
Conclusion: Total, cardiovascular, and lung cancer mortality were each positively associated with ambient PM2.5 concentrations. Reduced PM2.5 concentrations were associated with reduced mortality risk.
Keywords: air pollution, cohort studies, follow-up studies, mortality
An extensive epidemiologic literature has documented an association of fine particulate air pollution with mortality (1, 2). Most of this research consists of time-series studies of the effects of particle exposures experienced in the few days before death. The estimated effect of particulate air pollution has been shown to increase as longer exposure periods (up to 7 wk) are considered, indicating exposures in the month(s) before death may be important (3–6). Cohort studies have associated mortality with mean particulate air pollution concentrations over much longer periods. Three follow-up cohort studies in the United States (7–10) and a recent pilot study from Europe (11) evaluated the effects of long-term average ambient concentrations of fine particles and other air pollutants over many years. These cohort studies used annual or multiyear average pollution concentrations as the exposure index, but did not examine the time periods responsible for the observed association. Cohort studies with follow-up during periods of substantial change in air pollution can address this question. The linkage between improvements in air quality and improved health outcomes is of considerable public health interest.
A small number of studies have assessed the effect of reductions in air pollution on mortality. Mortality in Utah Valley decreased by 3% when average particulate air pollution (PM10) concentrations decreased by 15 μg/m3 as the result of a 13-mo strike at a local steel mill (12). Mortality in Dublin decreased by 8% after a 36-μg/m3 decrease in average particulate air pollution (black smoke) due to a ban on coal sales (13). Restrictions on the sulfur content of fuel oil in Hong Kong resulted in a 45% average reduction in SO2, and the average annual trend in deaths from all causes declined 2% and from respiratory causes declined 3.9% (14). In these studies, improvements in mortality were observed in the months after well-defined improvements in ambient air quality.
Dockery and colleagues (7) evaluated the effects of long-term pollution exposure on survival of adults participating in the Harvard Six Cities Study monitored for 14 to 16 yr during the 1970s and 1980s. Exposure to particulate matter smaller than 2.5 μm in aerodynamic diameter (PM2.5) was defined by the city-specific average during follow-up, ignoring the year-to-year fluctuations. The mortality rate ratio (RR) was 1.13 (95% confidence interval [CI], 1.04–1.73) for each 10-μg/m3 increase in city-specific PM2.5 concentrations. During follow-up, PM2.5 concentrations dropped in all cities, although the rank ordering of cities was unchanged. Evaluation of time-varying PM2.5 during this period showed slightly attenuated relative risk compared with estimates based on the average PM2.5 over the entire period (15).
In this analysis, we extended the follow-up period through 1998. We evaluated the robustness of the previous findings with additional years of follow-up and examined the extent to which changes in PM2.5 concentrations explain changes in mortality. Some of the results of this study have been previously reported in the form of an abstract (16, 17).
METHODS
Study Population and Follow-up
The study population consisted of 8,096 white participants residing in the following cities: Watertown, MA; Kingston and Harriman, TN; St. Louis, MO; Steubenville, OH; Portage, Wyocena, and Pardeeville, WI; and Topeka, KS. Participants were recruited between 1974 and 1977. The population and study design have been described previously (7), and additional details are provided in the online supplement. Date and cause of death were determined by searching the National Death Index for calendar years 1979 to 1998. Deaths between 1974 and 1978 were identified from next-of-kin reports and Social Security records (7). Survival times were calculated as death date (or December 31, 1998, for surviving participants) minus enrollment date.
Air Pollution Concentrations
Each participant's air pollution concentration was defined by city-specific mean concentrations of PM2.5. During the original Six Cities follow-up (1979–1987), daily ambient PM2.5 concentrations were measured at a centrally located air-monitoring station in each community (18). We estimated daily PM2.5 concentrations after the shutdown of the Six Cities monitoring (1985–1998) using city-specific regression equations based on extinction coefficient (humidity-corrected visibility data from local airports) (19), routinely collected PM10 concentrations (Environmental Protection Agency Aerometric Information Retrieval System [AIRS]) from representative monitors within 80 km, and indicators for season. (More details on the monitors selected and exposure metrics are provided in the online supplement) City-specific annual mean PM2.5 was calculated as the average of the quarterly mean of the estimated seasonal values. The Pearson correlation (r) between the estimated and observed annual mean PM2.5 from the Six Cities monitors during the years when both were available (1985–1987) was 0.93.
Statistical Analysis
We estimated mortality rate ratios associated with PM2.5 by Cox proportional hazards regression models (20), controlling for baseline individual risk factors and potential confounders. Time on study was measured by calendar date. Subjects were stratified by sex and 1-yr age groups, such that each sex/age group had its own baseline hazard. We controlled for current or former smoking, number of pack-years of smoking for former and current smokers separately, education, and body mass index (linear and squared terms). We first modeled exposure using period-specific (1974–1989 vs. 1990–1998) indicators for city. The end date of the original National Death Index search (1,989) was chosen as the cut-off. (Note that the Dockery and colleagues analysis [7] included several months of follow-up in 1990, which we have assigned to Period 2.) Portage, the city with the lowest PM2.5 levels, was the reference. To adjust for temporal trends in mortality, we included an indicator for period. We then assessed the association of mortality with average city-specific PM2.5 for the entire period of follow-up (pollution averaged from 1980–1998) and with the period-specific average PM2.5. We tested for a difference in association between the two periods with an interaction term (period by PM2.5) in the model. To evaluate the effect of change in mean PM2.5 between the two periods, we estimated the associations of period-specific mortality by including both the mean PM2.5 in Period 1 (1980–1985) and the change in mean PM2.5 between Period 1 and Period 2 (Period 2 [1990–1998] minus Period 1) in the model simultaneously. Finally, we treated city-specific yearly mean PM2.5 levels as a time-varying exposure variable to evaluate the effect of particle exposures in the year of death. All analyses were performed using SAS software (version 8; SAS Institute, Cary, NC).
RESULTS
Characteristics of the Dataset
The cohort has been described in detail elsewhere (7). In brief, the average age of participants at the beginning of the study was 50 yr (range, 25–74 yr) and 55% were female. Average body mass index was 25.8 kg/m2 (standard deviation, 4.5). Current smoking on enrollment ranged from 33% in Topeka to 40% in Watertown, and former smoking ranged from 21% in Harriman to 25% in both Topeka and Watertown. Education varied between cities; 12% of participants in Topeka and 45% in St. Louis had less than a high school education.
There were 104,243 person-years of follow-up and 1,364 deaths between 1974 and 1989 (Period 1) and an additional 54,735 person-years of follow-up and 1,368 deaths between 1990 and 1998 (Period 2; Table 1). The overall death rate was 13.1 deaths per 1,000 person-years in Period 1 and 25.0 in Period 2, reflecting the aging of this cohort. As in previous analyses, crude mortality rates were highest in Steubenville and St. Louis (Table 1).
TABLE 1.
NUMBER OF PERSON-YEARS OF FOLLOW-UP AND TOTAL DEATHS IN SIX CITIES: PERIOD 1 (1974–1989 FOLLOW-UP) AND PERIOD 2 (1990–1998 FOLLOW-UP)
| Characteristics | Portage | Topeka | Watertown | Harriman | St. Louis | Steubenville | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. participants | 1,630 | 1,238 | 1,332 | 1,258 | 1,292 | 1,346 | ||||||
| Period 1* (1,364 deaths; 104,243 person-yr) | ||||||||||||
| Person-yr | 20,224 | 14,967 | 18,640 | 16,991 | 16,572 | 16,849 | ||||||
| No. deaths | 212 | 149 | 238 | 219 | 267 | 279 | ||||||
| Deaths/1,000 person-yr | 10.5 | 10.0 | 12.8 | 12.9 | 16.1 | 16.6 | ||||||
| Average PM2.5 (μg/m3) | 11.4 | 12.4 | 15.4 | 20.9 | 19.2 | 29.0 | ||||||
| Period 2 (1,368 deaths; 54,735 person-yr) | ||||||||||||
| Person-yr | 11,658 | 9,062 | 8,979 | 8,363 | 8,172 | 8,501 | ||||||
| No. deaths | 264 | 184 | 194 | 229 | 251 | 246 | ||||||
| Deaths/1,000 person-yr | 22.6 | 20.3 | 21.6 | 27.4 | 30.7 | 28.9 | ||||||
| Average PM2.5, μg/m3 | 10.2 | 13.1 | 12.1 | 18.1 | 13.4 | 22.0 | ||||||
Period 1 is restricted to 1,974 through 1989, whereas the original Dockery and colleagues (7) analysis included person-years of follow-up through June 1991 for a total of 111,076 person-years and 1,430 deaths. In Period 1, average PM2.5 (μg/m3) is the mean concentration in 1980–1985, the years when there are monitoring data for all cities (18). In Period 2, average PM2.5 is the mean concentrations of the estimated PM2.5 in 1990–1998.
Trends in PM2.5 Concentrations
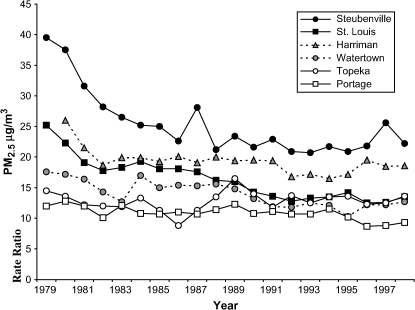
Annual mean PM2.5 concentrations decreased during the study period in all cities (Figure 1) but most dramatically in the dirtiest cities. Fitting a straight line to the annual means, PM2.5 declined on average 7 μg/m3 per decade in Steubenville, 5 μg/m3 in St. Louis, 3 μg/m3 in Watertown, 2 μg/m3 in Harriman, 1 μg/m3 in Portage, and less than 1 μg/m3 in Topeka.
Figure 1.
Annual average concentrations of PM2.5 in the Harvard Six Cities Study. (Six Cities monitoring data for available years 1980–1988 and PM2.5 estimated from Aerometric Information Retrieval System and extinction data for years where Six Cities data were not available.)
Association of PM2.5 with Mortality
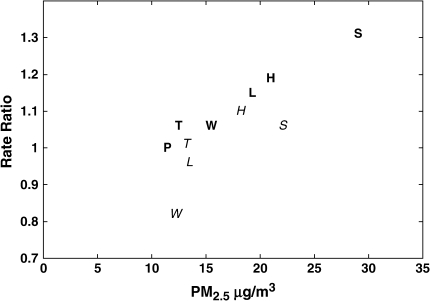
We calculated city-specific adjusted all-cause mortality rate ratios for Period 1, Period 2, and the complete period of follow-up compared with Portage (Table 2). City-specific rate ratios decreased with decreasing PM2.5 (Figure 2). Similar results were found for cardiovascular mortality (see online supplement).
TABLE 2.
ADJUSTED TOTAL MORTALITY RATE RATIOS AND 95% CONFIDENCE INTERVALS ESTIMATED FROM COX PROPORTIONAL HAZARDS MODEL FOR EACH FOLLOW-UP PERIOD (1974–1989 AND 1990–1998) AND THE COMPLETE FOLLOW-UP (1974–1998)
| Period 1 | Period 2 | Complete | |
|---|---|---|---|
| Person-Yr of Follow-up | 104,243 | 54,735 | 158,978 |
| Deaths | 1,364 | 1,368 | 2,732 |
| RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| City-specific model* | |||
| Portage | 1.00 | 1.00 | |
| Topeka | 1.06 (0.86–1.31) | 1.01 (0.83–1.22) | 1.03 (0.89–1.19) |
| Watertown | 1.06 (0.87–1.28) | 0.82 (0.67–1.00) | 0.95 (0.83–1.08) |
| Harriman | 1.19 (0.98–1.44) | 1.10 (0.91–1.33) | 1.15 (1.01–1.32) |
| St. Louis | 1.15 (0.96–1.38) | 0.96 (0.80–1.15) | 1.05 (0.93–1.20) |
| Steubenville | 1.31 (1.10–1.57) | 1.06 (0.89–1.27) | 1.18 (1.04–1.34) |
| Period | 1.00 | 0.97 (0.70–1.35) |
Definition of abbreviations: CI = confidence interval; RR = rate ratio.
Rate ratios have been adjusted for age in 1-yr categories, sex, current smoker, current pack-years of smoking, former smoker, former pack-years of smoking, less than high school education, and linear and quadratic terms for body mass index.
City-specific rate ratios are all expressed in relation to Portage.
Figure 2.
Estimated adjusted rate ratios for total mortality and PM2.5 levels in the Six Cities Study by period. P denotes Portage, WI (reference for both periods); T = Topeka, KS; W = Watertown, MA; L = St. Louis, MO; H = Harriman, TN; S = Steubenville, OH. A term for Period 1 (1 if Period 2, 0 if Period 1) was included in the model. Bold letters represent Period 1 (1974–1989) and italicized letters represent Period 2 (1990–1998). In Period 1, PM2.5 (μg/m3) is defined as the mean concentration during 1980–1985, the years where there are monitoring data for all cities (18). In Period 2, PM2.5 is defined as the mean concentrations of the estimated PM2.5 in 1990–1998.
The effect of each 10-μg/m3 increase in average annual PM2.5 pollution was comparable in Period 1 (RR, 1.17; 95% CI, 1.08–1.26; p = 0.0001) and Period 2 (RR, 1.13; 95% CI, 1.01–1.27; p = 0.03, interaction p = 0.82). Controlling for exposure in Period 1, each 10-μg/m3 reduction in Period 2 mean PM2.5 was associated with a reduction in risk (RR, 0.73; 95% CI, 0.57–0.95; p = 0.019; Table 3). We found an increased risk of total mortality associated with each 10-μg/m3 increase in average PM2.5 over the entire follow-up period (RR, 1.16; 95% CI, 1.07–1.26; p = 0.0004; Table 3). We found essentially the same association of total mortality with the annual mean PM2.5 level in the year of death (RR, 1.14; 95% CI, 1.06–1.22; p = 0.0003). These results were not substantially changed in sensitivity analyses, removing one city at a time from the analysis (data not shown).
TABLE 3.
ADJUSTED PROPORTIONAL HAZARD MORTALITY RATE RATIOS AND 95% CONFIDENCE INTERVALS FOR A 10-μg/m3 INCREASE IN AVERAGE AMBIENT PM2.5 OVER THE ENTIRE FOLLOW-UP (1974–1998) AND THE RATE RATIOS FOR AVERAGE PM2.5 IN PERIOD 1 AND THE DECREASE IN LEVELS BETWEEN THE TWO PERIODS
| RR (95% CI)
|
||||
|---|---|---|---|---|
| Model 1
|
Model 2
|
|||
| Cases | Entire Follow-Up Average PM2.5† | Period 1 Average PM2.5‡ | Decrease in Average PM2.5‡ | |
| Total mortality | 2,732 | 1.16 (1.07–1.26) | 1.18 (1.09–1.27) | 0.73 (0.57–0.95) |
| Cardiovascular* | 1,196 | 1.28 (1.13–1.44) | 1.28 (1.14–1.43) | 0.69 (0.46–1.01) |
| Respiratory* | 195 | 1.08 (0.79–1.49) | 1.21 (0.89–1.66) | 0.43 (0.16–1.13) |
| Lung cancer* | 226 | 1.27 (0.96–1.69) | 1.20 (0.91–1.58) | 1.06 (0.43–2.62) |
| Other | 1,115 | 1.02 (0.90–1.17) | 1.05 (0.93–1.19) | 0.85 (0.56–1.27) |
For definition of abbreviations, see Table 2.
Rate ratios have been adjusted for age in 1-yr categories, sex, current smoker, current pack-years of smoking, former smoker, former pack-years of smoking, less than high school education, and linear and quadratic terms for body mass index.
Cardiovascular disease (International Classification of Disease, 9th edition [ICD-9] codes 400–440); nonmalignant respiratory disease (ICD-9 codes 485–496); lung cancer (ICD-9 code 162).
Average PM2.5 calculated as the average of Six Cities monitoring data for available years 1980–1988 and PM2.5 estimated from Aerometric Information Retrieval System and extinction data for years where Six Cities data were not available.
Average PM2.5 in Period 1 calculated as the average from 1980–1985, the years where there are monitoring data for all cities, decrease in average PM2.5 (average Period 2 (1990–1998) − average Period 1).
Cardiovascular mortality was positively associated with average PM2.5 over the entire follow-up (p < 0.0001; Table 3). We found lung cancer mortality positively associated with average PM2.5 (p = 0.10; Table 3). Respiratory mortality also was positively associated with average PM2.5 (Table 3), but the association was not statistically significant (p = 0.63). There was no association (p = 0.71) with other causes of death (Table 3). There were stronger reductions in cardiovascular and respiratory mortality risk with each 10-μg/m3 improvement in city-specific mean PM2.5 in Period 2 compared with Period 1 (Table 3), but little evidence of reductions in lung cancer risk (Table 3).
DISCUSSION
With approximately 50% more person-years of follow-up and twice the number of deaths compared with the original Six Cities chronic mortality air pollution analysis (7), we observed significant associations of fine particulate air pollution with mortality. More importantly, we were able to evaluate the effect of changing average ambient PM2.5 concentrations since the original follow-up. Covariate adjusted mortality rates declined between 1974 and 1989 (Period 1) and 1990 and 1998 (Period 2), consistent with the general increase in adult life expectancy in the United States. However, the drop in the adjusted mortality rate was largest in the cities with the largest reductions in PM2.5 after controlling for such a period effect. The proportional hazards rate ratio for a 10-μg/m3 increase in PM2.5 was comparable in both of these periods. However, we found a reduction in risk: 0.73 for each 10-μg/m3 decrease in mean PM2.5 between periods. This reduction was observed specifically for deaths due to cardiovascular and respiratory disease and not from lung cancer, a disease with a longer latency period and less reversibility. These findings suggest that the mortality effects of long-term air pollution may be at least partially reversible over periods of a decade.
We found equivalent, statistically significant increased risk in overall mortality associated with each 10-μg/m3 increase in PM2.5 modeled either as average over the entire follow-up (RR, 1.16; 95% CI, 1.07–1.26) or as average in the year of death (RR, 1.14; 95% CI, 1.06–1.22). These findings also suggest that mortality effects may be partially reversible, but over time periods possibly as short as a year.
Exposure to PM2.5 was statistically significantly associated with deaths due to cardiovascular disease, and the association with lung cancer mortality was of borderline significance. The number of nonmalignant respiratory deaths was small (although comparable to numbers for lung cancer), but the PM2.5-associated risk was positive, although weak.
Chronic exposure studies have observed increased mortality rates associated with PM. However, the evidence is limited mainly to the Harvard Six Cities Study and three other studies. The American Cancer Society Study, a cohort of 552,138 adults with 7 yr of follow-up, assessed risk for 151 U.S. metropolitan statistical areas (8). With an additional 9 yr of follow-up, statistically significant elevations in risk associated with PM2.5 were observed for all-cause, lung cancer, and cardiopulmonary mortality (10). In analyses of cause-specific mortality, each 10-μg/m3 increase in PM2.5 was associated with 8 to 18% increases in cardiovascular mortality, but only weak associations were found with nonmalignant respiratory deaths (21). In the Adventist Health Study of Smog, a 15-yr follow-up of 6,338 nonsmoking Californians, Abbey and coworkers found mean PM10 associated with increased lung cancer mortality in men and women, and nonsignificantly increased all-cause and cardiopulmonary mortality in men (9). A pilot prospective study of 4,466 participants monitored for 8 yr in the Netherlands concluded that long-term exposure to traffic-related particulate air pollution measured by black smoke was associated with increased all-cause mortality (11).
Although a large body of literature has shown associations between particulate air pollution and mortality, the relative contributions of acute and chronic exposures are not known. Effect estimates from prospective studies are substantially greater than those indicated by daily time-series studies (22). The majority of this difference may be explained by expanding the exposure period from days to months. Two independent studies have assessed the mortality effects over 40 d rather than 1 or 2 d after particle exposure. In both studies, the extended PM effects for periods of up to 6 wk were at least twice the short-term effects (3, 5). Schwartz showed in a time-series study in Boston that moving the time scale from days to months (i.e., 60 d) increased the estimated PM effect and captured approximately half the difference between the time-series and long-term cohort studies (4). He concluded that decades of exposure are not required to develop most of the risk increase seen in cohort studies. Our results show that PM-associated mortality decreased in the decade of the 1990s compared with the mid-1970s and 1980s, consistent with the decrease in ambient PM2.5 concentrations. Furthermore, the similarity of effect for the annual air pollution metric compared with the mean over the study period (1980–1998) suggests that air pollution during the last year may be important. At least part of the PM2.5-associated mortality may be reversible, suggesting ambient PM2.5 is likely associated with exacerbation of existing disease. However, there also appears to be a second independent effect that could be described as development of chronic disease.
Our ability to assess the appropriate time scale is limited because, although PM2.5 levels declined, the ranking of cities did not change substantially over most of the study period. However, the largest improvements in PM2.5 concentrations were in cities with the highest initial concentrations. There was also some variation in city-specific annual mean PM2.5 concentrations. We did not examine time periods shorter than 1 yr in this analysis.
The original Six Cities Study mortality analysis has undergone an extensive reanalysis performed by an independent group of researchers (23). The original data were validated, the original findings reproduced, and these estimates were found to be robust to alternative models and to inclusion of other posited city-specific confounders. Alternative metrics of PM2.5 were not found to alter risk of all-cause mortality during the original period of follow-up (15).
Cardiovascular mortality rates have decreased in the United States over the course of this study (24). However, this improvement in cardiovascular mortality should affect all cities, and should not be larger in cities with the greatest improvement in PM2.5 concentrations. Moreover, PM2.5 concentrations fluctuated year to year, including increases as well as decreases from the preceding year. Yet, using PM2.5 as a time-varying covariate did not noticeably change the association. Thus, long-term secular trends are unlikely to explain our results.
This analysis lacked continuous monitoring of PM2.5 levels during the extended follow-up period. Six Cities monitoring of air pollutants ended in 1987 in most cities. The AIRS monitoring network began collecting PM10 data in 1985. PM2.5 measurements did not start until 1999, and even then did not include monitoring in all of the Six Cities or in the original monitoring sites. Therefore, Period 2 is completely dependent on estimated PM2.5 levels. We estimated the levels and patterns of PM2.5 during the missing years using city-specific regression of the original Six Cities PM2.5 measurements against the relative humidity–adjusted extinction coefficients from nearby airports and routine PM10 measurements from multiple nearby monitors. We assumed that the local change in PM2.5 would follow the local PM10 and extinction coefficient measurements, and that differences due to siting of the monitors and methodologies would have remained constant. Differences in measurement techniques and measurement locations preclude comparisons with current observations. Estimating the pattern of PM2.5 over time using the actual measured PM10 and extinction data has its limitations, but it is likely to be closer to reality than extrapolating levels beyond the available sampling data, as has been done previously (15).
Follow-up information on individual risk factors was available during the course of the first 12 yr of follow-up. Three follow-up questionnaires were administered to the participants. There was no updated information available on individual risk factors or residence during the extended period of follow-up. In the original study, baseline characteristics were used to control for confounding factors (7). Although these factors were significantly associated with mortality, they did not substantially confound the relationship with air pollution. In the reanalysis, Krewski and colleagues (23) evaluated the effect of updating smoking status and body mass index during the course of the original study. They restricted the study population to the 81.5% of the people who did not move from their original cities at any time during the study period. These alternative analyses did not change the conclusions about the association of air pollution and mortality. Therefore, we elected to use baseline characteristics in this analysis. We acknowledge that this modeling choice may lead to misclassification of confounders such as smoking status and body mass index, and that the associations of these factors and air pollution may have changed. For example, trends in smoking cessation are different in different parts of the country (25). Although these factors were significantly associated with mortality, they did not substantially confound the relationship with air pollution. In addition, censoring movers as defined in Krewski and colleagues' analysis (23) at the start of the continued follow-up or excluding all movers from the analysis did not change our results (data not presented). A limitation of this analysis is that individual level covariates were not available for this population in the second period of follow-up.
In this extended follow-up during a time of air pollution reductions, we had a unique opportunity to assess the effect of recent versus past exposures. City-specific average PM2.5 levels were lower in the extended follow-up during the 1990s than in the first follow-up (1974–1989) and mortality risk ratios in this period also were lower. This suggests that the PM2.5-associated mortality in this 25-yr follow-up was at least in part reversible.
Supplementary Material
Acknowledgments
The authors thank William Cormack Ramsey, Elizabeth Solomon, and Martha Fay for their work in tracking participants and Jaime Hart and Allan Heff for their help with manuscript preparation.
Supported by grants from the U.S. Environmental Protection Agency (EPA; R827353) and the National Institute of Environmental Health Sciences (ES00002).
This analysis has not been formally reviewed by EPA; the views expressed are solely those of the authors, and no official EPA endorsement should be inferred.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200503-443OC on January 19, 2006
Conflict of Interest Statement: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Pope CA III, Dockery DW. Epidemiology of particle effects. In: Holgate ST, Samet JM, Koren HS, Maynard RL, editors. Air pollution and health. San Diego, CA: Academic Press; 1999. pp. 673–706.
- 2.U.S. Environmental Protection Agency. Air quality criteria for particulate matter. Washington, DC: U.S. Environmental Protection Agency, Office of Research and Development; 1996. Publication No. EPA/600/P-95/001cF.
- 3.Goodman PG, Dockery DW, Clancy L. Cause-specific mortality and the extended effects of particulate pollution and temperature exposure. Environ Health Perspect 2004;112:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz J. Harvesting and long term exposure effects in the relation between air pollution and mortality. Am J Epidemiol 2000;151:440–448. [DOI] [PubMed] [Google Scholar]
- 5.Zanobetti A, Schwartz J, Samoli E, Gryparis A, Touloumi G, Atkinson R, Le Tertre A, Bobros J, Celko M, Goren A, et al. The temporal pattern of mortality responses to air pollution: a multicity assessment of mortality displacement. Epidemiology 2002;13:87–93. [DOI] [PubMed] [Google Scholar]
- 6.Zeger SL, Dominici F, Samet J. Harvesting-resistant estimates of air pollution effects on mortality. Epidemiology 1999;10:171–175. [PubMed] [Google Scholar]
- 7.Dockery DW, Pope CA III, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG Jr, Speizer FE. An association between air pollution and mortality in six US cities. N Engl J Med 1993;329:1753–1759. [DOI] [PubMed] [Google Scholar]
- 8.Pope CA III, Thun MJ, Namboodiri MM, Dockery DW, Evans JS, Speizer FE, Heath CW Jr. Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. Am J Respir Crit Care Med 1995;151:669–674. [DOI] [PubMed] [Google Scholar]
- 9.Abbey DE, Nishino N, McDonnell WF, Burchette RJ, Knutsen SF, Lawrence Beeson W, Yang JX. Long-term inhalable particles and other air pollutants related to mortality in nonsmokers. Am J Respir Crit Care Med 1999;159:373–382. [DOI] [PubMed] [Google Scholar]
- 10.Pope CA III, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 2002;287:1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoek G, Brunekreef B, Goldbohm S, Fischer P, van den Brandt PA. Association between mortality and indicators of traffic-related air pollution in the Netherlands: a cohort study. Lancet 2002;360:1203–1209. [DOI] [PubMed] [Google Scholar]
- 12.Pope CA III, Schwartz J, Ransom M. Daily mortality and PM10 pollution in Utah Valley. Arch Environ Health 1992;42:211–217. [DOI] [PubMed] [Google Scholar]
- 13.Clancy L, Goodman P, Sinclair H, Dockery DW. Effect of air-pollution control on death rates in Dublin, Ireland: an intervention study. Lancet 2002;360:1210–1214. [DOI] [PubMed] [Google Scholar]
- 14.Hedley AJ, Wong CM, Thach TQ, Ma S, Lam TH, Anderson HR. Cardiorespiratory and all-cause mortality after restrictions on sulphur content of fuel in Hong Kong: an intervention study. Lancet 2002;360:1646–1652. [DOI] [PubMed] [Google Scholar]
- 15.Villeneuve PJ, Goldberg MS, Krewski D, Burnett RT, Chen Y. Fine particulate air pollution and all-cause mortality within the Harvard Six-Cities Study: variations in risk by period of exposure. Ann Epidemiol 2002;12:568–576. [DOI] [PubMed] [Google Scholar]
- 16.Laden F, Schwartz J, Speizer FE, Dockery DW. Air pollution and mortality: a continued follow-up in the Harvard Six Cities Study. Epidemiology 2001;12:S81. [Google Scholar]
- 17.Laden F, Schwartz J, Speizer FE, Dockery DW. Continued follow-up of air pollution and mortality in the Harvard Six Cities Study. Health Effects Institute Annual Conference, Health Effects Institute, Washington, DC, 2001.
- 18.Schwartz J, Dockery DW, Neas LM. Is daily mortality associated specifically with fine particles? J Air Waste Manage Assoc 1996;46:927–939. [PubMed] [Google Scholar]
- 19.Abbey DE, Ostro BE, Fraser G, Vancuren T, Burchette RJ. Estimating fine particulates less than 2.5 microns in aerodynamic diameter (PM2.5) from airport visibility data in California. J Expo Anal Environ Epidemiol 1995;5:161–180. [PubMed] [Google Scholar]
- 20.Cox DR. Regression models and life-tables. J Roy Stat Soc (B) 1972;34:187–220. [Google Scholar]
- 21.Pope CA III, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation 2004;109:71–77. [DOI] [PubMed] [Google Scholar]
- 22.Kunzli N, Medina S, Kaiser R, Quenel P, Horak F Jr, Studnicka M. Assessment of deaths attributable to air pollution: should we use risk estimates based on time series or on cohort studies? Am J Epidemiol 2001;153:1050–1055. [DOI] [PubMed] [Google Scholar]
- 23.Krewski D, Burnett RT, Goldberg MS, Hoover K, Siemiatycki J, Jerrett M, Abrahamowicz M, White WH. Reanalysis of the Harvard Six Cities Study and the American Cancer Society Study of particulate air pollution and mortality: a special report of the institute's Particle Epidemiology Reanalysis Project. Cambridge, MA: Health Effects Institute; 2000.
- 24.Davis DL, Dinse GE, Hoel DG. Decreasing cardiovascular disease and increasing cancer among whites in the United States from 1973 through 1987: good news and bad news. JAMA 1994;271:431–437. [PubMed] [Google Scholar]
- 25.National Center for Chronic Disease Prevention and Health Promotion [Internet]. STATE system http://apps.nccd.cdc.gov/statesystem/ [accessed March 1, 2005]. Centers for Disease Control and Prevention; 2004.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.