Abstract
Rationale: Airway inflammation in asthma is accompanied by structural changes, including goblet cell metaplasia, smooth muscle cell layer thickening, and subepithelial fibrosis. This allergen-induced airway remodeling can be replicated in a mouse asthma model.
Objectives: The study goal was to determine whether established airway remodeling in a mouse asthma model is reversible by administration of the cysteinyl leukotriene (CysLT)1 receptor antagonist montelukast, the corticosteroid dexamethasone, or the combination montelukast + dexamethasone.
Methods: BALB/c mice, sensitized by intraperitoneal ovalbumin (OVA) as allergen, received intranasal OVA periodically Days 14–73 and montelukast or dexamethasone or placebo from Days 73–163.
Measurements and Main Results: Allergen-induced trafficking of eosinophils into the bronchoalveolar lavage fluid and lung interstitium and airway goblet cell metaplasia, smooth muscle cell layer thickening, and subepithelial fibrosis present on Day 73 persisted at Day 163, 3 mo after the last allergen challenge. Airway hyperreactivity to methacholine observed on Day 73 in OVA-treated mice was absent on Day 163. In OVA-treated mice, airway eosinophil infiltration and goblet cell metaplasia were reduced by either montelukast or dexamethasone alone. Montelukast, but not dexamethasone, reversed the established increase in airway smooth muscle mass and subepithelial collagen deposition. By immunocytochemistry, CysLT1 receptor expression was significantly increased in airway smooth muscle cells in allergen-treated mice compared with saline-treated controls and was reduced by montelukast, but not dexamethasone, administration.
Conclusions: These data indicate that established airway smooth muscle cell layer thickening and subepithelial fibrosis, key allergen-induced airway structural changes not modulated by corticosteroids, are reversible by CysLT1 receptor blockade therapy.
Keywords: eosinophils, fibrosis, mucus, smooth muscle
In patients with asthma, persistent allergen-induced inflammation is accompanied by structural changes in the airways. These airway remodeling changes observed in both children and adults include increased airway goblet cells, smooth muscle mass, and blood vessels (1–3). The increased smooth muscle mass present in the airways of patients with asthma (4) may result from hyperplasia and hypertrophy of smooth muscle cells and myofibroblasts (5, 6). Prominent in the remodeling process is thickening of the airway wall with development of subepithelial fibrosis from deposition of extracellular matrix (ECM) proteins (e.g., collagen, laminin, fibronectin, tenascin) in the lamina reticularis beneath the basement membrane (1, 7). The increased subepithelial layer thickness and vascularity are seen in both patients with newly diagnosed asthma and patients with long-standing disease duration (3, 8).
A key question is whether the airway structural changes in patients with asthma are reversible by drug intervention. Current therapeutic approaches to reverse established structural airway changes have had limited effect in both animal asthma models and patients with asthma (9, 10). In mouse (11, 12) and rat (13) asthma models, although concomitant treatment of corticosteroids during the allergen challenge period blocked the airway inflammatory response and structural changes including subepithelial fibrosis, dexamethasone treatment initiated after allergen challenge had only limited (11) or no effect (13) on reversing the established airway remodeling changes. In transgenic mice, transforming growth factor (TGF)-β1–induced lung fibrosis is reversible after cessation of transgene expression indicating that the fibrotic response in the airways is not inevitably irreversible (14).
Using an in vivo model of allergen-induced airway remodeling in mice, we have found cysteinyl leukotrienes (CysLTs) to be important in the development of the airway structural changes. In this asthma model, mice sensitized by intraperitoneal administration of ovalbumin (OVA), when challenged over a 2-mo period with intranasal OVA, develop airway eosinophilia, goblet cell metaplasia, smooth muscle cell layer thickening, and subepithelial fibrosis with deposition of collagen and laminin ECM proteins (12, 15). Administration of the specific CysLT1 receptor antagonist montelukast (MK) during the intranasal OVA challenge period blocked eosinophil trafficking/degranulation, Th2 cytokine release, and structural changes in the lungs (15).
In the present study, we determined the effect of the CysLT1 receptor antagonist MK, in comparison to the corticosteroid dexamethasone, on airway remodeling when administered after development of structural airway changes in a chronic mouse asthma model. MK and dexamethasone each partially reduced established airway inflammatory cell infiltration and goblet cell metaplasia, but only MK reversed the established increase in airway smooth muscle mass and subepithelial fibrosis. These data indicate that important structural features of allergen-induced airway remodeling resistant to corticosteroid treatment are reversible by CysLT1 receptor blockade.
METHODS
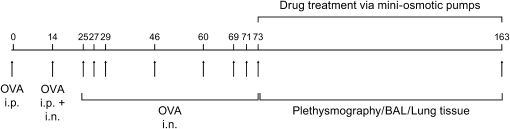
A detailed description of these methods can be found in the online supplement. Animal use procedures were approved by the University of Washington Animal Care Committee. Female BALB/c mice (6–8 wk; Jackson Laboratory, Bar Harbor, ME) received intraperitoneal injections of 100 μg OVA (0.2 ml of 0.5 mg/ml) complexed with alum on Days 0 and 14 and intranasal doses of 100 μg OVA (0.05 ml of 2 mg/ml) on Day 14 and 50 μg OVA (0.05 ml of 1 mg/ml) on Days (± 1–2 d) 25, 27, 29, 46, 60, 69, 71, and 73 (Figure 1). On Day 73, randomized treatment groups of mice had subcutaneous placement of miniosmotic pumps (200-μl Alzet Model 2004 osmotic pumps; 6 μl/d delivery rate; Durect Corporation, Cupertino, CA) containing MK sodium (1 mg/kg; Merck and Co., Inc., Rehway, NJ), dexamethasone (1 mg/kg; dexamethasone–water soluble; Sigma Chemical Co., St. Louis, MO), MK (1 mg/kg) + dexamethasone (1 mg/kg), or saline control (four to eight mice per study group) with pump replacement on Days 103 and 133. The control group received intraperitoneal saline with alum on Days 0 and 14 and intranasal saline without alum on Days (± 1–2 d) 14, 25, 27, 29, 46, 60, 69, 71, and 73. In preliminary studies (see Results), the 1 mg/kg dexamethasone dose inhibited airway eosinophil trafficking. OVA-sensitized mice challenged with intranasal OVA on Days 14, 25, 27, and 29 received either intraperitoneal dexamethasone (1 mg/kg) or saline 30 min before each intranasal OVA challenge on Days 25, 27, and 29 with bronchoalveolar lavage (BAL) performed on Day 30; controls were saline-treated mice.
Figure 1.
Study design. BAL = bronchoalveolar lavage; OVA = ovalbumin.
On Days 73 (30 min after OVA) and 163 (3 mo after the last allergen challenge), airway hyperreactivity to aerosolized methacholine was performed in conscious, freely moving, spontaneously breathing mice using whole-body plethysmography (Model PLY 3211; Buxco Electronics, Inc., Sharon, CT) (15, 16). The degree of bronchoconstriction was expressed as enhanced pause (Penh), a calculated dimensionless value that correlates with measurements of airway resistance, impedance, and intrapleural pressure. After plethysmography, mice were exsanguinated by cardiac puncture, BAL was performed on the right lung for cell counts (17), and left lung tissue was obtained for light microscopy and morphometry (15).
The lung sections were stained with hematoxylin and eosin to determine the airway inflammatory cell infiltrate (0–4+ scale) (17), eosinophils per unit airway (2,200 μm2) (17), and smooth muscle thickness in μm (15), Alcian blue, pH 2.5, with nuclear fast red counterstaining to identify goblet cells (as percent of total airway epithelial cells) (17), and Masson's trichrome to determine collagen deposition/fibrosis (0–4+ scale) (15); 10 airways (∼ 0.4–0.7 mm in diameter and surrounded by smooth muscle cells) per mouse were examined. Airway CysLT1 receptor expression was localized by immunocytochemistry (18) using polyclonal goat anti-CysLT1 receptor–specific primary antibody (19) (provided by Jilly F. Evans, Merck and Co., Inc.), followed by rabbit anti-goat secondary antibody conjugated to horseradish peroxidase (Vector Laboratories, Burlingame, CA).
The data are reported as the mean ± SE of the combined experiments. Differences were analyzed for significance (p < 0.05) by analysis of variance using the protected least significance difference method.
RESULTS
Effect of CysLT1 Receptor Antagonist and Corticosteroid Treatment on Reversal of Allergen-induced Airway Inflammation and Remodeling
The study design is shown in Figure 1. On (1) Day 73 after the final intranasal OVA or saline challenge and before initiation of randomized drug therapy and (2) Day 163, after a 3-mo treatment period with MK (1 mg/kg), dexamethasone (1 mg/kg), MK (1 mg/kg) + dexamethasone (1 mg/kg), or saline, BAL was performed, and lung tissue obtained to determine airway inflammation and remodeling. The 1 mg/kg/d dose of MK was based on our prior study (15) using the same chronic asthma model in which this dose significantly inhibited airway eosinophil infiltration and degranulation, production of Th2 cytokines interleukin 4 (IL-4) and IL-13, goblet cell metaplasia, smooth muscle cell layer thickening, and subepithelial collagen deposition/fibrosis when administered during the period of intranasal OVA challenge in sensitized mice (15). The 1-mg/kg/d dose of dexamethasone was chosen because it abrogated the influx of eosinophils into the airways by Day 30. Compared with the saline group, OVA-treated mice had a 349-fold increase in total eosinophils recovered in BAL fluid to 2.4 ± 0.6 × 105 (p = 0.003, OVA vs. saline; n = 5 each group) on Day 30. Dexamethasone given by intraperitoneal administration (1 mg/kg/day) before OVA on Days 25, 27, and 29 reduced the total number of eosinophils by 93.0% to 0.2 ± 0.0 3 × 105 (p = 0.01, OVA/dexamethasone vs. OVA; n = 5 each group). The 1-mg/kg/d dexamethasone dose is the same or higher dose as shown effective in other chronic mouse asthma models in blocking allergen-induced airway inflammation and remodeling when administered during the allergen challenge period (11, 20–26).
Airway inflammatory cell infiltration.
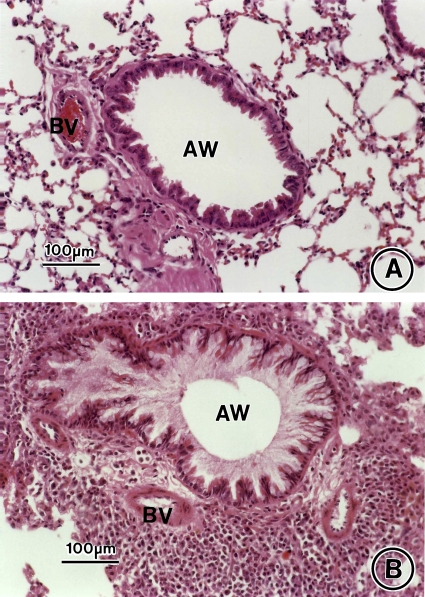
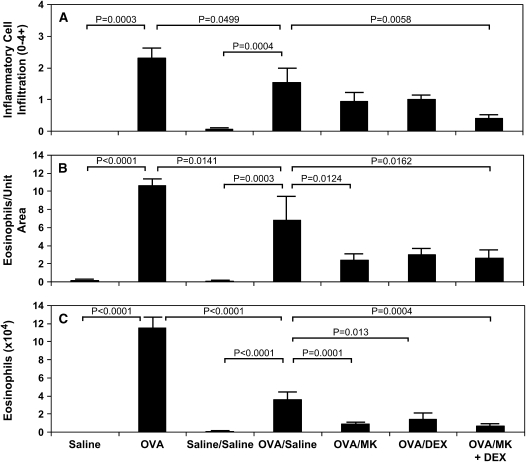
On Day 73, an extensive infiltrate of eosinophils, macrophages, and other inflammatory cells around the airways and blood vessels and influx of eosinophils into the BAL fluid occurred in the lungs of OVA-sensitized/challenged mice that was not seen in saline controls (Figures 2B vs. 2A and 3) in agreement with our previous work using this chronic asthma model (15). In OVA-treated mice, the (1) inflammatory cell and (2) eosinophil infiltrate in the lung interstitium and (3) eosinophil influx in BAL fluid persisted on Day 163 (Figures 4B vs. 4A), although each was significantly reduced by 33.5, 35.8, and 68.7%, respectively, compared with Day 73 (Figure 3). By morphometry, the number of inflammatory cells in the lung interstitium of the OVA-treated mice was significantly increased compared with the saline-treated controls on Day 163 (Figure 3A; p = 0.0004, OVA/saline vs. saline/saline). Infiltration of the airway interstitium by eosinophils increased approximately 68-fold from 0.1 ± 0.1 eosinophils/2,200 μm2 lung tissue in control animals to 6.8 ± 2.8 eosinophils/2,200 μm2 lung tissue in the OVA-sensitized/challenged mice on Day 163 (Figure 3B; p = 0.0003, OVA/saline vs. saline/saline). Compared with the saline-treated control animals on Day 163, the OVA-treated mice had an approximate 40-fold increase in eosinophils recovered in the BAL fluid to 3.6 ± 0.1 × 104 eosinophils/ml (Figure 3C; p < 0.0001, OVA/saline vs. saline/saline).
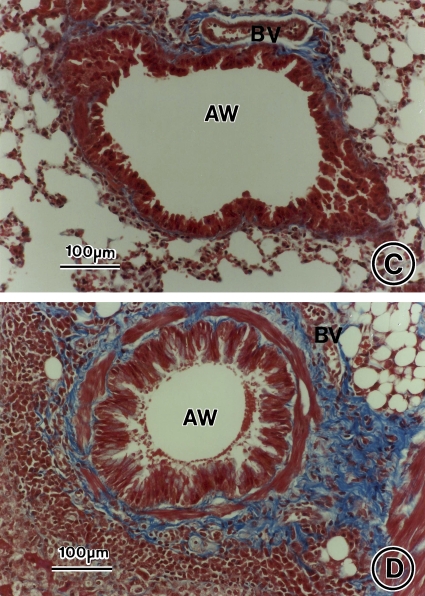
Figure 2.
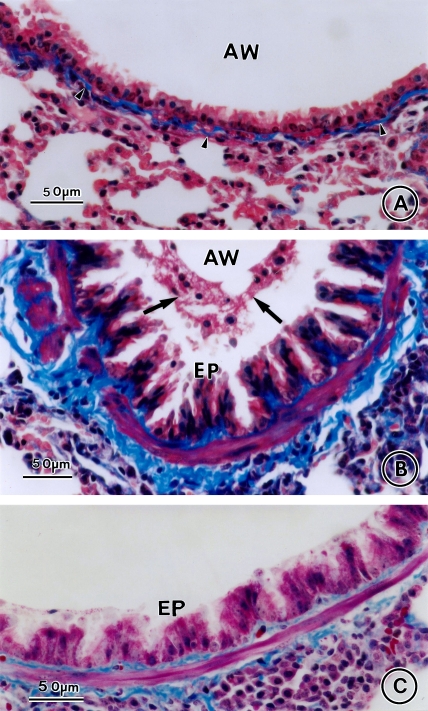
Allergen-induced airway inflammation and remodeling. Lung tissue was obtained on Day 73 from saline control animals (A, C) and OVA-sensitized/challenged mice (B, D) and stained with hematoxylin and eosin (A, B) or Masson's trichrome stain (C, D). AW = airway; BV = blood vessel. Bars = 100 μm.
Figure 3.
Effect of CysLT1 receptor blockade and corticosteroid treatment on airway inflammatory cell infiltration in OVA-treated mice. Lung tissue (A, B) and BAL fluid (C) were obtained on Day 73 from saline-treated mice (saline; n = 4) and OVA-sensitized/challenged mice (OVA; n = 4) and Day 163 from saline-treated mice (saline/saline; n = 7) and OVA-treated mice in the absence (OVA/saline; n = 6) or presence of treatment from Days 73–163 with 1 mg/kg of montelukast (OVA/MK; n = 8), 1 mg/kg of dexamethasone (OVA/DEX; n = 8), or 1 mg/kg of montelukast + 1 mg/kg of dexamethasone (OVA/MK + DEX; n = 4). The total inflammatory cell infiltrate (0–4+ scale; A) and number of eosinophils per unit area (2,200 μm2; B) in the lung interstitium, and number of eosinophils (× 104) per ml of BAL fluid (C) were determined by morphometry; p values are shown where significant (p < 0.05).
Figure 4.
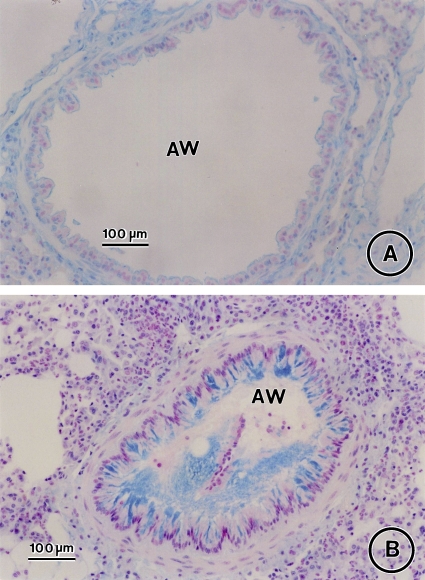
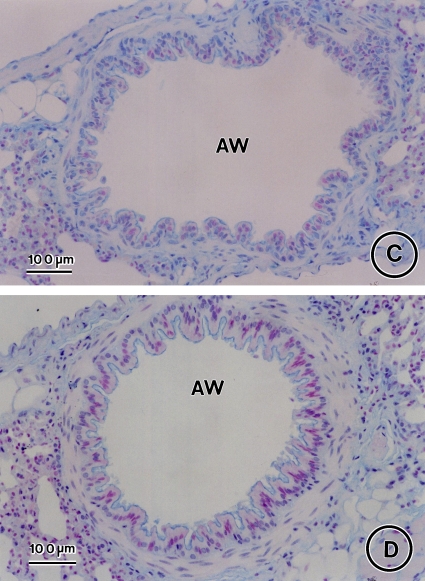
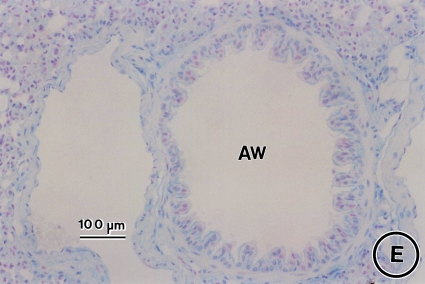
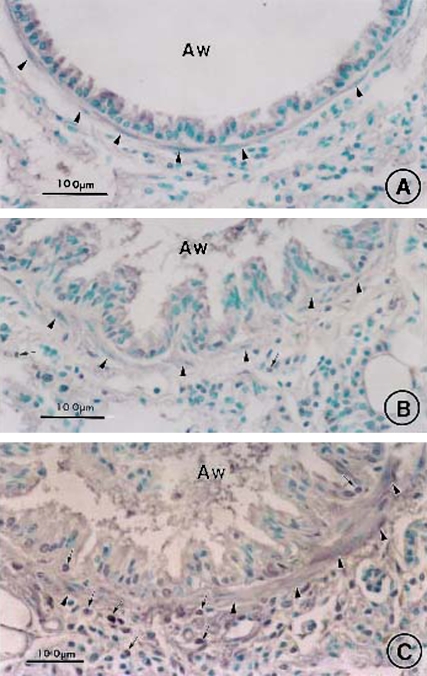
Effect of CysLT1 receptor blockade and corticosteroid treatment on allergen-induced airway goblet cell metaplasia. Lung tissue was obtained on Day 163 from saline controls (A) and OVA-treated mice in the absence (B) or presence of MK (C), dexamethasone (D), or MK + dexamethasone (E) and stained with Alcian blue with nuclear fast red counterstaining. AW = airway. Bars = 100 μm.
In OVA-treated mice on Day 163, MK significantly decreased the eosinophil infiltrate into the lung interstitium (Figure 3B; p = 0.0124, OVA/MK vs. OVA/saline and Figures 4C vs. 4B) and BAL fluid (Figure 3C; p = 0.0001, OVA/MK vs. OVA/saline); dexamethasone significantly reduced the number of BAL fluid eosinophils in OVA-treated mice (Figure 3C; p = 0.013, OVA/dexamethasone vs. OVA/saline). In OVA-treated mice, the combination MK + dexamethasone significantly reduced the total inflammatory cell (Figure 3A; p = 0.0058, OVA/MK + dexamethasone vs. OVA/saline) and eosinophil (Figure 3B; p = 0.0162, OVA/MK + dexamethasone vs. OVA/saline) infiltration of the lung tissue (Figures 4E vs. 4B) and eosinophil influx into BAL fluid (Figure 3C; p = 0.0004, OVA/MK + dexamethasone vs. OVA/saline).
Airway goblet cell metaplasia.
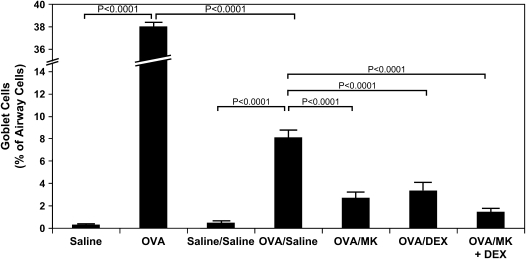
Airway goblet cell metaplasia seen in OVA-treated mice compared with saline controls on Day 73 (Figure 5; p < 0.0001, OVA vs. saline) persisted at Day 163, 3 mo after the last allergen challenge (Figures 4B vs. 4C and 5; p < 0.0001, OVA/saline vs. saline/saline). However, partial resolution of the allergen-induced mucus metaplasia observed on Day 73 occurred by Day 163 with goblet cells 8.1 ± 1.0% of airway epithelial cells on Day 163 vs. 38.0 ± 0.4% of airway epithelial cells on Day 73 (Figure 5; p < 0.0001, OVA/saline vs. OVA).
Figure 5.
Reduction in allergen-induced airway goblet cell metaplasia by CysLT1 receptor blockade and corticosteroid treatment. Lung tissue was obtained on Day 73 from saline-treated mice (saline; n = 4) and OVA-treated mice (n = 4) and on Day 163 from saline-treated mice (saline/saline; n = 7) and OVA-treated mice in the absence (OVA/saline; n = 6) or presence of MK (OVA/MK; n = 8), dexamethasone (OVA/DEX; n = 8), or MK + dexamethasone (OVA/MK + DEX; n = 4). The percentage of airway cells positive for mucus glycoproteins was determined by morphometry; p values are shown where significant (p < 0.05).
Administration of MK alone, dexamethasone alone, and the combination MK + dexamethasone for a 3-mo period after the last allergen challenge each significantly reduced airway goblet cell metaplasia (Figures 4C, 4D, and 4E, respectively, vs. Figures 4B and 5; 66.5% decrease, p < 0.0001, OVA/MK vs. OVA/saline; 58.9% decrease, p < 0.0001, OVA/dexamethasone vs. OVA/Saline; 82.0% decrease, p < 0.0001, OVA/MK + dexamethasone vs. OVA/saline). However, the goblet cell metaplasia observed in the OVA-sensitized/challenged mice was not completely reversed by any treatment because the number of goblet cells in the MK, dexamethasone, and MK + dexamethasone groups were each significantly greater than in the saline controls (Figure 5).
Airway smooth muscle cell layer thickening.
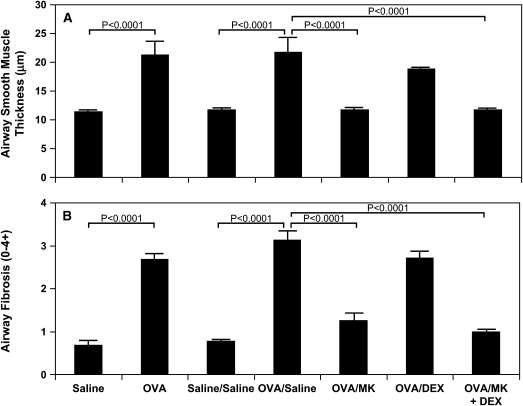
Increased airway smooth muscle thickness was seen on Day 73 in OVA-sensitized/challenged mice compared with saline controls (Figures 2B vs. 2A). This increase in airway smooth muscle mass persisted without diminution at Day 163, 3 mo after the last OVA challenge (Figures 6B vs. 6A, Figures E1B vs. E1A of the online supplement, and Figure 7A; p < 0.0001, OVA vs. saline). On both Days 73 and 163, the thickness of the airway smooth muscle cell layer was approximately twofold greater in the OVA-treated mice compared with control animals (Figure 7A; p < 0.0001, OVA vs. saline and p < 0.0001, OVA/saline vs. saline/saline).
Figure 6.
Effect of CysLT1 receptor blockade and corticosteroid treatment on allergen-induced increased airway smooth muscle mass and collagen deposition. Lung tissue was obtained on Day 163 from saline controls (A) and OVA-sensitized/challenged mice in the absence (B) or presence of MK (C), dexamethasone (D), or MK + dexamethasone (E), and stained with Masson's trichrome stain. Arrowheads indicate collagen deposition, arrows airway mucus. AW = airway; EP = epithelial cell. Bars = 50 μm.
Figure 7.
Reduction in allergen-induced increased airway smooth muscle mass and subepithelial collagen deposition by CysLT1 receptor blockade but not by corticosteroid treatment. Lung tissue was obtained on Day 73 from saline-treated mice (saline; n = 4) and OVA-treated mice (OVA; n = 4) and Day 163 from saline-treated mice (saline/saline; n = 7) and OVA-treated mice in the absence (OVA/saline; n = 6) or presence of MK (OVA/MK; n = 8), dexamethasone (OVA/DEX; n = 6), or MK + dexamethasone (OVA/MK + DEX; n = 4), and thickness of the airway smooth muscle cell layer in μm (A) and collagen deposition/fibrosis (0–4+ scale; B determined; p values are shown where significant (p < 0.05).
MK alone (Figures 6C vs. 6B) and the combination MK + dexamethasone (Figures 6E vs. 6B) treatment for a 3-mo period after the final OVA challenge each significantly reduced the increased smooth muscle mass by 99.2% (Figure 7A; p < 0.0001, OVA/MK vs. OVA/saline; p < 0.0001, OVA/MK + dexamethasone vs. OVA/saline). There was no statistical difference observed in airway smooth muscle thickness between the saline group and the OVA-treated groups administered either MK or the combination MK + dexamethasone (Figure 7A). Dexamethasone alone had no significant effect on the increased airway smooth muscle mass observed in OVA-treated mice on Day 163 (Figures 6D vs. 6B and 7A).
Airway collagen deposition/fibrosis.
On Day 73, collagen deposition markedly increased in the lung interstitium around airways and blood vessels in OVA-treated mice compared with saline-treated control animals as assessed by Masson's trichrome staining (Figures 2B vs. 2A and 7B; fourfold increase, p < 0.0001, OVA vs. saline). This increase in airway collagen deposition persisted without reduction at Day 163, 3 mo after the last allergen challenge (Figures 6B vs. 6B, Figures E1B vs. E1A, and Figure 7B). As determined by morphometry, the mean airway collagen deposition/fibrosis score remained approximately fourfold greater in OVA-treated mice compared with controls at Day 163 (Figure 7B; p < 0.0001, OVA/saline vs. saline/saline).
Administration of MK alone (Figures 6C vs. 6B, and Figures E1C vs. E1B) or in combination with dexamethasone (Figures 6E vs. 6B, Figures E1E vs. E1B) significantly decreased collagen deposition beneath the airway epithelial cell layer (Figure 7B; 79.6% decrease, p < 0.0001, OVA/MK vs. OVA/saline; 90.2% decrease, p < 0.0001, OVA/MK + dexamethasone vs. OVA/saline). No statistical difference in airway collagen was observed between the saline group and the OVA-treated group administered MK + dexamethasone. Corticosteroid treatment with dexamethasone alone had no significant effect on subepithelial airway fibrosis in OVA-sensitized/challenged mice (Figures 6D vs. 6B, Figures E1D vs. E1B, and Figure 7B).
Airway CysLT1 receptor expression.
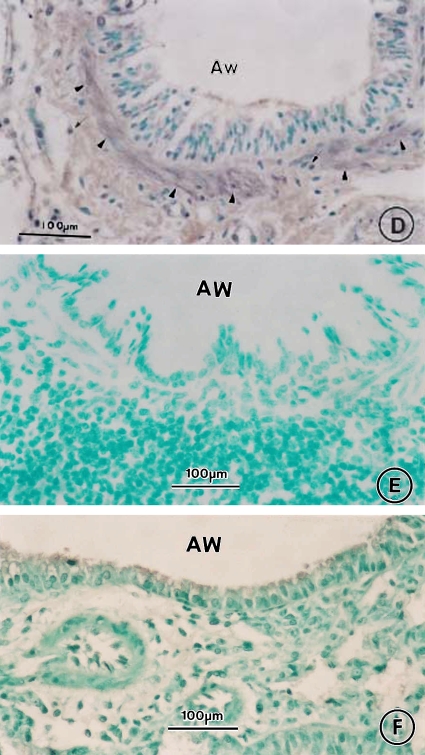
CysLT1 receptor expression was significantly increased in airway smooth muscle cells in the lungs of OVA-treated mice compared with saline-treated controls on Day 163 (Figures 8C vs. 8A). Eosinophils and other inflammatory cells infiltrating the airways of OVA-treated mice had marked expression of CysLT1 receptor (Figure 8C). The smooth muscle cells and inflammatory cells in the airways of OVA-treated mice did not exhibit immunostaining for CysLT1 receptor when phosphate-buffered saline (Figure 8E) or nonimmunized goat IgG (Figure 8F) was substituted for goat anti-CysLT1 receptor antibody as controls. In the OVA-treated mice, administration of MK significantly reduced the increased CysLT1 receptor expression in airway smooth muscle cells (Figures 8B vs. 8C). In contrast, dexamethasone treatment had no significant effect on the increased CysLT1 receptor expression in airway smooth muscle cells of OVA-treated mice (Figures 8D vs. 8C). Both MK (Figures 8B vs. 8C) and dexamethasone (Figures 8D vs. 8C) decreased the influx of CysLT1 receptor-positive eosinophils into the airways of OVA-sensitized/challenged mice.
Figure 8.
Effect of CysLT1 receptor blockade on CysLT1 receptor expression in OVA-treated mice. Lung tissue obtained on Day 163 from saline-treated mice (A) and OVA-treated mice in the absence (C, E, F) or presence of MK (B) or dexamethasone (D) underwent immunocytochemistry for CysLT1 receptor expression using goat anti-CysLT1 receptor antibody (A–D) or for controls, incubation with either phosphate-buffered saline (E) or nonimmunized goat IgG (F) instead of incubation with goat anti-CysLT1 receptor primary antibody. Arrowheads indicate smooth muscle cell layer, arrows indicate inflammatory cells. Bars = 100 μm.
Effect of CysLT1 Receptor Antagonist and Corticosteroid Treatment on Allergen-induced Airway Hyperreactivity to Methacholine
Noninvasive in vivo plethysmography.
Airway hyperreactivity to aerosolized methacholine was evaluated on Days 73 and 163 by noninvasive plethysmography (Figure 9). On Day 73, Penh (% of air) was significantly increased in the OVA-treated mice compared with saline controls after challenge with methacholine at 5 and 20 mg/ml (Figure 9A). However, 3 mo after the final allergen challenge on Day 163, no significant increase was seen in Penh in OVA-treated mice compared with the saline group (Figure 9B). Administration of MK, dexamethasone, or MK + dexamethasone did not significantly change the airway responses to methacholine on Day 163 in OVA-sensitized/challenged animals (Figure 9B).
Figure 9.
Effect of CysLT1 receptor blockade and corticosteroid treatment on pulmonary mechanics to aerosolized methacholine in OVA-treated mice. The degree of bronchoconstriction (expressed as Penh [% of air]) to aerosolized methacholine (0, 5, and 20 mg/ml) was determined on Day 73 in saline-treated mice (saline; n = 4) and OVA-treated mice (OVA; n = 4; *p < 0.05, OVA vs. saline) (A) and Day 163 in saline-treated mice (saline/saline; n = 7) and OVA-treated mice in the absence (OVA/saline; n = 8) or presence of MK (OVA/MK; n = 8), dexamethasone (OVA/DEX; n = 8), or montelukast + dexamethasone (OVA/MK + DEX; n = 4; B).
DISCUSSION
In this chronic asthma model, we found spontaneous partial resolution of the influx of eosinophils into the lung interstitium and BAL fluid and airway goblet cell metaplasia and loss of the airway hyperreactivity to methacholine in OVA-treated mice 3 mo after the last intranasal allergen challenge. In contrast, the established increase in airway smooth muscle mass and subepithelial collagen deposition established by Day 73 in this model persisted without diminution for the 3-mo period after the last OVA administration. Treatment with the CysLT1 receptor antagonist MK or the corticosteroid dexamethasone for the 3-mo period after the final allergen challenge each partially reduced the allergen-induced airway eosinophil trafficking and goblet cell metaplasia. MK, but not corticosteroid, treatment reversed the established airway smooth muscle cell layer thickening and subepithelial collagen deposition/fibrosis suggesting that cysLTs are important in the maintenance of these allergen-induced airway structural changes.
In other chronic asthma models in mice, a progressive diminution in the increased levels of BAL fluid eosinophils from 6 d to 8 wk after final OVA challenge has been observed (24, 27, 28). The rate and extent of attenuation of the airway inflammatory response after allergen challenge likely reflect differences in mouse strains and airway challenge procedures employed (29, 30). In a mouse asthma model without systemic immunization, persistent airway eosinophilia develops in A/J mice, to a lesser extent in BALB/c mice, but not in C57BL/6 or C3H/HeJ mice (31). In these studies, chronic allergen challenge by intranasal delivery led to airway remodeling changes but by aerosol delivery did not (31). In our model, using repeated intranasal allergen challenge over a 73-d period in systemically immunized mice, total BAL fluid eosinophils decreased 68.7% from Day 73 to Day 163, 3 mo after the last allergen challenge. Similarly, eosinophils infiltrating the lung interstitium decreased 35.8% from Day 73 to Day 163. Despite this spontaneous attenuation in airway eosinophilia, the levels of eosinophils in the BAL fluid and lung interstitium remained significantly greater in OVA-treated mice than saline control animals.
MK administered for a 3-mo period after the last OVA challenge significantly reduced the persistent eosinophil influx into the BAL fluid and decreased the infiltration of CysLT1 receptor-positive eosinophils into the lungs of OVA-treated mice. CysLTs promote eosinophil trafficking and retention in the airways by effects on eosinophil differentiation (32), adhesion proteins (33), locomotion (34, 35), and survival (36). Corticosteroids exert their well-documented effect of decreasing influx of eosinophils and other inflammatory cells into the blood (28), lung tissue (13, 26, 37, 38), and BAL fluid (13, 20, 21, 37) when given either before (13, 20, 21, 26, 28, 37, 39–42) or after allergen challenge in animal asthma models (11). In our study, although MK and dexamethasone produced similar reductions in airway eosinophils, only CysLT1 receptor antagonist administration significantly decreased established airway smooth muscle cell layer thickening and subepithelial fibrosis consistent with the hypothesis that airway wall remodeling and inflammation may occur in part as parallel rather than sequential pathways (43).
Spontaneous regression in allergen-induced airway goblet cell metaplasia has been observed 14 d after the last OVA challenge in a mouse asthma model (20). We found a nearly fivefold reduction in airway goblet cells at Day 163, compared with the last allergen challenge day on Day 73. Although spontaneously diminished, the percentage of airway goblet cells in OVA-treated mice remained significantly greater than in saline-treated controls. Administration of MK or dexamethasone separately or in combination for the 3-mo period after the final OVA challenge caused significant reductions in the persistent goblet cell metaplasia. We have previously shown that airway mucus release is blocked by specific inhibitors of 5-lipoxygenase and 5-lipoxygenase–activating protein that prevent leukotriene formation when administered before allergen challenge in a short-term mouse asthma model (17). We have also demonstrated in a long-term mouse asthma model in which airway remodeling occurs that MK, administered during the period of chronic allergen challenge, significantly reduces the airway goblet cell metaplasia (15). When administered after the last allergen challenge, dexamethasone decreases airway mucus-producing epithelial cell proliferation but does not completely reverse the mucus hypersecretory phenotype, despite blocking the airway inflammatory cell response in a mouse asthma model (41). Both CysLT1 receptor blockade and corticosteroids inhibit production of Th2 cytokines IL-4 and IL-13 that are key regulators of airway mucus release (15, 44). Overexpression of IL-4 in transgenic mice results in mucus hypersecretion and increased expression of the mucin gene MUC5AC (45). IL-4, in the absence of IL-5, IL-9, and IL-13, can induce goblet cell metaplasia in mice after allergen challenge (46). IL-4 blockade by administration of soluble IL-4 receptor after development of airway goblet cell metaplasia inhibits mucus hypersecretion in OVA-sensitized/challenged mice (47). IL-13, a key mediator of mucus metaplasia in mouse asthma models (48, 49), increases expression of MUC5AC (50) and Gob-5 (51) and induces goblet cell differentiation of tracheal epithelium (50). However, IL-13-induced airway MUC5AC overexpression and goblet cell metaplasia in mouse airways are unaffected by dexamethasone in a mouse asthma model (25).
In our chronic asthma model, we found that the increase in airway smooth muscle mass and collagen deposition that develops by Day 73 persists without resolution by Day 163, 3 mo after the last allergen challenge. In this chronic asthma model, hypertrophy and hyperplasia of smooth muscle cells and myofibroblasts likely contribute to the increased airway smooth muscle mass. We previously demonstrated that MK, when administered during the period of airway allergen challenge, markedly reduced the increased airway smooth muscle cell layer (15). We now show that CysLT1 receptor blockade administered for the 3-mo period after the last allergen challenge reverses the persistent established increase in airway smooth muscle mass. CysLTs promote human airway smooth muscle hyperplasia increasing epidermal growth factor–induced proliferation of these cells (52). Transforming growth factor (TGF)-β and IL-13 each increase expression of the CysLT1 receptor in human airway smooth muscle cells (52, 53). Our data suggest that CysLT1 receptor blockade treatment may reverse the established increase in airway smooth muscle mass in part by reducing the increased CysLT1 receptor expression on the smooth muscle cells in the lungs. Prior work has shown that concomitant treatment of dexamethasone during the period of allergen challenge in sensitized mice inhibits thickening of the airway smooth muscle layer without affecting epithelial thickening or subepithelial fibrosis (28). In a rat chronic asthma model with development of airway wall thickening, the increased fibronectin deposition persisted after the final OVA challenge and was not reversed by fluticasone treatment (13). In this report, we found that dexamethasone did not reduce either the increased airway smooth muscle cell CysLT1 receptor expression or the established increase in airway smooth muscle mass.
We have previously shown that MK prevents subepithelial collagen deposition/fibrosis in a long-term mouse asthma model when given during OVA challenge in sensitized mice (15). We now report a marked reversal in this fibrotic process when the CysLT1 receptor antagonist is given after the last airway allergen challenge. In vitro studies indicate that CysLTs induce release of collagen (54) and fibroblast growth factor from mouse alveolar macrophages (55). In human fetal lung fibroblasts transformed into myofibroblasts by TGF-β, CysLT1 receptor expression is increased (56). LTD4 alone stimulates collagen production by the TGF-β–transformed cells, and this LTD4-induced augmentation of collagen release is blocked by MK (56) suggesting a potential role of CysLTs in the deposition of ECM proteins in vivo.
In the present study, allergen-induced airway hyperresponsiveness demonstrated on Day 73 after the final airway allergen challenge was absent on Day 163, 3 mo after the final OVA challenge in OVA-sensitized/challenged mice, despite persistent airway inflammation and structural changes. In other models, with shorter periods after final allergen challenge before assessment of pulmonary mechanics, airway hyperreactivity persisted at the end of 2-wk (13) and 8-wk (27) allergen-free intervals, despite resolution of the airway eosinophilic response. In these models, the airway hyperreactivity correlated with increased airway thickness (13) and airway contractile tissue (27). However, our data suggest that airway hyperreactivity can be dissociated from the allergen-induced airway structural changes because the hyperresponsiveness to methacholine resolved despite the persistent increased airway smooth muscle mass and subepithelial collagen deposition seen 3 mo after the final allergen challenge. The relationship of airway wall thickness and other features of remodeling to airway reactivity in patients with asthma remains unclear (9). Whereas a positive correlation of airway wall thickness with airway hyperresponsiveness has been reported (57, 58), other studies have found either no significant relationship (59) or a negative correlation of airway reactivity with airway wall thickness (60, 61). A bronchial biopsy study to determine airway structural changes associated with severe asthma demonstrated that increases in collagen type III deposition, airway smooth muscle cell size, fibroblasts, and mucus glands selectively correlated with severe asthma, whereas mucosal eosinophilia and subepithelial basement membrane thickness did not (4). Thus the relationship of airway inflammation and remodeling with asthma severity and lung function is complex and in need of future research (for review, see Reference 9) to determine the clinical relevance of remodeling and benefit of therapeutic modalities controlling particular features of the airway injury/repair/remodeling response in asthma.
In summary, our studies indicate that CysLT1 receptor antagonism has a significant anti-airway remodeling effect in an animal model of human asthma. The reversal of established airway structural changes (i.e., increased smooth muscle mass and subepithelial fibrosis) by MK, but not dexamethasone, suggests an important role for cysLTs in the immunopathogenesis of the remodeling process that is not modulated by corticosteroids. Clinical studies in patients with asthma to determine the alterability of established airway structural changes by CysLT1 receptor blockade will be of great interest.
Supplementary Material
Acknowledgments
The authors thank Jilly F. Evans for providing anti–CysLT1 receptor antisera, Falaah Jones for excellent technical assistance, and Rachel Norris for typing this manuscript.
Supported by NIH grants AI42989 and HL73722 and by a Merck and Co., Inc., Medical School grant.
This article has an online supplement, which is accessible from this issue's table of contents online at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200501-088OC on December 30, 2005
Conflict of Interest Statement: W.R.H. has given lectures (2002–2004) in sessions at regional, national, and international meetings, which were funded in part by Merck & Co., Inc., and received research support from a Merck & Co. Medical School grant awarded to the University of Washington in 2002; G.K.S.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; Y.-T.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; E.Y.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med 2001;164:S28–S38. [DOI] [PubMed] [Google Scholar]
- 2.Payne DN, Rogers AV, Adelroth E, Bandi V, Guntupalli KK, Bush A, Jeffery PK. Early thickening of the reticular basement membrane in children with difficult asthma. Am J Respir Crit Care Med 2003;167:78–82. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka H, Yamada G, Saikai T, Hashimoto M, Tanaka S, Suzuki K, Fujii M, Takahashi H, Abe S. Increased airway vascularity in newly diagnosed asthma using a high-magnification bronchovideoscope. Am J Respir Crit Care Med 2003;168:1495–1499. [DOI] [PubMed] [Google Scholar]
- 4.Benayoun L, Druilhe A, Dombret MC, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med 2003;167:1360–1368. [DOI] [PubMed] [Google Scholar]
- 5.Ebina M, Takahashi T, Chiba T, Motomiya M. Cellular hypertrophy and hyperplasia of airway smooth muscles underlying bronchial asthma: a 3-D morphometric study. Am Rev Respir Dis 1993;148:720–726. [DOI] [PubMed] [Google Scholar]
- 6.Zhou L, Li J, Goldsmith AM, Newcomb DC, Giannola DM, Vosk RG, Eves EM, Rosner MR, Solway J, Hershenson MB. Human bronchial smooth muscle cell lines show a hypertrophic phenotype typical of severe asthma. Am J Respir Crit Care Med 2004;169:703–711. [DOI] [PubMed] [Google Scholar]
- 7.Hoshino M, Nakamura Y, Sim JJ. Expression of growth factors and remodeling of the airway wall in bronchial asthma. Thorax 1998;53:21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chetta A, Foresi A, Del Donno M, Bertorelli G, Pesci A, Olivieri D. Airways remodeling is a distinctive feature of asthma and is related to severity of disease. Chest 1997;111:852–857. [DOI] [PubMed] [Google Scholar]
- 9.Busse W, Banks-Schlegel S, Noel P, Ortega H, Taggart V, Elias J. Future research directions in asthma: an NHLBI Working Group report. Am J Respir Crit Care Med 2004;170:683–690. [DOI] [PubMed] [Google Scholar]
- 10.Inman M. Is there a place for anti-remodelling drugs in asthma which may not display immediate clinical efficacy? Eur Respir J 2004;24:1–2. [DOI] [PubMed] [Google Scholar]
- 11.Blyth DI, Wharton TF, Pedrick MS, Savage TJ, Sanjar S. Airway subepithelial fibrosis in a murine model of atopic asthma: suppression by dexamethasone or anti-interleukin-5 antibody. Am J Respir Cell Mol Biol 2000;23:241–246. [DOI] [PubMed] [Google Scholar]
- 12.Christie PE, Jonas M, Tsai C-H, Chi EY, Henderson WR Jr. Increase in laminin expression in allergic airway remodeling and decrease by dexamethasone. Eur Respir J 2004;24:107–115. [DOI] [PubMed] [Google Scholar]
- 13.Vanacker NJ, Palmans E, Kips JC, Pauwels RA. Fluticasone inhibits but does not reverse allergen-induced structural airway changes. Am J Respir Crit Care Med 2001;163:674–679. [DOI] [PubMed] [Google Scholar]
- 14.Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee PJ, Noble PW, Yehualaeshet T, Lu B, Flavell RA, Milbrandt J, et al. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor β1-induced pulmonary fibrosis. J Exp Med 2004;200:377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henderson WR Jr, Tang L-O, Chu S-J, Tsao S-M, Chiang GKS, Jones F, Jonas M, Pae C, Wang H, Chi EY. A role for cysteinyl leukotrienes in airway remodeling in a mouse asthma model. Am J Respir Crit Care Med 2002;165:108–116. [DOI] [PubMed] [Google Scholar]
- 16.Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, Gelfand EW. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med 1997;156:766–775. [DOI] [PubMed] [Google Scholar]
- 17.Henderson WR Jr, Lewis DB, Albert RK, Zhang Y, Lamm WJE, Chiang GKS, Jones F, Eriksen P, Tien Y, Jonas M, et al. The importance of leukotrienes in airway inflammation in a mouse model of asthma. J Exp Med 1996;184:1483–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson WR Jr, Chi EY, Albert RK, Chu S-J, Lamm WJE, Rochon Y, Christie PE, Harlan JM. Blockade of CD49d (α4 integrin) on intrapulmonary but not circulating leukocytes inhibits airway inflammation and hyperresponsiveness in a mouse model of asthma. J Clin Invest 1997;100:3083–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Figueroa DJ, Breyer RM, Defoe SK, Kargman S, Daugherty BL, Waldburger K, Liu Q, Clements M, Zeng Z, O'Neill GP, et al. Expression of the cysteinyl leukotriene 1 receptor in normal human lung and peripheral blood leukocytes. Am J Respir Crit Care Med 2001;163:226–233. [DOI] [PubMed] [Google Scholar]
- 20.Blyth DI, Pedrick MS, Savage TJ, Bright H, Beesley JE, Sanjar S. Induction, duration, and resolution of airway goblet cell hyperplasia in a murine model of atopic asthma: effect of concurrent infection with respiratory syncytial virus and response to dexamethasone. Am J Respir Cell Mol Biol 1998;19:38–54. [DOI] [PubMed] [Google Scholar]
- 21.Li XM, Huang CK, Zhang TF, Teper AA, Srivastava K, Schofield BH, Sampson HA. The Chinese herbal medicine formula MSSM-002 suppresses allergic airway hyperreactivity and modulates TH1/TH2 responses in a murine model of allergic asthma. J Allergy Clin Immunol 2000;106:660–668. [DOI] [PubMed] [Google Scholar]
- 22.Walter MJ, Morton JD, Kajiwara N, Agapov E, Holtzman MJ. Viral induction of a chronic asthma phenotype and genetic segregation from the acute response. J Clin Invest 2002;110:165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dorscheid DR, Low E, Conforti A, Shifrin S, Sperling AI, White SR. Corticosteroid-induced apoptosis in mouse airway epithelium: effect in normal airways and after allergen-induced airway inflammation. J Allergy Clin Immunol 2003;111:360–366. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda RK, Nayar J, Cho JY, Miller M, Rodriguez M, Raz E, Broide DH. Resolution of airway inflammation following ovalbumin inhalation: comparison of ISS DNA and corticosteroids. Am J Respir Cell Mol Biol 2003;28:655–663. [DOI] [PubMed] [Google Scholar]
- 25.Kibe A, Inoue H, Fukuyama S, Machida K, Matsumoto K, Koto H, Ikegami T, Aizawa H, Hara N. Differential regulation by glucocorticoid of interleukin-13-induced eosinophilia, hyperresponsiveness, and goblet cell hyperplasia in mouse airways. Am J Respir Crit Care Med 2003;167:50–56. [DOI] [PubMed] [Google Scholar]
- 26.Kumar RK, Herbert C, Thomas PS, Wollin L, Beume R, Yang M, Webb DC, Foster PS. Inhibition of inflammation and remodeling by roflumilast and dexamethasone in murine chronic asthma. J Pharmacol Exp Ther 2003;307:349–355. [DOI] [PubMed] [Google Scholar]
- 27.Leigh R, Ellis R, Wattie J, Southam DS, De Hoogh M, Gauldie J, O'Byrne PM, Inman MD. Dysfunction and remodeling of the mouse airway persist after resolution of acute allergen-induced airway inflammation. Am J Respir Cell Mol Biol 2002;27:526–535. [DOI] [PubMed] [Google Scholar]
- 28.Pitchford SC, Riffo-Vasquez Y, Sousa A, Momi S, Gresele P, Spina D, Page CP. Platelets are necessary for airway wall remodeling in a murine model of chronic allergic inflammation. Blood 2004;103:639–647. [DOI] [PubMed] [Google Scholar]
- 29.Kumar RK, Foster PS. Modeling allergic asthma in mice: pitfalls and opportunities. Am J Respir Cell Mol Biol 2002;27:267–272. [DOI] [PubMed] [Google Scholar]
- 30.Shore SA. Modeling airway remodeling: the winner by a nose? Am J Respir Crit Care Med 2003;168:910–911. [DOI] [PubMed] [Google Scholar]
- 31.Shinagawa K, Kojima M. Mouse model of airway remodeling: strain differences. Am J Respir Crit Care Med 2003;168:959–967. [DOI] [PubMed] [Google Scholar]
- 32.Braccioni F, Dorman SC, O'Byrne PM, Inman MD, Denburg JA, Parameswaran K, Baatjes AJ, Foley R, Gauvreau GM. The effect of cysteinyl leukotrienes on growth of eosinophil progenitors from peripheral blood and bone marrow of atopic subjects. J Allergy Clin Immunol 2002;110:96–101. [DOI] [PubMed] [Google Scholar]
- 33.Fregonese L, Silvestri M, Sabatini F, Rossi GA. Cysteinyl leukotrienes induce human eosinophil locomotion and adhesion molecule expression via a CysLT1 receptor-mediated mechanism. Clin Exp Allergy 2002;32:745–750. [DOI] [PubMed] [Google Scholar]
- 34.Spada CS, Nieves AL, Krauss AHP, Woodward DF. Comparison of leukotriene B4 and D4 effects on human eosinophil and neutrophil motility in vitro. J Leukoc Biol 1994;55:183–191. [DOI] [PubMed] [Google Scholar]
- 35.Laitinen LA, Laitinen A, Haahtela T, Vilkka V, Spur BW, Lee TH. Leukotriene E4 and granulocytic infiltration into asthmatic airways. Lancet 1993;341:989–990. [DOI] [PubMed] [Google Scholar]
- 36.Lee E, Robertson T, Smith J, Kilfeather S. Leukotriene receptor antagonists and synthesis inhibitors reverse survival in eosinophils of asthmatic individuals. Am J Respir Crit Care Med 2000;161:1881–1886. [DOI] [PubMed] [Google Scholar]
- 37.Eum SY, Maghni K, Hamid Q, Eidelman DH, Campbell H, Isogai S, Martin JG. Inhibition of allergic airways inflammation and airway hyperresponsiveness in mice by dexamethasone: role of eosinophils, IL-5, eotaxin, and IL-13. J Allergy Clin Immunol 2003;111:1049–1061. [DOI] [PubMed] [Google Scholar]
- 38.Mathur M, Herrmann K, Li X, Qin Y, Weinstock J, Elliott D, Monahan J, Padrid P. TRFK-5 reverses established airway eosinophilia but not established hyperresponsiveness in a murine model of chronic asthma. Am J Respir Crit Care Med 1999;159:580–587. [DOI] [PubMed] [Google Scholar]
- 39.Hellings PW, Kasran A, Liu Z, Vandekerckhove P, Wuyts A, Overbergh L, Mathieu C, Ceuppens JL. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am J Respir Cell Mol Biol 2003;28:42–50. [DOI] [PubMed] [Google Scholar]
- 40.Fujitani Y, Trifilieff A. In vivo and in vitro effects of SAR 943, a rapamycin analogue, on airway inflammation and remodeling. Am J Respir Crit Care Med 2003;167:193–198. [DOI] [PubMed] [Google Scholar]
- 41.Trifilieff A, El Hashim A, Bertrand C. Time course of inflammatory and remodeling events in a murine model of asthma: effect of steroid treatment. Am J Physiol Lung Cell Mol Physiol 2000;279:L1120–L1128. [DOI] [PubMed] [Google Scholar]
- 42.Dorscheid DR, Wojcik KR, Sun S, Marroquin B, White SR. Apoptosis of airway epithelial cells induced by corticosteroids. Am J Respir Crit Care Med 2001;164:1939–1947. [DOI] [PubMed] [Google Scholar]
- 43.Holgate ST, Peters-Golden M, Panettieri RA, Henderson WR Jr. Roles of cysteinyl leukotrienes in airway inflammation, smooth muscle function, and remodeling. J Allergy Clin Immunol 2003;111:S18–S34. [DOI] [PubMed] [Google Scholar]
- 44.Barnes PJ. Corticosteroids, IgE, and atopy. J Clin Invest 2001;107:265–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Temann U-A, Prasad B, Gallup MW, Basbaum C, Ho SB, Flavell RA, Rankin JA. A novel role for murine IL-4 in vivo: induction of MUC5AC gene expression and mucin hypersecretion. Am J Respir Cell Mol Biol 1997;16:471–478. [DOI] [PubMed] [Google Scholar]
- 46.Fallon PG, Jolin HE, Smith P, Emson CL, Townsend MJ, Fallon R, Smith P, McKenzie AN. IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9, and IL-13. Immunity 2002;17:7–17. [DOI] [PubMed] [Google Scholar]
- 47.Henderson WR Jr, Chi EY, Maliszewski CR. Soluble IL-4 receptor inhibits airway inflammation following allergen challenge in a mouse model of asthma. J Immunol 2000;164:1086–1095. [DOI] [PubMed] [Google Scholar]
- 48.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science 1998;282:2258–2261. [DOI] [PubMed] [Google Scholar]
- 49.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 1998;282:2261–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kondo M, Tamaoki J, Takeyama K, Nakata J, Nagai A. Interleukin-13 induces goblet cell differentiation in primary cell culture from guinea pig tracheal epithelium. Am J Respir Cell Mol Biol 2002;27:536–541. [DOI] [PubMed] [Google Scholar]
- 51.Lee CG, Homer RJ, Cohn L, Link H, Jung S, Craft JE, Graham BS, Johnson TR, Elias JA. Transgenic overexpression of interleukin (IL)-10 in the lung causes mucus metaplasia, tissue inflammation, and airway remodeling via IL-13-dependent and -independent pathways. J Biol Chem 2002;277:35466–35474. [DOI] [PubMed] [Google Scholar]
- 52.Panettieri RA, Tan EM, Ciocca V, Luttmann MA, Leonard TB, Hay DW. Effects of LTD4 on human airway smooth muscle cell proliferation, matrix expression, and contraction in vitro: differential sensitivity to cysteinyl leukotriene receptor antagonists. Am J Respir Cell Mol Biol 1998;19:453–461. [DOI] [PubMed] [Google Scholar]
- 53.Espinosa K, Bosse Y, Stankova J, Rola-Pleszczynski M. CysLT1 receptor upregulation by TGF-β and IL-13 is associated with bronchial smooth muscle cell proliferation in response to LTD4. J Allergy Clin Immunol 2003;111:1032–1040. [DOI] [PubMed] [Google Scholar]
- 54.Phan SH, McGarry BM, Loeffler KM, Kunkel SL. Binding of leukotriene C4 to rat lung fibroblasts and stimulation of collagen synthesis in vitro. Biochemistry 1988;27:2846–2853. [DOI] [PubMed] [Google Scholar]
- 55.Phan SH, McGarry BM, Loeffler KM, Kunkel SL. Regulation of macrophage-derived fibroblast growth factor release by arachidonate metabolites. J Leukoc Biol 1987;42:106–113. [DOI] [PubMed] [Google Scholar]
- 56.Asakura T, Ishii Y, Chibana K, Fukuda T. Leukotriene D4 stimulates collagen production from myofibroblasts transformed by TGF-β. J Allergy Clin Immunol 2004;114:310–315. [DOI] [PubMed] [Google Scholar]
- 57.Boulet L, Belanger M, Carrier G. Airway responsiveness and bronchial-wall thickness in asthma with or without fixed airflow obstruction. Am J Respir Crit Care Med 1995;152:865–871. [DOI] [PubMed] [Google Scholar]
- 58.Chetta A, Foresi A, Del DM, Consigli GF, Bertorelli G, Pesci A, Barbee RA, Olivieri D. Bronchial responsiveness to distilled water and methacholine and its relationship to inflammation and remodeling of the airways in asthma. Am J Respir Crit Care Med 1996;153:910–917. [DOI] [PubMed] [Google Scholar]
- 59.Little SA, Sproule MW, Cowan MD, Macleod KJ, Robertson M, Love JG, Chalmers GW, McSharry CP, Thomson NC. High resolution computed tomographic assessment of airway wall thickness in chronic asthma: reproducibility and relationship with lung function and severity. Thorax 2002;57:247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Milanese M, Crimi E, Scordamaglia A, Riccio A, Pellegrino R, Canonica GW, Brusasco V. On the functional consequences of bronchial basement membrane thickening. J Appl Physiol 2001;91:1035–1040. [DOI] [PubMed] [Google Scholar]
- 61.Niimi A, Matsumoto H, Takemura M, Ueda T, Chin K, Mishima M. Relationship of airway wall thickness to airway sensitivity and airway reactivity in asthma. Am J Respir Crit Care Med 2003;168:983–988. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.