Abstract
Rationale: Serotonin is a pulmonary vasoconstrictor and smooth muscle cell mitogen. The serotonin transporter (SERT) is abundant in pulmonary vascular smooth muscle. Compared with the short (S) allele, the long (L) SERT promoter allele is associated with increased SERT transcription and more severe pulmonary hypertension in a cohort of patients with chronic obstructive pulmonary disease, and was more prevalent in a cohort with idiopathic pulmonary arterial hypertension (IPAH), compared with control subjects.
Objective: We hypothesized that the SERT L allele would associate with an earlier age at diagnosis and/or shorter survival interval in pulmonary arterial hypertension (PAH) than the S allele.
Methods: SERT promoters from 166 familial PAH (FPAH), 83 IPAH, and 125 control subjects were sequenced. One hundred twenty-seven of the patients with FPAH had a known mutation in bone morphogenetic protein receptor 2 (BMPR2).
Results: The mean age at diagnosis was 35.8 yr in patients with FPAH and 41.1 yr in patients with IPAH (p = 0.02). There were no significant differences in distribution of the LL, LS, or SS genotypes in IPAH, FPAH, or unaffected BMPR2 mutation carriers. In FPAH, the LL genotype was associated with an earlier age at diagnosis (p < 0.02).
Conclusions: In patients with IPAH, these SERT genotypes do not correlate with age at diagnosis or survival interval. In patients with FPAH, the LL genotype correlates with an earlier age at diagnosis than SL or SS, although survival among the groups was similar. The correlation of the SERT promoter polymorphism with age at diagnosis in FPAH suggests a possible relationship between the SERT and BMPR2.
Keywords: familial pulmonary arterial hypertension, 5-HT, 5-HTT, idiopathic pulmonary arterial hypertension, primary pulmonary hypertension, serotonin transporter
Much of what is known about the genetic basis of pulmonary arterial hypertension (PAH) is related to mutations in bone morphogenetic protein receptor 2 (BMPR2), which have been identified in over 75% of patients with familial PAH (FPAH) (1–3), but are likely to be responsible for over 90% of FPAH. This discovery has provided a genetic explanation for PAH as an inherited disease; however, only 20% of family members with a BMPR2 mutation develop disease (1, 4). In idiopathic PAH (IPAH), the frequency of mutations in BMPR2 is not well defined but is reported to be between 9 and 26% in small cohorts of patients (1, 5). These findings suggest that additional genes or possibly environmental factors are associated with the development of PAH, or may be related to the modification of disease expression. Although there are currently no known modifier genes, there are several possible candidates. In particular, common genetic variations (polymorphisms) in specific genes, such as the serotonin transporter (SERT), may influence development of PAH in the presence or absence of mutations in BMPR2.
Serotonin (5-HT) is a neurotransmitter that is a potent pulmonary vasoconstrictor and pulmonary artery smooth muscle cell (PASMC) mitogen (6, 7). Herve and coworkers reported elevated plasma 5-HT levels in a patient with a platelet storage disease who developed IPAH (8) and, later, in 16 patients with PAH of various etiologies (9). Recent studies have shown that cultured PASMCs from patients with IPAH demonstrate a greater proliferative response to 5-HT in comparison to cells from subjects without PAH, and that this proliferation is augmented in the setting of hypoxia (10). In addition, 5-HT can interact with cells either by binding to specific cell surface receptors, which transduce intracellular signaling, or they can be transported into the cell through the SERT. The pulmonary vasoconstrictor effects of 5-HT are transmitted via binding to receptors, and the mitogenic actions of 5-HT are transduced via the SERT pathway (6, 11, 12).
The SERT gene is localized to 17q11.1–q12, spans 31 kb, and consists of 14 exons (13). The long (L)/short (S) functional polymorphism within the promoter region, which has allelic variants of different transcriptional efficiencies, is located 1 kb upstream of the transcriptional initiation site and consists of 16 repeat elements. The polymorphism consists of a 44-bp insertion or deletion involving repeat elements 6 to 8 (14). A frequently observed A to G transition (HTT-179AG) observed upstream of the start codon within the L allele, was predicted to change the L allele phenotype (rs25531; http://www.ncbi.nlm.nih.gov/SNP).
There is evidence suggesting a correlation of the SERT L/S polymorphism with the development of PAH. The L allele is associated with a two- to threefold higher rate of gene transcription compared with the S allele (14). In one report, the L allele was found to be more prevalent in patients with IPAH than in control subjects (7, 15). In a study of patients with chronic obstructive pulmonary disease, 56% of patients had the LL genotype, which was associated with increased SERT transcription and more severe pulmonary hypertension (PH) (16). The role of SERT polymorphisms in the penetrance and severity of PAH is unknown.
The purpose of this investigation was to study whether polymorphisms in the SERT gene would correlate with disease variables, such as age at diagnosis and/or survival interval in IPAH and FPAH. In addition, we sought to define the prevalence of the SERT genotypes in our patients with PAH and control subjects, and to determine whether the prevalence of the SERT genotypes would correlate with penetrance of disease in FPAH. To reduce the risk of a type II error, we chose to test the SERT genotype prevalence in two additional IPAH cohorts from separate geographic regions: Columbia University in New York and Université Paris-Sud in Clamart, France. Some results of this study were previously reported in abstract form at the American Thoracic Society meeting in May 2005 (17).
METHODS
Study Population
The diagnosis of PAH was made by mean pulmonary arterial pressure of more than 25 mm Hg with a pulmonary capillary or left atrial pressure of less than 15 mm Hg and exclusion of other causes of PAH in accordance with accepted international standards of diagnostic criteria (1, 2, 4, 5, 21). Although BMPR2 mutations have been found in some sporadic cases, in our IPAH cohort, there were no known exonic BMPR2 mutations. In the Columbia and French IPAH groups, those with a known BMPR2 mutation were excluded. In the 166 subjects with FPAH, 127 individuals had a known BMPR2 mutation at the time of analysis. We now know that more than 90% of our patients with FPAH have either exonic or intronic mutations in BMPR2 that cause transcriptional errors (3).
In this study, DNA and clinical information was obtained from cohorts of patients with IPAH or FPAH. Individuals with secondary PH, PAH related to hereditary hemorrhagic telangiectasia, PAH related to anorexigen use, and pulmonary venoocclusive disease were excluded. The remaining samples are from BMPR2 mutation carriers without PAH and control subjects. The married-in control subjects are a cohort that is closely matched with patients in age and for environmental and social factors. The remaining control subjects are unrelated, healthy, 1:1 male to female, and primarily white, similar to our patients. The DNA was obtained from the Human Variation Panel at the Coriell Institute (Camden, NJ). Informed consent was obtained from all patients and family members before sample collection. Samples were obtained at the time of clinic visits or hospitalization or by mail via a kit for collection of DNA and whole blood. The institutional review board at Vanderbilt University Medical Center approved the protocol, and informed consent was obtained from all participants. The DNA bank is housed in the Center for Human Genetics Research at Vanderbilt University School of Medicine. DNA from a French IPAH population from Université Paris-Sud was sent directly to our lab for genotype analysis. Genotypic information from an IPAH cohort at Columbia University was sent to our institution after institutional review board approval.
Study population demographics and hemodynamic information, as well as genotype analysis, are detailed in the online supplement.
Statistical Analysis
The age at diagnosis was defined as the age at which the patient underwent diagnostic right heart catheterization. Survival interval was the time period, in years, from year of diagnostic catheterization to year of death. Patients who were alive at the time of survival measure were censored in the survival analysis. Analyses were performed with SERT genotypes categorized as LL versus non-LL, and LL versus LS versus SS. Because functional data show similar transport activity for LS and SS genotypes (7), we chose to categorize our individuals and report our results as LL versus non-LL (SL or SS). The Kaplan-Meier method was used to assess the cumulative proportion of disease-free persons based on SERT genotypes, in relation to age at diagnosis or survival interval. The log-rank test was used to obtain p values comparing the survival curves. p values less than 0.05 with two-tailed tests were considered statistically significant. The Hardy-Weinberg equation tested allelic equilibrium/disequilibrium for the SERT L/S polymorphism. A χ2 test or Fisher's exact test was used to assess categoric comparisons of data. Differences in the means of variables between groups were measured by a nonparametric t test (Wilcoxon rank-sum test). Statistical analyses were performed using SPSS for Windows (version 13.0; SPSS, Inc., Chicago, IL).
RESULTS
We determined the genotypes of 374 individuals: 83 patients with IPAH, 99 patients with FPAH from 66 families, 67 unaffected carriers of a BMPR2 mutation, and 125 control subjects. Patients with FPAH had an earlier age at diagnosis in comparison to IPAH (35.8 ± 14.1 [SD] vs. 41.1 ± 14.4 yr, p = 0.02), although survival intervals did not differ (4.9 ± 0.5 [SEM] vs. 5.7 ± 0.6 yr, p = 0.3; Table 1).
TABLE 1.
AGE AT DIAGNOSIS AND SURVIVAL IN PATIENTS WITH FAMILIAL PULMONARY ARTERIAL HYPERTENSION VERSUS PATIENTS WITH IDIOPATHIC PULMONARY ARTERIAL HYPERTENSION
| Age at Diagnosis (yr ± SD)* | Survival Interval (yr ± SD)† | |
|---|---|---|
| FPAH | 35.8 ± 14.1 | 4.9 ± 0.5 |
| IPAH | 41.1 ± 14.4 | 5.7 ± 0.6 |
Definition of abbreviations: FPAH = familial pulmonary arterial hypertension; IPAH = idiopathic pulmonary arterial hypertension.
p = 0.02.
p = 0.3.
The SERT genotypes were not significantly different among these groups (Table 2). All cohorts were in Hardy-Weinberg equilibrium. Among the three cohorts of patients with IPAH (from Vanderbilt University, Columbia University, and Hôpital Antoine Beclere), the SERT genotype distribution was similar among groups (Table 3). SERT genotype did not appear to affect disease penetrance, as the distribution of genotypes was not different between patients with FPAH and those unaffected family members who carry a BMPR2 mutation (p = 0.5; Table 2).
TABLE 2.
DISTRIBUTION OF SERT GENOTYPES
| SERT Group
|
||||
|---|---|---|---|---|
| Group | LL | SL | SS | Total |
| Vanderbilt IPAH | 31 (37.3%) | 38 (45.8%) | 14 (16.9%) | 83 (100.0%) |
| FPAH obligate | 25 (37.3%) | 29 (43.4%) | 13 (19.4%) | 67 (100.0%) |
| FPAH affected | 29 (29.3%) | 49 (49.5%) | 21 (21.2%) | 99 (100.0%) |
| Control subjects | 43 (34.4%) | 63 (50.4%) | 19 (15.2%) | 125 (100.0%) |
| Total | 128 (34.2%) | 179 (47.9%) | 67 (17.9%) | 374 (100.0%) |
Definition of abbreviations: FPAH = familial pulmonary arterial hypertension; IPAH = idiopathic pulmonary arterial hypertension; SERT = serotonin transporter.
For all values, p = 0.81.
TABLE 3.
DISTRIBUTION OF SERT GENOTYPES AMONG THREE DISTINCT POPULATIONS
| SERT Group
|
||||
|---|---|---|---|---|
| Group | LL | SL | SS | Total |
| IPAH from Vanderbilt | 31 (37.8%) | 37 (45.1%) | 14 (17.1%) | 82 (100.0%) |
| IPAH from Columbia | 22 (33.8%) | 30 (46.2%) | 13 (20.0%) | 65 (100.0%) |
| IPAH from France | 25 (32.9%) | 32 (42.1%) | 19 (25.0%) | 76 (100.0%) |
| Total | 78 (35.0%) | 99 (44.4%) | 46 (20.6%) | 223 (100.0%) |
For definition of abbreviations, see Table 2.
For all values, p = 0.80.
The prevalence of the L single nucleotide polymorphism (L-SNP; HTT-179AG) was 12% in both IPAH and FPAH and was not statistically different among the control subjects or the unaffected mutation carriers.
In IPAH, SERT genotype did not show a statistically significant association with age at diagnosis, in any allelic comparison: LL versus non-LL, or LL versus LS versus SS. In fact, there was a trend for those with the LL genotype to have a later age at diagnosis than those with the SL and SS genotypes (mean age at diagnosis, respectively: 44.8 ± 2.7 [SD] vs. 39.1 ± 2.0 yr, p = 0.13). Because the age at diagnosis for three geographically distinct IPAH cohorts was not statistically different (Vanderbilt, 41.1; Columbia, 42.3; and French, 41.2 yr; p = 0.87), the groups were combined to increase statistical power for age at diagnosis analysis for SERT association. In this larger IPAH group of 205 individuals, SERT genotype still did not correlate with age at diagnosis (Figure 1). Survival interval was not available for all three groups; however, in the Vanderbilt IPAH group, SERT genotype did not have a statistically significant association with survival (Figure 2).
Figure 1.
Correlation of serotonin transporter (SERT) promoter L/S genotype with age at diagnosis in IPAH from Vanderbilt University, Columbia University, and Clamart, France (p = 0.35). LL versus SL or SS: LL, black line; SL or SS, gray line.
Figure 2.
Correlation with SERT promoter L/S genotype with survival interval in subjects with idiopathic pulmonary arterial hypertension (IPAH; Vanderbilt). There was no significant correlation with SERT genotype with survival interval in IPAH (p = 0.26). LL versus SL and SS: LL (n = 27), black line; SL or SS (n = 51), gray line.
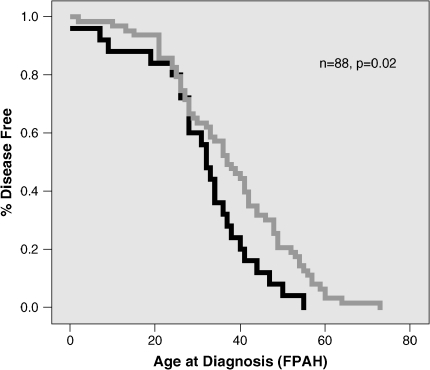
In patients with FPAH, however, the LL genotype did correlate with an earlier age at diagnosis (p = 0.02; Figure 3), although survival intervals did not differ among the SERT genotypes (p = 0.9; Figure 4). When analyzing the groups after correction of the SERT L/S genotype for the L-SNP (HTT-179AG), there was no statistically significant association with age at diagnosis or survival interval.
Figure 3.
Correlation of SERT promoter L/S genotype with age at diagnosis in subjects with familial PAH (FPAH). The LL genotype associated with an earlier age of disease onset in FPAH (p = 0.02). LL versus SL and SS: LL (n = 25), black line; SL or SS (n = 63), gray line.
Figure 4.
Correlation of SERT promoter L/S genotype with survival interval in FPAH. There was no significant correlation with SERT genotype with survival interval in FPAH (p = 0.92). LL versus SL and SS: LL (n = 22), black line; SL or SS (n = 51), gray line.
DISCUSSION
A number of studies have implicated 5-HT in the development of PH, through receptor and/or transporter signaling. The SERT is believed to be responsible for the mitogenic effects of 5-HT, and increased levels of SERT mRNA have been demonstrated in cultured PASMCs in rats subjected to long-term hypoxia (6). Knockout mice for the SERT gene are protected from developing hypoxia-induced PAH, despite greater acute vasoconstriction than wild-type mice (10). Anorexigens have been associated with the development of PAH and the metabolites of these drugs bind to the SERT acting as 5-HT substrates, and are translocated into smooth muscle cells causing intracellular effects similar to or greater than 5-HT itself (18, 19). Infusion of fluoxetine, a selective SERT inhibitor, completely reverses the PH in rats induced by monocrotaline (20). In addition to these studies, the SERT promoter L/S polymorphism has been associated with more severe PH in patients with chronic obstructive pulmonary disease (16), and the LL genotype was previously reported as more prevalent in patients with IPAH than in control subjects (15). Given the evidence implicating this SERT polymorphism in the pathogenesis of PAH, we aimed to describe the prevalence of the SERT L/S promoter polymorphism in our population of patients with IPAH and FPAH, and we hypothesized that, in PAH, the SERT LL genotype would associate with earlier age at diagnosis and/or shorter survival after diagnosis than the LS or SS genotype. We also predicted that L alleles containing the HTT-179AG SNP within the insertion/deletion site would behave phenotypically like S alleles.
In our study, patients with FPAH had an earlier age at diagnosis in comparison to those with IPAH, although survival intervals did not differ. This association supports a recent publication by Sztrymf and colleagues who found a mean age at diagnosis in patients with FPAH of 31 ± 15 yr versus a mean age at diagnosis patients with IPAH of 45 ± 18 yr (p = 0.002) (21). Past studies have suggested that IPAH and FPAH are histologically and clinically identical (1, 4). Perhaps the difference in age at diagnosis could be explained by an ascertainment bias, as those who are in families with PAH are educated about the disease, may be aware of their possible risk, and are likely to seek medical evaluation for symptoms of disease sooner. They are thus apt to undergo diagnostic testing earlier in the course of their illness than patients with nonfamilial disease. Despite the earlier age at diagnosis in patients with FPAH, the survival intervals between patients with FPAH and IPAH were not different. It is not clear why those individuals who are diagnosed earlier would not have better access to treatment and therefore live longer, as it has been shown that epoprostenol improves survival in patients with IPAH (22). Many of the patients in this cohort with FPAH were diagnosed before the availability of epoprostenol or other more recently approved PAH medications, and information on how they were treated is not known. Therefore, the effects of therapeutic interventions on the survival data are also not known. However, the lack of difference in survival between the two groups suggests that no systematic effect biased these results.
Another interesting finding is the lack of difference in SERT genotype distribution among patients with IPAH, patients with FPAH, unaffected BMPR2 mutation carriers, and control subjects. In a previous report, the LL genotype was found in 65% of French patients with IPAH (n = 89) compared with only 27% of control subjects (n = 84) (15, 16). Given this discrepancy, we sought to confirm our findings and examined other cohorts of patients with IPAH from Columbia University and a second cohort from France (Table 3). Each of these cohorts had similar prevalence data, confirming a similar distribution of SERT genotypes in patients with IPAH and control subjects. All groups were in Hardy-Weinberg equilibrium. We do not have another explanation for the difference in genotype distribution in the original French IPAH cohort, except that it is possible that a false-positive association related to small sample bias size occurred. This phenomenon is common in small cohorts and in rare diseases. Alternatively, true differences between their population and our three geographically separate IPAH cohorts may exist that have yet to be identified.
In addition, because the SERT genotypes in patients with FPAH and unaffected BMPR2 mutation carriers did not differ, it appears that this polymorphism does not affect disease penetrance. We did not evaluate hemodynamic or other clinical information that could be influenced by SERT due to inaccessible data, but given our current findings, positive association seems unlikely. There are other polymorphisms within SERT—an intron 2 variable nucleotide tandem repeat and a G/T SNP in the 3′ untranslated region, for example—and whether these polymorphisms may enable SERT to act as a modifier gene has yet to be determined.
In IPAH, the SERT L/S polymorphism did not associate with age at diagnosis or survival interval. In fact, there was a trend for the LL to associate with a later age at diagnosis than the SL and SS in both our Vanderbilt and in the merged IPAH cohort, which was contrary to our hypothesis. In FPAH, however, the LL genotype did correlate with an earlier age at diagnosis. The only obvious difference between the patients with IPAH and those with FPAH is the presence of a BMPR2 mutation in patients with FPAH. New data from our center suggest that the true prevalence of BMPR2 mutations in FPAH is greater than 90%. Southern blot analysis and reverse transcriptase–polymerase chain reaction assays have helped to identify large gene alterations, such as insertions, deletions, inversions, and rearrangements, not discovered by conventional exon sequencing (3). These results suggest that the true prevalence of BMPR2 mutations in families is much higher than initially believed. Our observation in the FPAH group suggests that there may be some interaction with SERT and BMPR2. How these two transmembrane proteins may interact is unclear, although it is possible that they may share downstream signaling pathways.
More work to investigate the possible relationship between BMPR2 and SERT needs to be pursued. Functional studies measuring 5-HT transport in cells with and without a BMPR2 mutation may be one avenue for this pursuit, although, due to the possibility of a positive correlation due to chance alone, these findings require additional validation.
Supplementary Material
Supported by National Institutes of Health grants NHLBI PO1 072058 and GCRC 00095.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200509-1361OC on December 9, 2005
Conflict of Interest Statement: E.D.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.H.N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.E.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. I.M.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L.A.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.A.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.C.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.A.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.R.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Columbia University required J.A.M. to submit a patent on BMPR2 mutations, but there is no conflict here. J.A.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.E.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.J.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.J.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.A.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Newman JH, Trembath RC, Morse JA, Grunig E, Loyd JE, Adnot S, Coccolo F, Ventura C, Phillips JA III, Knowles JA, et al. Genetic basis of pulmonary arterial hypertension: current understanding and future directions. J Am Coll Cardiol 2004;43(12, Suppl S):33S–39S. [DOI] [PubMed] [Google Scholar]
- 2.Newman JH, Fanburg BL, Archer SL, Badesch DB, Barst RJ, Garcia JG, Kao PN, Knowles JA, Loyd JE, McGoon MD, et al. Pulmonary arterial hypertension: future directions: report of a National Heart, Lung and Blood Institute/Office of Rare Diseases workshop. Circulation 2004;109:2947–2952. [DOI] [PubMed] [Google Scholar]
- 3.Cogan JD, Vnencak-Jones CL, Phillips JA III, Lane KB, Wheeler LA, Robbins IM, Garrison G, Hedges LK, Loyd JE, et al. Gross BMPR2 gene rearrangements constitute a new cause for primary pulmonary hypertension. Genet Med 2005;7:169–174. [DOI] [PubMed] [Google Scholar]
- 4.Loyd JE, Primm RK, Newman JH. Familial primary pulmonary hypertension: clinical patterns. Am Rev Respir Dis 1984;129:194–197. [DOI] [PubMed] [Google Scholar]
- 5.Thomson JR, Machado RD, Pauciulo MW, Morgan NV, Humbert M, Elliott GC, Ward K, Yacoub M, Mikhail G, Rogers P, et al. Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding BMPR-II, a receptor member of the TGF-beta family. J Med Genet 2000;37:741–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eddahibi S, Fabre V, Boni C, Martres MP, Raffestin B, Hamon M, Adnot S. Induction of serotonin transporter by hypoxia in pulmonary vascular smooth muscle cells: relationship with the mitogenic action of serotonin. Circ Res 1999;84:329–336. [DOI] [PubMed] [Google Scholar]
- 7.Marcos E, Fadel E, Sanchez O, Humbert M, Dartevelle P, Simonneau G, Hamon M, Adnot S, Eddahibi S. Serotonin-induced smooth muscle hyperplasia in various forms of human pulmonary hypertension. Circ Res 2004;94:1263–1270. [DOI] [PubMed] [Google Scholar]
- 8.Herve P, Drouet L, Dosquet C, Launay JM, Rain B, Simonneau G, Caen J, Duroux P. Primary pulmonary hypertension in a patient with a familial platelet storage pool disease: role of serotonin. Am J Med 1990;89:117–120. [DOI] [PubMed] [Google Scholar]
- 9.Herve P, Launay JM, Scrobohaci ML, Brenot F, Simonneau G, Petitpretz P, Poubeau P, Cerrina J, Duroux P, Drouet L. Increased plasma serotonin in primary pulmonary hypertension. Am J Med 1995;99:249–254. [DOI] [PubMed] [Google Scholar]
- 10.Eddahibi S, Hanoun N, Lanfumey L, Lesch KP, Raffestin B, Hamon M, Adnot S. Attenuated hypoxic pulmonary hypertension in mice lacking the 5-hydroxytryptamine transporter gene. J Clin Invest 2000;105:1555–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacLean MR, Sweeney G, Baird M, McCulloch KM, Houslay M, Morecroft I. 5-Hydroxytryptamine receptors mediating vasoconstriction in pulmonary arteries from control and pulmonary hypertensive rats. Br J Pharmacol 1996;119:917–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eddahibi S, Raffestin B, Hamon M, Adnot S. Is the serotonin transporter involved in the pathogenesis of pulmonary hypertension? J Lab Clin Med 2002;139:194–201. [DOI] [PubMed] [Google Scholar]
- 13.Lesch KP, Balling U, Gross J, Strauss K, Wolozin BL, Murphy DL, Riederer P. Organization of the human serotonin transporter gene. J Neural Transm Gen Sect 1994;95:157–162. [DOI] [PubMed] [Google Scholar]
- 14.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996;274:1527–1531. [DOI] [PubMed] [Google Scholar]
- 15.Eddahibi S, Humbert M, Fadel E, Raffestin B, Darmon M, Capron F, Simonneau G, Dartevelle P, Hamon M, Adnot S. Serotonin transporter overexpression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension. J Clin Invest 2001;108:1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eddahibi S, Chaouat A, Morrell N, Fadel E, Fuhrman C, Bugnet AS, Dartevelle P, Housset B, Hamon M, Weitzenblum E, et al. Polymorphism of the serotonin transporter gene and pulmonary hypertension in chronic obstructive pulmonary disease. Circulation 2003;108:1839–1844. [DOI] [PubMed] [Google Scholar]
- 17.Willers ED, Newman JH, Loyd JE, Robbins IM, Wheeler LA, Prince MA, Cogan JD, Phillips JA III. Serotonin transporter (SERT) promoter polymorphisms and age of onset in familial and idiopathic pulmonary arterial hypertension (PAH). Poster presented at the American Thoracic Society conference, San Diego, CA, May 22, 2005.
- 18.Rothman RB, Ayestas MA, Dersch CM, Baumann MH. Aminorex, fenfluramine, and chlorphentermine are serotonin transporter substrates: implications for primary pulmonary hypertension. Circulation 1999;100:869–875. [DOI] [PubMed] [Google Scholar]
- 19.Lee SL, Wang WW, Fanburg BL. Dexfenfluramine as a mitogen signal via the formation of superoxide anion. FASEB J 2001;15:1324–1325. [DOI] [PubMed] [Google Scholar]
- 20.Guignabert C, Raffestin B, Benferhat R, Raoul W, Zadigue P, Rideau D, Hamon M, Adnot S, Eddahibi S. Serotonin transporter inhibition prevents and reverses monocrotaline-induced pulmonary hypertension in rats. Circulation 2005;111:2812–2819. [DOI] [PubMed] [Google Scholar]
- 21.Sztrymf B, Francoual J, Sitbon O, Labrune P, Jambou M, Pous C, Simonneau G, Humbert M. Clinical, haemodynamic and genetic features of familial pulmonary arterial hypertension. Rev Mal Respir 2004;21:909–915. [DOI] [PubMed] [Google Scholar]
- 22.Kuhn KP, Byrne DW, Arbogast PG, Doyle TP, Loyd JE, Robbins IM. Outcome in 91 consecutive patients with pulmonary arterial hypertension receiving epoprostenol. Am J Respir Crit Care Med 2003;167:580–586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.