Abstract
The labeling of over-the-counter (OTC) drugs is critical to their safe and effective use, and certain warnings are meant to be read at the point of purchase (POP). Examples include (i) warnings that alert consumers to the fact that the package is not child-resistant and (ii) warnings that alert consumers to potential product tampering. U.S. law mandates these warnings be “conspicuous” and “prominent” so that it is likely that consumers will read them before leaving the store. Our objective was to quantify the relative prominence and conspicuousness of these warnings. Sixty-one participants reviewed the packages of 5 commercially available analgesics to evaluate the prominence and conspicuousness of these warnings. Evaluated data included (i) the time spent examining the warnings compared with other areas of the label (using a bright pupil eye tracker), (ii) the ability to recall information from the OTCs viewed, and (iii) the legibility of the warnings relative to other elements of the labels (as measured by ASTM D7298-06). Eye-tracking data indicated that warnings were viewed by fewer participants and for less time than other elements of the packages. Recall and legibility data also indicated that the warning statements compared unfavorably with other elements of the labels tested. Evidence presented in this study suggests that 2 required warnings on 5 different OTCs are not prominent or conspicuous when compared with other elements of tested labels.
Keywords: conspicuous, drug labels, labeling, OTC drugs, prominent
It has been proposed that information processing occurs in a stage-like progression and includes 4 steps (1). To be effective, (i) information must be noticed; (ii) information must be encoded into memory, most commonly through visual routes (for this to occur, text must be legible); (iii) the encoded message must then be comprehended; and (iv) the encoded message must, finally, move the reader to action or compliance.
For some products, successful completion of the 4 steps is optional. However, much of the information contained on the packaging of medical products is critical for their safe and effective use. As such, for these products, completion of the 4 steps is compulsory.
The ability of patients and consumers to read and interpret pharmaceutical labels, both prescription and over-the-counter (OTC), has long been recognized as important (2–30). However, most research has been quite fragmented in approach, separating the steps of information processing. Some studies examine the ability of consumers to notice information on drug labels (step 1) (27, 31, 32), and others look at a consumer's ability to read (step 2) (21, 27, 29, 30, 33) or interpret (step 3) (7, 8, 21, 34) specific elements of drug labels. Few, if any, consider the entire process when analyzing informational layouts.
Because branding plays a critical role in consumer purchasing decisions, OTC manufacturers are motivated to ensure that consumers attend to brand information, as opposed to other information (e.g., warnings), at the point of purchase (POP). However, warnings that alert the consumer to the potential tampering of a product [tamper evident [TE] warnings) or the fact that the package does not have a child-resistant feature (CR warnings) are intended to be read in the store to inform purchase decisions (35).
As such, U.S. law requires TE and CR warnings to be “prominent” (36) and “conspicuous” (37). However, there is limited operational guidance from the federal government regarding what constitutes a “prominent” or “conspicuous” label statement (38). This is despite the fact that these terms are used in the U.S. documents that require TE and CR warnings (36, 37) as well as other official mandates for the labels of various products (39–43).
The objective of this study was to determine the “conspicuousness” and “prominence” of the CR and TE warnings relative to other label elements (referred to as gaze zones) for 5 OTC products that contain acetaminophen (Fig. 1 and Table S1). Acetaminophen was targeted because 1 in 5 American adults consume this drug in a given week, making it one of the most commonly used drugs in the United States (44–47).
Fig. 1.
Stimulus material, with gaze zones identified. Note that packages 1–2 drug facts zones (and a portion of the drug facts zone on 3 and 4) are present on faces not pictured.
The standardized time a participant spent attending to a given gaze zone and the number of people that failed to record any time in the warning zones, as measured by eye tracking, were considered to be quantification of step 1 of the information-processing model, or the “noticeability” of a given gaze zone. To evaluate step 2 of the model, we used a Lockhart Legibility Instrument, a tool that quantifies relative legibility (ASTM D7298-06). Additionally, participants were asked to recall any information that they could regarding the pain relievers they viewed in the course of the study. This was intended to serve as a rough measure of the ability of participants to navigate steps 1–2.
Results
Participants.
Usable data were obtained from 43 men and 18 women. The average age of participants was 25 years, with an SD of 6.3 years, with ages ranging from 20 to 51 years. Forty participants (average age, 21 years; SD, 1.3 years) did not have children younger than 10 years living in their homes. Twenty-one participants (average age, 31 years; SD, 6.7 years) did have children living in their homes; the average age of the children was 35 months (SD, 24.4 months).
Eye Tracking.
Standardized time spent in each label zone.
The statistical model for time spent in a given zone included the fixed effect of label zone, the random effect of package, and their mutual interaction. Additional factors considered for model selection included age and the presence of children in a household. In addition, heterogeneity of residual variances across label zones was identified, and zone-specific variance components were estimated. Neither age nor the presence of children proved to improve the model fit, and thus these were excluded from the model.
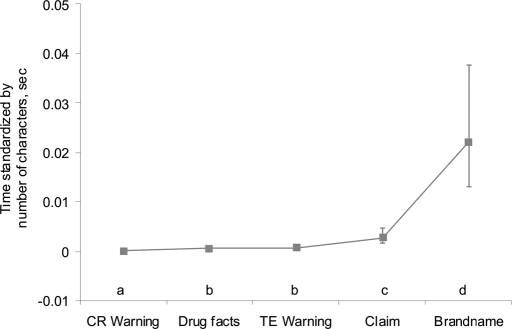
Fig. 2 depicts estimated time (standardized by dividing by the number of typographical characters present in the gaze zone) spent in each of the 5 label gaze zones of interest for all OTCs tested.
Fig. 2.
Time (estimated least-square means) in seconds, standardized by number of characters, for each gaze zone across all packages. Bars indicate the extent of the estimated 95% confidence interval for each gaze zone. Letters indicate differences in standardized time (P < 0.05) between gaze zones (a, b, c, and d).
Results indicated a significant difference in standardized time (P < 0.05) spent in gaze zones. As such, pairwise comparisons between gaze zones were conducted (Fig. 2).
Subjects attended to the CR warning significantly less standardized time than any other zone tested (P < 0.05). The TE warning and drug facts zones were attended for longer standardized time than the CR warning but significantly less than the claim statement or brand name. Not surprisingly, the standardized time that subjects spent attending to brand name was significantly greater than for any other zone tested.
Failure to record time in gaze zones.
The proportion of individuals that failed to record time in each gaze zone for each package is also presented (Figs. 3 and 4). The statistical model included the fixed effects of gaze zone and the presence of children, as well as their 2-way interaction. The random blocking factor of package was included in the model. No interaction was identified between label zones and the presence of children (P > 0.50). Significant differences in the proportion of failures to record time were identified between gaze zones (P < 0.0001). However, the evidence did not support a relationship between the proportion of failures to record time in a gaze zone and the presence of children in the home (P > 0.50).
Fig. 3.
Number and percentage of study participants that failed to see a gaze zone for all packages tested. CR, child resistant; TE, tamper evident; BN, brand name; DF, drug facts.
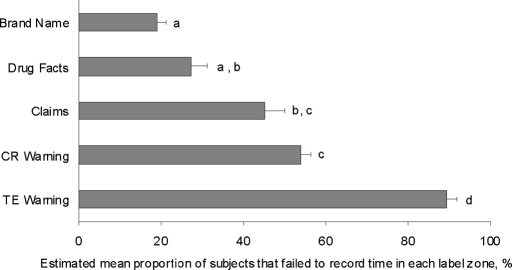
Fig. 4.
Estimated mean proportion of participants that failed to see a label gaze zone (across all packages). Letters (a, b, and c) indicate differences between gaze zones (P < 0.05). Bars represent standard errors for each estimated mean.
More than 80% of the participants failed to record time in the TE warning gaze zones across all packages (Figs. 3 and 4). This means <20% of participants registered any time in the warning zone (step 1 of the information-processing model). In fact, the TE warning registered the greatest proportion of failure to record time compared with any other gaze zone (P < 0.05; Fig. 4). The CR warning zone had the second greatest proportion of participants failing to record any time (more than 50%). Failures to record time in the CR warning were greater than for brand name and drug facts zones (P < 0.05 in both cases).
Recall.
Recall data were coded for each participant for 11 researcher-identified categories. The statistical model included the fixed effects of researcher-identified category and the presence of children in the home. The interaction between category and child presence did not improve the model fit, and thus was excluded from the final model.
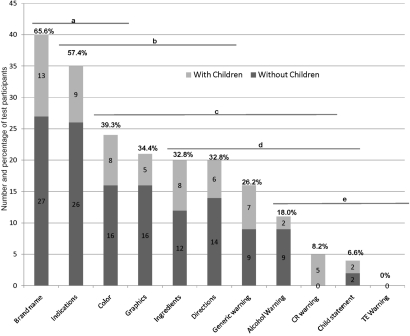
Fig. 5 indicates the number and percentage of participants that indicated an affirmative response in each of the 11 categories. Recall ability varied by information category (P < 0.05; Fig. 5) but was not associated with the presence of children (P = 0.30). The warning categories (alcohol warning, CR warning, child statement, and TE warning) were the least frequently recalled categories and ranged from 0% (estimated least-square means for recall of TE warnings) to 18% (estimated least-square means for recall of the alcohol warning). In contrast, brand name, indications, and package color were the most frequently recalled pieces of information, with ≈66% of the participants recalling the brand name and almost 40% recalling the package color.
Fig. 5.
Number and percentage of test participants that recalled 1 or more items in researcher-identified categories. Lines and letters are indicative of statistical significance. For instance, the proportion of people recalling brand name, indications, and color showed no evidence of statistical significance when compared (a), but brand name was recalled significantly more frequently than graphics (bc), ingredients (bcd), etc.
Legibility.
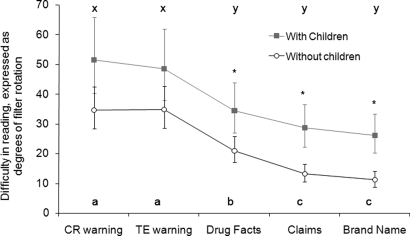
Legibility index was recorded in degrees of the rotation of a polarizing filter inside the instrument; the greater the degree of rotation, the more light that reaches the subject. As such, the more difficulty a participant has deciphering the message, the higher his or her degree of rotation (an indicator of step 2 of the information-processing model). The statistical model of best fit included the fixed effects of gaze zone and the presence of children, as well their 2-way interactions. The model included visual acuity for each participant as a covariate. A significant interaction was identified between gaze zone and the presence of children in the home (P < 0.01; Fig. 6).
Fig. 6.
Difficulty in reading, expressed as estimated least-square mean degrees of filter rotation by label gaze zone and the presence of children. Bars indicate the extent of the 95% confidence interval for each group. Asterisks (*) indicate differences in degrees of filter rotation between the presence of children in the home within the corresponding label zones. Letters indicate differences in degrees of filter rotation between label zones within participants with children (x and y) and participants without children (a, b, and c), respectively.
The estimated least-square means and associated 95% confidence intervals of degrees of filter rotation across gaze zones, by the presence of children, are presented in Fig. 6.
The TE and CR warning gaze zones required the greatest degrees of rotation for participants both with and without children (P < 0.05, respectively; labeled as a, b, c or x, y in Fig. 6). This implies that the warnings were the least legible of the gaze zones examined. In turn, the zones for brand name and claims required the least degrees of rotation to be legible among the gaze zones examined (P < 0.05, respectively; Fig. 6). This indicates that brand name and claims were more legible than all other tested zones for both groups of participants. Additionally, the degrees of filter rotation for the brand name, claims, and drug facts were significantly greater for participants with children compared with those without children (P < 0.05, respectively; Fig. 6, asterisk).
Discussion
The study presented here used 3 methods to quantify participants' ability to notice and encode 2 warnings (CR and TE) relative to 3 other label elements (drug facts, brand name, and claims statements) on the packages of OTC drugs containing acetaminophen (Fig. 1 and Table S1). The CR and TE warnings are required by current U.S. law (37) and regulation (36) to be conspicuous and prominent, yet there is little operational guidance available from the federal government regarding what constitutes prominence or conspicuousness.
Although many pieces of information are critical for the safe and effective use of OTC products, these warnings were targeted for study because they are intended to be read at the POP. The U.S. Consumer Product Safety Commission (CPSC) indicates that the availability of non-CR packages for OTC medications is one of the reasons that the unintentional poisoning of children still occurs (48). If a product without a CR feature is purchased and brought into an environment where children are present, the warning has failed. Likewise, the Food and Drug Administration has indicated that, “It is important that the consumer view the tamper-evident statement before purchase and use of the product” (35).
For this reason, study participants were provided a trigger text that involved a POP scenario and allowed to view each stimulus package (Fig. 1) for a period of 10 s while wearing the eye tracker (see Methods). Research has indicated that consumers spend an average of 5–7 s at the POP when making a purchase decision (49); 10 s represented a conservative estimate for the POP scenario. It is important to note that different trigger texts (e.g., an emergency situation or use scenario) would likely have changed the results.
Data collected with the eye tracker indicate study participants spent very little time, if any, attending the TE and CR warnings, whereas most of the attention was drawn to the brand name (Fig. 2). In viewing this information, it is important to remember that the TE zone contained appreciably fewer characters than the drug facts zone. So, despite the fact that subjects spent more total time in the drug facts zone (compared with the TE zone; see Table S1), no statistical difference was identified because of the standardization technique. (Time was standardized by dividing by the number of typographical characters per zone; see Table S1.)
Eye-tracking data were also analyzed in an attribute fashion. Of particular interest were the results obtained for the TE warning. A majority of study participants failed to record ANY time in the TE gaze zone for all 5 products tested (Fig. 3), and the proportion of people that examined the TE gaze zone across all products was found to be significantly less than any of the other gaze zones tested (Fig. 4).
Recall data further corroborated the findings of the eye-tracking study; warning information of all types (alcohol-related warnings, CR warnings, child statements, and the TE warning) were recalled significantly less frequently than other elements. Notably, the TE information was not mentioned by a single subject (0%; Fig. 5).
Legibility was also quantified. The CR warning and the TE warning were found to be significantly less legible than the other gaze zones (drug facts, claims, and brand name) (Fig. 6). This was true for both groups of test subjects: those with children in their homes and those without children in their homes.
Data suggest some information was less legible for people that resided with children than for subjects without (Fig. 6). This difference was found to be statistically significant when the results for drug facts, claims, and brand name were analyzed (Fig. 6, asterisk). However, it is not likely that the presence of children in the house was the cause of lowered legibility. It is more likely that this is because people with children were older (average age, 31 years; SD, 6.7 years) than those without children in their homes (average age, 21 years; SD, 1.3 years). Physiological changes in the older groups' eyes demanded the need for more light than the younger group. Nonetheless, the 2 groups followed the same general pattern when comparing the legibility of the gaze zones of interest (Fig. 6 and Limitations for further discussion).
Forward Path.
Evidence presented here suggests that 2 required warnings (TE and CR) on 5 different OTC analgesics are not prominent or conspicuous relative to other elements of labels that are used for marketing purposes. This implores exploration of the reason for the failure (i.e., Is it as simple as changing the graphic design of OTC packages? Is it an issue of consumer characteristics, or a combination of graphical design and consumer?).
Work done by Vigilante and Wogalter (50) would suggest that the consumer plays an important role in the apparent lack of prominence of these label zones. Their research indicated that people consider the TE warning to be a relatively low priority when compared with other label information. Similar findings can be found with regard to the CR warnings, which indicate 60% of people are not aware that OTC medications can be purchased without a CR feature (51).
It is possible that because people believe these pieces of information to be of relatively low importance or are altogether unaware, they tend not to fixate on the warnings or report them in the recall test. This result is counter to official U.S. documents, which have indicated that these warnings need to be heeded at the POP (35) and require text to be conspicuous (37) and prominent (36). If so, the solution may not simply entail changing graphic elements of the warning, but require a more comprehensive approach. Not only does this study call into question the current design practice regarding these warning labels, it also raises questions regarding the need to educate consumers about the importance of these particular warnings, which are intended to save lives.
The genesis of 21 CFR 211.132, the TE requirement, was the deaths of 7 people who ingested cyanide-laced Tylenol in Chicago in 1982. The CR warning is intended to prevent people from bringing packages that do not have CR features into environments where children can gain access to them. The presence of non-CR packages continues to be listed by the CPSC among the reasons (48) that children die as the result of unintentional poisoning; (96 in the United States in 2001, with children ages 4 years and under accounting for more than 45% of deaths) (52).
This information is imperative, and it is mandated to be conspicuous and prominent. Despite this, its effectiveness is debatable.
Methods
Research was conducted in accordance with procedures approved by the Michigan State University Social Science/Behavioral/Education Institutional Review Board as IRB no. 03-056. The labels of 5 OTCs available for purchase during the summer of 2003 (Fig. 1) were tested. Analysis was conducted on 5 gaze zones of interest, namely (i) the TE warning, required by 21 CFR 211.132 to be “prominent”; (ii) the CR warning, required by the Poison Prevention Packaging Act of 1970 to be “conspicuous”; (iii) claims statements, e.g., “extra strength,” “maximum strength,” and “aspirin free”; (iv) the brand name; and (v) the drug facts box, required by 21 CFR201.66.
Participants.
Participants were recruited via nonprobability, network sampling from an undergraduate class and in the employee pool at Michigan State University (East Lansing, MI). These recruitment avenues were chosen in an attempt to recruit people with and without children residing in their homes in the form of a network sample.
Demographic information collected from study participants included their biological sex and age. Participants were also asked if they had children under the age of 10 residing in their homes. If they indicated that they did, they were asked to record the number of children and the ages of these children.
Eye-Tracking Procedure.
The head-mounted optics of an Applied Science Laboratories 501 eye tracker were calibrated to a pane of glass positioned at a comfortable reading distance from the participant. Fixing the reader at a set distance from the viewing plane during the eye-tracking study allowed for a very accurate track of the participant over the 25 small zones (5 products × 5 zones; Fig. 1 and Table S1) of interest in the study.
After a participant was noted to be accurately calibrated, he or she was provided with a POP scenario and randomly handed a series of 10 packages, one at a time. Participants were told that this was a study of the information people seek from products while shopping and that they would have 10 s to view each package before being handed another. Participants were not informed of the study's emphasis on warnings or OTC products. As such, 5 “dummy” packages (batteries, powdered laundry soap, a prepackaged lunch, laundry soap tablets, and a single-serving cereal box) were randomly interspersed with the 5 OTC packages. Dummy packages were chosen as products commonly available for purchase and because they were similar in size and shape to the OTCs.
Each participant was instructed that he or she could view any face of the package when seeking information (Fig. 1 and Table 1) and to hold the package against the pane of glass as he or she viewed it. This ensured that the participant viewed the item in the calibrated plane, minimizing parallax error. Participants were permitted 10 s to view each of the packages; at the end of 10 s, they were asked to put down the package and were handed a new one.
The dependent variables of interest for the eye-tracking data were the amount of time a participant spent in a label's zone divided by the number of typographical characters in that zone. This served to standardize collected data; because all zones did not contain the same amount of information, time spent per zone was expected to vary based on the amount of information present. The standardized variable, expressed as the amount of time per typographical character, was intended to provide a measure of relative conspicuousness of the label zones viewed.
Researchers were also interested in the number of people that failed to view the warnings; that is, those that had zero time in a given gaze zone. As such, the proportion of participants that spent zero time in the gaze zones on each of the packages was also recorded for later analysis.
Recall.
After a participant had viewed all 10 packages while wearing the eye tracker, he or she was asked to record any information that he or she could recall with regard to the OTC medications. None of the packages he or she had viewed were visible to them at this time.
For the purpose of summarizing the recall data, 11 categories of information were defined (Fig. 5). If the participant recorded at least 1 comment with regard to a category, this category was coded as “recalled.” If no comments in a category were recorded, recall response was coded as “not recalled.”
Several participants indicated statements such as “I remember that you should keep out of reach of children.” Although this is similar to the tested CR warning, “Not intended for households with young children,” it is not the same. As such, 2 categories were coded: “child statement” and “CR warning.” Those that indicated that the drugs should be kept away from children had a positive recall coded in the child statement category, and those that indicated that the product was not for households that had children were coded affirmatively in the warning category. In the event that a participant made both such statements, both categories were coded affirmatively.
Visual Acuity.
Following the collection of recall data, each participant's Snellen Near Point visual acuity (20/20, 20/30, etc.) was determined by using a Dow Corning Ophthalmics Near Point Visual Acuity card and recorded.
Legibility.
The legibility of each zone on each package was measured one time for all participants in accordance with ASTM D7298-06, “Standard Test Method of Comparative Legibility by Means of a Polarizing Filter.” Participants were instructed to rotate the handle of the instrument until the first point that they “could easily read” the message of the gaze zone. Rotation of the instrument's handle correlates with rotation of the first of a pair of polarizing filters in series; the more the filter is rotated, the more light is allowed through. As such, information that requires a larger degree of rotation is expected to be more difficult for a participant to decipher than information that requires a lesser degree of rotation. As with the eye tracker, order of package presentation was randomized to minimize any effect of run order.
Statistical Analyses.
General linear mixed models were fit to the continuous outcome variables (i.e., standardized time spent in a gaze zone and legibility, in degrees of rotation) by using the MIXED procedure of the statistical software SAS (version 9.1; SAS Institute). To ensure that model assumptions were appropriately met, variables were subjected to a double log transformation whenever needed. In turn, generalized linear mixed models were fit to the binary outcome variables (i.e., failure to record time in a gaze zone and information recall for each zone) by using the GLIMMIX procedure of SAS. Overall, fixed effects of interest considered for statistical models were gaze zone and the presence of children 10 years or younger in the home. Participants were included in the statistical models as random blocks. Model specifications are provided on a case-by-case basis in Results. Results are presented in the original scale as least-square mean estimates and estimated 95% confidence intervals. In all cases, posthoc pairwise comparisons were performed by using Bonferroni's adjustment to avoid inflation of type I error rates.
Limitations.
Further research is needed to analyze a broader range of population sectors than the study sample. Participants that did not have children were primarily students recruited as part of a network sample at Michigan State Universtiy (East Lansing, MI). Participants that had children were recruited from the employee pool at the same university and were older than their counterparts who did not have children.
Previous research has indicated a significant effect of age on the legibility index (29). This is likely due to physiological changes of aging, which result in the requirement for more light and presbyopia. Changes that have the potential to affect legibility results include: muscle weakness, stiffening of the lens, diminished ability of the pupil to dilate, and yellowing of the retina and vitreous humor (30). As such, it is impossible to know whether the differences the model used to analyze the legibility data are due to the presence of children in the home or a matter of age. Despite the fact that there were significant differences in the result of the 2 groups (children living in the home or not), the overall pattern of the data across the gaze zones followed the same trend (Fig. 6).
The research is also limited by the fact that the authors failed to collect data regarding participants' past history of use and familiarity with products containing acetaminophen. This is an important factor that could have been analyzed for behavioral differences. It is recommended that future research include this factor.
Additionally, only 5 OTC products, representing 2 companies, were used in this study (Fig. 1). It should be mentioned that comparisons between those specific products or their companies were not of primary interest. Rather, these OTC products were chosen as a random sample from the variety of OTCs available on the market. However, the warnings present on the packages tested here are very typical of those found on myriad other OTC products.
Supplementary Material
Acknowledgments.
We are grateful for the contribution of many student workers, including Greg Alvarado, Shannon Arnold, Shellie Berkesch, Javier de la Fuente, Kristi Radadovic, Rebecca Schaeff, Chris Steckler, and Audrey Whaling. Additionally, we thank the reviewers for insights that have greatly improved the content of this article. We gratefully acknowledge the Center for Food and Pharmaceutical Packaging Research, which partially funded this work.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810665106/DCSupplemental.
References
- 1.Rousseau G, Lamson N, Rodgers W. Designing warnings to compensate for age-related changes in perceptual and cognitive abilities. Psychol Mark. 1998;15:643–662. [Google Scholar]
- 2.Food and Drug Administration. Over-the-counter human drugs: Proposed labeling requirements. 62 Federal Register. 1997;39(1997):9024–9062. [Google Scholar]
- 3.Sansgiry S, Cady P, Shubhada P. Readability of over-the-counter medication labels. J Am Pharm Assoc. 1997;37:522–528. doi: 10.1016/s1086-5802(16)30244-3. [DOI] [PubMed] [Google Scholar]
- 4.Wechsler J. Information please: Patient education as a cost cutter. Pharma Exec. 1991;11(7):16–19. [Google Scholar]
- 5.Food and Drug Administration. Over-the-counter Human drugs: Labeling requirement. Final rule. Fed Regist. 1999;64:13254–13303. [PubMed] [Google Scholar]
- 6.Alsobrook H. An overview of liability for OTC drugs. Drug Info J. 1992;26:317–328. [Google Scholar]
- 7.Davis T, et al. Low literacy impairs comprehension of prescription drug warning labels. J Gen Intern Med. 2006;21:847–851. doi: 10.1111/j.1525-1497.2006.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis T, et al. Literacy and misunderstanding prescription drug labels. Ann Intern Med. 2006;145:887–895. doi: 10.7326/0003-4819-145-12-200612190-00144. [DOI] [PubMed] [Google Scholar]
- 9.Gore P, Madhavan S, McClung G, Riley D. Consumer involvement in nonprescription medicine purchases. J Health Care Mark. 1994;14:16–23. [PubMed] [Google Scholar]
- 10.Kalsher M, Wogalter M, Racicot B. Pharmaceutical container labels: Enhancing preference perceptions with alternative designs and pictorials. Int J Ind Ergon. 1996;18:83–90. [Google Scholar]
- 11.Lehto M, Miller J. The effectiveness of warning labels. J Prod Liability. 1988;11:225–270. [Google Scholar]
- 12.Lumpkin J, Strutton D, Lim C, Lowrey S. A shopping orientation based prescription for the treatment of OTC medication misuse among the elderly. Health Marke Q. 1990;8:95–110. [Google Scholar]
- 13.Mansoor L, Dowse R. Design and evaluation of a new pharmaceutical pictogram sequence to convey medicine usage. J Ergon Soc S Afr. 2004;16(2):29–41. [Google Scholar]
- 14.Rogers W, Rousseau G, Lamson N. In: Maximizing the Effectiveness of the Warning Process: Understanding the Variables That Interact with Age. Park DC, Roger W, Morrell R, Shifren K, Park D, editors. London: Psychology Press; 1999. [Google Scholar]
- 15.Morrow D, Leier V. Designing medication instructions for older adults. In: Park D, Morrell R, Shifren K, editors. Processing of Medical Information in Aging Patients. London: Lawrence Erlbaum and Associates; 1999. [Google Scholar]
- 16.Nabors L, Lehmkuhl H, Parkins I, Drury A. Reading about over-the-counter medications. Issues Compr Pediatr Nursing. 2004;27:297–305. doi: 10.1080/01460860490884192. [DOI] [PubMed] [Google Scholar]
- 17.Reisenwitz T, Wimbish J. The purchase decision process and involvement of the elderly regarding nonprescription products. Health Mark Q. 1997;15:49–68. doi: 10.1300/j026v15n01_04. [DOI] [PubMed] [Google Scholar]
- 18.Robinson D, Stewart R. Elderly: Understanding their prescription needs. Am Pharm. 1981;21:48. doi: 10.1016/s0160-3450(16)31498-2. [DOI] [PubMed] [Google Scholar]
- 19.Rogers W, Rousseau G, Lamson N. Morrell R, Park D, editors. Maximizing the effectiveness of the warning process: Understanding the variables that interact with age. Aging and Medication Information Processing. 1998 [Google Scholar]
- 20.Roumie C, Griffin M. Over-the-counter analgesics in older adults: A call for improved labelling and consumer education. Drugs Aging. 2004;21:485–498. doi: 10.2165/00002512-200421080-00001. [DOI] [PubMed] [Google Scholar]
- 21.Rousseau G, Lamson N, Rogers W. Designing warnings to compensate for age-related changes in perceptual and cognitive abilities. Psychol Mark. 1998;15:643–662. [Google Scholar]
- 22.Sansgiry S, Cady P. Can picture use effectively enhance the understanding of nonprescription drug labels in older adults? J Geriatr Drug Ther. 1996;10:51–69. [Google Scholar]
- 23.Sansgiry S, Cady P. How the elderly and young adults differ in the decision making process of nonprescription medication purchases. Health Mark Q. 1996;14:3–22. doi: 10.1300/J026v14n01_02. [DOI] [PubMed] [Google Scholar]
- 24.Sansgiry S, Cady P, Sansgiry S. Consumer involvement: Effect on information processing from over-the-counter medication labels. Health Mark Q. 2001;19:61–78. doi: 10.1300/J026v19n01_05. [DOI] [PubMed] [Google Scholar]
- 25.Tennesen M. Before you play doctor. Health Mark Q. 1999;13:98–100. 102–103. [Google Scholar]
- 26.Vanderplas J, Vanderplas J. Some factors affecting legibility of printed materials for older adults. Percept Mot Skills. 1980;50:923–932. [Google Scholar]
- 27.Wogalter MS, Dietrich DA. Enhancing label readability for over-the-counter pharmaceuticals by elderly consumers. Proceedings of the Human Factors and Ergonomics Society 39th Annual Meeting 1995; Santa Monica, CA: HFES; 1995. pp. 143–147. [Google Scholar]
- 28.Wogalter M, Magurno A, Scott K, Dietrich D. Facilitating information acquistion for over-the-counter drugs using supplemental labels. Proceedings of the Human Factors and Ergonomics Society 40th Annual Meeting 1996; Santa Monica, CA: HFES; 1996. pp. 732–736. [Google Scholar]
- 29.Bix L. The effect of subject age on legibility. East Lansing, MI: Michigan State Univ; 1998. MSc thesis. [Google Scholar]
- 30.Bix L. PhD dissertation. East Lansing, MI: Michigan State Univ; 2001. Toward a performance standard for typeface legibility: The lockhart legibility instrument. [Google Scholar]
- 31.Bojko A, Buffardi K, Lew G, Israelski E. Eye tracking study on the impact of the manufacturer's logo and multilingual descrption on drug selection performance. Proceedings of the Human Factors and Ergonomics Society 50th Annual Meeting 2006; Santa Monica, CA: HFES; 2006. pp. 1112–1116. [Google Scholar]
- 32.Bojko A, Gaddy C, Lew G, Quinn A, Israelski E. Evaluation of drug label designs using eye tracking. Proceedings of the Human Factors and Ergonomics Society 49th Annual Meeting 2005; Santa Monica, CA: HFES; 2005. pp. 1033–1037. [Google Scholar]
- 33.Harris Interactive Market Research Firm. (Prepared for the National Council on Patient Information and Education). Attitudes and beliefs about the use of over-the-counter medicines: A dose of reality. [Accessed March 3, 2009];2002 Jan; Available at http://www.bemedwise.org/survey/final_survey.pdf.
- 34.Johnson M, Drungle S. Purchasing over-the-counter medications: The influence of age and familiarity. Exp Aging Res. 2000;26:245–261. doi: 10.1080/036107300404886. [DOI] [PubMed] [Google Scholar]
- 35.Food and Drug Administration. Guidance for the Industry: Labeling OTC Human Drug Products Questions and Answers. 2005 [Google Scholar]
- 36.Food and Drugs Chapter I Subchapter C. Tamper-Evident Packaging Requirements for Over-The-Counter (OTC) Human Drug Products. 63 Federal Register. 213:59463–59471. (1998) [PubMed] [Google Scholar]
- 37.Poison Prevention Packaging Act. Public L no. 91-601, 84 Stat 1670 (1970) [Google Scholar]
- 38.Food and Drug Administration. Compliance with section 301 of the Medical Device User Fee and Modernization Act of 2002, as amended: Prominent and conspicuous mark of manufacturers on single-use devices. [Accessed March 10, 2009];2006 Available at http://www.fda.gov/CDRH/comp/guidance/1217.html. [Google Scholar]
- 39.Food and Drug Administration. Drugs and Devices; National Drug Code Numbers. 21 Code of Federal Regulations. 75:143–144. section 201.2. [Google Scholar]
- 40.Medical Device User Fee and Modernization Act of 2002. Public Law 107-250 (116 Stat, 1588-162) Section 302. [Google Scholar]
- 41.Alcohol Labeling Beverage Act of 1988. 27 USC Chapter 8 Subchapter II Subsection 215 (1998) [Google Scholar]
- 42.Environmental Protection Agency 40 Code of Federal Regulations 23. :61–67. section 156.10. Revised as of July 1, 2008. [Google Scholar]
- 43.Food and Drug Administration 21 Code of Federal Regulations 4. :14–15. section 201.15. Revised as of April 1, 2008. [Google Scholar]
- 44.Boston University Epidemiology Center. The Slone Survey: Patterns of Medication Use in the United States. Boston: Boston Univ; 2004. [Google Scholar]
- 45.Boston University Epidemiology Center. The Slone Survey: Patterns in Medication Use in the United States. Boston: Boston Univ; 2005. [Google Scholar]
- 46.Boston University Epidemiology Center. The Slone Survey: Patterns of Medication Use in the United States: A Report from the Slone Survey. Boston: Boston Univ; 2006. [Google Scholar]
- 47.Fair Packaging and Labeling Act of 1966. Public Law 89-755. Title 15; Chapter 39. USC (1966) [Google Scholar]
- 48.U.S. Consumer Products Safety Commission. [Accessed March 2009, 10];Poison Prevention Packaging: A Text for Pharmacists and Physicians. 1999 Available at http://www.cpsc.gov/cpscpub/pubs/384.pdf.
- 49.Young S. Applying an architecture: Label viewing patterns suggest easy ways to prioritize package messages. [Accessed March 10, 2009];Package Design Magazine. 2007 Available at http://www.packagedesignmag.com/cgi-bin.
- 50.Vigilante W, Wogalter M. The preferred order of over-the-counter (OTC) pharmaceutical label components. Drug Inf J. 1997;31:973–988. [Google Scholar]
- 51.Kou EY. Child Resistant Drug Packaging and Arthritis: Can Older Consumers Access Their Medications? East Lansing, MI: Michigan State Univ; 2006. [Google Scholar]
- 52.National SAFE KIDS Campaign (NSKC) [Accessed March 10, 2009];Poisoning Fact Sheet. 2004 Available at http://www.preventinjury.org/PDFs/POISONING.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.