Abstract
The income–achievement gap is a formidable societal problem, but little is known about either neurocognitive or biological mechanisms that might account for income-related deficits in academic achievement. We show that childhood poverty is inversely related to working memory in young adults. Furthermore, this prospective relationship is mediated by elevated chronic stress during childhood. Chronic stress is measured by allostatic load, a biological marker of cumulative wear and tear on the body that is caused by the mobilization of multiple physiological systems in response to chronic environmental demands.
A large, robust literature demonstrates a pervasive income–achievement gap. Family income is a strong and consistent predictor of multiple indices of achievement, including standardized test scores, grades in school, and educational attainment. Family income matters to children's cognitive development (1–3), with more enduring economic hardship particularly harmful (4, 5). The income–achievement gap is already present by kindergarten and accelerates over time (6, 7). The longer the duration of childhood exposure to poverty, the worse achievement levels become. Achievement test scores and school performance, however, do not inform us about what neurocognitive processes are influenced by childhood poverty. Furthermore, the voluminous income–achievement gap literature is silent on underlying biological explanations.
Here, we test 2 hypotheses. One is that childhood poverty will interfere with working memory in young adults. Working memory is the temporary storage mechanism that enables us to hold a small amount of information active over a short interval and to manipulate it. Working memory is essential to language comprehension, reading, and problem solving, and it is a critical prerequisite for long-term storage of information. The second hypothesis we test is that the prospective relationship between childhood poverty and adult working memory will be mediated by chronic stress exposure, (i.e., poverty → chronic stress → working memory). Farah and colleagues (8) found significant deficits in working memory between low- and middle-socioeconomic status (SES) kindergarten children and, in a second sample, between low- and middle-SES 11-year-olds (9). In a third study of first-graders, SES was a significant predictor of working memory (10). An important, missing component of this groundbreaking work is the underlying biological mechanisms to account for the SES–neurocognitive link.
Both animal and human studies reveal that working memory resides in the prefrontal cortex, although it is clearly influenced by hippocampal, and possibly amygdala, interactions with the prefrontal cortex as well (11–14). The human hippocampus and prefrontal cortex are each disrupted by chronic physiological stress (14–17). Chronically elevated physiological stress is a plausible model for how poverty could get into the brain and eventually interfere with achievement.
We measure chronic physiological stress by using allostatic load. Allostatic load is an index of cumulative wear and tear on the body caused by repeated mobilizations of multiple physiological systems over time in response to environmental demands (16, 18–24). Allostasis is a dynamic and interactive set of multiple physiological systems of bodily equilibrium maintenance. According to allostasis theory, the body continuously adjusts its normal operating range in response to external requirements. These dynamic adjustments reflect downward regulation to maintain the organism's internal stability, but at levels more congruent with environmental conditions. The active, ongoing maintenance of internal equilibrium increases allostatic load, which reflects chronic wear and tear caused by the mobilization of resources to meet changing environmental exigencies. Overexposure to a combination of multiple, activated bodily response systems (e.g., neuronal, endocrine, cardiovascular) alters the ability of the body to respond efficiently to environmental demands. Longer, more frequent exposure to environmental stressors accelerates bodily wear and tear. Chronic and more intensive environmental stressors cause the body to mobilize multiple physiological systems to meet those demands, but at higher levels of activity. Conversely, when environmental demands are low, individuals who have had a higher allostatic load burden will be less efficient in turning off the multiple physiological resources marshaled to deal with chronic demands.
Interest in allostasis has risen primarily for 2 reasons. First, whereas singular physiological markers of adaptation to environmental demands (e.g., blood pressure) are modestly linked to various disease endpoints (e.g., coronary heart disease), the combined effect of singular physiological changes across multiple biological systems captured by allostatic load is substantially more predictive of disease outcomes (18–24). Second, in addition to contributing to physical morbidity, chronically elevated allostatic load also influences neurological processes, particularly in the hippocampus and prefrontal cortex, that are capable of disrupting cognitive functioning. These neurological processes include altered neurotransmitter activity (e.g., dopamine, norepinephrine, glutamate), suppression of neurogenesis as well as elevated neurotoxicity, alterations in receptor binding sites (e.g., mineral corticoid, glucocorticoid), and morphological changes, such as dendritic remodeling and smaller hippocampal and prefrontal cortex volumes (14–17). Thus, chronically elevated allostatic load could lead to disturbances in working memory in human beings. To date, however, this has not been tested.
Thus, in this paper we bring together 2 separate research literatures, neurocognition and physiological stress, to address a major societal problem, namely, the income–achievement gap. Numerous investigators employing a wide array of study designs have uncovered consistent evidence of an income–achievement gap. Missing in this voluminous literature is evidence of underlying neurocognitive and biological mechanisms. We hypothesize that a plausible contributor to the income–achievement gap is working memory impairments in lower-income adults caused by stress-related damage to the brain during childhood.
Results
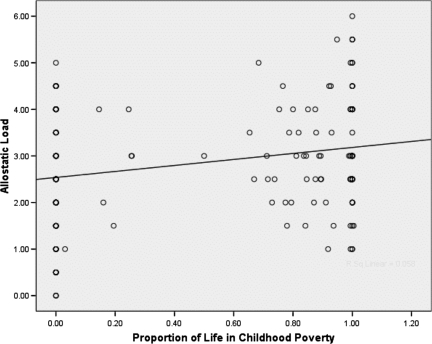
Fig. 1 depicts the relationship between the proportion of childhood from birth through age 13 years spent in poverty and chronic stress as measured by allostatic load over the same developmental period. Allostatic load is a marker of cumulative wear and tear on the body. As described in Materials and Methods, each singular indicator of physiological mobilization is dichotomized (0 = no risk and 1 = risk), with risk defined as the upper half of the values for the specific risk factor. Allostatic load is the simple sum of the 6 risk factors (resting blood pressure, overnight cortisol and catecholamines, and body mass index). As can be seen in Fig. 1, the greater the proportion of a child's life growing up in poverty, the higher the degree of cumulative wear and tear on the body during his or her early lifetime [b = 0.49 (SE = 0.18), P < 0.01].
Fig. 1.
Duration of childhood poverty and children's levels of chronic stress.
The average levels of allostatic load (0–6) at ages 9 and 13 years were 2.59 and 3.08, respectively, for children who had never lived in poverty compared with those who had. This information and the data shown in Table 1 are for descriptive purposes only. All of the inferential analyses here maintained the continuous nature of the proportion of childhood growing up in poverty from birth through age 13 years.
Table 1.
Descriptive data on components of allostatic load
| Not poor | Poor | |
|---|---|---|
| Wave 1, age 9 years | ||
| Resting diastolic blood pressure, mmHg | 58.64 | 60.14 |
| Resting systolic blood pressure, mmHg | 100.97 | 103.77 |
| Overnight cortisol, μg/mg creatinine | 0.02 | 0.03 |
| Overnight epinephrine, ng/mg creatinine | 3.62 | 5.19 |
| Overnight norepinephrine, ng/mg creatinine | 31.17 | 33.29 |
| Body mass index, kg/m2 | 17.90 | 18.86 |
| Allostatic load, 0–6 | 2.63 | 3.21 |
| Wave 2, age 13 years | ||
| Resting diastolic blood pressure, mmHg | 63.90 | 63.94 |
| Resting systolic blood pressure, mmHg | 109.65 | 112.26 |
| Overnight cortisol, μg/mg creatinine | 0.12 | 0.11 |
| Overnight epinephrine, ng/mg creatinine | 5.00 | 4.91 |
| Overnight norepinephrine, ng/mg creatinine | 21.45 | 20.78 |
| Body mass index, kg/m2 | 21.41 | 23.57 |
| Allostatic load, 0–6 | 2.62 | 2.93 |
Allostatic load was calculated with 0 = no risk and 1 = risk, wherein risk is defined as scoring in the upper 50th percentile for each of the following 6 components: Mean of 6 resting systolic BP readings after discarding the initial reading, Mean of 6 resting diastolic BP readings after discarding the initial reading, 12 hour overnight urinary cortisol/creatinine, 12 hour overnight urinary epinephrine/creatinine, 12 hour overnight urinary norepinephrine/creatinine, and BMI.
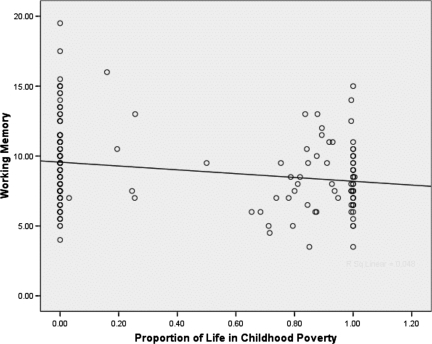
As shown in Fig. 2, the proportion of early childhood spent in poverty is also significantly related to working memory in young adulthood [b = −1.01 (SE = 0.44); P < 0.02]. The greater the proportion of life growing up in poverty from birth to age 13 years, the shorter the span of sequential information 17-year-old adults can accurately hold in their working memory. On average, adults raised in middle-income families could hold in working memory a sequence of 9.44 items, whereas adults who grew up in poverty had a working memory capacity of 8.50 items.
Fig. 2.
Duration of childhood poverty and working memory in young adults.
We then tested whether the significant relationship between childhood poverty and adult working memory could be explained, at least in part, by elevated chronic stress accompanying childhood poverty. The previously significant relationship between childhood poverty and adult working memory (b = −1.01) became nonsignificant [b = −0.77 (SE = 0.45)] when childhood allostatic load was added to the regression equation for adult working memory. This reduction in the poverty b weight is highly significant [t (193) = 2.57; P < 0.01]. Childhood poverty no longer predicts young adults' working memory capacity once chronic stress exposure is partialed from the covariance between childhood poverty and adult working memory. As expected, allostatic load during childhood significantly predicts working memory in young adulthood [b = −0.47 (SE = 0.16); P < 0.01].
Discussion
The vast literature on the income–achievement gap (1–7) raises important theoretical and practical questions. Grades and standardized test scores are distal indicators of underlying neurocognitive processes but offer no insight into the underlying biological mechanisms that may account for the income–achievement gap. We integrated research from 2 research areas, neurocognition and physiological stress, to demonstrate that the greater the duration of childhood poverty from birth to age 13 years, the worse one's working memory as a young adult. This finding is consistent with work by the Farah laboratory showing that concurrent SES among elementary school children (8, 10) and middle school children (9) is inversely related to working memory. We build on this work in 2 respects. One, we demonstrate that the duration of childhood poverty is related prospectively to working memory performance later in life among young adults. Two, we show that allostatic load, an index of chronic stress (16, 18–24), conveys a significant proportion of the covariation between childhood deprivation and an adult's working memory performance. The longer the period of childhood poverty, the higher the levels of allostatic load during childhood, and the greater the reductions in young adults' subsequent working memory (Figs. 1 and 2). Furthermore, elevated childhood allostatic load predicts working memory in young adults and, in turn, largely explains the prospective relationship between childhood poverty and these working memory deficits.
The finding that the longer the duration of childhood poverty, the greater the elevation in allostatic load (Fig. 1), is congruent with previous research on SES and physical health. SES is inversely associated with cardiovascular and neuroendocrine markers of physiological stress in children (25–27). Among adults, SES is consistently negatively associated with health across a wide range of diseases (28, 29). Furthermore, of particular interest given the present results, emerging data suggest that the duration of childhood spent in poverty accumulates over time to adversely affect morbidity and mortality in later adulthood (30–32).
Because the data in the present study are not from an experiment, it is important to consider alternative explanations for the pattern of results uncovered. One possibility is reverse causation, wherein memory loss mediates the poverty–allostatic load link (i.e., poverty → working memory → allostatic load) rather than allostatic load mediating the poverty → working memory relationship, as hypothesized in the current study. This alternative is unlikely. The relationship between duration of childhood poverty and working memory performance in young adulthood was significantly mediated by allostatic load, whereas the relationship between duration of childhood poverty and allostatic load was not attenuated when working memory was partialed from the equation. Subject selection effects are another alternative explanation worthy of consideration. Perhaps some omitted personal characteristic influences both working memory and economic circumstances. We evaluated the plausibility of selection effects by progressively adding into the initial poverty–working memory regression equation (Fig. 2) invariant personal characteristics, including sex, birth weight, and maternal age at childbirth, as well as maternal education and marital status at study onset. If selection bias was operating, then the magnitude of change in the b weight for poverty and working memory performance would be large as each respective invariant personal characteristic was added to the original regression model. However, we found negligible shifts in the b for poverty duration with the addition of each personal variable into the model.
Another strategy to strengthen causal inference with the present research design would be to include a measure of working memory earlier in childhood as an additional variable in the regression model. This type of research design would enable us to examine residual changes in memory performance at age 17 years as a function of childhood poverty. Because we assessed working memory in the third wave only (i.e., age 17 years) of this longitudinal study (Table 2), we cannot conduct a longitudinal, prospective regression analysis, which would strengthen the causal evidence for the model we propose. Such a design could also inform us whether the adverse effects of early childhood poverty on working memory were already in place in early childhood and simply persisted throughout early adulthood, or whether the deficits continued to worsen into adulthood as the duration of poverty exposure increased over time. Farah and colleagues' work would suggest that poverty-related deficits were already in place during early childhood in the data here, because they uncovered significant associations as early as kindergarten (8–10). Some indirect evidence is available in our data suggesting that the duration of early childhood poverty exposure is important for working memory deficits later on in life. Poverty status at either the first (age 9 years) or second (age 13 years) wave of data collection did not significantly predict working memory deficits at age 17 years. Only the duration of poverty during early childhood predicted subsequent working memory in young adulthood.
Table 2.
Study design
| Factors | Age |
|||
|---|---|---|---|---|
| Birth | 9 years | 13 years | 17 years | |
| Duration of poverty | X | X | X | X |
| Allostatic load (0–6) | — | X | X | — |
| Working memory | — | — | — | X |
Duration of poverty was measured every 6 months. For details regarding the calculation of allostatic load, see Table 1. Working memory was calculated using the mean of 2 trials on the Simon game. X, age at which data was collected; —, no data collection.
A third possibility is that some other underlying mechanism(s) in addition to allostatic load might account for the linkages uncovered between childhood poverty and young adult working memory. We were able to test 2 theoretically plausible alternatives, and neither proved tenable. First, we examined whether the significant relationship between duration of poverty and working memory could be accounted for by 2 different measures of parenting. Neither maternal sensitivity (i.e., responsiveness to child's emotional and instrumental needs) nor a standard classification of parenting styles (i.e., authoritarian, indulgent, indifferent, authoritative) mediated the poverty–working memory link. Second, we examined whether maternal stress might explain the poverty–working memory association. It did not. Nevertheless, other pathways in addition to chronic stress might also account for the working memory sequelae of childhood poverty uncovered in the present study.
In addition to testing for alternative mechanisms that might help explain the link between childhood poverty and adult working memory, an important extension of the present study would be to examine in more detail how early disadvantage gets into the brain to disrupt neurological mechanisms (33–35). Animal models and human brain imaging work in organisms exposed to chronic stress show altered neurotransmitter activity and suppression of neurogenesis, as well as dendritic remodeling and volume reduction in the hippocampus and prefrontal cortex (14–17). Given the functional and morphological interactions between the hippocampus and prefrontal cortex, it is difficult to parse out the specific role of each of these brain structures in the income-related deficits in working memory uncovered in the current study. An important adjunct to the present study would be an examination of chronic poverty, allostatic load, and long-term, declarative memory, which resides in the hippocampus. Work indicates that allostatic load among elderly individuals predicts declines in general cognitive functioning, although this composite index did not include working memory (21). Additional research is necessary to more precisely model which neurological processes are disrupted by chronic physiological stress and how these disruptions may, in turn, produce specific neurocognitive deficits.
The income–achievement gap is an important societal problem. Childhood poverty is a well-established risk factor for cognitive competency as well as for physical morbidity throughout the life course. We show that these 2 outcomes of childhood poverty are interrelated. The prospective association between the duration of childhood poverty and adult working memory appears to be explained in part by elevated chronic stress during childhood.
Materials and Methods
Participants were 195 Caucasian young adults [mean (M) = 17.29 years, 50% female] with complete data on duration of childhood poverty exposure, allostatic load, and working memory. They were part of a longitudinal study on rural poverty, cumulative risk, and children's development (36). Approximately half of the sample grew up below the poverty line (an income-to-needs ratio ≤1, which is the U.S. official poverty line based on an annually adjusted, per-capita index), and the other half grew up at levels ≈2–4 times the poverty line, the income level of the majority of American families.
Duration of childhood poverty was defined as the proportion of months from birth through age 13 years the participant had grown up at or below the poverty line. Poverty was operationalized in this way because the duration of childhood deprivation, rather than the timing of poverty exposure, appears particularly critical for cognitive achievement (4, 5). Furthermore, rural poverty, unlike urban poverty, tends to be quite stable, thus precluding the assessment of specific critical periods for childhood exposure to material deprivation. Finally, we assessed poverty exposure in this manner because allostasis theory emphasizes the duration of chronic environmental demands as the key precipitating factor in wear and tear on the body.
Allostatic load was calculated to capture physiological activity across a range of physiological response systems and included cardiovascular, hypothalamic pituitary adrenocortical axis, sympathetic adrenal medullary system, and metabolic activity. Resting blood pressure was calculated with automated readings (Dinamap Model Pro 100, Critikon) taken every 2 minutes while the subject sat quietly. The mean of the second through seventh readings was used as the index of basal blood pressure (37). Overnight urine was collected from 8 PM on the evening of the experimental protocol to 8 AM the next morning. Epinephrine and norepinephrine were assayed by HPLC with electrochemical detection (38) and cortisol with a radioimmunoassay (39). Creatinine was included as a statistical control for the neuroendocrine assays. Body mass index was calculated as kg/m2. Allostatic load (0–6) was calculated by summing the number of physiological parameters (resting diastolic and systolic blood pressure, overnight epinephrine, norepinephrine, cortisol, and body mass index) for which the participant scored above the median (0 = –1st to 50th percentile; 1 = >50th percentile).* Empirical investigation has shown that this simple, additive model of allostatic load across multiple physiological systems predicts morbidity and mortality endpoints better than either singular components of allostatic load (e.g., resting systolic blood pressure) or compared with other ways to measure aggregate activity across multiple physiological systems, such as weighted models or profiles of various combinations of the different components of allostatic load (18–24). Each individual physiological component of allostatic load was measured at age 9 years and again at age 13 years, with allostatic load calculated separately at each age. Chronic childhood allostatic load was then calculated as the mean of allostatic load at ages 9 and 13 years. The mean was used to best capture the accumulative effect of chronic stress during childhood on subsequent working memory in young adulthood.
Working memory was assessed at age 17 years only and was not included in either of the experimental protocols at age 9 years or 13 years. Working memory was measured by the subject's ability to recall a sequence of stimuli presented on a touch pad divided into 4 quadrants. Each sequence began with 1 stimulus signal. In the initial trial, 1 of 4 monochromatic panels on the touch pad was lit with a corresponding unique tone for 500 msec. The subject's task was to recall, in order, each of the stimuli that had been displayed thus far on the touch pad. The subject did this by tapping each panel in the correct sequential order as he or she recalled it. Thus, in trial 1, the subject tapped the panel location of the single signal (light and tone) he or she had just been exposed to immediately after the light and tone were extinguished. For each subsequent trial, 1 additional panel was activated randomly. Thus, in trial 2, the subject had to recall the correct sequential order of 2 prior stimuli. The maximum number of stimuli the subject could recall in the correct sequential order was the index of working memory. Before the onset of the test session, subjects completed a practice run to ensure they understood the procedure. Participants then completed 2 different sessions of the working memory task, with a 1-hour interval between the 2 sessions. Working memory was the average performance across these 2 separate sessions. Table 2 summarizes the design and methods of the study.
Acknowledgments.
We are grateful to the families who participated throughout this research. We thank Jana Cooperman, Kim English, Missy Globerman, Matt Kleinman, Rebecca Kurland, Melissa Medoway, Tina Merilees, Chanelle Richardson, Adam Rohksar, and Amy Schreier for their assistance with data collection. Pilyoung Kim and Anthony Ong provided constructive feedback on earlier drafts. This work was supported by the W. T. Grant Foundation and the John D. and Catherine T. MacArthur Foundation Network on Socioeconomic Status and Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
*Typically, allostatic load is the summation of each physiological risk factor defined as 1 > upper 25th percentile of values, and 0 = all other values of the variable. We defined risk here as >50th percentile because approximately half of the individuals in our sample are low-income children. If we calculate allostatic load by using the traditional upper quartile cutoff, the same statistically significant patterns of results occur.
References
- 1.Bradley RH, Corwyn R. Socioeconomic status and child development. Annu Rev Psych. 2002;53:371–399. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- 2.Conger RD, Donnellan MB. An interactionist perspective on the socioeconomic context of human development. Annu Rev Psych. 2007;58:175–199. doi: 10.1146/annurev.psych.58.110405.085551. [DOI] [PubMed] [Google Scholar]
- 3.McLoyd VC. Socioeconomic disadvantage and child development. Am Psychol. 1998;53:185–204. doi: 10.1037//0003-066x.53.2.185. [DOI] [PubMed] [Google Scholar]
- 4.National Institute of Child Health and Human Development Early Child Care Research Network. Duration and developmental timing of poverty and children's cognitive and social development. Child Dev. 2005;76:795–810. doi: 10.1111/j.1467-8624.2005.00878.x. [DOI] [PubMed] [Google Scholar]
- 5.Korenman S, Miller JE, Sjaastad JE. Long-term poverty and child development: Evidence from the NLSY. Child Youth Serv Rev. 1995;17:127–155. [Google Scholar]
- 6.Heckman JJ. Skill formation and the economics of investing in disadvantaged children. Science. 2006;312:1900–1902. doi: 10.1126/science.1128898. [DOI] [PubMed] [Google Scholar]
- 7.Pungello EP, Kupersmidt JB, Burchinal MR, Patterson CJ. Environmental risk factors and children's achievement from middle childhood to early adolescence. Dev Psychol. 1996;32:755–767. [Google Scholar]
- 8.Noble KG, Norman MF, Farah MJ. Neurocognitive correlates of socioeconomic status in kindergarten children. Dev Sci. 2005;8:74–87. doi: 10.1111/j.1467-7687.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- 9.Farah MJ, et al. Childhood poverty: Specific associations with neurocognitive development. Brain Res. 2006;110:166–174. doi: 10.1016/j.brainres.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 10.Noble KG, McCandiliss BD, Farah MJ. Socioeconomic gradients predict individual differences in neurocognitive abilities. Dev Sci. 2007;10:464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- 11.LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lupien SJ, Gillin C, Hauger RL. Working memory is more sensitive than declarative memory to the acute effects of corticosteroids: A dose-response study. Behav Neurosci. 1999;113:420–430. doi: 10.1037//0735-7044.113.3.420. [DOI] [PubMed] [Google Scholar]
- 13.Smith EE, Jonides J, Marshuetz C, Koeppe RA. Components of verbal memory: Evidence from neuroimaging. Proc Natl Acad Sci USA. 1998;95:876–882. doi: 10.1073/pnas.95.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lupien SJ, Maheu F, Tu M, Fiocco A, Shrmaek TE. The effect of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain Cogn. 2007;65:209–237. doi: 10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Lupien S, et al. Beyond the stress concept: Allostatic load, a developmental biological and cognitive perspective. In: Cicchetii D, Cohen D, editors. Developmental Psychopathology. New York: Wiley; 2006. [Google Scholar]
- 16.McEwen BS. The neurobiology of stress: From serendipity to clinical relevance. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- 17.Sapolsky R. Stress and cognition. In: Gazzaniga M, editor. The Cognitive Neurosciences. Vol 3. Cambridge, MA: MIT Press; 2004. [Google Scholar]
- 18.McEwen BS. The End of Stress as We Know It. Washington, DC: John Henry Press; 2002. [Google Scholar]
- 19.McEwen BS, Seeman TE. Protective and damaging effects of mediators of stress: Elaborating and testing the concepts of allostasis and allostatic load. In: Adler NE, Marmot M, McEwen BS, Stewart J, editors. Socioeconomic Status and Health in Industrial Nations. New York: New York Academy of Sciences; 1999. [DOI] [PubMed] [Google Scholar]
- 20.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 21.Karlamangla AS, Singer BH, McEwen BS, Rowe JW, Seeman TE. Allostatic load as a predictor of functional decline: MacArthur studies of successful aging. J Clin Epidemiol. 2002;29:696–710. doi: 10.1016/s0895-4356(02)00399-2. [DOI] [PubMed] [Google Scholar]
- 22.Seeman TE, Singer BH, Rowe JW, Horwitz R, McEwen BS. Price of adaptation-allostatic load and its health consequences. Arch Intern Med. 1997;157:2259–2268. [PubMed] [Google Scholar]
- 23.Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci USA. 2001;98:4770–4775. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seeman TE, et al. Cumulative biological risk and socioeconomic differences in mortality: MacArthur studies of successful aging. Soc Sci Med. 2004;58:1985–1997. doi: 10.1016/S0277-9536(03)00402-7. [DOI] [PubMed] [Google Scholar]
- 25.Chen E, Matthews KA, Boyce WT. Socioeconomic differences in children's health: How and why do these relationships change with age? Psychol Bull. 2002;128:295–329. doi: 10.1037/0033-2909.128.2.295. [DOI] [PubMed] [Google Scholar]
- 26.Evans GW, English K. The environment of poverty: Multiple stressor exposure, psychophysiological stress and socioemotional adjustment. Child Dev. 2002;73:1238–1248. doi: 10.1111/1467-8624.00469. [DOI] [PubMed] [Google Scholar]
- 27.Lupien SJ, King S, Meaney MJ, McEwen BS. Child's stress hormones correlate with mother's socioeconomic status and depressive state. Biol Psychiatry. 2000;48:976–980. doi: 10.1016/s0006-3223(00)00965-3. [DOI] [PubMed] [Google Scholar]
- 28.Adler NE, Rehkopf DG. U.S. disparities in health: Descriptions, causes, and mechanisms. Annu Rev Public Health. 2008;29:235–252. doi: 10.1146/annurev.publhealth.29.020907.090852. [DOI] [PubMed] [Google Scholar]
- 29.Williams DR, Collins C. US socioeconomic and racial differences in health: Patterns and explanations. Annu Rev Public Health. 1995;21:349–386. [Google Scholar]
- 30.Power C, Manor O, Matthews S. The duration and timing of exposure: Effects of socioeconomic environment on adult health. Am J Public Health. 1999;89:1059–1065. doi: 10.2105/ajph.89.7.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lynch JW, Kaplan GA, Shema SJ. Cumulative impact of sustained economic hardship on physical, cognitive, psychological, and social functioning. N Engl J Med. 1997;337:1889–1895. doi: 10.1056/NEJM199712253372606. [DOI] [PubMed] [Google Scholar]
- 32.Chen E, Martin D, Matthews KA. Trajectories of socioeconomic status across children's lifetime predict health. Pediatrics. 2007;120:297–303. doi: 10.1542/peds.2006-3098. [DOI] [PubMed] [Google Scholar]
- 33.Knudsen EI, Heckman JJ, Cameron JL, Shonkoff JP. Economic, neurobiological, and behavioral perspectives on building America's future workforce. Proc Natl Acad Sci USA. 2006;103:10155–10162. doi: 10.1073/pnas.0600888103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shonkoff JP, Phillips DA. From Neurons to Neighborhoods: The Science of Early Childhood Development. Washington, DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- 35.Zuckerman B, Kahn R. Early child health and development: Pathways linking neurodevelopment to social context. In: Danziger S, Waldfogel J, editors. Securing the Future: Investing in Children from Birth to College. New York: Russell Sage Foundation; 2004. [Google Scholar]
- 36.Evans GW, Kim P. Childhood poverty and health: Cumulative risk exposure and stress dysregulation. Psychol Sci. 2007;18:953–957. doi: 10.1111/j.1467-9280.2007.02008.x. [DOI] [PubMed] [Google Scholar]
- 37.Kamarck T, Jennings R, Debski T. Reliable measures of behaviorally evoked cardiovascular reactivity from a PC-based test battery. Psychophysiology. 1992;29:17–28. doi: 10.1111/j.1469-8986.1992.tb02006.x. [DOI] [PubMed] [Google Scholar]
- 38.Riggin R, Kissinger P. Determination of catecholamines in urine by reverse phase chromatography with electrochemical detection. Anal Chem. 1977;49:2109–2111. doi: 10.1021/ac50021a052. [DOI] [PubMed] [Google Scholar]
- 39.Contreras LN, Hane S, Tyrrell JB. Urinary cortisol in the assessment of pituitary adrenal functioning: Utility of 24 hour spot determinations. J Clin Endocrinol Metab. 62:965–969. doi: 10.1210/jcem-62-5-965. [DOI] [PubMed] [Google Scholar]