Abstract
In autoimmune prone murine strains, sequential engagement of the B cell antigen receptor (BCR) on the cell surface and toll-like receptors (TLRs) in late endosomes is necessary and sufficient for secretion of autoantibodies. However, ubiquitous nucleoprotein self-antigens fail to elicit productive TLR activation, and break self-tolerance in anergic DNA-reactive B cells. The mechanisms limiting TLR activation in these cells are largely unknown. Here, we demonstrate that in anergic 3H9/Vκ8 and Ars/A1 B cells the normal endocytic transit of both the ligated BCR and TLR9 into late endosomes is abrogated. The BCR and TLR9 arrest together just outside late endosomes, indicating that they enter this compartment along a single, regulated endocytic route. Access to late endosomes could be restored by reversing anergy through several methods, including conferring genetic susceptibility to autoimmunity, complementing proximal BCR signaling or by preventing BCR binding to self-antigen. Downstream of the BCR, JNK, which is activated in naive but not anergic B cells, regulated entry into late endosomes. Restoration of BCR and TLR9 endocytic trafficking rescued TLR9 activation by BCR-captured ligands. These results indicate that B cell anergy is reinforced by the exclusion of both TLRs and their BCR captured ligands from subcellular environments necessary for TLR activation.
Keywords: endocytic trafficking, anergy
Immunogenic and tolerogenic stimuli induce different proximal B cell antigen receptor (BCR) signaling responses (1–3). Immunogenicity is associated with the coordinated activation of a network of signaling pathways, including those leading to rapid intracellular calcium elevation, ERK, and JNK activation. Most anergic B cells manifest aberrant BCR-induced calcium responses and attenuated JNK activation (4, 5).
Little is known about how immunogenic and tolerogenic ligands induce opposing cell fates. Initial investigations focused on differences in transcriptional programs (6). However, only a few transcription factors are consistently differentially regulated in anergic B cells (7). The apparent lack of correlation between transcriptional events and B cell anergy is not surprising, given the rapidity with which anergy can be reversed (5, 8–10).
BCR signaling is not the only determinant of peripheral B cell activation. The recognition of pathogen-associated molecular patterns by toll-like receptors (TLRs) is important in the loss of B cell tolerance. Deficiency of myeloid differentiation primary response gene-88 (MyD88) in MRL/lpr mice abrogates autoantibody production (11), whereas deficiencies in TLR7 or TLR9 inhibit the production of antibodies against RNP and dsDNA, respectively (12). B cell activation through TLR7 and TLR9 is coupled to BCR recognition of ligand containing antigenic complexes (13–15). Presumably, this is because the BCR is the primary conduit into the late endosomes where these TLRs reside (16, 17).
Given the frequency of DNA-binding B cells in the peripheral repertoire, it might be expected that polyclonal anti-DNA antibody secretion would be a usual feature of the immune system (18, 19). However, in nonautoimmune prone strains, TLR ligands fail to overcome anergy. In anergic HEL-specific B cells, chronic activation of ERK inhibits CpG DNA-induced plasma cell differentiation in vitro (20). However, ERK activation is not a universal feature of B cell anergy (5). Therefore, other mechanism(s) must attenuate TLR activation in anergic B cells.
Results
Aberrant Endocytic Antigen Receptor Trafficking in Anergic 3H9/Vκ8 Splenic B Cells.
We first examined whether BCR endocytic trafficking was attenuated in peripheral anergic B cells from gene-targeted mice expressing 3H9/Vκ8, a BCR specific for ssDNA and cardiolipin (21, 22). Splenic B cells expressing either Vκ8, 3H9/Vκ8, or nontransgenic C57BL/6 WT B cells were stimulated with FITC-conjugated F(ab′)2 goat anti-mouse IgG/M (heavy and light chain) antibodies for 30 min at 37 °C then fixed, counterstained with anti-Lamp-1 antibodies (ID4B), and visualized by confocal microscopy (23). As we show in Fig. 1A, BCR aggregation on WT splenic B cells induced internalization and endocytic targeting of aggregated receptor complexes to Lamp-1+ late endosomes. Substantial colocalization of the internalized BCR with Lamp-1 was detected after 30 min of stimulation in 87% (SD = ±12.2%) of B cells from 3 independent experiments (Fig. 1B). A similar pattern of BCR endocytic trafficking was seen in splenic B cells expressing Vκ8 and a WT heavy-chain repertoire. In contrast, in 3H9/Vκ8 splenic B cells, the aggregated BCR was cleared from the cell surface, but failed to colocalize with Lamp-1+ late endosomes. In single plane confocal images, colocalization between the BCR and Lamp-1+ was detected in 7.7% (±6.4%) of cells. The difference in colocalization between WT and anergic B cells was highly significant (P = 3.38 × 10−12). Also, 3D reconstruction of several anergic cells demonstrating apparent colocalization on single optical sections revealed that the internalized BCR was adjacent to Lamp-1+ late endosomes, and not within them [Movie S1]. Therefore, it is likely that analysis of single-plane images overestimates the degree of colocalization between BCR and Lamp-1 in anergic B cells. A similar block in BCR endocytic trafficking was observed 60 min after BCR stimulation (Fig. S1).
Fig. 1.
Anergic B cells have a block in BCR endocytic trafficking, which is reversed on an MRL.Faslpr/lpr autoimmune background. (A) BCR endocytic trafficking after 30 min of stimulation with anti-BCR antibodies (BCR) or antigen (CG50) to Lamp-1+ late endosomes was examined in splenic B cells from WT C57BL/6, Vκ8, or anergic 3H9/Vκ8 mice. Results are representative of those obtained from 5 independent experiments. (B) Quantification, from single plane confocal images, of experiments represented in A. Shown are the mean and SD derived from 3 independent experiments, in which at least 50 cells were scored in each experimental group. (C) Ligation-induced BCR endocytic trafficking to late endosomes was evaluated as in A for splenic B cells from MRL.Faslpr/lpr mice expressing Vκ8 or 3H9/Vκ8. (D) Quantification of colocalization between endocytosed BCR and late endosomes evaluated as in B.
To confirm our results in anergic B cells using antigen, we stimulated 3H9/Vκ8 splenic cells as above with the biotinylated synthetic DNA ligand, CG50 (13) complexed to streptavidin-Qdot655 (Fig. 1A). Cells were then counterstained with anti-Lamp-1 antibodies, and visualized by confocal microscopy. Similar to what was observed with cross-linking antibodies, there was a distinct block in BCR entry into Lamp-1+ late endosomes (7.8 ± 2.2%; n = 3). As was the case when stimulatory antibodies were used, 3D reconstructions revealed that, in cells demonstrating apparent colocalization on single plane confocal images, the endocytosed BCR was adjacent to Lamp-1+ late endosomes (Movie S2). CG50 did not bind detectably to Vκ8 C57BL/6 splenic B cells (Fig. S2). These data indicate that, in anergic 3H9/Vκ8 B cells, there is a block in the delivery of ligated BCR complexes to late endosomes.
To examine whether aberrant BCR endocytic trafficking in 3H9/Vκ8 B cells was an inherent property of anergy, Vκ8 and 3H9/Vκ8 were bred onto the MRL.Faslpr/lpr background, and splenic B cells from these mice assayed for BCR trafficking to late endosomes. As demonstrated in Fig. 1 C and D, the MRL.Faslpr/lpr background restored efficient BCR endocytic trafficking and entry into Lamp-1+ late endosomes. These data indicate that aberrant BCR trafficking in anergic 3H9/Vκ8 B cells is associated with anergy, and is not an intrinsic characteristic of the 3H9/Vκ8 receptor.
Reversible Block in BCR Endocytic Trafficking in Ars/A1 Splenic B Cells.
We next examined splenic B cells from Ars/A1 transgenic mice in which the expressed Ig heavy and light chains (Ig μδ/κ) bind ssDNA with low affinity and confer high-affinity binding to p-azophenylarsonate (Ars) (24). B cells from these mice are anergic. In this model, anergy can be reversed within minutes in ex vivo cultured Ars/A1 splenic B cells by incubation with the monovalent ligand, arsonate-tyrosine (Ars-Tyr) (10). Therefore, splenic Ars/A1 specific or κ transgenic only B cells were cultured in the presence or absence of Ars-Tyr for 30 min, and then stimulated with anti-BCR F(ab)2 antibodies and analyzed as in Fig. 1A. As we show in Fig. 2 A and B, in the absence of Ars-Tyr, anergic Ars/A1 B cells manifested a block in BCR endocytic trafficking to Lamp-1+ late endosomes that was similar to that observed in 3H9/Vκ8 B cells (4.2 ± 1.6%; n = 3); 3D reconstructions of apparent regions of colocalization between endocytosed BCRs and late endosomes were revealed to be examples of close juxtaposition (Movie S3). In contrast, preincubation of Ars/A1 B cells for 30 min with Ars-Tyr restored normal BCR endocytic trafficking (91.2 ± 8.8%). The difference in BCR endocytic trafficking between cells cultured with or without hapten was highly significant (P = 9.24 × 10−6). These results indicate that aberrant BCR endocytic trafficking is associated with the maintenance of anergy. Also, comparison of the results obtained from the 3H9/Vκ8 and Ars/A1 anergic models indicate that aberrant endocytic BCR trafficking is a common feature of anergy.
Fig. 2.
Aberrant BCR trafficking in ssDNA-specific anergic B cells is reversible on removal of self-ligand by hapten competition. (A) Anergic Ars/A1 splenic B cells and κ Tg only B cells were examined for BCR trafficking to Lamp-1+ late endosomes. Cells were treated with FITC-F(ab′)2 anti-BCR antibodies for 30 min, in the presence or absence of Ars-Tyr. Cells were then visualized as in Fig. 1. Results are representative of 3 independent experiments. (B) Quantification of A, calculated as for Fig. 1B.
Normal BCR Internalization, Ubiquitinylation, and Early Endosomal Sorting in Anergic 3H9/Vκ8 B Cells.
We next sought to determine where the observed block in BCR endocytic trafficking was occurring. When we examined aggregation-induced BCR internalization using a flow cytometric assay, it was similar between B cells from 3H9, Vκ8, or 3H9/Vκ8 mice (Fig. S3A) (23). Also, transit of the ligated BCR through TfR+ early endosomes was similar between all 3 populations of splenic B cells (Fig. S4). Last, ubiquitinylation of Igβ, which is required for early endosomal sorting (23), was similar in Vκ8, 3H9, and 3H9/Vκ8 splenic B cells (Fig. S3B). From these observations, we conclude that, in anergic B cells, there is a post-early endosomal sorting trafficking defect.
JNK Activation Is Necessary for BCR Entry into Late Endosomes.
Initial experiments indicated that complementing proximal signaling with phorbol 12-myristate 13-acetate (PMA) could restore normal BCR endocytic trafficking in anergic 3H9/Vκ8 B cells (Fig. S5). Therefore, we sought to determine which specific signaling pathways, lacking in anergic B cells, were required for BCR endocytic trafficking.
We next assayed the ability of the BCR on anergic 3H9/Vκ8 B cells to induce the activation of the MAPKs, including the ERKs, JNKs, and p38 MAP kinase. Splenic B cells were stimulated with anti-IgM/G (H+L) F(ab′)2 antibodies for 0, 2, or 5 min, and whole cell lysates were subjected to Western immunoblotting with the indicated phospho-MAPK antibodies, and then reprobed with antibodies against ERK1/2, JNK1, or p38, respectively (Fig. 3A). In WT B cells, antigen mimetics induced ERK phosphorylation (5-min post-BCR stimulation) to a greater extent than in 3H9/Vκ8 B cells. Notably, basal ERK phosphorylation was not elevated in 3H9/Vκ8 B cells. Basal and BCR-induced p38 MAP kinase phosphorylation was similar in WT and anergic B splenocytes. However, expression of p38 MAP kinase was higher in anergic B cells (n = 3). Therefore, the fraction total p38 MAP kinase activated in anergic cells was diminished. BCR stimulation of WT cells induced phosphorylation of JNK1 at 2 min, whereas this pathway failed to be activated in 3H9/Vκ8 B cells (Fig. 3A).
Fig. 3.
BCR induced JNK activation is required for efficient receptor trafficking to late endosomes. (A) Splenic B cells from WT and 3H9/Vκ8 mice were assayed for the induction of MAPK activation after BCR stimulation with anti-IgG/M F(ab′)2 antibodies, and total cell lysates probed in Western blottings with the phosphorylated and total pools of the indicated MAPKs. The normalized ratio of the density for each phospho-MAPK band to the corresponding total MAPK band is provided. (B) Purified B cells were preincubated for 30 min with inhibitors to p38 (SB202190, 0.3 μM; Calbiochem), JNK (SP600125, 10 μM; Invitrogen), or ERK1/2 (25 μM; Calbiochem). Indicated samples were incubated for 18 h with JNK Inhibitor III (100 μM; Calbiochem). Aliquots were subsequently stimulated with FITC-conjugated anti-IgG/M (H+L) F(ab′)2 antibodies (green) for 30 min. Samples were then fixed, counterstained with ID4B (red), and visualized by confocal microscopy. (C) WT splenic B cells were cultured in the presence of a pan-JNK inhibitor (10 μM, SP600125) for 30 min, and then stimulated with FITC-conjugated anti-BCR antibodies and PMA for 30 min. Samples were then fixed, stained with ID4B, and visualized by confocal microscopy. Results are representative of 3 independent experiments. (D) Quantitation of results provided in C performed as described in Fig. 1.
To determine whether activation of MAPKs were required for internalization and endocytic trafficking of the aggregated BCR, WT C57BL/6 B splenocytes were preincubated with pharmacological inhibitors of p38, ERK, or JNK, and then stimulated with anti-IgM/G F(ab′)2 antibodies (Fig. 3B). In these experiments, inhibition of JNK had no effect on BCR internalization, but blocked the ability of internalized BCR complexes to enter late endosomes. Inhibition of p38 had no discernable effect on the BCR endocytic trafficking, whereas ERK inhibition blocked BCR internalization. These data implicate JNK in controlling BCR endocytic trafficking.
We next examined whether blocking JNK activation could inhibit the ability of PMA to restore normal BCR endocytic trafficking. Anergic 3H9/Vk8 splenic B cells were first incubated in the presence or absence of JNK inhibitor, and subsequently stimulated through the BCR with anti-receptor antibodies in the presence or absence of PMA. Cells were then assayed for BCR endocytic transit by counterstaining for Lamp-1 followed by confocal microscopy (Fig. 3 C and D). In BCR-stimulated 3H9/Vκ8 cells, the internalized BCR arrested outside Lamp-1+ late endosomes (4.5 ± 2.5% colocalization; n = 3), whereas PMA restored normal endocytic transit (93.8 ± 8.3%). In contrast, inhibiting JNK blocked the ability of PMA to rescue normal BCR trafficking (20 ± 14.1% at 10 μm; P = 4.48 × 10−5, when compared with +PMA alone; and P = NS, when compared with −PMA). These data indicate that JNK is a necessary downstream effector of PMA (4).
Regulation of TLR9 Access and Activation in Anergic 3H9/Vκ8 Cells.
In naive B cells, signaling through the BCR induces the translocation of TLR9 into late endosomes (25). Therefore, we next examined the spatial relationships between the ligated BCR, TLR9, and late endosomes in WT and anergic B cells. In both resting Vκ8 and 3H9/Vκ8 splenic B cells, TLR9 resided in a compartment distinct from Lamp-1+ endosomes (Fig. 4A). No staining of TLR9 was observed in splenic B cells from TLR9−/− mice (Fig. S6). Stimulation of the BCR on Vκ8 splenic B cells induced the rapid transit of both the BCR and TLR9 to Lamp-1+ late endosomes with strong colocalization of all 3 transmembrane proteins observed in 85.9% (±14.8%; n = 3) of cells (Fig. 4A). In contrast, in stimulated 3H9/Vκ8 splenic B cells, the BCR and TLR9 were excluded from Lamp-1+ late endosomes with only 4.5% (±2.9%) of BCR stimulated cells demonstrating any colocalization of TLR9 with Lamp-1+ late endosomes on single plane confocal images. The observed difference between WT and anergic B cells was highly significant (P = 3.34 × 10−7). However, colocalization between the BCR and TLR9 was observed in 91.3% (±12.8%) of single-plane confocal images from stimulated 3H9/Vκ8 splenic B cells, indicating that these receptors were arrested at a similar point along the endocytic pathway. Similar results were observed in anergic Ars/A1 splenic B cells. Stimulation of 3H9/Vκ8 splenic B cells with PMA reconstituted the tri-localization of Lamp-1, BCR, and TLR9 (87.5 ± 14.1%; n = 3) (Fig. S7). These observations suggest that one of the consequences of altered signaling in anergic cells is to prevent BCR-captured nucleotide containing antigenic complexes and TLR9 receptors from meeting within the acidic and processive environment of late endosomes.
Fig. 4.
Entry of TLR9 into late endosomes, and TLR9 activation, is abrogated in anergic 3H9/Vκ8 B cells. (A) Vκ8 and 3H9/Vκ8 splenic B cells were either left unstimulated or were stimulated with FITC-conjugated anti-IgG/M F(ab′)2 antibodies (green) ±PMA for 30 min. Samples were then fixed and counterstained with anti-Lamp1 (red) and anti-TLR9 antibodies (blue). Cells were visualized by confocal microscopy. Magnified single and triple stains for boxed regions of interest are shown (Upper Left, merged images; Upper Right, Lamp-1; Lower Left, BCR; and Lower Right, TLR9). Arrows indicate regions of colocalization between BCR and TLR9. (B) Anergic 3H9/Vκ8 B cells were stimulated with CG50 (5, 25, or 50 ng/mL) in the presence or absence of PMA. Cell samples were then harvested after 6 h, and T-bet/β actin mRNA was assayed by quantitative PCR. Data are representative of 3 independent experiments.
We next examined whether reconstituting BCR and TLR9 entry into late endosomes enabled TLR9 activation by BCR captured DNA. The CG50 ligand contains 50 CpG motifs that optimally activate murine TLR9 receptors (13). A unique downstream target of TLR9 is the transcription factor T-box expressed in T cells (T-bet), which can be directly activated by CpG via TLR9 in B cells through an IFNγR/STAT1-independent pathway (26). Anergic 3H9/Vκ8 B cells were isolated and treated with CG50 at 5, 25, or 50 ng/mL for 6 h in the presence or absence of PMA. IFN-γ was used as a positive control (26). Total RNA was isolated from stimulated B cells, and T-bet mRNA expression assayed by quantitative PCR. In the absence of PMA, CG50 alone did not significantly increase T-bet mRNA expression at any concentration tested (Fig. 4B). However, when CG50 was used to stimulate B cells through the BCR in the presence of PMA, T-bet expression was significantly up-regulated (P = 0.032 at 5 ng/mL, and P = 0.0062 at 50 ng/mL), similar to the level seen with IFN-γ stimulation (Fig. 4B).
Discussion
There is a large body of literature describing both the phenotypes of anergic B cells and the aberrations they manifest in BCR signaling. However, it has remained unclear as to how altered proximal signaling dictated cellular unresponsiveness. In this article, we demonstrate that in anergic B cells, the BCR is uncoupled from proximal signaling pathways necessary for the delivery of antigen-engaged BCR complexes to late endosomes (Fig. 5). Preventing receptor endocytic transit uncouples the sequential linkage between BCR and TLR engagement necessary to induce B cells to secrete anti-DNA antibodies (12, 13, 15, 19). Thus, our results identify an important functional consequence of the alterations in signaling observed in anergic B cells.
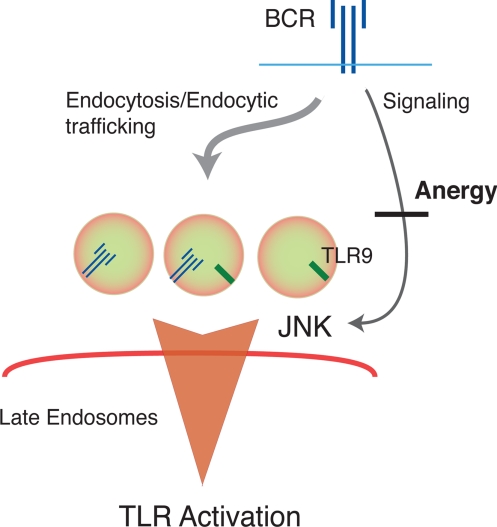
Fig. 5.
Anergy and the regulation of BCR/TLR9 entry into late endosomes. Antigen engagement of the BCR initiates 2 concurrent processes, signal transduction and receptor internalization and trafficking to late endosomes. Our data demonstrate that these 2 processes are interrelated, and that BCR-mediated JNK activation is required for both the BCR and TLR9 to enter late endosomal structures. In anergy, defective JNK activation leads to the nonproductive accumulation of BCR and TLR9 containing vesicles outside late endosomes and the uncoupling of adaptive and innate immune responses.
Signals transduced through the BCR not only regulate entry of the BCR into late endosomes, they also regulate the entry of TLR9. This finding is consistent with recent observations that BCR signaling, but not TLR9-dependent signaling pathways (27), control the transit of TLR9 from early to late endosomes (25). The observed colocalization of the ligated BCR and TLR9 outside late endosomes in anergic cells suggests that the BCR and TLR9 meet in the endocytic pathway and transit together into late endosomes (Fig. 5). Such temporal and spatial coordination of BCR and TLR9 endocytic trafficking may facilitate the delivery of BCR captured ligands to TLR9 (28). Our data, and previous observations, indicate that this transfer of nucleotide ligands from BCR to TLR9 must occur in the acidic and cathespsin-rich environment of late endosomes (29–31).
Treatment with PMA reversed the endocytic trafficking defect in anergic B cells within minutes, indicating that endocytic transit is directly initiated by proximal signaling, and not indirectly by changes in transcriptional programs. Such direct regulation would be expected, given the time scale of receptor endocytosis and endocytic transit, which are complete within 30 min of receptor ligation.
We would predict that arrested BCR endocytic transit prevents the efficient processing of antigen for cognate presentation to T cells. The Lamp-1+ late endosomes targeted by antigen/BCR complexes are the primary site of antigen processing in B cells (32, 33). However, exclusion of antigen from the MIIC is just one potential mechanism that may inhibit the productive presentation of antigen to T cells. Failure to up-regulate CD86 (34) and other intrinsic mechanisms limit the ability of anergic T cells to provide help (35). Last, in vivo, anergic B cells are excluded from follicular environments necessary for efficient activation (36).
Importantly, our data demonstrate that BCR and TLR9 endosomal trafficking are regulated in a way that has not been appreciated in studies of other mammalian receptors. Specifically, we observed that receptor entry into late endosomes depends on the receptor-initiated signals including JNK activation. A role for JNK is consistent with observations that the B cell linker protein (BLNK) (37), and BLNK recruitment to the BCR, is required for receptor entry into late endosomes (38). The downstream targets of JNK that control BCR endocytic trafficking are not known. However, p38 has been demonstrated to regulate rab proteins involved in receptor endocytosis (39, 40). It remains to be determined whether JNK has a similar role in controlling rab proteins involved in late endosomal transit.
Our findings are in apparent contrast to what has been reported for BCR internalization and endocytic trafficking in HEL-specific anergic B cells (41). In these cells, anergy is associated with accelerated BCR internalization and recycling of endocytosed BCRs to the cell surface. Neither of these features were observed in either anergic 3H9/Vκ8 or Ars/A1 B cells (Fig. 3). Also, when we assessed MD4xML5 anergic splenic B cells (42) by confocal microscopy, there was no detectable defect in BCR endocytic trafficking to Lamp-1+ late endosomes (Fig. S8). These findings are not necessarily surprising, because anergy appears to be a heterogeneous state in which different proximal signaling defects can give rise to unresponsiveness (5). However, common to all of the anergic models so far examined are mechanisms to limit TLR-mediated activation (20).
In summary, our observations identify a unique layer of control determining B cell responses to antigen. Previous studies have focused on how proximal signaling, transcriptional programs, and cellular trafficking determine cell fate (43). Our data demonstrate that within the cell, control of the subcellular location of adaptive and innate immune receptors can determine cellular responses to complex antigens. These results also demonstrate that in B cells, endocytic trafficking is not a constitutive, homeostatic process, but one that is determined by the quality of signals delivered through the BCR. It is likely that the basic constituents of endocytic trafficking in B cells are similar to those described in other cells. However, our data indicate that lymphocytes use unique mechanisms to regulate endocytic transport and influence cell fate.
Methods
Mice.
Vκ8 and VH3H9 (3H9) gene-targeted mice on a C57BL/6 background were provided by M. Weigert (University of Chicago). For some experiments, Vκ8 and 3H9 mice were bred onto the MRL.Faslpr/lpr background (8 generations). The ArsA1 and κ transgenic controls were on a C57BL/6 background (24). B cells were isolated from the spleens of 6- to 10-week-old mice as described in ref. 23.
Signal Transduction Assays.
Western blot assays for MAPK activation and assays of intracellular calcium were performed as previously described (44). Band densities were quantitated by using ImageJ (National Institutes of Health).
Confocal Microscopy.
Confocal microscopy was performed as previously described (23). Splenic B cells were labeled with either FITC-conjugated goat anti-mouse IgG+IgM (heavy and light chain) F(ab′)2 antibodies (Jackson Immunoresearch) or CG50-biotin linked to streptavidin-Qdot655, and then warmed to 37 °C for the indicated times. Purified B cells from Ars/A1 or κ transgenic mice were cultured on coverslips in the presence or absence of Ars/Tyr (100 μM) for 30 min at 37 °C before stimulation. Images were collected by using a Leica TCS SP2 AOBS confocal microscope. To quantitate the extent of colocalization between 2 or 3 fluorescent markers in individual cells, we used the JACoP plug-in of ImageJ. The specific algorithm used was based on the Mander's coefficient with a threshold of 40 (45). For colocalization of TLR9 with the BCR (Fig. 4) in a cell, a value >10% was considered positive. For all other experiments, the cut-off was 25%. For each experiment, at least 50 randomly selected cells were scored.
Quantitative Real-Time PCR.
RNA was isolated with Tri-Reagent (Molecular Research Center). Reverse transcription was performed with random hexamers and SuperScript II (Invitrogen). PCR for T-bet mRNA was as described (26). PCR amplification for murine β actin was performed on each sample as a control. Relative differences between samples were determined by calculating ΔΔCT (Applied Biosystems). The ΔΔCT values were converted to fold differences compared with control by raising 2 to the −ΔΔCT power (2-ΔΔCT).
Statistical Analyses.
A Student's t test was used to compare categorical findings.
Supplementary Material
Acknowledgments.
We thank Martin Weigert (University of Chicago) for providing us with the 3H9/Vκ8 mice on both the C57BL/6 and MRL.Faslpr/lpr backgrounds, and Ann Marshak-Rothstein for many helpful discussions. M.R.C. was supported by National Institutes of Health Grant R01 GM0677772.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812922106/DCSupplemental.
References
- 1.Vilen BJ, Famigelietti SJ, Carbone AM, Kay BK, Cambier JC. B Cell antigen receptor desensitization. J Immunol. 1997;159:231–243. [PMC free article] [PubMed] [Google Scholar]
- 2.Cooke MP, et al. Immunoglobulin signal transduction guides the specificity of B cell-T cell interactions and is blocked in tolerant self-reactive B cells. J Exp Med. 1994;179:425–438. doi: 10.1084/jem.179.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jun JE, Goodnow CC. Scaffolding of antigen receptors for immunogenic versus tolergenic signaling. Nat Immunol. 2003;4:1057–1064. doi: 10.1038/ni1001. [DOI] [PubMed] [Google Scholar]
- 4.Healy JI, et al. Different nuclear signals are activated by the B cell receptor during positive versus negative signaling. Immunity. 1997;6:419–428. doi: 10.1016/s1074-7613(00)80285-x. [DOI] [PubMed] [Google Scholar]
- 5.Cambier JC, Gauld SB, Merrell KT, Vilen BJ. B-cell anergy: From transgenic models to naturally occurring anergic B cells? Nature Rev. 2007;7:633–643. doi: 10.1038/nri2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glynne R, et al. How self-tolerance and the immunosuppressive drug FK506 prevent B-cell mitogenesis. Nature. 2000;403:672–676. doi: 10.1038/35001102. [DOI] [PubMed] [Google Scholar]
- 7.Merrell KT, et al. Identification of anergic B cell within a wild-type repertoire. Immunity. 2006;25:953–962. doi: 10.1016/j.immuni.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 8.Cyster JG, Goodnow CC. Antigen-induced exclusion from follicles and anergy are separate and complementary processes that influence peripheral B cell fate. Immunity. 1995;3:691–701. doi: 10.1016/1074-7613(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 9.Goodnow CC, Brink R, Adams E. Breakdown of self-tolerance in anergic B lymphocytes. Nature. 1991;352:532–536. doi: 10.1038/352532a0. [DOI] [PubMed] [Google Scholar]
- 10.Gauld SB, Benschop RJ, Merrell KT, Cambier JC. Maintenance of B cell anergy requires constant antigen receptor occupancy and signaling. Nat Immunol. 2005;6:1160–1167. doi: 10.1038/ni1256. [DOI] [PubMed] [Google Scholar]
- 11.Sadanaga A, et al. Protection against autoimmune nephritis in MyD88-deficient MRL/lpr mice. Arthritis Rheum. 2007;56:1618–1628. doi: 10.1002/art.22571. [DOI] [PubMed] [Google Scholar]
- 12.Christensen SR, et al. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Viglianti GA, et al. Activation of autoreactive B cells by CpG dsDNA. Immunity. 2003;19:837–847. doi: 10.1016/s1074-7613(03)00323-6. [DOI] [PubMed] [Google Scholar]
- 14.Lau CM, et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leadbetter EA, et al. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:595–598. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 16.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defense. Nat Rev Immunol. 2006;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 17.Siemasko K, Clark MR. The control and facilitation of MHC class II antigen processing by the BCR. Curr Opin Immunol. 2001;13:32–36. doi: 10.1016/s0952-7915(00)00178-3. [DOI] [PubMed] [Google Scholar]
- 18.Yurasov S, et al. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201:703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herlands RA, Christensen S, Sweet RA, Hershberg U, Shlomchik MJ. T cell-independent and toll-like receptor-dependent antigen-driven activation of autoreactive B cells. Immunity. 2008;29:249–260. doi: 10.1016/j.immuni.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rui L, Vinuesa CG, Blasioli J, Goodnow CC. Resistance to CpG DNA-induced autoimmunity through tolerogenic B cell antigen receptor ERK signaling. Nat Immunol. 2003;4:594–600. doi: 10.1038/ni924. [DOI] [PubMed] [Google Scholar]
- 21.Erikson J, et al. Expression of anti-DNA immunoglobulin transgenes in non-autoimmune mice. Nature. 1991;349:331–334. doi: 10.1038/349331a0. [DOI] [PubMed] [Google Scholar]
- 22.Radic MZ, Weigert M. Genetic and structural evidence for antigen selection of anti-DNA antibodies. Ann Rev Immunol. 1994;12:487–506. doi: 10.1146/annurev.iy.12.040194.002415. [DOI] [PubMed] [Google Scholar]
- 23.Zhang M, et al. Ubiquitinylation of Ig-beta dictates the endocytic fate of the B cell antigen receptor. J Immunol. 2007;179:4435–4443. doi: 10.4049/jimmunol.179.7.4435. [DOI] [PubMed] [Google Scholar]
- 24.Benschop RJ, et al. Activation and anergy in bone marrow B cells of a novel immunoglobulin transgenic mouse that is both hapten specific and autoreactive. Immunity. 2001;14:33–43. doi: 10.1016/s1074-7613(01)00087-5. [DOI] [PubMed] [Google Scholar]
- 25.Chaturvedi A, Dorward D, Pierce SK. The B cell receptor governs the subcellular location of Toll-like receptor 9 leading to hyperresponses to DNA-containing antigens. Immunity. 2008;28:799–809. doi: 10.1016/j.immuni.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu N, Ohnishi N, Ni L, Akira S, Bacon KB. CpG directly induces T-bet expression and inhibits IgG1 and IgE switching in B cells. Nat Immunol. 2003;4:687–693. doi: 10.1038/ni941. [DOI] [PubMed] [Google Scholar]
- 27.Latz E, et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5:190–198. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 28.Clark MR, Massenburg D, Siemasko K, Hou P, Zhang M. B-cell antigen receptor signaling requirements for targeting antigen to the MHC class II presentation pathway. Cur Opin Immunol. 2004;16:382–387. doi: 10.1016/j.coi.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto F, et al. Cathepsins are required for Toll-like receptor 9 responses. Biochem Biophys Res Commun. 2008;367:693–699. doi: 10.1016/j.bbrc.2007.12.130. [DOI] [PubMed] [Google Scholar]
- 30.Rutz M, et al. Toll-like receptor 9 binds single-stranded CpG-DNA in a sequence- and pH-dependent manner. Eur J Immunol. 2004;34:2541–2550. doi: 10.1002/eji.200425218. [DOI] [PubMed] [Google Scholar]
- 31.Asagiri M, et al. Cathepsin K-dependent toll-like receptor 9 signaling in experimental arthritis. Science. 2008;319:624–627. doi: 10.1126/science.1150110. [DOI] [PubMed] [Google Scholar]
- 32.Qiu Y, Xu X, Wandinger-Ness A, Dalke DP, Pierce SK. Separation of subcellular compartments containing distinct functional forms of MHC class II. J Cell Biol. 1994;125:595–605. doi: 10.1083/jcb.125.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferrari G, Knight AM, Watts C, Pieters J. Distinct intracellular compartments involved in invariant chain degradation and antigenic peptide loading of major histocompatibility complex (MHC) class II molecules. J Cell Biol. 1997;139:1433–1446. doi: 10.1083/jcb.139.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rathmell JC, Fournier S, Weintraub BC, Allison JP, Goodnow CC. Repression of B7.2 on self-reactive B cells is essential to prevent proliferation and allow Fas-mediated deletion of CD4+ T cells. J Exp Med. 1998;188:651–659. doi: 10.1084/jem.188.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heissmeyer V, et al. Calcineurin imposes T cell unresponsiveness through target proteolysis of signaling proteins. Nat Immunol. 2004;5:255–265. doi: 10.1038/ni1047. [DOI] [PubMed] [Google Scholar]
- 36.Cyster JG, Hartley SB, Goodnow CC. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature. 1994;371:389–395. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- 37.Ishiai M, et al. BLNK required for coupling Syk to PLCγ2 and Rac1-JNK in B Cells. Immunity. 1999;10:117–125. doi: 10.1016/s1074-7613(00)80012-6. [DOI] [PubMed] [Google Scholar]
- 38.Siemasko K, et al. Receptor-facilitated antigen presentation requires the recruitment of B cell linker protein to Igα. J Immunol. 2002;168:2127–2138. doi: 10.4049/jimmunol.168.5.2127. [DOI] [PubMed] [Google Scholar]
- 39.Cavalli V, et al. The stress-induced MAP kinase p38 regulates endocytic trafficking via the GDI:Rab5 complex. Mol Cell. 2001;7:421–432. doi: 10.1016/s1097-2765(01)00189-7. [DOI] [PubMed] [Google Scholar]
- 40.Zwang Y, Yarden Y. p38 MAP kinase mediates stress-induced internalization of EGFR: Implications for cancer chemotherapy. EMBO J. 2006;25:4195–4206. doi: 10.1038/sj.emboj.7601297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blery M, Tze LE, Miosge LA, Jun JE, Goodnow CC. Essential role of membrane cholesterol in accelerated BCR internalization and uncoupling from NF-kappa B in B cell clonal anergy. J Exp Med. 2006;203:1773–1783. doi: 10.1084/jem.20060552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goodnow CC, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 43.Goodnow CC, Sprent J, de St Groth BF, Vinuesa CG. Cellular and genetic mechanisms of self -tolerance and autoimmunity. Nature. 2005;435:590–597. doi: 10.1038/nature03724. [DOI] [PubMed] [Google Scholar]
- 44.Kabak S, et al. The direct recruitment of BLNK to immunoglobulin alpha couples the B-cell antigen receptor to distal signaling pathways. Mol Cell Biol. 2002;22:2524–2535. doi: 10.1128/MCB.22.8.2524-2535.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microscopy. 2006;224:213–232. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.