Abstract
The molecular pathways that promote the proliferation and maintenance of pituitary somatotrophs and other cell types of the anterior pituitary gland are not well understood at present. However, such knowledge is likely to lead to the development of novel drugs useful for the treatment of various human growth disorders. Although muscarinic cholinergic pathways have been implicated in regulating somatotroph function, the physiological relevance of this effect and the localization and nature of the receptor subtypes involved in this activity remain unclear. We report the surprising observation that mutant mice that selectively lack the M3 muscarinic acetylcholine receptor subtype in the brain (neurons and glial cells; Br-M3-KO mice) showed a dwarf phenotype associated with a pronounced hypoplasia of the anterior pituitary gland and a marked decrease in pituitary and serum growth hormone (GH) and prolactin. Remarkably, treatment of Br-M3-KO mice with CJC-1295, a synthetic GH-releasing hormone (GHRH) analog, rescued the growth deficit displayed by Br-M3-KO mice by restoring normal pituitary size and normal serum GH and IGF-1 levels. These findings, together with results from M3 receptor/GHRH colocalization studies and hypothalamic hormone measurements, support a model in which central (hypothalamic) M3 receptors are required for the proper function of hypothalamic GHRH neurons. Our data reveal an unexpected and critical role for central M3 receptors in regulating longitudinal growth by promoting the proliferation of pituitary somatotroph cells.
Keywords: anterior pituitary gland, dwarfism, knockout mice
Various neurotransmitter systems have been implicated in regulating somatotroph function and growth hormone (GH) secretion, either by acting on the anterior pituitary gland directly or by modulating the release of GH-releasing hormone (GHRH) or somatostatin from the hypothalamus (for a comprehensive review, see ref. 1). However, the physiological relevance of the individual pathways and the identity and localization of the receptor subtypes involved in mediating these effects are not well understood in most cases.
Pharmacological studies suggest that central muscarinic cholinergic pathways play a role in stimulating GH release in experimental animals and humans (1–9). The identification of the molecular pathways and the specific muscarinic ACh receptor (mAChR) subtypes involved in mediating these responses should be of considerable potential therapeutic interest. However, work in this area has been complicated by the existence of 5 molecularly distinct mAChR subtypes (M1–M5) that are difficult to distinguish by classical pharmacological tools (10, 11). Moreover, virtually all brain regions express multiple mAChRs in a complex, overlapping pattern (12–15).
The M3 mAChR subtype is expressed at relatively high levels in the hypothalamus but is also found in many other brain regions (16–18). At present, little is known about the physiological relevance of these neuronal M3 mAChRs. To shed light on this issue, we used Cre/loxP technology to generate a set of mutant mice, referred to as Br-M3-KO mice, that selectively lacked the M3 mAChR subtype in the brain (neurons and glial cells). Strikingly, Br-M3-KO mice showed a dwarf phenotype, associated with a dramatic reduction in the size of the anterior pituitary gland, a marked decrease in pituitary GH and prolactin levels, and significantly reduced serum GH, insulin-like growth factor-1 (IGF-1), and prolactin levels. Remarkably, treatment of Br-M3-KO mice with CJC-1295, a synthetic GHRH analog (19–21), restored normal pituitary size, serum GH and IGF-1 levels, and longitudinal growth. These data reveal an unexpected and critical role for central M3 receptors in promoting longitudinal growth.
Results
Generation of Brain-Specific M3 mAChR Knockout (KO) Mice (Br-M3-KO Mice).
We reported the generation of a mutant mouse strain that harbored a floxed (fl) version of the M3 mAChR gene (22). To obtain mutant mice that lack M3 mAChRs only in the brain (neurons and glial cells), we mated the floxed M3 receptor mice with a mouse strain carrying a nestin-Cre (NesCre) transgene (23). In this strain, expression of Cre recombinase occurs only in those cells that give rise to neurons and glial cells. To generate mutant mice that were homozygous for the floxed M3 receptor allele and carried the NesCre transgene (for the sake of simplicity, these mice are referred to as Br-M3-KO mice in the following), we crossed M3 fl/+ mice with M3 fl/+ mice that were hemizygous for the NesCre transgene. This mating strategy also produced 3 littermate control groups, fl/fl, +/+, and +/+ Cre mice. All mouse genotypes were obtained at the expected Mendelian frequency.
To confirm the absence of M3 receptor protein in the brain of Br-M3-KO mice, we used a previously developed combined radioligand binding/immunoprecipitation strategy (17). This analysis indicated that M3 receptor expression was virtually eliminated in the brain of Br-M3-KO mice (Fig. 1A). In contrast, brains derived from the 3 control groups showed similar M3 receptor expression levels (Fig. 1A). For control purposes, we carried out similar studies with salivary gland membranes, which are known to express M3 receptors at comparable levels (24). Salivary gland membranes prepared from Br-M3-KO mice did not show a decrease in M3 receptor density, confirming that Br-M3-KO mice selectively lacked M3 receptors in the brain (Fig. 1A). In the experiments reported below, floxed M3 receptor mice lacking the NesCre transgene were used as control mice, unless stated otherwise.
Fig. 1.
Body weight and length and food intake of Br-M3-KO mice and control littermates. (A) Lack of M3 mAChRs in brains from Br-M3-KO mice. M3 mAChR densities in mouse brain and salivary glands (submandibular gland) were determined by using a combined radioligand binding/immunoprecipitation approach. For these studies, 4-month-old male mice (littermates) of the indicated genotypes were used. Quinuclidinyl [3H]benzilate-labeled M3 receptors were solubilized from membrane preparations and immunoprecipitated with an M3 receptor-specific antiserum (17). Each bar represents the mean ± SEM of at least 3 independent experiments. (B) Growth curves of Br-M3-KO mice and their control littermates (males). Starting from postnatal week 4, Br-M3-KO mice weighed significantly less than their control littermates (10–12 mice per group). (C) Physical appearance of a representative Br-M3-KO and control (fl/fl) mouse (20-week-old males). (D) Body length (distance from the tip of the nose to the base of the tail) of Br-M3-KO and control littermates (24-week-old males; control, n = 29; Br-M3-KO, n = 10). (E) Food intake studies (16-week-old males; control, n = 18; Br-M3-KO, n = 6). All measurements were carried out with 20-week-old males (control, n = 17; Br-M3-KO, n = 6). In D and E, the 3 control strains (+/+, +/+ NesCre, and fl/fl) gave results that did not differ significantly from each other. The data obtained with these mice were therefore pooled for the sake of clarity. Data are given as means ± SEM. *, P < 0.05; **, P < 0.01, as compared with the corresponding control group(s).
Metabolic Studies.
We reported previously that whole-body M3 receptor KO mice display a series of pronounced metabolic phenotypes, including decreased food intake and body fat content, and increased metabolic rate, locomotor activity, body temperature, glucose tolerance, and insulin sensitivity (17, 25). Interestingly, Br-M3-KO mice did not display any significant changes in food intake (Fig. 1E), metabolic rate (Fig. S1A), locomotor activity (Fig. S1B), and body composition (Fig. S1 C and D). Moreover, blood glucose and insulin levels, glucose tolerance, insulin sensitivity, and body temperature were also unaltered in Br-M3-KO mice (Fig. S2 and Table S1).
Br-M3-KO Mice Are Dwarfs and Show Reduced Serum GH and IGF-1 Levels.
Strikingly, growth curves showed that Br-M3-KO mice weighed considerably less than their corresponding littermate control groups (Fig. 1B). This difference in body weight was not observed during the first 3 postnatal weeks but became highly significant when the mice were ≈4 weeks old. On average, adult Br-M3-KO mice weighed ≈25% less than their control littermates.
Fig. 1C shows that the Br-M3-KO mice exhibited reduced longitudinal growth, displaying a dwarf-like appearance. Specifically, adult mutant mice were ≈10% shorter than their control littermates (Fig. 1D).
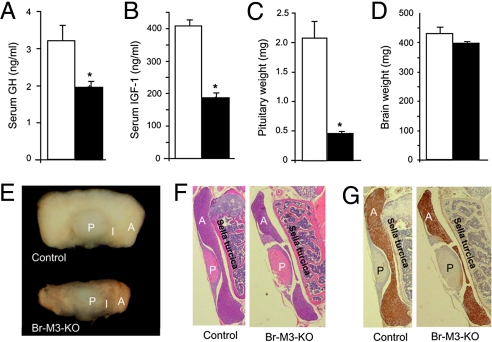
To explore the mechanisms underlying the dwarf phenotype displayed by the Br-M3-KO mice, we next measured serum levels of key hormones involved in somatic growth. Strikingly, Br-M3-KO mice showed a significant reduction in the serum levels of GH (Fig. 2A) and insulin-like growth factor I (IGF-1; Fig. 2B). In contrast, the serum levels of the thyroid hormones, T3 and T4, and the sex hormones, testosterone and estradiol (E2), were not significantly different between Br-M3-KO and control littermates (Table 1). Br-M3-KO mice also showed unchanged serum levels of ghrelin, a hormone that stimulates pituitary GH release via activation of central GH secretagogue receptors (GHSR) (26). However, serum corticosterone levels were found to be significantly increased in Br-M3-KO mice (Table 1). Consistent with this finding, Br-M3-KO mice also exhibited significantly elevated serum levels of ACTH, the pituitary hormone that simulates corticosterone synthesis and release from the adrenal cortex (Table 1).
Fig. 2.
Serum GH and IGF-1 levels and pituitary gland weight and morphology of Br-M3-KO mice and control littermates. (A and B) Serum GH and IGF-1 levels. Hormone levels were measured by RIA (20-week-old males; GH: control, n = 31; Br-M3-KO, n = 20; IGF-1: n = 6 per group). (C) Total weight of pituitary glands (20-week-old males; n = 10 per group). (D) Total brain weights (16-week-old males; n = 10 per group). (E) Gross morphology of pituitary glands. Representative glands from adult male Br-M3-KO and control mice are shown. A, anterior pituitary; I, intermediate lobe; P, posterior pituitary. (F and G) H&E staining and GH immunostaining, respectively, of pituitary sections. Pituitary glands from Br-M3-KO and control littermates (20-week-old males) were sectioned and stained with H&E or incubated with a mouse GH antibody as described in Materials and Methods. Note the pronounced hypoplasia of the anterior pituitary of Br-M3-KO mice. Data are given as means ± SEM. **, P < 0.01 as compared with the corresponding control group.
Table 1.
Serum hormone levels of Br-M3-KO mice and control littermates
| Hormone | Control | Br-M3-KO |
|---|---|---|
| T3, ng/dL | 64.5 ± 3.7 | 66.4 ± 5.6 |
| T4, ng/dL | 3.35 ± 0.30 | 3.49 ± 0.35 |
| Testosterone, ng/mL | 0.75 ± 0.23 | 2.75 ± 1.10 |
| Estradiol (E2), pg/mL | 9.8 ± 3.3 | 15.6 ± 3.4 |
| Corticosterone, ng/mL | 107 ± 25 | 189 ± 20* |
| Ghrelin, ng/mL | 2,794 ± 311 | 2,791 ± 343 |
| ACTH, ng/mL | 285 ± 38 | 497 ± 53** |
| Prolactin, ng/mL | 82.3 ± 22.2 | 23.1 ± 7.3* |
| LH, ng/mL | 0.11 ± 0.01 | 0.15 ± 0.02 |
| FSH, ng/mL | 1.27 ± 0.06 | 1.02 ± 0.20 |
| TSH, ng/mL | 2.19 ± 0.14 | 2.65 ± 0.22 |
All data were obtained with 16- to 24-week-old male mice (littermates). Data represent means ± SEM (n = 6–9 per group). *, P < 0.05; **, P < 0.01 compared with the corresponding control value.
GH is released into the blood stream from the anterior pituitary gland and binds to specific receptors in the liver, where it triggers the secretion of IGF-1. Circulating IGF-1 is considered the major factor that mediates the stimulatory effects of GH on longitudinal growth (27, 28). It is therefore likely that the observed decreases in serum IGF-1 and GH levels displayed by the Br-M3-KO mice are responsible for the observed growth deficit.
Br-M3-KO Mice Show a Pronounced Hypoplasia of the Anterior Pituitary Gland.
We next examined whether the lack of central M3 mAChRs affected the morphology of the pituitary gland, where GH and several other important hormones are synthesized and stored. We found that the pituitary gland of Br-M3-KO mice was significantly smaller than that of their control littermates (Fig. 2E). Fig. 2C shows that the pituitary glands from Br-M3-KO mice weighed ≈75% less than those of control mice. In contrast, total brain weight was similar in control and Br-M3-KO mice (Fig. 2D). Fig. 2E indicates that the pituitary hypoplasia displayed by the Br-M3-KO mice was primarily caused by a dramatic decrease in the size of the anterior pituitary (the size of posterior pituitary appeared essentially unchanged). Besides the pituitary hypoplasia displayed by the M3 receptor mutant mice, we did not observe any noticeable differences in overall brain morphology between the mutant and control mice (Fig. S3). Interestingly, the deficits in pituitary size observed with adult Br-M3-KO mice (Fig. 2E) were not observed with neonatal Br-M3-KO mice (Fig. S4), consistent with the observation that Br-M3-KO mice and control littermates showed similar body weight during the first 3 postnatal weeks (Fig. 1B).
To examine the morphology of the pituitary glands from Br-M3-KO mutant mice and control littermates in greater detail, we prepared pituitary gland sections and stained them with either hematoxylin and eosin (H&E) (Fig. 2F) or an antibody directed against GH (Fig. 2G). We noted that the size of the posterior pituitary, which stores and releases only 2 major hormones, vasopressin and oxytocin, was similar in Br-M3-KO and control mice (also see Fig. 2E). In striking contrast, the size of the anterior pituitary gland/area of GH staining was greatly reduced in the Br-M3-KO mice compared with control littermates (Fig. 2 F and G) [note that most cells of the anterior pituitary are GH cells (somatotrophs) (29)].
Selective Reduction of Pituitary GH and Prolactin Levels in Br-M3-KO Mice.
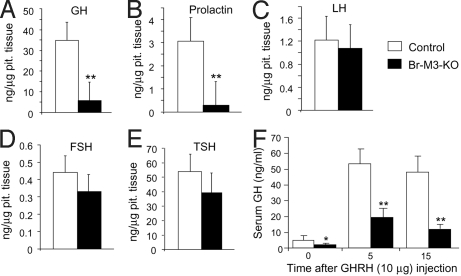
Besides GH, the anterior pituitary synthesizes and releases several other physiologically important hormones, including LH, FSH, TSH, and prolactin. We found that pituitary GH and prolactin levels were drastically reduced in Br-M3-KO mice (Fig. 3 A and B). Consistent with this observation, serum prolactin levels, like serum GH and IGF-1 levels, were significantly decreased (by ≈70%) in Br-M3-KO mice, compared with control littermates (Table 1). The major physiological function of prolactin is to stimulate milk production in females (30). However, female Br-M3-KO mice did not display any obvious impairments in their ability to nurse their young, suggesting that low prolactin levels are sufficient to maintain this function in female mutant mice.
Fig. 3.
Hormone levels in pituitary gland extracts from Br-M3-KO mice and control littermates and acute GHRH challenge test. (A–E) GH, prolactin, LH, FSH, and TSH levels, respectively, in pituitary extracts from Br-M3-KO and control mice (adult males). Pituitary extracts were prepared and hormone levels were determined as described in Materials and Methods (n = 6 per group). (F) Effect of GHRH administration on serum GH levels in Br-M3-KO mice and control littermates. After i.p. administration of GHRH (10 μg per animal), serum GH levels were measured by RIA at the indicated times (30-week-old males; n = 8 per group). Data are given as means ± SEM. *, P < 0.05; **, P < 0.01 compared with the corresponding control group.
In contrast to pituitary GH and prolactin amounts, the pituitary levels (concentrations) of LH, FSH, and TSH (expressed in nanograms of hormone per microgram of pituitary tissue) were similar in control and Br-M3-KO mice (Fig. 3 C–E). In agreement with this observation, serum LH, FSH, and TSH levels did not differ significantly between the M3 receptor mutant mice and control littermates (Table 1).
The secretion of GH from somatotrophs and the proliferation and maintenance of these cells are under the stimulatory control of the hypothalamic GH-releasing hormone (GHRH) (1). To examine whether the GHRH-GH pathway was still functional in Br-M3-KO mice, we injected Br-M3-KO mice and control littermates with a single dose of GHRH (10 μg per mouse, i.p.) and measured serum GH levels 5 and 15 min after GHRH administration. As expected, GHRH administration led to a robust increase in serum GH levels in the control mice (Fig. 3F). In contrast, the corresponding GH responses were greatly reduced in Br-M3-KO mice (Fig. 3F), consistent with the finding that pituitary GH levels were very low in Br-M3-KO mice (Fig. 3A).
GHRH Neurons Express M3 mAChRs.
In contrast to the posterior pituitary, the anterior pituitary is not of neuronal origin; it is derived from the oral ectoderm (31). As a result, the NesCre transgene is not expressed in the anterior pituitary (23, 32). It seemed therefore reasonable to assume that the defect leading to the hypoplasia of the anterior pituitary in Br-M3-KO-mice does not reside in the pituitary itself but involves other brain regions that stimulate the growth of the anterior pituitary.
The hypothalamus synthesizes several hormones that are known to stimulate the proliferation of specific cell types of the anterior pituitary. Studies with mice that lack hypothalamic GHRH neurons or that have been subjected to manipulations that impair the function of these neurons suggest that GHRH stimulates the proliferation of both GH- and prolactin-expressing cells of the anterior pituitary (33, 34). It is well known that the hypothalamus expresses relatively high levels of M3 receptors (17, 18). Since Br-M3-KO-mice showed a selective reduction in pituitary GH and prolactin levels, it is possible that the activity of hypothalamic M3 receptors is required for the proper function of GHRH neurons.
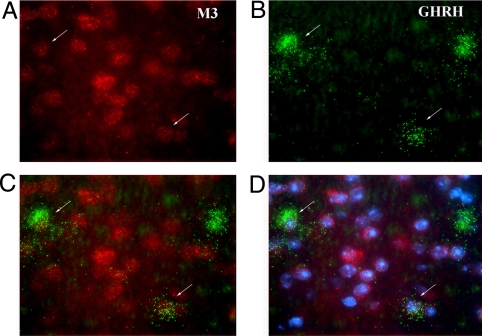
In the mouse hypothalamus, GHRH is primarily expressed by specialized cells of the arcuate nucleus (35). To examine whether the GHRH-containing cells of the arcuate nucleus also express M3 mAChRs, we carried out a series of in situ mRNA hybridization studies combining radioactive and nonradioactive detection techniques. We found that most (but not all) of the hypothalamic GHRH neurons coexpress M3 mAChR mRNA (Fig. 4).
Fig. 4.
Colocalization of M3 mAChR and GHRH mRNA in neurons of the arcuate nucleus of WT mice. (A) Labeling of M3 receptor mRNA-positive cells (red Alexafluor-594 signal). (B) Labeling of GHRH mRNA-positive cells by using a GHRH-specific 35S-labeled riboprobe. Autoradiographic silver grains (originally black) were colored green by using Adobe Photoshop. (C) Overlay of M3 receptor mRNA (A) and GHRH mRNA (B) expression. (D) Overlay of A, B, and DAPI (nuclear chromosomal) stain. For details, see Materials and Methods. Arrows point at cells that express both M3 receptor and GHRH mRNAs.
Hypothalamic GHRH and Somatostatin Levels Are Significantly Reduced in Br-M3-KO Mice.
To examine whether hypothalamic GHRH levels were altered in Br-M3-KO mice, we measured total hypothalamic GHRH content by ELISA. We found that GHRH levels were reduced by ≈40–50% in Br-M3-KO mice, as compared with control littermates (Fig. 5A). GHRH and GH release are known to be under the inhibitory control of somatostatin, which is stored in different subsets of hypothalamic neurons (1). ELISA studies showed that hypothalamic somatostatin levels were reduced by ≈35% in Br-M3-KO mice (Fig. 5B). Since somatostatin generally exerts an inhibitory effect on longitudinal growth (via inhibition of GH release), it is likely that the decrease in hypothalamic somatostatin levels displayed by Br-M3-KO mice is a compensatory event triggered by reduced peripheral GH and IGF-1 levels (1).
Fig. 5.
Hypothalamic GHRH and somatostatin levels in Br-M3-KO mice and control littermates. Hypothalamic GHRH (A) and somatostatin (SS) (B) levels were measured by ELISA. Data are presented as means ± SEM (n = 6 per group; 24-week-old males). **, P < 0.01 compared with the corresponding control group.
CJC-1295 Rescue Experiments.
Given the findings presented above, we next tested the hypothesis that the hypoplasia of the anterior pituitary gland and the resulting decreases in serum GH and IGF-1 levels and longitudinal growth exhibited by Br-M3-KO mice were due to reduced hypothalamic GHRH release. Specifically, we examined whether chronic administration of CJC-1295, a synthetic GHRH analog (19–21), was able to prevent the various hormonal and morphological deficits characteristic of Br-M3-KO mice. CJC-1295 selectively and covalently binds to endogenous albumin after injection, thereby extending its half-life and duration of action. Br-M3-KO mice and control littermates were treated with CJC-1295 (one daily s.c. injection of 2 μg per mouse) for 8 weeks. CJC-1295 treatment was initiated when mice were 1 week old and Br-M3-KO mice did not yet display any significant growth retardation (Fig. 1B). Nontreated Br-M3-KO mice and control littermates served as additional controls.
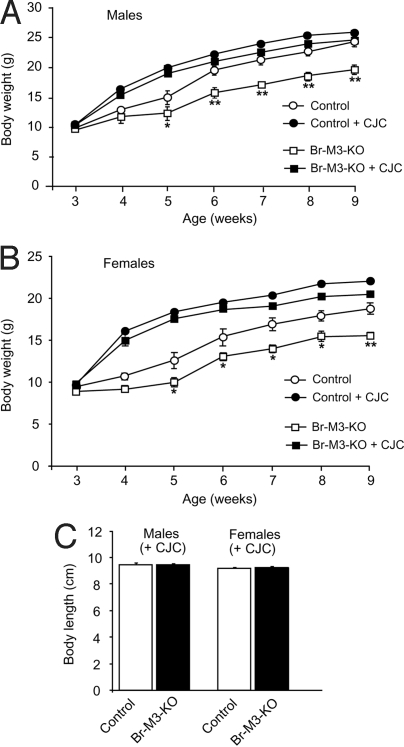
Strikingly, CJC-1295 treatment of male or female Br-M3-KO mice was able to fully rescue the growth deficit associated with the lack of central M3 receptors (Fig. 6). Fig. 6 A and B shows that the growth curves of CJC-1295-treated Br-M3-KO mice were similar to those of CJC-1295-treated control mice. It should be noted that CJC-1295 also significantly increased the body weight of control mice compared with nontreated control animals (Fig. 6 A and B). At the end of the 8-week treatment period, the body lengths of CJC-1295-treated Br-M3-KO mice were not significantly different from those of CJC-1295-treated control mice (males or females) (Fig. 6C).
Fig. 6.
CJC-1295 administration restores normal growth in Br-M3-KO mice. (A and B) Growth curves of male (A) and female (B) Br-M3-KO mice and control littermates. Mice were either injected with CJC-1295 (2 μg s.c. per mouse per day; week 1 to 9) or left untreated. (C) Body length of CJC-1295-treated Br-M3-KO and control mice (9-week-old males and females). Data are presented as means ± SEM (n = 5–8 per group). *, P < 0.05; **, P < 0.01 compared with the corresponding littermates.
CJC-1295 treatment also rescued the hypoplasia of the anterior pituitary gland displayed by nontreated Br-M3-KO mice (Fig. S5 A, C, and D). Fig. S5A shows that the size of the anterior pituitary gland was similar in CJC-1295-treated Br-M3-KO mice and control littermates, consistent with the outcome of H&E staining and GH immunostaining studies of pituitary sections (Fig. S5 C and D). Moreover, total pituitary weight did not differ significantly between CJC-1295-treated Br-M3-KO and control mice (Fig. S5B).
Finally, we also measured serum GH and IGF-1 levels in CJC-1295-treated and nontreated Br-M3-KO mice and control littermates (males and females). We found that CJC-1295-treated Br-M3-KO mice showed a ≈2- to 4-fold increase in serum GH levels compared with nontreated Br-M3-KO mice (SI Materials and Methods and Fig. S6 A and C). In fact, the serum GH levels of CJC-1295-treated Br-M3-KO mice were not significantly different from those measured in CJC-1295-treated (or nontreated) control littermates (Fig. S6 A and C). CJC-1295 treatment of Br-M3-KO mice also led to a ≈2–3-fold increase in serum IGF-1 levels as compared with nontreated Br-M3-KO mice (Fig. S6 B and D). The serum IGF-1 levels observed with CJC-1295-treated Br-M3-KO mice were not significantly different from those measured in nontreated control mice, but somewhat lower (P < 0.01) than those displayed by CJC-1295-treated control mice (Fig. S6 B and D).
Discussion
In the present study, we demonstrated that Br-M3-KO mice displayed a dramatic hypoplasia of the anterior pituitary gland, associated with greatly reduced pituitary GH and prolactin levels. Moreover, serum GH and IGF-1 levels, the major endocrine regulators of postnatal growth in mammals, were significantly decreased in these mutant mice, leading to greatly reduced longitudinal growth.
The anterior pituitary, in contrast to the posterior pituitary, is not of neuronal origin but is derived from the oral ectoderm. As a result, the NesCre transgene used in the present study is not expressed in the anterior pituitary (23, 32). It is therefore unlikely that the defect leading to the hypoplasia of the anterior pituitary in the Br-M3-KO mice resides in the pituitary itself. It is well established that GHRH, which is released from specific hypothalamic neurons, plays a key role in stimulating the proliferation of pituitary somatotroph cells (1, 33). Because M3 mAChRs are expressed in the hypothalamus at relatively high density (17, 18), we speculated that hypothalamic M3 receptor activity might be required for the proper function of GHRH neurons, resulting in the observed hypoplasia of the anterior pituitary and reduced longitudinal growth of Br-M3-KO mice. Consistent with this notion, we found that treatment of Br-M3-KO mice with CJC-1295, a synthetic GHRH analog (19–21), restored normal pituitary size and serum GH and IGF-1 levels, and normal longitudinal growth. The ability of CJC-1295 to rescue the various hormonal and morphological deficits of Br-M3-KO mice supports the concept that the primary defect underlying the dwarf phenotype of these mutant mice does not reside in the pituitary gland itself but most likely involves impaired function of hypothalamic GHRH neurons.
We found that hypothalamic GHRH levels were greatly reduced in Br-M3-KO mice. Moreover, in situ mRNA hybridization studies showed that GHRH neurons of the arcuate nucleus, the primary site of GHRH synthesis and storage in mice (35), also express M3 mAChRs. In agreement with this observation, a recent electrophysiological study demonstrated that GHRH neurons are endowed with functional mAChRs, activation of which leads to enhanced action potential firing (36).
Interestingly, selective ablation of GHRH neurons in transgenic mice results in phenotypic changes very similar to those observed with Br-M3-KO mice, including a selective reduction in pituitary levels of GH and prolactin (34). GHRH itself has little effect on prolactin synthesis or release (37), consistent with the observation that GHRH KO mice showed normal pituitary prolactin content (38). These findings support a model in which hypothalamic GHRH neurons synthesize 1 or more additional factors or hormones that stimulate the proliferation of pituitary lactotrophs (ref. 34; also see ref. 39). Taken together, these findings are consistent with a model in which M3 mAChR activity is critical for the proper function of GHRH neurons. However, we cannot completely exclude the possibility that M3 receptors expressed by other hypothalamic neurons or other brain regions affect the activity of GHRH neurons through a more indirect mechanism.
While GHRH stimulates GH release from the pituitary gland, another hypothalamic hormone, somatostatin, exerts an inhibitory function on the secretion of GH (but not its biosynthesis) (1). We found that hypothalamic somatostatin levels were significantly reduced in Br-M3-KO mice, excluding the possibility that increased central somatostatin signaling is responsible for the observed decrease in circulating GH and IGF-1 levels. It is likely that the observed decrease in hypothalamic somatostatin content is due to feedback inhibition triggered by low serum GH and IGF-1 levels (1). It should be noted in this context that mutant mice deficient in somatostatin show growth curves identical to their WT littermates (40), suggesting that the GHRH/GH system plays the dominant role in the regulation of postnatal growth in mice. Taken together, these findings strongly suggest that it is unlikely that changes in hypothalamic somatostatin synthesis or release make a significant contribution to the growth deficit displayed by the Br-M3-KO mice.
Interestingly, whole-body dopamine transporter (41) and brain-specific Gq/11 KO mice (32) show phenotypic changes similar to those described here for Br-M3-KO mice, indicating that dopamine and Gq-type G proteins, like ACh, also play important roles in regulating the proliferation and maintenance of specific cell types of the anterior pituitary gland.
We previously reported that whole-body M3 receptor KO mice show normal (17) or only slightly reduced (25) body length and display serum IGF-1 levels similar to their control littermates (17). One possible explanation for the observed differences in growth phenotypes between whole-body M3 receptor KO and Br-M3-KO mice is that whole-body M3 receptor KO mice lack M3 receptors throughout development, raising the possibility that compensatory pathways are able to evolve that mimic the growth-promoting effects of M3 receptor activity. In contrast, in Br-M3-KO mice, NesCre-mediated deletion of the M3 receptor gene occurs relatively late during embryonic development (42).
In comparison with GHRH KO mice (38, 43) or mice with a nonfunctional GHRH receptor (little mice) (29), Br-M3-KO mice showed a milder degree of growth reduction and pituitary hypoplasia. One possible explanation for this observation is that not all GHRH neurons appear to express M3 receptors.
In conclusion, the phenotypic analysis of Br-M3-KO mice revealed an unexpected and critical role of central M3 receptors in the proliferation of the anterior pituitary and the stimulation of longitudinal growth. Our results suggest that central M3 receptors represent a potential pharmacological target to enhance or inhibit GH release in the treatment of various forms of human growth disorders.
Materials and Methods
Mouse Maintenance and Diet.
Mice were housed in a specific pathogen-free barrier facility, maintained on a 12 hr light/dark cycle. Mice were fed ad libitum with a standard mouse chow [4% (wt/wt) fat content; Zeigler). All experiments were approved by the Animal Care and Use Committee of the National Institute of Diabetes and Digestive and Kidney Diseases.
Generation of Mutant Mice Lacking M3 mAChRs in Neuronal Precursor Cells (Br-M3-KO Mice).
We recently established a mutant mouse strain harboring a floxed version of the M3 mAChR gene (M3 fl/fl mice) (22). These mice were crossed to transgenic mice that express Cre recombinase under the control of the rat nestin promoter (23, 42). The NesCre transgenic mice were obtained from the The Jackson Laboratory (official strain name: B6.Cg-Tg(Nes-cre)1Kln/J; genetic background: C57BL/6J). The resulting M3 fl/+ NesCre mice were then crossed with M3 fl/+ mice to generate M3 fl/fl NesCre mice (referred to as Br-M3-KO mice throughout the text) and the 3 corresponding littermate control groups (M3 fl/fl, +/+, and +/+ Cre mice). Mice were genotyped by PCR analysis of tail DNA as described in ref. 22.
Quantification of M3 mAChR Expression Levels by Using an Immunoprecipitation Strategy.
To quantitate the expression of M3 mAChR protein in mouse tissues, we used a combined radioligand binding/immunoprecipitation strategy, as described in detail in ref. 17 (also see SI Materials and Methods).
Body Length and Composition, Food Intake, Metabolic Rate, and Locomotor Activity Measurements.
Body length was defined as the distance between the tip of the nose and the base of the tail. Body composition, food intake, metabolic rate, and locomotor activity measurements were carried out at room temperature, as described previously (25).
Hormone Measurements.
Serum hormone concentrations were determined by RIA by the Vanderbilt University Hormone Assay and Analytical Services Core or by Ani Lytics. Samples for the measurement of serum GH levels were usually collected between 8 and 10 a.m.
To determine pituitary hormone levels (GH, prolactin, LH, FSH, and TSH), pituitary extracts were obtained by sonicating individual pituitary glands in 0.01 M NaHCO3. Pituitary hormones levels were measured by RIA in the laboratory of the National Hormone and Peptide Program (Torrance, CA). To quantitate the hypothalamic levels of GHRH and somatostatin, hypothalami were removed surgically and GHRH and somatostatin levels were measured in hypothalamic extracts by ELISA (Phoenix Pharmaceuticals). Hypothalamic extracts were prepared by sonicating individual pituitary glands in the buffer provided with the ELISA kits.
Acute GHRH Injection Studies.
To measure GHRH-induced GH release, Br-M3-KO mice and control littermates were injected i.p. with 10 μg of human GHRH (Phoenix Pharmaceuticals). Blood samples for serum GH determinations were collected 5 and 15 min after GHRH administration by means of retro-orbital sinus puncture.
CJC-1295 Rescue Experiments.
One-week-old Br-M3-KO mice and control littermates (males and females) were treated for 8 weeks with CJC-1295 (one single s.c. injection of 2 μg per mouse per day; time of injection: 9–10 a.m.). CJC-1295 was a gift from ConjuChem (Montreal).
Histology.
Whole brains or pituitary glands were harvested from control and Br-M3-KO mice. Tissue sections were processed and sectioned for H&E and/or GH immunostaining by standard techniques (see SI Materials and Methods for details).
In situ mRNA Hybridization Studies. To examine whether mouse GHRH neurons express M3 mAChRs, coronal hypothalamic sections (12 μm thick) were hybridized with digoxigenin-labeled M3 mAChR-specific and 35S-labeled GHRH-specific riboprobes (see SI Materials and Methods for details).
Statistics.
Data are expressed as means ± SEM for the indicated number of observations. Statistical significance of difference between groups was determined by using 2-tailed Student's t test or one-way ANOVA followed by appropriate post hoc tests.
Supplementary Material
Acknowledgments.
We thank Drs. Karen Thibaudeau and Tom Ulich (ConjuChem, Montreal) for providing us with the CJC-1295 compound. This work was supported by the Intramural Research Programs of the National Institute of Diabetes and Digestive and Kidney Diseases and the National Institute of Dental and Craniofacial Research, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900977106/DCSupplemental.
References
- 1.Giustina A, Veldhuis JD. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev. 1998;19:717–797. doi: 10.1210/edrv.19.6.0353. [DOI] [PubMed] [Google Scholar]
- 2.Locatelli V, et al. Cholinergic agonist and antagonist drugs modulate the growth hormone response to growth hormone-releasing hormone in the rat: Evidence for mediation by somatostatin. J Endocrinol. 1986;111:271–278. doi: 10.1677/joe.0.1110271. [DOI] [PubMed] [Google Scholar]
- 3.Torsello A, et al. Involvement of the somatostatin and cholinergic systems in the mechanism of growth hormone autofeedback regulation in the rat. J Endocrinol. 1988;117:273–281. doi: 10.1677/joe.0.1170273. [DOI] [PubMed] [Google Scholar]
- 4.Arce VM, Cella SG, Locatelli V, Müller EE. Studies of growth hormone secretion in calorically restricted dogs: Effect of cholinergic agonists and antagonists, glucose and thyrotropin-releasing hormone. Neuroendocrinology. 1991;53:467–472. doi: 10.1159/000125759. [DOI] [PubMed] [Google Scholar]
- 5.Delitala G, et al. Cholinergic receptor control mechanisms for L-dopa, apomorphine, and clonidine-induced growth hormone secretion in man. J Clin Endocrinol Metab. 1983;57:1145–1149. doi: 10.1210/jcem-57-6-1145. [DOI] [PubMed] [Google Scholar]
- 6.Casanueva FF, Villanueva L, Cabranes JA, Cabezas-Cerrato J, Fernandez-Cruz A. Cholinergic mediation of growth hormone secretion elicited by arginine, clonidine, and physical exercise in man. J Clin Endocrinol Metab. 1984;59:526–530. doi: 10.1210/jcem-59-3-526. [DOI] [PubMed] [Google Scholar]
- 7.Taylor BJ, Smith PJ, Brook CG. Inhibition of physiological growth hormone secretion by atropine. Clin Endocrinol (Oxford) 1985;22:497–501. doi: 10.1111/j.1365-2265.1985.tb00149.x. [DOI] [PubMed] [Google Scholar]
- 8.Massara F, et al. Cholinergic involvement in the growth hormone releasing hormone-induced growth hormone release: Studies in normal and acromegalic subjects. Neuroendocrinology. 1986;43:670–675. doi: 10.1159/000124602. [DOI] [PubMed] [Google Scholar]
- 9.Peters JR, et al. Cholinergic muscarinic receptor blockade with pirenzepine abolishes slow wave sleep-related growth hormone release in normal adult males. Clin Endocrinol (Oxford) 1986;25:213–217. doi: 10.1111/j.1365-2265.1986.tb01684.x. [DOI] [PubMed] [Google Scholar]
- 10.Wess J. Molecular biology of muscarinic acetylcholine receptors. Crit Rev Neurobiol. 1996;10:69–99. doi: 10.1615/critrevneurobiol.v10.i1.40. [DOI] [PubMed] [Google Scholar]
- 11.Caulfield MP, Birdsall NJM. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50:279–290. [PubMed] [Google Scholar]
- 12.Levey AI. Immunological localization of m1–m5 muscarinic acetylcholine receptors in peripheral tissues and brain. Life Sci. 1993;52:441–448. doi: 10.1016/0024-3205(93)90300-r. [DOI] [PubMed] [Google Scholar]
- 13.Vilaro MT, Mengod G, Palacios JM. Advances and limitations of the molecular neuroanatomy of cholinergic receptors: The example of multiple muscarinic receptors. Prog Brain Res. 1993;98:95–101. doi: 10.1016/s0079-6123(08)62385-7. [DOI] [PubMed] [Google Scholar]
- 14.Wolfe BB, Yasuda RP. Development of selective antisera for muscarinic cholinergic receptor subtypes. Ann NY Acad Sci. 1995;757:186–193. doi: 10.1111/j.1749-6632.1995.tb17474.x. [DOI] [PubMed] [Google Scholar]
- 15.Wess J, Eglen RM, Gautam D. Muscarinic acetylcholine receptors: Mutant mice provide new insights for drug development. Nat Rev Drug Discov. 2007;6:721–733. doi: 10.1038/nrd2379. [DOI] [PubMed] [Google Scholar]
- 16.Levey AI, Edmunds SM, Heilman CJ, Desmond TJ, Frey KA. Localization of muscarinic m3 receptor protein and M3 receptor binding in rat brain. Neuroscience. 1994;63:207–221. doi: 10.1016/0306-4522(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 17.Yamada M, et al. Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature. 2001;410:207–212. doi: 10.1038/35065604. [DOI] [PubMed] [Google Scholar]
- 18.Oki T, et al. Quantitative analysis of binding parameters of [3H]N-methylscopolamine in central nervous system of muscarinic acetylcholine receptor knockout mice. Mol Brain Res. 2005;133:6–11. doi: 10.1016/j.molbrainres.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Jetté L, et al. Human growth hormone-releasing factor (hGRF)1–29-albumin bioconjugates activate the GRF receptor on the anterior pituitary in rats: Identification of CJC-1295 as a long-lasting GRF analog. Endocrinology. 2005;146:3052–3058. doi: 10.1210/en.2004-1286. [DOI] [PubMed] [Google Scholar]
- 20.Alba M, et al. Once-daily administration of CJC-1295, a long-acting growth hormone-releasing hormone (GHRH) analog, normalizes growth in the GHRH knockout mouse. Am J Physiol. 2006;291:E1290–E1294. doi: 10.1152/ajpendo.00201.2006. [DOI] [PubMed] [Google Scholar]
- 21.Teichman SL, et al. Prolonged stimulation of growth hormone (GH) and insulin-like growth factor I secretion by CJC-1295, a long-acting analog of GH-releasing hormone, in healthy adults. J Clin Endocrinol Metab. 2006;91:799–805. doi: 10.1210/jc.2005-1536. [DOI] [PubMed] [Google Scholar]
- 22.Gautam D, et al. A critical role for β cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab. 2000;3:449–461. doi: 10.1016/j.cmet.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Tronche F, et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- 24.Gautam D, et al. Cholinergic stimulation of salivary secretion studied with M1 and M3 muscarinic receptor single- and double-knockout mice. Mol Pharmacol. 2004;66:260–267. doi: 10.1124/mol.66.2.260. [DOI] [PubMed] [Google Scholar]
- 25.Gautam D, et al. Beneficial metabolic effects of M3 muscarinic acetylcholine receptor deficiency. Cell Metab. 2006;4:363–375. doi: 10.1016/j.cmet.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci USA. 2004;101:4679–4684. doi: 10.1073/pnas.0305930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yakar S, et al. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest. 2002;110:771–781. doi: 10.1172/JCI15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nilsson O, Marino R, De Luca F, Phillip M, Baron J. Endocrine regulation of the growth plate. Horm Res. 2005;64:157–165. doi: 10.1159/000088791. [DOI] [PubMed] [Google Scholar]
- 29.Lin SC, et al. Molecular basis of the little mouse phenotype and implications for cell type-specific growth. Nature. 1993;364:208–213. doi: 10.1038/364208a0. [DOI] [PubMed] [Google Scholar]
- 30.Ostrom KM. A review of the hormone prolactin during lactation. Prog Food Nutr Sci. 1990;14:1–43. [PubMed] [Google Scholar]
- 31.Cohen LE, Radovick S. Molecular basis of combined pituitary hormone deficiencies. Endocr Rev. 2002;23:431–442. doi: 10.1210/er.2001-0030. [DOI] [PubMed] [Google Scholar]
- 32.Wettschureck N, et al. Loss of Gq/11 family G proteins in the nervous system causes pituitary somatotroph hypoplasia and dwarfism in mice. Mol Cell Biol. 2005;25:1942–1948. doi: 10.1128/MCB.25.5.1942-1948.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frohman LA, Kineman RD. Growth hormone-releasing hormone and pituitary development, hyperplasia and tumorigenesis. Trends Endocrinol Metab. 2002;13:299–303. doi: 10.1016/s1043-2760(02)00613-6. [DOI] [PubMed] [Google Scholar]
- 34.Le Tissier PR, et al. Hypothalamic growth hormone-releasing hormone (GHRH) deficiency: Targeted ablation of GHRH neurons in mice using a viral ion channel transgene. Mol Endocrinol. 2005;19:1251–1262. doi: 10.1210/me.2004-0223. [DOI] [PubMed] [Google Scholar]
- 35.Suhr ST, Rahal JO, Mayo KE. Mouse growth-hormone-releasing hormone: Precursor structure and expression in brain and placenta. Mol Endocrinol. 1989;3:1693–1700. doi: 10.1210/mend-3-11-1693. [DOI] [PubMed] [Google Scholar]
- 36.Baccam N, et al. Dual-level afferent control of growth hormone-releasing hormone (GHRH) neurons in GHRH-green fluorescent protein transgenic mice. J Neurosci. 2007;27:1631–1641. doi: 10.1523/JNEUROSCI.2693-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore JP, Jr, et al. Pituitary hormone gene expression and secretion in human growth hormone-releasing hormone transgenic mice: Focus on lactotroph function. Endocrinology. 2000;141:81–90. doi: 10.1210/endo.141.1.7262. [DOI] [PubMed] [Google Scholar]
- 38.Alba M, Salvatori R. A mouse with targeted ablation of the growth hormone-releasing hormone gene: A new model of isolated growth hormone deficiency. Endocrinology. 2004;145:4134–4143. doi: 10.1210/en.2004-0119. [DOI] [PubMed] [Google Scholar]
- 39.Li H, Zeitler PS, Valerius MT, Small K, Potter SS. Gsh-1, an orphan Hox gene, is required for normal pituitary development. EMBO J. 1996;15:714–724. [PMC free article] [PubMed] [Google Scholar]
- 40.Low MJ, et al. Somatostatin is required for masculinization of growth hormone-regulated hepatic gene expression but not of somatic growth. J Clin Invest. 2001;107:1571–1580. doi: 10.1172/JCI11941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bossé R, et al. Anterior pituitary hypoplasia and dwarfism in mice lacking the dopamine transporter. Neuron. 1997;19:127–138. doi: 10.1016/s0896-6273(00)80353-0. [DOI] [PubMed] [Google Scholar]
- 42.Zimmerman L, et al. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron. 1994;12:11–24. doi: 10.1016/0896-6273(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 43.Alba M, Schally AV, Salvatori R. Partial reversibility of growth hormone (GH) deficiency in the GH-releasing hormone (GHRH) knockout mouse by postnatal treatment with a GHRH analog. Endocrinology. 2005;146:1506–1513. doi: 10.1210/en.2004-1044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.