Abstract
Rationale: Excessive deposition of extracellular matrix occurs in proximal airways of individuals with asthma, but fibrosis in distal lung has not been observed. Whether differing fibrotic capacities of fibroblasts from these two regions contribute to this variability is unknown.
Objectives: We compared morphologic and functional characteristics of fibroblasts isolated from proximal airways and distal lung parenchyma to determine phenotypic differences.
Methods: Concurrent proximal airway and distal lung biopsies were obtained by bronchoscopy from subjects with asthma to isolate airway and distal lung fibroblasts, respectively. The following characteristics were compared: morphology, proliferation, α-smooth muscle actin expression, and synthesis of procollagen type I and eotaxin-1.
Results: Airway fibroblasts (AFs) are morphologically distinct from distal lung fibroblasts (DLFs): they are larger (2.3-fold greater surface area vs. matched DLFs; p = 0.02), stellate in appearance, and with more cytoplasmic projections compared with the spindle-shaped DLFs. AFs synthesized more procollagen type I than did DLFs at baseline (twofold higher; p = 0.003) and after transforming growth factor-β stimulation (1.4-fold higher; p = 0.02). Similarly, AFs produced more eotaxin-1 than did DLFs at baseline (2.5-fold higher; p = 0.004) and after interleukin-13 stimulation (13-fold higher; p = 0.0001). In contrast, DLFs proliferate more than AFs with serum stimulation (about sixfold greater; p = 0.03). Unstimulated DLFs also expressed more α-smooth muscle actin than did corresponding AFs (p = 0.006).
Conclusions: These studies suggest that at least two phenotypes of fibroblast exist in the lung. These phenotypic differences may partially explain the variable responses to injury and repair between proximal airways and distal lung/parenchyma in asthma and other respiratory diseases.
Keywords: asthma, fibroblast, interleukin 13, remodeling, transforming growth factor β
Increased airway fibrosis is a commonly described feature of remodeling in asthma (1–3). More specifically, excessive deposition of collagen and other extracellular matrix (ECM) components occurs in the subepithelial–basement membrane region of proximal airways (2, 4–6). Although studies are limited, distal lung parenchyma or alveolar tissue appears to be nearly devoid of fibrosis. Rather, data suggest abnormal loss of ECM in the alveolar interstitium adjacent to small airways in the distal lung tissue of individuals with fatal asthma (7, 8). The cellular mechanisms of such contrasting matrix patterns in proximal airways versus distal lung tissue of individuals with asthma remain to be determined.
The fibroblast–myofibroblast cell type is believed to be the major cell responsible for production of ECM (9, 10). In addition to their fibrotic potential, fibroblasts release other mediators that are important in the pathogenesis of asthma (11–13). For instance, fibroblasts are an important source of eotaxin, a potent eosinophil chemoattractant (13, 14). Aberrant or abnormal phenotypes of fibroblasts have been described in several fibrotic disorders (15). In vitro studies of fibroblasts isolated from the skin of patients with systemic sclerosis indicate an activated phenotype with increased capability for collagen synthesis (16, 17). This enhanced potential is likely due to an abnormal response to transforming growth factor β (TGF-β), an important profibrotic signaling molecule (18). Similarly, altered fibroblast phenotypes have been described in fibrotic diseases of the lung (19). Raghu and coworkers have reported that fibroblasts isolated from regions of lungs with early fibrosis in patients with idiopathic pulmonary fibrosis have greater proliferative potential than those obtained from regions of dense fibrosis or from normal lungs (20). However, there are no comparative studies of fibroblasts from distinct anatomic regions of the lung (airway vs. alveolar/parenchyma) in either diseased or normal lungs.
We hypothesized that fibroblasts isolated from the proximal airways are phenotypically distinct from distal lung/parenchymal fibroblasts. Early-passage fibroblasts were cultured from tissue samples of proximal airways and from distal lung parenchyma of subjects with asthma (and two normal subjects) and compared for differences in morphology, proliferation, and differentiation. In addition, we speculated that airway fibroblasts disproportionately contribute to ECM production and recruitment of eosinophils in response to a profibrotic stimulus (TGF-β) and a helper T-cell type 2 cytokine, interleukin 13 (IL-13), as compared with distal lung fibroblasts. Therefore, we also examined the capacity of matched proximal airway and distal lung fibroblasts to synthesize collagen and eotaxin in response to TGF-β and IL-13. Some of the results of these studies have been reported previously in the form of an abstract (21).
METHODS
Subjects and Biopsies
Overall, 18 subjects with asthma of varying severity participated in the study (22, 23). Endobronchial biopsies (of large airways, typically from third- to fifth-order subcarinae) and transbronchial biopsies (2–3 cm from the pleura) were obtained by bronchoscopy as described previously (24, 25). Atopic status was determined by skin testing to a panel of indoor and outdoor antigens. The Institutional Review Board of the National Jewish Medical and Research Center (Denver, CO) approved the study and all subjects gave informed consent. As transbronchial biopsies in normal subjects are not ethically feasible, we evaluated airway and distal lung tissue from two patients without known preexisting lung disease (and within 24 h of traumatic death), to provide data on regional fibroblast differences in normal lung. The location of sampling was similar to that of bronchoscopically obtained biopsies; pieces of airway tissue were excised from proximal large airways and distal lung tissue was obtained from the lung periphery/parenchyma.
Fibroblast Culture and Experimentation
The biopsy (and postmortem) lung tissue was processed as described previously (14). Briefly, the biopsy pieces were cultured until fibroblasts advanced out from the tissue. Fibroblasts were passaged during the proliferative state and were studied at the third or fourth passage. Endobronchial samples produced fibroblasts termed “airway” fibroblasts (AFs) whereas transbronchial biopsies produced “distal lung” fibroblasts (DLFs). To contrast AFs and DLFs, parallel experiments were performed on paired fibroblasts (therefore, AFs were compared with DLFs from the same subject). Although a total of 18 subjects with asthma took part in the study, sample size varied for each experimental system on the basis of availability of cells and the extent of the responses seen.
Morphologic Assessment
Fibroblasts were plated without serum for 24 h followed by stimulation with 0.5% fetal bovine serum (FBS). Twenty-four and 48 h after plating, the medium was removed and cells were washed with phosphate-buffered saline and then stained according to a modified Wright-Giemsa method (Hema 3; Fisher Scientific, Pittsburgh, PA). Fibroblasts were visualized by direct light microscopy at ×200, using an Olympus BX51 microscope (Olympus America, Melville, NY). To avoid observer bias, the first 20 cells (5 in each quadrant of the well) that were distinctly visible and not in significant contact with other cells were identified in a blinded fashion. Electronic images of these cells were analyzed and cell surface area was measured with Scion Image Analysis software (National Institutes of Health, Bethesda, MD).
Proliferation Assay
Fibroblast proliferation was estimated by measuring DNA synthesis. Paired fibroblasts were plated for 24 h, followed by stimulation with 0.5 and 10% FBS in the presence of [3H]thymidine (10 μCi/ml). After 24 h, cells were harvested and [3H]thymidine incorporation was measured with a β-scintillation counter (Beckman Coulter, Fullerton, CA) and recorded as counts per minute. Each condition was performed in triplicate and data were normalized to the untreated sample (i.e., fold increase relative to control).
α-Smooth Muscle Actin Western Blot Assay
Paired third-passage fibroblasts were plated without serum for 24 h followed by addition of 0.5% FBS alone or with TGF-β (0.5 ng/ml) for 48 h. The cells were lysed and equal amounts of cell lysate protein were electrophoresed as described previously (14). Immunoblotting was performed for α-smooth muscle actin (α-SMA) (M0851; Dako, Carpinteria, CA) and specific bands at 42 kD were scanned and semiquantitated by densitometry (Scion software). Equal protein loading was confirmed by probing for β-actin on stripped blots.
Enzyme-linked Immunoassay for Human Procollagen Type I and Eotaxin-1
Paired fibroblasts were plated and stimulated with 0.5% FBS alone or with the addition of TGF-β, IL-13, or both for 48 h as described above. Procollagen type I and eotaxin-1 levels in fibroblast supernatants were measured by sandwich ELISA performed by ELISA Tech LLC (Aurora, CO). When eotaxin-1 measurements were below the assay detection limit (8 pg/ml) they were assigned an arbitrary value of 4 pg/ml.
Statistics
Data were checked for normality of distribution. Data that were normally distributed are presented as means (± SEM). Heavily right-skewed data were log transformed to produce variables normally distributed. Responses of the two fibroblast types with stimulation as well as differences between fibroblast groups based on location were analyzed by paired t tests or analysis of variance. p ⩽ 0.05 was deemed statistically significant. Log-transformed data were converted back into linear units for presentation in text and figures. Given the smaller sample size of the nondiseased matched pairs of AFs and DLFs, 95% prediction intervals (mean ± 2 SD × [(1 + 1/n)1/2]) of measurements were used from subjects with asthma to infer whether the responses of nondiseased fibroblasts were distributed like those obtained from subjects with asthma (26).
RESULTS
Subject Characteristics
Overall, 18 subjects with asthma took part in the study, although the number of fibroblast pairs used in each experiment varied. At the time of bronchoscopy, their average age was 39 yr (range, 20–59 yr) with a mean duration of disease of 28 yr (range, 3–49 yr). Eight of the 18 subjects were women. Most subjects (72%) had severe asthma based on criteria proposed by the American Thoracic Society Workshop on Refractory Asthma (22). Taken as a whole, the subjects had moderate to severe airflow limitation; their FEV1 averaged 63% of predicted values (measured values, 2.33 ± 0.12 L [mean ± SEM]). Although measurement of bronchial hyperresponsiveness was not required for inclusion in the study, 15 of the 18 subjects had a methacholine PC20 (provocative concentration causing a 20% fall in FEV1) of 0.54 ± 0.14 mg/ml. Eight of the subjects used oral corticosteroids, with an average dose equivalent of 31 mg (range, 14–60 mg) of prednisone per day. All subjects used inhaled corticosteroids. Thirteen of the 18 subjects were atopic on the basis of skin testing. For more details of subject characteristics, see Table E1 of the online supplement.
Morphologic Differences
In six matched pairs, airway fibroblasts were morphologically distinct from distal lung fibroblasts. Figures 1A and 1B show representative images of fibroblasts from each location from the same subject at the same magnification. At baseline 24 h after plating without serum, AFs were significantly larger than DLFs. The mean (± SEM) surface area of AFs was 2,167 ± 334 μm2 whereas the area of DLFs averaged 937 ± 72 μm2 (p = 0.02). Other descriptive morphologic differences were also identified, as evident from Figure 1. Adherent airway fibroblasts were stellate in appearance with several cytoplasmic projections. In contrast, the DLFs were thinner and more spindle shaped with fewer projections. On average, AFs had 4.05 ± 0.15 cytoplasmic projections per cell, whereas the DLFs had 2.85 ± 0.06 projections per cell (p = 0.0002 between AFs and DLFs). Adherent AFs were approximately 40% wider than DLFs (cell width at the center: AFs, 19.8 ± 1.4 μm as compared with DLFs, 11.9 ± 0.8 μm; p = 0.005). The cytoplasm:nucleus ratio, measured at the widest part of the nucleus, was also significantly greater for AFs compared with DLFs, consistent with their bigger size (1.64 ± 0.06 vs. 1.22 ± 0.03; p = 0.001). Even after 24 h of serum stimulation, these differences persisted (data not shown). These morphologic observations indicate significant morphologic distinctions between fibroblasts isolated from the proximal airways and distal lung of subjects with asthma.
Figure 1.
Morphologic differences between primary airway and distal lung fibroblasts from subjects with asthma. (A and B) Photomicrographs of airway and distal lung fibroblasts, respectively, from the same subject taken at an original magnification of ×200. Insets: Enlarged images for greater detail. (C and D) Comparison of cell size and number of cytoplasmic projections per cell, respectively, from each location (n = 6 matched pairs).
Proliferation Differences
The proliferative potential of eight matched pairs of fibroblasts under subconfluent culture conditions was assessed by [3H]thymidine uptake for DNA synthesis (Figure 2). After serum starvation and subsequent stimulation with 10% FBS for 24 h, AFs had a twofold increase in incorporation of thymidine compared with cells proliferating without FBS (basal, 365 ± 92 cpm vs. 10% FBS, 718 ± 220 cpm; p = 0.09). Serum-stimulated DNA synthesis increased fourfold in DLFs (basal, 350 ± 121 cpm vs. 10% FBS, 1,421 ± 247 cpm; p = 0.002). The change in proliferation with and without serum was significantly different (p = 0.03) between the matched pairs (AFs vs. DLFs). Likewise, 24 h of stimulation with 0.5% FBS led to significantly greater 3H incorporation by DLFs than AFs (1,474 ± 424 vs. 589 ± 166 cpm, respectively; p = 0.06). Addition of [3H]thymidine in the last 4 h of serum stimulation before harvest showed similar results, with greater uptake by DLFs compared with AFs in four matched pairs, but the difference did not reach statistical significance. This suggests that the cells were still in the proliferative or growth phase of the cell cycle at the time of harvest. Visual assessment of confluence at the time of harvest of all cells and conditions was between 70 and 90%, suggesting that contact inhibition was not likely to have influenced the results.
Figure 2.
Differences in proliferation of primary airway and distal lung fibroblasts (n = 8), measured by uptake of [3H]thymidine (counts per minute [cpm]) in response to serum stimulation. Data are presented as means and SEM without stimulation (control) and after 10% serum stimulation for 24 h.
α-SMA Expression
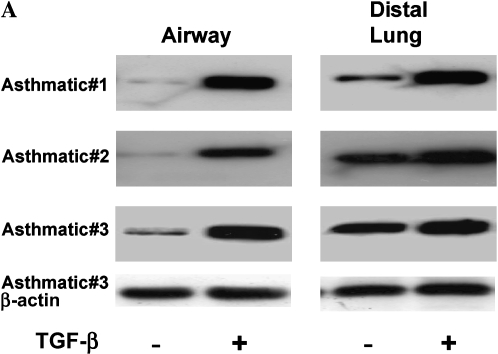
Estimation of the amount of α-SMA was used to identify the level of myofibroblast differentiation at baseline and after stimulation with TGF-β in seven matched pairs of fibroblasts. Figure 3A shows the Western blots of three matched pairs of asthmatic fibroblasts. In all seven subjects with asthma studied, unstimulated DLFs expressed higher amounts of α-SMA than did AFs, indicating greater basal differentiation toward a myofibroblast phenotype. Densitometry of the blots revealed 10-fold higher expression of α-SMA by DLFs compared with AFs (Figure 3B; p = 0.006). TGF-β increased α-SMA expression in all fibroblasts, as expected. After 48 h of TGF-β stimulation, the level of α-SMA expression increased 12-fold in AFs (p = 0.001 compared with unstimulated controls). This response was less robust in the DLFs; α-SMA expression increased 3-fold after 48 h of TGF-β stimulation (p = 0.03), although from higher baseline levels. Despite individual variations between matched pairs, overall α-SMA expression tended to be higher in DLFs after TGF-β stimulation (p = 0.09). However, in four of the seven fibroblast pairs, overall α-SMA expression after TGF-β stimulation between the two cell types was identical. These data suggest that although unstimulated AFs do not exhibit a myofibroblast phenotype, they are capable of differentiation in response to TGF-β.
Figure 3.
Differences in α-smooth muscle actin (α-SMA; molecular mass, 42 kD) expression in primary airway and distal lung fibroblasts as determined by immunoblotting. (A) Western blots of matched pairs of airway fibroblasts (AFs) and distal lung fibroblasts (DLFs) obtained from three subjects with asthma at baseline and after stimulation with transforming growth factor β (TGF-β). (B) Expression of α-SMA in relation to β-actin from seven matched pairs of asthmatic fibroblasts was semiquantified by densitometry, and mean (and SEM) values are shown relative to expression in airway fibroblasts at baseline.
Procollagen Type I Production
The fibrotic potential of AFs and DLFs was assessed by measuring procollagen type I (PC-I) production at baseline and after 48 h of stimulation with TGF-β and/or IL-13 in 12 matched pairs (Figure 4). The ELISA measurements were right-skewed and required log transformation to establish normal distribution. In airway fibroblasts, baseline production of PC-I was 812 ± 147 ng/ml. Basal production of PC-I in distal lung fibroblasts was significantly lower compared with AFs (DLF baseline, 432 ± 87 ng/ml; p = 0.003 [AFs vs. DLFs]). Stimulation with TGF-β increased PC-I synthesis in airway fibroblasts (post–TGF-β AFs: 1,901 ± 344 ng/ml; p < 0.0001 compared with baseline AFs) and in distal lung fibroblasts (post–TGF-β DLFs: 1,339 ± 283 ng/ml; p = 0.002 compared with baseline DLFs; p = 0.02 for comparison of post–TGF-β AFs vs. DLFs). The TGF-β–stimulated increase in PC-I production was greater in DLFs compared with AFs, likely because of the lower baseline (fold increase with TGF-β compared with baseline: AFs, 2.3; DLFs, 3.1; p = 0.03). Stimulation with IL-13 alone did not affect PC-I production by fibroblasts from either location. However, stimulation with TGF-β and IL-13 resulted in a synergistic increase in PC-I in each group (post–TGF-β plus IL-13: AFs, 2,186 ± 373 ng/ml; DLFs, 1,556 ± 282 ng/ml; p < 0.001 comparing each group with their respective baseline; p = 0.03 for comparison of post–TGF-β plus IL-13 AFs vs. DLFs). The increase in PC-I by costimulation with TGF-β plus IL-13 was greater in DLFs compared with AFs (fold increase with TGF-β plus IL-13 compared with baseline: AFs, 2.7; DLFs, 5.11; p = 0.02). These observations indicate an increased basal ability of airway fibroblasts to produce PC-I when compared with DLFs. Although DLFs are capable of increasing collagen production with stimulation by TGF-β alone or in combination with IL-13, poststimulation levels remain higher in airway fibroblasts.
Figure 4.
Procollagen type I production by primary asthmatic airway and distal lung fibroblasts (n = 12) at baseline and after stimulation with TGF-β plus interleukin 13 (IL-13). Mean (and SEM) values were transformed back into normal units (ng/ml) for presentation. The p values shown are for the matched pair comparisons of absolute procollagen type I levels in airway and distal lung fibroblast supernatants at baseline and after stimulation. Comparisons of relative differences in response with stimulation between fibroblast types are given in text.
Eotaxin-1 Production
Similar to our observations with PC-I, eotaxin-1 was measured in the supernatants of the same 12 matched pairs of fibroblasts (Figure 5). At baseline, unstimulated AFs produced eotaxin-1 at 30 ± 5 pg/ml. Unstimulated DLFs produced eotaxin-1 at 11 ± 2 pg/ml (p = 0.004 compared with unstimulated AFs). Addition of TGF-β alone did not significantly impact eotaxin production in either group. IL-13 stimulation induced a 10-fold increase in eotaxin in AF supernatants (post–IL-13 AFs, 287 ± 112 pg/ml; p < 0.001 compared with AFs at baseline) and only a 2-fold increase in DLFs (post–IL-13 DLFs, 22 ± 7 pg/ml; p = 0.04 compared with DLFs at baseline and p = 0.0001 in comparing post–IL-13 AFs and DLFs). The IL-13–induced increase in eotaxin-1 was significantly greater in AFs compared with DLFs (p = 0.009). In combination with TGF-β, eotaxin-1 synthesis increased synergistically (post–TGF-β plus IL-13: AFs, 4,024 ± 1,872 pg/ml; DLFs, 351 ± 125 pg/ml; p < 0.0001 in comparing each group with its respective baseline, and p = 0.001 in comparing post–IL-13 plus TGF-β AFs and DLFs). Whereas the absolute post–TGF-β plus IL-13 levels were higher in AFs compared with DLFs (p = 0.001), the increase in eotaxin-1 among AFs was not different from that among DLFs (p = 0.11). These results indicate an enhanced ability of AFs compared with DLFs to produce eotaxin at baseline and in response to IL-13.
Figure 5.
Eotaxin-1 production by primary asthmatic airway and distal lung fibroblasts (n = 12) at baseline and after stimulation with IL-13 and TGF-β. Mean (and SEM) values were transformed back into normal units (pg/ml) for presentation. The ordinate is presented as a logarithmic scale because of the marked increase in values after stimulation. The p values shown are for the matched pair comparisons of absolute eotaxin-1 levels in airway and distal lung fibroblast supernatants at baseline and after stimulation. Comparisons of relative differences in response with stimulation between fibroblast types are given in text.
Comparison of AFs and DLFs from Nondiseased Lungs
Similar to studies with asthmatic airway and distal lung fibroblasts, experiments were performed on two matched pairs of nondiseased airway and distal lung fibroblasts. The pattern of responses is similar to those noted with asthmatic fibroblasts. In both distal lung fibroblasts, the basal expression of α-SMA exceeded that of AFs. Figure 6A shows the Western blot of a matched pair of AFs and DLFs at baseline and after 48 h of stimulation with TGF-β. Furthermore, nonasthmatic AFs produced more PC-I at baseline compared with matched DLFs (mean values: AFs, 1,333 ng/ml; DLFs, 959 ng/ml). Stimulation with TGF-β alone or in combination with IL-13 resulted in greater augmentation of PC-I in AFs compared with DLFs (mean values post–TGF-β: AFs, 2,371 ng/ml; DLFs, 2,045 ng/ml; mean values post–TGF-β plus IL-13: AFs, 2,793 ng/ml; DLFs, 2,360 ng/ml). Eotaxin-1 levels in the supernatants of normal AFs and DLFs at baseline were undetectable (less than 8 pg/ml). IL-13 alone or in combination with TGF-β increased eotaxin-1 production in normal AFs to a greater extent than in their matched DLFs (mean values post–IL-13: AFs, 345 pg/ml; DLFs, 46 pg/ml; mean values post–TGF-β plus IL-13: AFs, 13,667 pg/ml; DLFs, 2,779 pg/ml). Given that PC-I and eotaxin-1 levels are not normally distributed, log-transformed data were compared with 95% prediction intervals of measurements made with matched pairs of asthmatic fibroblasts (Figures 6B and 6C). Except for the undetectable level of eotaxin in AFs at baseline, all other unstimulated and stimulated procollagen type I and eotaxin-1 measurements were within the 95% confidence intervals of the asthmatic fibroblasts, suggesting that the individual values of nondiseased fibroblasts fit within the distribution of the asthmatic fibroblast data. These limited data suggest that phenotypic differences in fibroblast differentiation, fibrotic potential, and eotaxin production between AFs and DLFs are not limited to cells derived from asthmatic lungs.
Figure 6.
Comparison of matched pairs of airway and distal lung fibroblasts obtained from nondiseased lungs (n = 2). (A) Western blot for α-SMA expression in fibroblasts obtained from proximal airways and distal lung of normal subjects. (B and C) Comparison of procollagen type I and eotaxin-1 production, respectively, by nonasthmatic fibroblasts and asthmatic fibroblasts. The measured values for the two nonasthmatic fibroblasts are indicated by circles and triangles. Bars indicate the 95% prediction intervals of the measurements obtained for fibroblasts from subjects with asthma. Except for the undetectable level of eotaxin in AFs at baseline (asterisk), all other unstimulated and stimulated procollagen type I and eotaxin-1 measurements were well within these intervals.
DISCUSSION
Our observations indicate that phenotypic differences exist in lung fibroblasts obtained from different lung regions. Airway fibroblasts are morphologically distinct from parenchymal fibroblasts and have different functional characteristics that are likely important to the pathologic mechanisms of asthma. Fibroblasts isolated from distal lung tissue are more myofibroblast-like and proliferate faster, whereas proximal airway fibroblasts in the basal state synthesize more collagen and eotaxin than their corresponding distal lung fibroblasts. Moreover, whereas DLFs respond to cytokines relevant in asthma (TGF-β and IL-13), the overall absolute procollagen and eotaxin responses of AFs to these stimuli exceed those of DLFs. To the best of our knowledge, this is the first report of phenotypic differences in airway and distal lung/parenchyma fibroblasts, which could provide a cellular basis for the regional differences in extracellular matrix and eosinophil infiltration observed in the asthmatic lung.
Previous attempts to characterize phenotypes of airway fibroblasts in asthma (and other diseases) have compared “diseased state” with normal airway fibroblasts (27–30). In asthma, these studies have yielded conflicting results. Dube and coworkers showed that asthmatic bronchial fibroblasts have a lower proliferative potential than normal AFs and did not differ in collagen synthesis in response to platelet-derived growth factor (27). However, more recently, Lewis and coworkers suggested that platelet-derived growth factor stimulation alone induced enhanced procollagen type I production in fibroblasts obtained from patients with severe asthma as compared with those obtained from subjects with milder asthma and normal subjects (28). In each of these previous studies, the fibroblasts studied were obtained from large airway endobronchial biopsies.
In our study, using matched pairs of airway and parenchymal fibroblasts from the same subject, we were able to observe regional phenotypic differences in fibroblasts obtained from different compartments of the lung. It is difficult to simultaneously obtain airway and parenchymal tissue from normal individuals. The relative safety and usefulness of transbronchial biopsies in understanding distal lung changes in asthma have been demonstrated (25). However, ethical considerations preclude use of this technique in normal individuals given that these subjects have little to gain from such experiments. We were able to access proximal and distal lung tissue from two “nondiseased” lungs excised postmortem. Analogous to the bronchoscopic samples, fibroblasts were cultured from large airways and distal lung tissue and studied in a similar manner. In those two subjects, similar regional phenotypic and functional differences were seen, suggesting that the described heterogeneity is more likely related to location than to disease. However, further studies with more subjects are required to determine whether the differences in proximal and distal lung fibroblasts exist in normal subjects as well as subjects with asthma, and whether there are differences between those two groups, as well.
A potential caveat of our study is that distal lung sampling via transbronchial biopsies may contain small airways. These tissue samples are obtained from the lung periphery, about 2–3 cm from the pleural surface, and are composed primarily of alveolar tissue. The presence of small airways in these samples is relatively rare (one in four to eight biopsies) and even when present, the small airway component of the transbronchial biopsy constitutes a small portion of the entire tissue. Therefore we are confident that the fibroblasts obtained represent primarily alveolar or parenchymal fibroblasts.
Other studies have suggested different fibroblast subpopulations in asthma. Larsen and coworkers have reported the culture of fibroblast-like cells from bronchoalveolar lavage fluid (BALF) of about 40% of subjects with asthma studied (31). Typically, fibroblasts are not free floating, but are anchored to extracellular matrix or other structural cells. Therefore the exact origin of these cells “outside the airway epithelium” remains unclear. It is possible that the fibroblasts isolated by these investigators represent parenchymal fibroblasts that were released during the trauma of lavage of actively inflamed distal asthmatic lung. A lack of such cells in the BALF of normal subjects and of subjects with asthma with low BALF cellularity would be consistent with this argument. Interestingly, the authors also reported increased migratory capacity of these “BALF fibroblasts” compared with proximal airway fibroblasts obtained from endobronchial biopsies. This would also support our observations that the basal phenotype of distal/parenchymal fibroblasts is in fact more differentiated toward myofibroblasts
Although an obvious potential explanation for differences in airway and parenchymal fibroblast phenotypes is the inflammatory milieu in which these respective fibroblasts are resident, this is probably not the cause of the differences observed. The fibroblasts studied in these experiments were cultured under exactly the same conditions ex vivo for at least 1 mo before experimental conditions. The prolonged expression of these observed differences (third to fourth generation ex vivo) suggests no pronounced phenotypic differences between normal and asthmatic fibroblasts from either compartment. For these two reasons, it is unlikely that environmental factors in the lung explain the observed phenotypic differences. In addition, these same reasons make it unlikely that the fibroblast differences are a result of regional differences in exposure to corticosteroids. Also, we did not observe any impact of steroid dose or the use of systemic steroids on fibroblast differences.
It may appear paradoxical that the myofibroblastic cells are more likely to be those obtained from the distal lung/parenchyma of subjects with asthma, because asthma is often regarded as a large airway disease. In contrast to asthma, however, myofibroblasts are identified frequently in models of interstitial lung disease and are seen only rarely in asthma (19, 32). The benefit of having these myofibroblasts in the distal lung is not clear, but with their known increased contractile properties and increased proliferative capacity they are likely to be critical to wound repair. One study suggested that patients who died of an asthma attack had evidence for disruption of alveolar attachments with incomplete repair (7). Although the directionality of the wound-healing process is not known (airway to alveolar or alveolar to airway), primary involvement by airway and parenchymal fibroblasts could have considerable implications for repair.
Excessive fibrosis can result from either a hyperproliferative state (too many fibroblasts) or a hypersynthetic state (more collagen/ECM synthesis per fibroblast) (16, 17, 20). In the case of asthmatic airways, it has long been appreciated that a specific increase in matrix deposition occurs just underneath the basement membrane (1, 2). One of the major components of this subepithelial fibrosis is collagen type I, formed from the cleavage of procollagen type I (measured in this study). Although the number of airway fibroblasts is almost impossible to quantify, our observed in vitro differences in procollagen type I production and proliferation between airway fibroblasts and distal lung fibroblasts suggest that the fibrotic defect is more likely the result of increased collagen production, rather than a “hyperproliferation” of fibroblasts. The lack of fibrosis in distal lung of subjects with asthma despite the presence of fibroblasts that have a greater proliferative capacity in this region also supports that conclusion.
These data also illustrate the need for studies with primary human cells, and bring out a note of caution in interpreting data derived from immortalized, and even purchased primary human cell lines. Fibroblast lines from the lung are readily available and easy to use. However, the exact origins (airway vs. parenchymal) of these fibroblasts are not reported. Our results suggest there are profound differences in lung fibroblasts depending on their location of origin. Therefore, the use of cell lines without considering their location of origin may be highly misleading.
In summary, our observations indicate the existence of heterogeneous fibroblast phenotypes in asthmatic airways and distal lung. These differences may be responsible for the alterations of extracellular matrix in the airways and alveolar attachments in asthma. Future studies, including microarray analyses of proximal and distal fibroblasts, are required to further elucidate the phenotypic differences.
Supplementary Material
This work was supported by grants HL-69174, AI-60400, and RR-00051.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200508-1218OC on March 16, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Jeffery PK. Morphology of the airway wall in asthma and in chronic obstructive pulmonary disease. Am Rev Respir Dis 1991;143:1152–1158. [DOI] [PubMed] [Google Scholar]
- 2.Chu HW, Halliday JL, Martin RJ, Leung DY, Szefler SJ, Wenzel SE. Collagen deposition in large airways may not differentiate severe asthma from milder forms of the disease. Am J Respir Crit Care Med 1998;158:1936–1944. [DOI] [PubMed] [Google Scholar]
- 3.Vignola AM, Chanez P, Bonsignore G, Godard P, Bousquet J. Structural consequences of airway inflammation in asthma. J Allergy Clin Immunol 2000;105:S514–S517. [DOI] [PubMed] [Google Scholar]
- 4.Chakir J, Shannon J, Molet S, Fukakusa M, Elias J, Laviolette M, Boulet LP, Hamid Q. Airway remodeling–associated mediators in moderate to severe asthma: effect of steroids on TGF-β, IL-11, IL-17, and type I and type III collagen expression. J Allergy Clin Immunol 2003;111:1293–1298. [DOI] [PubMed] [Google Scholar]
- 5.Hocking DC. Fibronectin matrix deposition and cell contractility: implications for airway remodeling in asthma. Chest 2002;122:275S–278S. [DOI] [PubMed] [Google Scholar]
- 6.James AL, Maxwell PS, Pearce-Pinto G, Elliot JG, Carroll NG. The relationship of reticular basement membrane thickness to airway wall remodeling in asthma. Am J Respir Crit Care Med 2002;166:1590–1595. [DOI] [PubMed] [Google Scholar]
- 7.Mauad T, Silva LF, Santos MA, Grinberg L, Bernardi FD, Martins MA, Saldiva PH, Dolhnikoff M. Abnormal alveolar attachments with decreased elastic fiber content in distal lung in fatal asthma. Am J Respir Crit Care Med 2004;170:857–862. [DOI] [PubMed] [Google Scholar]
- 8.Mauad T, Xavier AC, Saldiva PH, Dolhnikoff M. Elastosis and fragmentation of fibers of the elastic system in fatal asthma. Am J Respir Crit Care Med 1999;160:968–975. [DOI] [PubMed] [Google Scholar]
- 9.Desmouliere A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen 2005;13:7–12. [DOI] [PubMed] [Google Scholar]
- 10.Rennard SI, Bitterman PB, Crystal RG. Pathogenesis of the granulomatous lung diseases. IV. Mechanisms of fibrosis. Am Rev Respir Dis 1984;130:492–496. [DOI] [PubMed] [Google Scholar]
- 11.Jordana M, Sarnstrand B, Sime PJ, Ramis I. Immune-inflammatory functions of fibroblasts. Eur Respir J 1994;7:2212–2222. [DOI] [PubMed] [Google Scholar]
- 12.Denburg JA, Gauldie J, Dolovich J, Ohtoshi T, Cox G, Jordana M. Structural cell–derived cytokines in allergic inflammation. Int Arch Allergy Appl Immunol 1991;94:127–132. [DOI] [PubMed] [Google Scholar]
- 13.Nonaka M, Pawankar R, Fukumoto A, Ogihara N, Sakanushi A, Yagi T. Induction of eotaxin production by interleukin-4, interleukin-13 and lipopolysaccharide by nasal fibroblasts. Clin Exp Allergy 2004;34:804–811. [DOI] [PubMed] [Google Scholar]
- 14.Wenzel SE, Trudeau JB, Barnes S, Zhou X, Cundall M, Westcott JY, McCord K, Chu HW. TGF-β and IL-13 synergistically increase eotaxin-1 production in human airway fibroblasts. J Immunol 2002;169:4613–4619. [DOI] [PubMed] [Google Scholar]
- 15.Fries KM, Blieden T, Looney RJ, Sempowski GD, Silvera MR, Willis RA, Phipps RP. Evidence of fibroblast heterogeneity and the role of fibroblast subpopulations in fibrosis. Clin Immunol Immunopathol 1994;72:283–292. [DOI] [PubMed] [Google Scholar]
- 16.Ohtsuka T, Koibuchi N, Sakai H, Yamakage A, Yamazaki S. Simultaneous analysis of [3H]-thymidine uptake and α1(I) procollagen mRNA expression in systemic sclerosis skin fibroblasts: an in situ hybridization study. Arch Dermatol Res 2000;292:248–255. [DOI] [PubMed] [Google Scholar]
- 17.Zurita-Salinas CS, Krotzsch E, Diaz de Leon L, Alcocer-Varela J. Collagen turnover is diminished by different clones of skin fibroblasts from early- but not late-stage systemic sclerosis. Rheumatol Int 2004;24:283–290. [DOI] [PubMed] [Google Scholar]
- 18.Ihn H, Yamane K, Kubo M, Tamaki K. Blockade of endogenous transforming growth factor β signaling prevents up-regulated collagen synthesis in scleroderma fibroblasts: association with increased expression of transforming growth factor β receptors. Arthritis Rheum 2001;44:474–480. [DOI] [PubMed] [Google Scholar]
- 19.Phan SH. The myofibroblast in pulmonary fibrosis. Chest 2002;122:286S–289S. [DOI] [PubMed] [Google Scholar]
- 20.Raghu G, Chen YY, Rusch V, Rabinovitch PS. Differential proliferation of fibroblasts cultured from normal and fibrotic human lungs. Am Rev Respir Dis 1988;138:703–708. [DOI] [PubMed] [Google Scholar]
- 21.Schoonover K, Barnes SM, Trudeau JB, Tilstra JA, Wenzel SE. The inflammatory/fibrotic phenotype of proximal airway fibroblasts differs from that of distal lung fibroblasts [abstract]. Am J Respir Crit Care Med 2003;167:A958. [Google Scholar]
- 22.Wenzel SE, Fahy JV, Irvin CG, Peters SP, Spector S, Szefler SJ. Proceedings of the ATS Workshop on Refractory Asthma: current understanding, recommendations and unanswered questions. Am J Respir Crit Care Med 2000;162:2341–2351. [DOI] [PubMed] [Google Scholar]
- 23.Wenzel SE, Schwartz LB, Langmack EL, Halliday JL, Trudeau JB, Gibbs RL, Chu HW. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med 1999;160:1001–1008. [DOI] [PubMed] [Google Scholar]
- 24.Wenzel SE, Szefler SJ, Leung DY, Sloan SI, Rex MD, Martin RJ. Bronchoscopic evaluation of severe asthma: persistent inflammation associated with high dose glucocorticoids. Am J Respir Crit Care Med 1997;156:737–743. [DOI] [PubMed] [Google Scholar]
- 25.Balzar S, Wenzel SE, Chu HW. Transbronchial biopsy as a tool to evaluate small airways in asthma. Eur Respir J 2002;20:254–259. [DOI] [PubMed] [Google Scholar]
- 26.Kleinbaum DG, Kupper LK, Muller KE, Nizam A. Applied regression analysis and other multivariable methods, 3rd ed. Pacific Grove, CA: Brooks/Cole Publishing; 1998. pp. 59–60.
- 27.Dube J, Chakir J, Laviolette M, Saint Martin S, Boutet M, Desrochers C, Auger F, Boulet LP. In vitro procollagen synthesis and proliferative phenotype of bronchial fibroblasts from normal and asthmatic subjects. Lab Invest 1998;78:297–307. [PubMed] [Google Scholar]
- 28.Lewis CC, Chu HW, Westcott JY, Tucker A, Langmack EL, Sutherland ER, Kraft M. Airway fibroblasts exhibit a synthetic phenotype in severe asthma. J Allergy Clin Immunol 2005;115:534–540. [DOI] [PubMed] [Google Scholar]
- 29.Laliberte R, Rouabhia M, Bosse M, Chakir J. Decreased capacity of asthmatic bronchial fibroblasts to degrade collagen. Matrix Biol 2001;19:743–753. [DOI] [PubMed] [Google Scholar]
- 30.Westergren-Thorsson G, Chakir J, Lafreniere-Allard MJ, Boulet LP, Tremblay GM. Correlation between airway responsiveness and proteoglycan production by bronchial fibroblasts from normal and asthmatic subjects. Int J Biochem Cell Biol 2002;34:1256–1267. [DOI] [PubMed] [Google Scholar]
- 31.Larsen K, Tufvesson E, Malmstrom J, Morgelin M, Wildt M, Andersson A, Lindstrom A, Malmstrom A, Lofdahl CG, Marko-Varga G, et al. Presence of activated mobile fibroblasts in bronchoalveolar lavage from patients with mild asthma. Am J Respir Crit Care Med 2004;170:1049–1056. [DOI] [PubMed] [Google Scholar]
- 32.White ES, Lazar MH, Thannickal VJ. Pathogenetic mechanisms in usual interstitial pneumonia/idiopathic pulmonary fibrosis. J Pathol 2003;201:343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.