Abstract
Rationale: There is growing evidence that alveolar cell apoptosis plays an important role in emphysema pathogenesis, a chronic inflammatory lung disease characterized by alveolar destruction. The association of α1-antitrypsin deficiency with the development of emphysema has supported the concept that protease/antiprotease imbalance mediates cigarette smoke–induced emphysema.
Objectives: We propose that, in addition to its antielastolytic effects, α1-antitrypsin may have broader biological effects in the lung, preventing emphysema through inhibition of alveolar cells apoptosis.
Methods, Measurements, and Main Results: Transduction of human α1-antitrypsin via replication-deficient adeno-associated virus attenuated airspace enlargement and emphysema caused by inhibition of vascular endothelial growth factor (VEGF) receptors with SU5416 in mice, a model of apoptosis-dependent emphysema lacking neutrophilic inflammation. The overexpressed human serine protease inhibitor accumulated in lung cells and suppressed caspase-3 activation and oxidative stress in lungs treated with the VEGF blocker or with VEGF receptor-1 and -2 antibodies. Similar results were obtained in SU5416-treated rats given human α1-antitrypsin intravenously.
Conclusions: Our findings suggest that inhibition of structural alveolar cell apoptosis by α1-antitrypsin represents a novel protective mechanism of the serpin against emphysema. Further elucidation of this mechanism may extend the therapeutic options for emphysema caused by reduced level or loss of function of α1-antitrypsin.
Keywords: antiprotease, caspase, chronic obstructive pulmonary disease, oxidative stress, serpin
Significant reductions in serum level of human α1-antitrypsin (hA1AT), a serine protease inhibitor (serpin) with potent inhibitory activity against neutrophil elastase, have been associated with the development of emphysema (1), a chronic obstructive pulmonary disease characterized by permanent destruction of the airways distal of the terminal bronchioles (2). A1AT deficiency occurs from the inheritance of two protease inhibitor deficiency alleles from the A1AT locus (designated Pi), predominantly in persons of European origin (3). Emphysema is the most prevalent clinical feature associated with A1AT deficiency and the primary cause of death. The main risk factor for emphysema in patients with A1AT deficiency is cigarette smoking, which triggers the disease decades earlier than in “usual” patients with chronic obstructive pulmonary disease with normal serum A1AT levels. Because A1AT is an effective elastase inhibitor, emphysema is believed to occur as a result of increased, unopposed destruction of lung matrix by smoking-activated neutrophil elastase/proteinase-3 (4). This paradigm leads to the introduction of systemic supplementation with A1AT, which slows the decline in lung function in these patients (5).
It is becoming increasingly evident that other mechanisms, in addition to matrix proteolysis induced by neutrophil elastase and other matrix proteases, are implicated in the development of emphysema, such as oxidative stress and apoptosis of alveolar septal cells (6). Understanding how these mechanisms are affected by serpins may provide more effective therapeutic interventions needed to halt the lung destruction in patients with emphysema. Decreased function or availability of A1AT in the lung may be common to deficiency states due to genetic mutations or post-translational modifications such as oxidation from cigarette smoke (7, 8).
Presently, a mouse knockout model for hA1AT deficiency is not available. Nevertheless, there is evidence that mouse strain susceptibility to cigarette smoke–induced emphysema (9, 10) correlates inversely with A1AT levels in the bronchoalveolar lavage (BAL) (11), in that even a 40% reduction of BAL A1AT renders C57Bl/6J mice sensitive to smoking-induced injury, when compared with more resistant strains ICR and DBA. Furthermore, the pallid mouse, characterized by low A1AT serum levels, develops spontaneous emphysema worsened by cigarette smoke (11, 12), closely resembling A1AT deficiency.
The role of apoptosis in alveolar destruction was uncovered in experimental emphysema caused by vascular endothelial growth factor receptor (VEGFR) blockade (13), followed by documentation of critical elements of the apoptosis hypothesis in lungs of patients with emphysema (14, 15) and in experimental cigarette smoke–induced emphysema (16, 17). Furthermore, novel roles for A1AT are becoming evident, including inhibition of apoptosis in models of ischemia–reperfusion injury (18, 19) and serum deprivation injury (20), in liver, kidney, and cell-culture studies. However, in these models, one cannot discriminate between a direct effect of A1AT on apoptosis and an indirect effect against excessive inflammation or serum deprivation, respectively. To investigate whether A1AT exerts an antiapoptotic, protective effect on pulmonary alveolar cells, our experimental approach relied on emphysema caused by VEGFR blockade in the C57/Bl6 mouse. Using this noninflammatory model of emphysema, we explored the effect of A1AT on apoptosis independent of its role in curbing inflammation and neutrophil elastase activity. Our results implicate an antiapoptotic effect of A1AT in alveolar cells. Some of the results of these studies have been previously reported in abstract form (21–23).
METHODS
Chemicals and reagents were from Sigma-Aldrich (St.Louis, MO), unless otherwise specified. Animal studies were approved by the Animal Care and Use Committee of Johns Hopkins University and University of Colorado Health Sciences Center and performed on male C57Bl/6 mice (3 mo old; Jackson Laboratory [Bar Harbor, ME]) or Sprague-Dawley rats (n = 4–6/group).
Adeno-associated Virus Administration
For experiments involving direct lung administration, mice were anesthetized and their trachea was instilled with 5 × 1010 particles of control adeno-associated virus (c-AAV; UF11-AAV5) or hA1AT-AAV (AAV5-CB-AAT) in 25 μl 0.9% NaCl. For intramuscular injection (hind leg), 9.6 × 1010 cAAV (UF11-622) or 1.2 × 1010 of A1AT-AAV (pTR2-CB-AAT) were injected in 40 μl H2O in experiments involving SU5416. For experiments involving VEGFR antibodies, mice received 9.6 × 1010 UF11-622 or 9.6 × 1010 A1AT-AAV.
Viral Constructs
All experiments were performed with recombinant AAV, rAAV-CB-AAT (24), which consists of AAV serotype 2 inverted terminal repeats flanking an expression cassette that drives hA1AT expression from a hybrid cytomegalovirus enhancer/β-actin promoter. For intramuscular experiments, we used the vector pseudotyped into AAV serotype 1 capsids (25). For intratracheal experiments, we used the vector pseudotyped into AAV serotype 5 (25, 26). All preparations were titrated using DNA dot-blot hybridization (25).
Administration of Agents Inducing Airspace Enlargement
SU5416 (Sugen, Inc., South San Francisco, CA) or carboxymethylcellulose (CMC) was administered 20 mg/kg, subcutaneously once in mice and rats (14). VEGFR-specific antibodies DC101 and MF1 (Imclone, New York, NY; 800 μg/mouse/injection) or dialyzed rat IgG were administered intraperitoneally three times per week.
A1AT Augmentation in Rat
Rats were divided into the following groups (each group, n = 6): (1) CMC + cell culture–grade bovine serum albumin (BSA; 30 mg/kg intravenously [i.v.] tiw), (2) SU5416 + BSA (30 mg/kg i.v. tiw), and (3) SU5416 + hA1AT (30 mg/kg intravenously three times weekly).
Morphometric analysis was performed on coded slides as described (27, 28).
Immunohistochemistry (IHC) (14) with A1AT antibody (Santa Cruz, Santa Cruz, CA) was followed by diamino benzidone or fluorescent-labeled secondary antibody (Molecular Probes, Eugene, OR). Cell-type markers were surfactant protein-C antibody (Chemicon, Temecula, CA), CD34 (Zymed, South San Francisco, CA), and smooth muscle actin (Sigma), followed by 4,6 diamidino-2 phenylindole (Molecular Probes) nuclear counterstaining.
Apoptosis was detected in lysates (15) or inflated lung, enabling focus on alveoli, rather than large airways and vessels (27), via active caspase-3 IHC (Abcam [Cambridge, MA] and Cell Signaling [Danvers, MA]) or in situ labeling of apoptotic DNA on murine lung (15), using rat serum as a negative control.
Measurements of oxidative stress included detection of nitrotyrosine and 4-hydroxynonenol (27) markers and of catalase activity (Cayman Chemical, Ann Arbor, MI).
Further detail on methods can be found in the online supplement.
Overexpression of Mouse A1AT
Mouse A1AT (isoform 1) cDNA was generated by reverse transcriptase– polymerase chain reaction using liver RNA from the C57BL/6 mouse. This cDNA was inserted into rAAV-CB-AAT vector plasmid to replace the hA1AT cDNA, thus creating rAAV-CB-mA1AT1 plasmid, which was transfected into 293 cells and medium containing mA1AT tested by immunoblot.
Rabbit anti-mouse A1AT antiserum was raised against all isoforms of mouse A1AT, by synthesizing and injecting a short peptide (15 aa) representing a conserved surface region of mA1AT into rabbits.
A sandwich ELISA assay detected hA1AT (24), and Western blotting was performed as described (29).
Statistical analysis was performed with SPSS software (SPSS, Inc., Chicago, IL) using unpaired Student's t test or one-way analysis of variance with Student-Newman-Keuls post hoc test. Statistical difference was accepted at p < 0.05.
RESULTS
Augmentation of A1AT Attenuated the Development of Apoptosis-dependent Emphysema in Mice and Rats
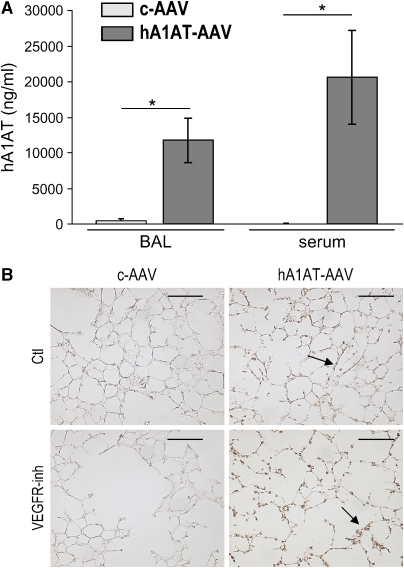
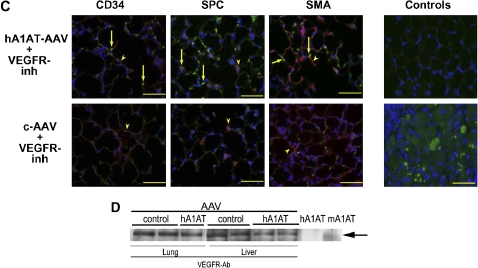
Blockade of VEGF receptors 1 (Flt) and 2 (Flk/KDR) with SU5416 causes murine emphysema with no evidence of neutrophilic or macrophage inflammation (13, 15). To address our hypothesis that A1AT augmentation is protective in this model, mice received hA1AT-carrying, replication-deficient, adeno-associated virus serotype 5 (hA1AT-AAV) or empty AAV as control (c-AAV) by two routes: intratracheal and intramuscular injection. Although the intramuscular route ensured sustained increased serum levels of hA1AT (30, 31), the intratracheal route also allowed for direct lung hA1AT production. The intratracheal transduction led to marked increases of hA1AT protein for up to 4 wk as detected by serum and BAL ELISA (Figure 1A). In an early set of experiments, intramuscular transduction of hA1AT with AAV serotype 1, known for less efficient transduction (26), also resulted in increased lung hA1AT (IHC; Figures 1B and 1C) and, in this setting, inhibition of VEGFR appeared to be associated with mildly enhanced lung hA1AT expression. The activity of the overexpressed lung hA1AT measured in the BAL from the hA1AT-transduced animals demonstrated a 30% increase in the elastase inhibitory activity, when compared with control animals (not shown). Species-specific Western blot assays (tested against purified hA1AT and mouse recombinant A1AT in Figure E1 of the online supplement) indicated that, in the setting of VEGFR blockade, the augmented hA1AT expression was not due to increased endogenous mouse A1AT (Figure 1D). Rather, the elevated hA1AT might reflect alterations of lung cell properties after VEGFR inhibition, of increased lung availability to serum h1A1AT, or of c-AAV uptake. Because hA1AT serum levels were similar in vehicle- and SU5416-treated mice, an effect of VEGFR blockade on the systemic c-AAV uptake and hA1AT expression was unlikely.
Figure 1.
Enhanced expression levels of human α1-antitrypsin (hA1AT) in the mouse lung and serum after adeno-associated virus (AAV) transduction. (A) hA1AT transduced by intratracheal instillation. hA1AT protein measured in bronchoalveolar lavage (BAL) and serum by ELISA 4 wk after hA1AT-AAV (n = 8) or empty virus (c-AAV, n = 8) transduction and SU5416 (vascular endothelial growth factor receptor inhibition [VEGFR-inh], 20 mg/kg subcutaneously) or vehicle control (Ctl) administration (*p < 0.01). (B) hA1AT transduced by intramuscular hA1AT expression by immunohistochemistry (brown, arrows; bar = 50 μm) in mouse lung 3 wk after hA1AT-AAV transduction and VEGFR-inh administration (n = 4/group). (C) Alveolar colocalization of hA1AT (in green) with alveolar cell–specific markers (in red, arrowheads): CD34 (endothelial cells), surfactant protein C (SPC; type II epithelial cells), or smooth muscle actin (SMA; interstitial myofibroblasts) in mice transduced with intramuscular hA1AT-AAV and treated with VEGFR-inh. Note colocalization in endothelial, epithelial, and interstitial fibroblasts (in yellow, arrows). Controls: (upper panel, negative control) hA1AT + VEGFR-inh–treated lung stained with goat serum and (lower panel, positive control) human liver with A1AT deficiency stained with hA1AT antibody. Note intense homogenously and granular fluorescence staining in hepatocytes, the latter representing polymerized A1AT, characteristic of A1AT deficiency (bars = 100 μm; nuclei blue, 4,6 diamidino-2 pheylindole [DAPI]). (D) Endogenous mouse A1AT levels in the lung and liver lysates by Western blotting with a specific mouse A1AT antiserum. Controls are purified hA1AT protein and mA1AT (recombinant mouse A1AT). Note the lack of significant increases in the mouse A1AT in response to hA1AT-AAV injection compared with empty virus–treated animals.
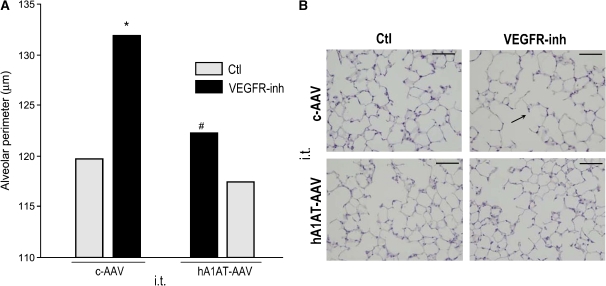
Transduction of hA1AT by intracheal injection significantly decreased alveolar septal enlargement induced by VEGFR blockade in mice, as measured by morphometry at 4 wk after the administration of the specific VEGFR inhibitor SU5416 (Figures 2A and 2B).
Figure 2.
Protective effect of hA1AT overexpression on the alveolar space enlargement triggered by VEGFR inhibition in mice. (A) Morphometric measurements of alveolar perimeters of VEGFR-blocked lungs performed 4 wk after the intratracheal delivery of hA1AT-AAV (median; *p < 0.05 vs. Ctl, #p < 0.05 vs. VEGFR-inh). (B) Representative histology after intratracheal transduction of hA1AT-AAV and VEGFR inhibitor SU5416. Note the alveolar space enlargement induced by the VEGFR blockade (arrows; bars = 50 μm) and attenuation by hA1AT-AAV.
The protective effects of hA1AT against emphysema were reproduced in rats treated with a single dose of SU5416 where intravenous treatment with hA1AT protein prevented the increase in mean linear intercept and the decrease in the surface–volume ratio (Figure E2) induced by the VEGFR blockade at 3 wk.
hA1AT Protected against VEGFR Blockade–induced Apoptosis of Alveolar Cells
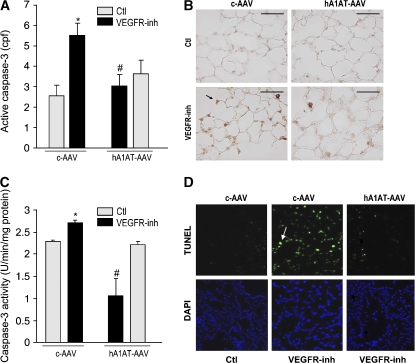
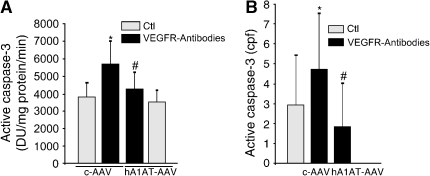
In line with our previous findings that emphysema caused by VEGFR inhibition involves both apoptosis and oxidative stress (27), hA1AT overexpression significantly attenuated VEGFR blockade–triggered lung cellular apoptosis, as measured by caspase-3 IHC (Figures 3A and 3B), caspase-3 activity assay (Figure 3C), and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) (Figure 3D) in treated lungs. The protection afforded by AAV-transduced hA1AT was specific, because overexpression of the empty virus did not decrease SU5416-induced apoptosis and, in high titers (1013) intratracheally, the empty virus alone increased lung apoptosis (not shown). Similar antiapoptotic effects of A1AT were obtained in the rat lung, where intravenous administration of hA1AT decreased the abundance of active caspase-3–positive alveolar septal cells, typically seen with the VEGFR blockade (Figure E2). To ensure that the antiapoptotic effect of hA1AT was unrelated to an interaction of A1AT with SU5416, we blocked VEGFRs with specific rat monoclonal antibodies against VEGFR-2 (DC101 antibody) and VEGFR-1 (MF1 antibody) (32). In combination, the VEGFR-blocking antibodies induced significant alveolar cell apoptosis in mice at 4 wk. hA1AT overexpression via intracheal hA1AT-AAV markedly protected against VEGFR antibody–induced lung apoptosis measured by caspase-3 activity assay (Figure 4A) or active casapase-3 IHC (Figure 4B). Taken together, these results suggest that A1AT inhibits apoptosis in structural lung alveolar cells.
Figure 3.
Inhibitory effects of hA1AT augmentation on lung apoptosis in the VEGFR blockade model. (A–C) Effect of hA1AT on SU5416-induced apoptosis in mice. (A, B) Active caspase-3 expression (cells per field [cpf] normalized by alveolar perimeter, mean + SEM; p < 0.05 vs. Ctl* and vs. VEGFR-inh#) is shown in (B) as representative panel (positive cells in brown, arrows; bar = 50 μm) at 3 wk after SU5416 administration. (C) Lung caspase-3 activity (+ SEM; p < 0.05 vs. Ctl* and vs. VEGFR-inh#). Measurements performed 4 wk after SU5416 administration. (D) Apoptotic nuclei detected by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining (green, arrow) in the upper panels. Note increased apoptotic nuclei in the VEGFR-inh animals receiving the empty virus, compared with the hA1AT virus. Lower panels show total alveolar nuclear counterstaining with DAPI, in blue. Representative sections of n = 3 mice/group.
Figure 4.
Effect of hA1AT on VEGFR antibody–induced apoptosis in mice. (A) Lung caspase-3 activity (+ SEM; p < 0.05 vs. Ctl* and vs. VEGFR-inh#) and (B) active caspase-3 expressing cells (immunohistochemistry), positive cpf normalized by total number of DAPI-stained nuclei in mouse alveoli at 4 wk after intratracheal transduction of c-AAV or hA1AT-AAV and VEGFR inhibition with a combination of VEGFR-1 and -2 neutralizing antibodies (DC101 and MF1, respectively) or Ctl (rat IgG; mean + SD; *p < 0.05), 4 wk after DC101 and MF1 (VEGFR neutralizing) antibody administration (800 μg thrice weekly intraperitoneally; n = 3 in control groups, n = 4 in treatment groups).
hA1AT Augmentation Decreased Oxidative Stress Levels Induced by VEGFR Blockade
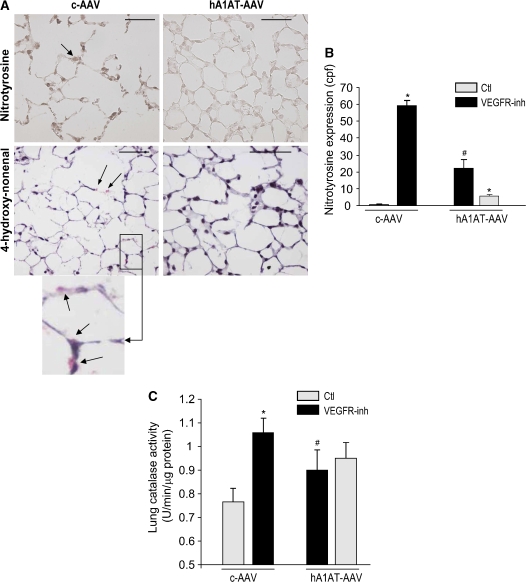
In the VEGFR blockade model, apoptosis and oxidative stress mutually interact as they cause disappearance of the alveolar cells (27). hA1AT-AAV (intramuscular), but not c-AAV, decreased levels of reactive oxygen species assessed by nitrotyrosine and 4-hydroxynonenal expression (Figures 5A and 5B). Consistent with enhanced reactive oxygen species production, the VEGFR inhibition caused an increased lung catalase activity, which was attenuated by hA1AT overexpression (intratracheal; Figure 5C).
Figure 5.
Lung antioxidative effects of hA1AT augmentation in the VEGFR blockade and caspase-3 instillation models. (A–B) Intramuscular transduction of hA1AT-AAV in the SU5416-treated mouse. (A) Representative immunohistochemistry (IHC) of nitrotyrosine (in brown, arrow, upper panel; bar = 50 μm) and 4-hydroxy-nonenal (in red, arrows, lower panel and inset) expression at 3 wk VEGFR blockade. (B) Nitrotyrosine expression (cpf by IHC, mean + SD; *p < 0.05 vs. Ctl #p < 0.05 vs. VEGFR-inh. (C) Intratracheal transduction of hA1AT-AAV in the SU5416-treated mouse. Lung catalase activity 4 wk after VEGFR-inh administration (mean + SEM; *p < 0.05 vs. Ctl #p < 0.05 vs. VEGFR-inh).
DISCUSSION
Our studies support a novel antiapoptotic function of hA1AT in the lung, uncoupled from its inhibition of neutrophil-generated serine proteases, and provide the framework for future studies addressing how A1AT is internalized in alveolar cells and inhibits alveolar structural (noninflammatory) cell apoptosis.
Prior experimental evidence of protection afforded by hA1AT to cigarette smoke exposure–induced lung injury (33) and liver and kidney ischemia–reperfusion injury (18, 19) could not dissect the particular contribution of a direct prosurvival effect of hA1AT vis-à-vis its effects on neutrophil elastase. Our experimental approach allowed us to demonstrate that the protection of hA1AT against apoptosis-dependent emphysema was achieved by hA1AT overexpressed either systemically or locally in lung. Because hA1AT supplementation was required to block apoptosis of alveolar cells, it became apparent that endogenous A1AT could not rescue the apoptosis and oxidative stress caused by emphysema triggers. Similar implications are derived from findings that even mild decreases in serum A1AT characteristic of Pi MZ individuals may increase their risk of lung disease (34, 35), and that impaired A1AT function (despite normal serum levels) from post-translational modifications by free radicals may contribute to acquired emphysema due to cigarette smoking (7, 8). Although our study was not specifically designed to test an hA1AT dose response against apoptosis-dependent emphysema, we observed clear beneficial effects of hA1AT at concentrations of up to 30 μg/ml. We noticed enhanced intracellular accumulation of hA1AT in septal cells (Figure 1), despite serum increases of only up to 1% of predicted mouse serum A1AT levels. It is conceivable that the intratracheal instillation resulted in alveolar cell AAV infection and intracellular accumulation of hA1AT. Because intramuscular or intravenous administration afforded similar lung protection, hA1AT may be preferentially internalized by alveolar cells as compared with mouse A1AT. This might be particularly relevant for C57Bl/6 mice, with increased susceptibility to emphysema attributed to their lower lung A1AT levels (36). Importantly, the 40% differences in BAL A1AT levels between susceptible and resistant strains suggest that even moderate increases in lung A1AT, as observed in our experiments, may protect against emphysematous lung injury (defined in our studies by a combination of alveolar cell apoptosis, alveolar space enlargement, and enhanced lung oxidative stress). A differential cell-entry or antiapoptotic function of mouse versus hA1AT could be explained by structural differences (60% overlap in amino acid sequence; Figure E1), which are supported by immunogenicity of hA1AT after prolonged administration in mice (31), and a possible lower antielastase activity of the mouse A1AT, compared with hA1AT (37). As mentioned, the oxidative stress from VEGFR blockade may induce localized decreases in mouse A1AT activity or intracellular availability.
Our findings broaden the lung protective roles of A1AT beyond its effects on neutrophil elastase, to include apoptosis blockade. A1AT is among several serum proteins that can rescue serum withdrawal-induced apoptosis (20), suggesting a tonic prosurvival effects of A1AT exerted by a yet unknown mechanism. When analyzed in the context of unpublished observations of direct interaction between hA1AT and active caspase 3 in vitro (38), the in vivo antiapoptotic effects of hA1AT may be direct, intracellular, and mediated by specific interactions with active caspase-3 in apoptosis, as we found in a parallel line of investigation of the mechanisms involved in caspase-3 inhibition of hA1AT (Petrache and colleagues, unpublished manuscript, 2006). These findings argue against a direct effect specifically linked to VEGFR blockade–initiated vascular injury. Three other serpins, poxvirus CrmA (39), the endogenous proteinase inhibitor-9 (40), and plasminogen activator inhibitor type-1 (41), have been shown to be endogenous caspase inhibitors, yet their mechanism of action remains unknown. Bona-fide mammalian inhibitors of caspase-3, such as the inhibitor of apoptosis XIAP, share the BIR2 domain, which is structurally unrelated to A1AT (NCBI blast). Similarly, a prosurvival effect of transduced A1AT may occur via inhibition of intracellular serine proteases implicated in apoptosis (42). Although our main hypothesis is that the protection afforded by the A1AT augmentation against oxidative stress is explained by a reduction in the burden of apoptotic cells in the lung, a direct antioxidative stress effect of A1AT cannot be excluded (see the increase in baseline catalase activity observed with the hA1AT-AAV in Figure 5).
An important development from our studies will be the investigation of mechanisms by which A1AT (native or polymerized) elicits intracellular responses in cells other than hepatocytes (the major synthetic source of A1AT). The nonserpin actions of hA1AT may involve receptor-dependent and/or -independent effects, and be part of a growing novel intracellular actions involved in cell signaling, as our results imply, in cell protection. Although the lung itself is not a major source of A1AT synthesis, local production or accumulation of A1AT (43) may exert important roles in the maintenance of alveolar cell survival.
There is increasing evidence of novel biological effects of A1AT beyond its inhibition of elastolysis, such as inhibition of proinflammatory response (44), and based on our studies, as inhibition of lung structural cell apoptosis, possibly independent of its inhibitory effects on neutrophil elastase. These properties of A1AT may broaden its role in the lung structural maintenance, its impact in emphysema development, and in systemic diseases also characterized by inflammation, oxidative stress, and apoptosis.
Supplementary Material
Acknowledgments
The authors thank Dr. Gordon Snider for his seminal contributions to COPD research and invaluable support throughout this work. They also thank Ugonma Chukwueke for technical expertise and Amy Richter who helped with immunohistochemistry studies.
This work was supported by the Alpha 1 Foundation Research Fund, K08 HL04396-04; an ATS/Alpha One Foundation Research Grant (I.P.); the Hart-Family Endowed Chair for Emphysema and NIH RO1 HL60913-01 (N.F.V.); NIH R01-HL69877 and P01-DK58327 (T.F.); and NIH RO1HL66554 (R.M.T.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200512-1842OC on March 2, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Wiedemann HP, Stoller JK. Lung disease due to alpha 1-antitrypsin deficiency. Curr Opin Pulm Med 1996;2:155–160. [DOI] [PubMed] [Google Scholar]
- 2.National Heart, Lung, and Blood Institute. The definition of emphysema: report of a National Heart, Lung, and Blood Institute, Division of Lung Diseases workshop. Am Rev Respir Dis 1985;132:182–185. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Alpha 1-antitrypsin deficiency: memorandum from a WHO meeting. Bull World Health Organ 1997;75:397–415. [PMC free article] [PubMed] [Google Scholar]
- 4.Turino GM, Senior RM, Garg BD, Keller S, Levi MM, Mandl I. Serum elastase inhibitor deficiency and alpha 1-antitrypsin deficiency in patients with obstructive emphysema. Science 1969;165:709–711. [DOI] [PubMed] [Google Scholar]
- 5.American Thoracic Society/European Respiratory Society. American Thoracic Society/European Respiratory Society statement: standards for the diagnosis and management of individuals with α1 antitrypsin deficiency. Am J Respir Crit Care Med 2003;168:818–900. [DOI] [PubMed] [Google Scholar]
- 6.Tuder RM, Petrache I, Elias JA, Voelkel NF, Henson PM. Apoptosis and emphysema: the missing link. Am J Respir Cell Mol Biol 2003;28:551–554. [DOI] [PubMed] [Google Scholar]
- 7.Taggart C, Cervantes-Laurean D, Kim G, McElvaney NG, Wehr N, Moss J, Levine RL. Oxidation of either methionine 351 or methionine 358 in alpha 1-antitrypsin causes loss of anti-neutrophil elastase activity. J Biol Chem 2000;275:27258–27265. [DOI] [PubMed] [Google Scholar]
- 8.Carp H, Miller F, Hoidal JR, Janoff A. Potential mechanism of emphysema: alpha 1-proteinase inhibitor recovered from lungs of cigarette smokers contains oxidized methionine and has decreased elastase inhibitory capacity. Proc Natl Acad Sci USA 1982;79:2041–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shapiro SD, Demeo DL, Silverman EK. Smoke and mirrors: mouse models as a reflection of human chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004;170:929–931. [DOI] [PubMed] [Google Scholar]
- 10.Guerassimov A, Hoshino Y, Takubo Y, Turcotte A, Yamamoto M, Ghezzo H, Triantafillopoulos A, Whittaker K, Hoidal JR, Cosio MG. The development of emphysema in cigarette smoke-exposed mice is strain dependent. Am J Respir Crit Care Med 2004;170:974–980. [DOI] [PubMed] [Google Scholar]
- 11.Cavarra E, Bartalesi B, Lucattelli M, Fineschi S, Lunghi B, Gambelli F, Ortiz LA, Martorana PA, Lungarella G. Effects of cigarette smoke in mice with different levels of α(1)-proteinase inhibitor and sensitivity to oxidants. Am J Respir Crit Care Med 2001;164:886–890. [DOI] [PubMed] [Google Scholar]
- 12.Martorana PA, Brand T, Gardi C, van Even P, de Santi MM, Calzoni P, Marcolongo P, Lungarella G. The pallid mouse: a model of genetic alpha 1-antitrypsin deficiency. Lab Invest 1993;68:233–241. [PubMed] [Google Scholar]
- 13.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest 2000;106:1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasahara Y, Tuder RM, Cool CD, Lynch DA, Flores SC, Voelkel NF. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am J Respir Crit Care Med 2001;163:737–744. [DOI] [PubMed] [Google Scholar]
- 15.Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, Hubbard WC, Berdyshev EV, Tuder RM. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med 2005;11:491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng T, Kang MJ, Crothers K, Zhu Z, Liu W, Lee CG, Rabach LA, Chapman HA, Homer RJ, Aldous D, et al. Role of cathepsin S-dependent epithelial cell apoptosis in IFN-γ-induced alveolar remodeling and pulmonary emphysema. J Immunol 2005;174:8106–8115. [DOI] [PubMed] [Google Scholar]
- 17.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest 2004;114:1248–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daemen MA, Heemskerk VH, van't Veer C, Denecker G, Wolfs TG, Vandenabeele P, Buurman WA. Functional protection by acute phase proteins alpha(1)-acid glycoprotein and alpha(1)-antitrypsin against ischemia/reperfusion injury by preventing apoptosis and inflammation. Circulation 2000;102(12):1420–1426. [DOI] [PubMed] [Google Scholar]
- 19.Ikebe N, Akaike T, Miyamoto Y, Hayashida K, Yoshitake J, Ogawa M, Maeda H. Protective effect of S-nitrosylated alpha(1)-protease inhibitor on hepatic ischemia-reperfusion injury. J Pharmacol Exp Ther 2000;295:904–911. [PubMed] [Google Scholar]
- 20.Ikari Y, Mulvihill E, Schwartz SM. Alpha 1-proteinase inhibitor, alpha 1-antichymotrypsin, and alpha 2-macroglobulin are the antiapoptotic factors of vascular smooth muscle cells. J Biol Chem 2001;276:11798–11803. [DOI] [PubMed] [Google Scholar]
- 21.Petrache I ZL, Medler TR, Skirball J, Fijalkowska I, Flotte T, Tuder RM. Alpha 1 antitrypsin (A1AT) inhibits pulmonary endothelial cell apoptosis by direct inhibition of caspase-3 activity in a mouse model of non-inflammatory emphysema. Cellular Senescence and Cell Death, Keystone, CO Symposia; March 2005.
- 22.Petrache IFI, Zhen L, Medler TR, Skirball J, Flotte T, Tuder RM. An antiapoptotic role for alpha 1 antitrypsin in the prevention of emphysema. Presented at the Thomas L. Petty Aspen Lung Conference, 48th annual meeting “Pathobiology of COPD,” Aspen, Colorado; 2005.
- 23.Petrache IFI, Zhen L, Medler TR, Brown E, Skirball J, Flotte T, Tuder RM. Alpha 1 antitrypsin (AAT) treatment inhibits alveolar cell apoptosis and lung destruction in a model of non-inflammatory emphysema. Presented at the ATS International Conference in San Diego, California; 2005.
- 24.Song S, Embury J, Laipis PJ, Berns KI, Crawford JM, Flotte TR. Stable therapeutic serum levels of human alpha-1 antitrypsin (AAT) after portal vein injection of recombinant adeno-associated virus (rAAV) vectors. Gene Ther 2001;8:1299–1306. [DOI] [PubMed] [Google Scholar]
- 25.Zolotukhin S, Potter M, Zolotukhin I, Sakai Y, Loiler S, Fraites TJ Jr, Chiodo VA, Phillipsberg T, Muzyczka N, Hauswirth WW, et al. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods 2002;28:158–167. [DOI] [PubMed] [Google Scholar]
- 26.Sirninger J, Muller C, Braag S, Tang Q, Yue H, Detrisac C, Ferkol T, Guggino WB, Flotte TR. Functional characterization of a recombinant adeno-associated virus 5-pseudotyped cystic fibrosis transmembrane conductance regulator vector. Hum Gene Ther 2004;15:832–841. [DOI] [PubMed] [Google Scholar]
- 27.Tuder RM, Zhen L, Cho CY, Taraseviciene-Stewart L, Kasahara Y, Salvemini D, Voelkel NF, Flores SC. Oxidative stress and apoptosis interact and cause emphysema due to vascular endothelial growth factor receptor blockade. Am J Respir Cell Mol Biol 2003;29:88–97. [DOI] [PubMed] [Google Scholar]
- 28.Aherne WA, Dunnill MS. Morphometry. London: E. Arnold; 1982.
- 29.Petrache I, Verin AD, Crow MT, Birukova A, Liu F, Garcia JG. Differential effect of MLC kinase in TNF-alpha-induced endothelial cell apoptosis and barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 2001;280:L1168–L1178. [DOI] [PubMed] [Google Scholar]
- 30.Flotte TR, Brantly ML, Spencer LT, Byrne BJ, Spencer CT, Baker DJ, Humphries M. Phase I trial of intramuscular injection of a recombinant adeno-associated virus alpha 1-antitrypsin (rAAV2-CB-hAAT) gene vector to AAT-deficient adults. Hum Gene Ther 2004;15:93–128. [DOI] [PubMed] [Google Scholar]
- 31.Song S, Morgan M, Ellis T, Poirier A, Chesnut K, Wang J, Brantly M, Muzyczka N, Byrne BJ, Atkinson M, et al. Sustained secretion of human alpha-1-antitrypsin from murine muscle transduced with adeno-associated virus vectors. Proc Natl Acad Sci USA 1998;95:14384–14388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGrath-Morrow SA, Cho C, Zhen L, Hicklin DJ, Tuder RM. Vascular endothelial growth factor receptor 2 blockade disrupts postnatal lung development. Am J Respir Cell Mol Biol 2005;32:420–427. [DOI] [PubMed] [Google Scholar]
- 33.Churg A, Wang RD, Xie C, Wright JL. α1-Antitrypsin ameliorates cigarette smoke-induced emphysema in the mouse. Am J Respir Crit Care Med 2003;168:199–207. [DOI] [PubMed] [Google Scholar]
- 34.Sandford AJ, Chagani T, Weir TD, Connett JE, Anthonisen NR, Pare PD. Susceptibility genes for rapid decline of lung function in the Lung Health Study. Am J Respir Crit Care Med 2001;163:469–473. [DOI] [PubMed] [Google Scholar]
- 35.Tattersall SF, Pereira RP, Hunter D, Blundell G, Pride NB. Lung distensibility and airway function in intermediate alpha 1-antitrypsin deficiency (Pi MZ). Thorax 1979;34:637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gardi C, Cavarra E, Calzoni P, Marcolongo P, de Santi M, Martorana PA, Lungarella G. Neutrophil lysosomal dysfunctions in mutant C57 Bl/6J mice: interstrain variations in content of lysosomal elastase, cathepsin G and their inhibitors. Biochem J 1994;299:237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De BP, Heguy A, Hackett NR, Ferris B, Leopold PL, Lee J, Pierre L, Gao G, Wilson JM, Crystal RG. High levels of persistent expression of alpha1-antitrypsin mediated by the nonhuman primate serotype rh.10 adeno-associated virus despite preexisting immunity to common human adeno-associated viruses. Mol Ther 2006;13:67–76. [DOI] [PubMed] [Google Scholar]
- 38.Skirball JFI, Tuder RM, Petrache I. Alpha 1 antitrypsin (AAT) is an effective inhibitor of caspase-3 activity in vitro. Presented at the ATS International Conference in San Diego, California; 2005
- 39.Stennicke HR, Ryan CA, Salvesen GS. Reprieval from execution: the molecular basis of caspase inhibition. Trends Biochem Sci 2002;27:94–101. [DOI] [PubMed] [Google Scholar]
- 40.Young JL, Sukhova GK, Foster D, Kisiel W, Libby P, Schonbeck U. The serpin proteinase inhibitor 9 is an endogenous inhibitor of interleukin 1beta-converting enzyme (caspase-1) activity in human vascular smooth muscle cells. J Exp Med 2000;191:1535–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y, Kelm RJ Jr, Budd RC, Sobel BE, Schneider DJ. Inhibition of apoptosis and caspase-3 in vascular smooth muscle cells by plasminogen activator inhibitor type-1. J Cell Biochem 2004;92:178–188. [DOI] [PubMed] [Google Scholar]
- 42.Stenson-Cox C, FitzGerald U, Samali A. In the cut and thrust of apoptosis, serine proteases come of age. Biochem Pharmacol 2003;66:1469–1474. [DOI] [PubMed] [Google Scholar]
- 43.Mulgrew AT, Taggart CC, Lawless MW, Greene CM, Brantly ML, O'Neill SJ, McElvaney NG. Z alpha1-antitrypsin polymerizes in the lung and acts as a neutrophil chemoattractant. Chest 2004;125:1952–1957. [DOI] [PubMed] [Google Scholar]
- 44.Aldonyte R, Jansson L, Janciauskiene S. Concentration-dependent effects of native and polymerised alpha1-antitrypsin on primary human monocytes, in vitro. BMC Cell Biol 2004;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.