Abstract
Rationale: Sepsis produces significant mitochondrial and contractile dysfunction in the heart, but the role of superoxide-derived free radicals in the genesis of these abnormalities is not completely understood.
Objectives: The study was designed to test the hypothesis that superoxide scavenger administration prevents endotoxin-induced cardiac mitochondrial and contractile dysfunction.
Methods: Four groups of rats were studied, and animals were injected with either saline, endotoxin, endotoxin plus polyethylene glycol-adsorbed–superoxide dismutase (PEG-SOD; a free-radical scavenger), or PEG-SOD alone. Animals were killed 48 h after injections. We then measured cardiac mitochondrial generation of reactive oxygen species (ROS), formation of free-radical reaction products (protein carbonyls, lipid aldehydes, nitrotyrosine), mitochondrial function, and cardiac contractile function.
Measurements and Main Results: Endotoxin elicited increases in cardiac mitochondrial ROS formation (p < 0.001), increases in cardiac levels of free-radical reaction products, reductions in mitochondrial ATP generation (p < 0.001), and decrements in cardiac pressure–generating capacity (p < 0.01). Administration of PEG-SOD blocked formation of free-radical reaction products, prevented mitochondrial dysfunction, and preserved cardiac contractility. For example, mitochondrial ATP generation was 923 ± 50, 392 ± 32, 753 ± 25, and 763 ± 36 nmol/min/mg, respectively, for control, endotoxin, endotoxin + PEG-SOD, and PEG-SOD groups (p < 0.001). In addition, cardiac systolic pressure generation at a diastolic pressure of 15 mm Hg averaged 110 ± 11, 66 ± 7, 129 ± 10 and 124 ± 5 mm Hg, respectively, for control, endotoxin, endotoxin + PEG-SOD, and PEG-SOD groups (p < 0.01).
Conclusion: These data indicate that superoxide-derived oxidants play a critical role in the development of cardiac mitochondrial and contractile dysfunction in endotoxin-induced sepsis.
Keywords: endotoxin, free radicals, heart, mitochondria, sepsis
Despite decades of work, current therapies have only a limited effectiveness in preventing the development of organ failure in patients with sepsis. In addition, considerable controversy still exists with regard to the cellular mechanisms responsible for the development of sepsis-induced organ dysfunction (1–8). Some work suggests that oxygen-derived free radicals may play a role in the induction of several types of organ failure after the development of sepsis and other forms of the systemic inflammatory response syndrome (9–11). In keeping with this concept, peroxynitrite scavengers have been shown to improve several types of organ dysfunction after sepsis (12). In addition, tempol, an agent with activity as a free-radical scavenger, has been shown to improve mortality and to lessen liver and renal damage in an animal model of sepsis (13). Other investigators, however, have reported contradictory observations, finding that antioxidant administration failed to improve mortality (14) and failed to maintain blood pressure (15) in two animal models of sepsis.
One of the major organs affected by sepsis is the heart. Cardiac dysfunction has been observed in several animal models of systemic inflammatory response syndrome, and is especially prominent after endotoxin administration (16, 17). Endotoxin induces large reductions in both cardiac pressure–generating capacity and cardiac mitochondrial function (16, 18). Recent studies have also shown that there is heightened free-radical generation in cardiac tissue in animal models of sepsis (19). No previous work, however, has demonstrated a beneficial effect of superoxide dismutase (SOD) administration on cardiac contractile or mitochondrial function in an animal model of sepsis. In addition, there has been no determination as to whether cardiac mitochondrial generation of reactive oxygen species is increased after endotoxin administration or in other animal models of sepsis.
The purpose of the present study, therefore, was to test the hypothesis that superoxide anions contribute to the induction of cardiac mitochondrial and contractile dysfunction in sepsis by comparing cardiac mitochondrial function and pressure–generating capacity in the following groups of rats: (1) saline-treated control animals; (2) animals in which sepsis was induced by the administration of endotoxin; (3) animals given both endotoxin and polyethylene glycol-adsorbed–SOD (PEG-SOD), a specific superoxide scavenger; and (4) animals given PEG-SOD alone. We measured multiple indices of mitochondrial function (i.e., State 3 respiration, respiratory control ratio [RCR], adenosine diphosphate/oxygen [ADP/O] ratio, NADH oxidase activity), cardiac pressure generation, cardiac mitochondrial free-radical generation, and several tissue free-radical reaction products (protein carbonyls, lipid aldehydes, nitrotyrosine). We expected endotoxin administration to reduce cardiac mitochondrial and contractile function and to induce protein and lipid oxidation; support for our hypothesis would be provided if we found that PEG-SOD administration prevented or blunted endotoxin-induced alterations in mitochondrial function, contractile function, and indices of tissue oxidation. Some of the results of these studies have been previously reported in the form of an abstract (20).
METHODS
Animal Protocol
Studies used adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing between 250 and 350 g. Rats were housed in the Medical College of Georgia Animal Resource Center; food and water were allowed ad libitum. Animal protocols were approved by the Institutional Animal Care and Use Committee.
In an initial group of studies, we examined basic parameters of cardiac mitochondrial function for control animals (n = 3) and for small groups of animals (n = 3/group) given endotoxin for varying periods of time (12, 24, 36, and 48 h) to characterize the time course of endotoxin-induced cardiac mitochondrial dysfunction. Endotoxin was administered as a daily intraperitoneal injection of 8 mg/kg/d (Escherichia coli endotoxin; Sigma Chemicals, St. Louis, MO) with the first dose given at time zero; all animals were also given subcutaneous doses of saline (60 mg/kg/d) to prevent dehydration. At the time of killing, animals were anesthetized with pentobarbital (50 mg/kg intraperitoneally); abdominal aortas were flushed with 60 ml of isolation buffer after cannulation, and hearts were removed. Animal weight and heart weight were recorded and cardiac mitochondria isolated and evaluated as described below.
After these initial studies, a second group of experiments was performed to determine if endotoxin administration increased mitochondrial free-radical generation and to determine if administration of SOD, a highly selective superoxide scavenger, could prevent endotoxin-induced alterations in cardiac mitochondrial and contractile function. For this second set of studies, animal groups included the following: (1) saline-injected control animals, (2) endotoxin-treated (8 mg/kg/d intraperitoneally) animals, (3) animals given endotoxin and PEG-SOD (PEG-SOD, 2,000 units/kg/d intraperitoneally), and (4) animals given PEG-SOD alone (2,000 units/kg/d intraperitoneally). All animals were given saline subcutaneously, 60 ml/kg, to prevent dehydration, and were killed at 48 h after the initial injection (because this time point produced the greatest mitochondrial dysfunction in initial studies). After animals were killed, hearts were removed and used for determination of either mitochondrial free-radical generation (n = 4, 5, 3, and 3, respectively, for control, endotoxin, endotoxin + PEG-SOD, and PEG-SOD groups), determination of mitochondrial function (n = 5, 5, 5, and 3, respectively, for these groups), or determination of cardiac pressure generation (n = 5, 5, 4, and 5, respectively, for these groups). Samples of mitochondrial isolates were also used to assess lipid aldehydes, protein carbonyls, nitrotyrosine protein modification, and Oxyblot (Chemicon International, Temecula, CA) determination of protein carbonylation.
Details for determination of mitochondrial free-radical generation (21, 22), mitochondrial respiration (21–23), cardiac pressure generation, and free-radical reaction markers (lipid aldehydes, protein carbonyls, nitrotyrosine protein modification, Oxyblots) (24) are provided in the online supplement.
Statistics
Calculations were performed using SigmaStat software (SPSS, Inc., Chicago, IL). Comparisons of parameters across experimental groups were made using an analysis of variance, with post hoc testing (Tukey's) to determine statistical differences between individual groups. A p value of less than 0.05 was taken to indicate statistical significance. Data are presented as mean ± 1 SEM.
RESULTS
Time Course of Endotoxin-induced Mitochondrial Dysfunction
We first determined the time-dependent effects of endotoxin administration (given as 8 mg/kg/d) on cardiac mitochondrial function (see Table 1). Cardiac mitochondrial isolates obtained at 12 and 24 h after an initial endotoxin dosage were not functionally different from samples obtained for saline-treated control animals. At the 36-h time point (i.e., after an initial endotoxin injection at time zero and a second injection at 24 h), State 3 respiration rates were lower than those obtained for saline-treated control animals. By 48 h into the endotoxin administration protocol, State 3 rates had declined by 56% when compared with saline-treated control animals (p < 0.001). ADP/O ratios were not altered by this regimen of endotoxin administration, and remained at the control level for all time points tested.
TABLE 1.
TIME COURSE OF ALTERATIONS IN CARDIAC MITOCHONDRIAL FUNCTION IN RESPONSE TO ENDOTOXIN ADMINISTRATION
| Control | 12-h LPS | 24-h LPS | 36-h LPS | 48-h LPS | |
|---|---|---|---|---|---|
| State 3 respiration, natom O/min/mg | 267 ± 17 | 272 ± 15 | 263 ± 19 | 202 ± 11* | 118 ± 8* |
| State 4 respiration, natom O/min/mg | 40 ± 3 | 49 ± 7 | 55 ± 5 | 41 ± 2 | 28 ± 2* |
| Respiratory control ratio | 6.9 ± 0.2 | 6.0 ± 0.4 | 5.0 ± 0.2 | 4.9 ± 0.2 | 4.9 ± 0.2* |
| ADP/O | 3.5 ± 0.1 | 3.2 ± 0.1 | 3.2 ± 0.1 | 3.3 ± 0.1 | 3.3 ± 0.1 |
| ATP production rate, nmol/min/mg | 923 ± 50 | 859 ± 40 | 826 ± 31 | 667 ± 43 | 392 ± 32* |
State 3 and State 4 respiration rates were measured with malate/pyruvate as substrate, with State 3 initiated by addition of adenosine diphosphate (ADP). The respiratory control ratio was calculated as the ratio of the State 3 to State 4 rate, and the ADP/oxygen (O) ratio determined by dividing the ADP added by the atoms of oxygen consumed during State 3 respiration. ATP production was calculated as the rate ATP was generated from ADP during State 3 respiration. Data for each time point represent the mean of determinations for n = 3 animals. Natom = nanoatom.
Different from control, p < 0.05
Effect of Endotoxin on Mitochondrial Generation of Reactive Oxygen Species
Because our time course experiments suggested that mitochondrial dysfunction was most severe at 48 h after endotoxin administration, all additional experiments were performed at the 48-h point after the initial injection of endotoxin or saline (for control animals).
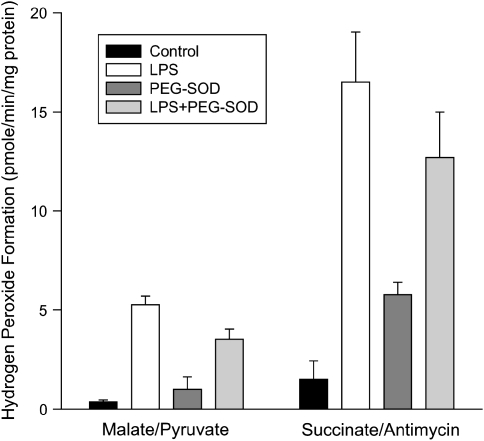
An Amplex red–based assay was used to assess mitochondrial formation of reactive oxygen species (Figure 1). This assay is based on the fact that mitochondrial glutathione peroxidase converts a substantial portion of the mitochondrially generated superoxide into hydrogen peroxide. Hydrogen peroxide readily diffuses across mitochondrial membranes into surrounding medium, where it can be detected with fluorescent indicators such as Amplex red. Cardiac mitochondria isolated from endotoxin-treated animals had large increases in hydrogen peroxide release, both when incubated with malate/pyruvate and with succinate/antimycin (p < 0.001 for both of these comparisons). As expected, levels of hydrogen peroxide generation by cardiac mitochondria isolated from animals given both PEG-SOD and endotoxin were similar to levels generated by animals given endotoxin alone, and levels for animals given PEG-SOD alone were close to levels for control animals given saline (i.e., because PEG-SOD does not reduce hydrogen peroxide levels).
Figure 1.
Mitochondrial hydrogen peroxide production. Formation of hydrogen peroxide by cardiac mitochondrial isolates incubated either with malate plus pyruvate (left) or succinate and antimycin (right). Comparison is made of samples from control, LPS-, LPS + polyethylene glycol-adsorbed–superoxide dismutase (PEG-SOD)–, and PEG-SOD-alone–treated animal groups. Hydrogen peroxide production was significantly greater for LPS samples than for control samples with both malate/pyruvate (p < 0.001) and succinate/antimycin (p < 0.001).
Free-Radical Reaction Products
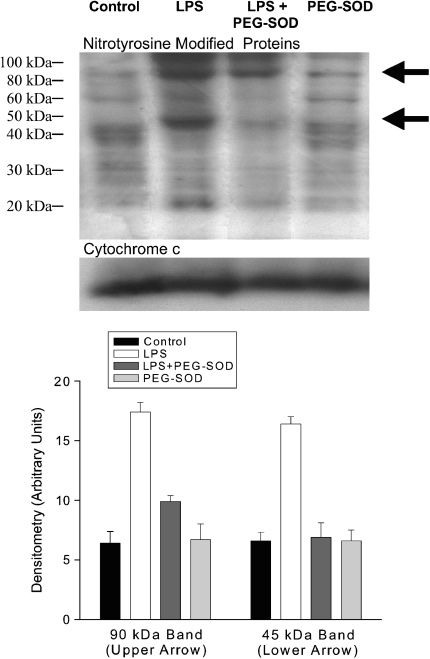
Reaction of superoxide-derived molecular species with tissues generates a variety of molecular modifications of cellular proteins and lipids. Several such markers (nitrotyrosine side-group formation, lipid aldehyde formation, protein carbonyls) were assessed on cardiac mitochondrial samples from control, endotoxin-, endotoxin + PEG-SOD–, and PEG-SOD-alone–treated animals as shown in Figures 2 through 5. We found that endotoxin elicited a marked increase in nitrotyrosine side-group levels for two mitochondrial proteins, as shown in Figure 2. One of these proteins, of approximately 90 kD, increased to 272% of control levels after endotoxin administration (top band in Figure 2), whereas the second protein, with a molecular weight of 45 kD (bottom band in Figure 2), increased to 248% of control levels. Administration of PEG-SOD prevented these endotoxin-induced cardiac mitochondrial protein nitrotyrosine modifications.
Figure 2.
Nitrotyrosine modification of mitochondrial proteins. Representative Western blot (top) probed with antinitrotyrosine antibody for cardiac mitochondrial samples from control, LPS-, LPS + PEG-SOD–, and PEG-SOD– treated animal groups. Samples were reprobed with anti–cytochrome c (bottom blot) as a protein-loading control. LPS was associated with a large increase in nitrotyrosine side-group formation for two proteins (arrows), including a protein of ∼ 90-kD molecular weight (top band) and a second protein with a 45-kD molecular weight (bottom band). Concomitant administration of PEG-SOD blocked LPS-induced increases in these two nitrotyrosine bands. Group mean data are shown in the lower graph; LPS increased nitrotyrosine content of the top and bottom protein bands; p < 0.001 for comparison of control to LPS for the top protein band, p < 0.001 for comparison of control to LPS for the bottom protein band. Both top and bottom protein bands were lower for the LPS + PEG-SOD group than for the LPS group; p < 0.001 and p < 0.001, respectively.
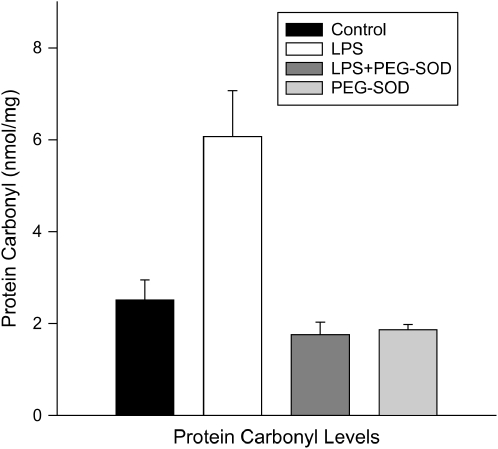
Figure 5.
Mitochondrial protein carbonyl levels. Protein carbonyl concentrations for mitochondrial samples from control, LPS-, LPS + PEG-SOD–, and PEG-SOD–treated animals. LPS administration resulted in an increase in protein carbonyl levels (p < 0.003), and administration of PEG-SOD prevented this alteration (p < 0.001).
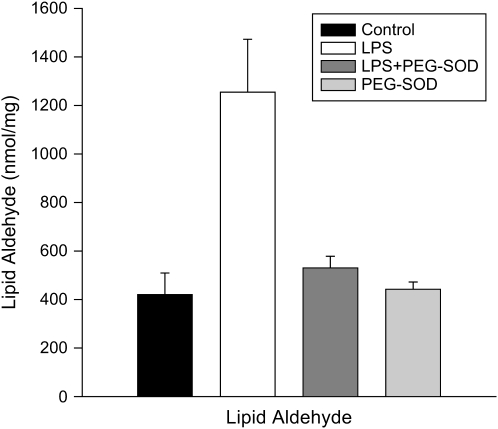
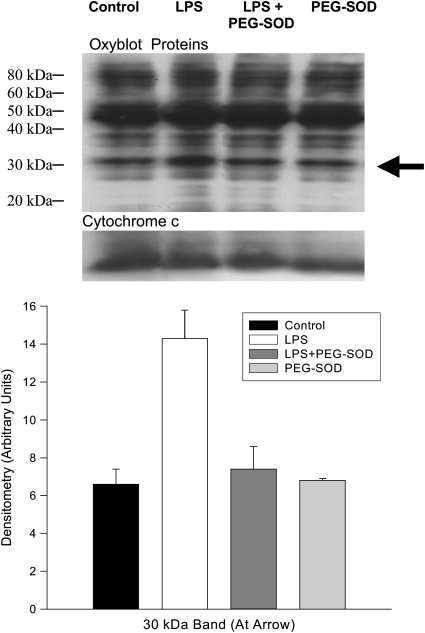
Lipid aldehyde levels (Figure 3) also increased in response to endotoxin administration (p < 0.004) and PEG-SOD ablated this endotoxin-induced increase (p < 0.012). We assessed protein carbonyl side-group formation both by measuring total levels for cardiac mitochondrial homogenates (Figure 5) and by using Western blots to identify individual proteins (Figure 4). Total carbonyl side-group levels were higher for cardiac homogenates from endotoxin-treated animals (p < 0.001). In addition, inspection of Western blots for protein carbonyl side groups (Figure 4) revealed a prominent increase in the level of carbonyl modification of a single protein band at 30 kD (p < 0.003). PEG-SOD administration prevented both the increase in total protein carbonyl content and modification of this 30-kD protein (p < 0.002 for both comparisons).
Figure 3.
Mitochondrial lipid aldehyde levels. Lipid aldehyde levels for cardiac mitochondrial samples from control, LPS-, LPS + PEG-SOD–, and PEG-SOD–treated animals. Lipid aldehyde formation increased in response to LPS, and administration of PEG-SOD prevented this increase (p < 0.004 and p < 0.02, respectively).
Figure 4.
Mitochondrial Oxyblots. Representative Western blot (top) probed with antibody to derived protein carbonyl side groups (i.e., the Oxyblot technique). A second Western blot probed with anti–cytochrome c (bottom blot) served as a loading control. Group mean data for densitometry from this group of blots are presented in the lower graph. LPS administration resulted in a large increase in the protein carbonyl content of a 30-kD protein (shown by arrow, p < 0.001 for group mean data). PEG-SOD administration prevented this increase; p < 0.002 for comparison of LPS to the LPS + PEG-SOD group.
Effect of PEG-SOD on Endotoxin-induced Mitochondrial Dysfunction
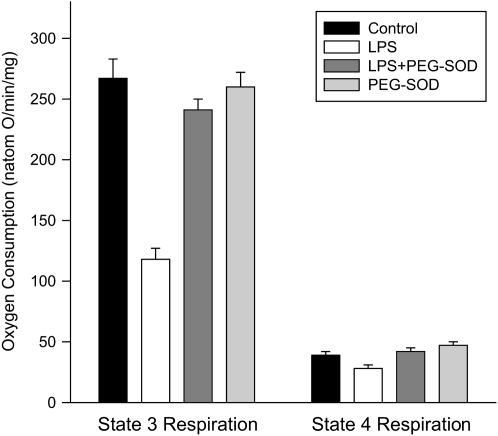
We found that endotoxin administration induced a large reduction in the mean State 3 cardiac mitochondrial respiration rate (Figure 6), and concomitant administration of PEG-SOD prevented this endotoxin-induced effect (p < 0.001 for comparison of State 3 respiration rates between control and endotoxin-treated groups; p < 0.001 comparing endotoxin with endotoxin + PEG-SOD groups). State 3 rates for animals given PEG-SOD alone were similar to rates for control animals. State 4 respiration rates were also statistically lower for samples from endotoxin-treated animals than for control animals (p < 0.05), albeit the absolute differences among State 4 rates for the different experimental groups were small.
Figure 6.
Mitochondrial oxygen consumption. Mean data for State 3 respiration rates (left) and State 4 respiration rates (right) for samples from control, LPS-, LPS + PEG-SOD–, and PEG-SOD-alone–treated animals. State 3 rates were lower for the LPS-treated group than for controls (p < 0.001); samples from LPS + PEG-SOD–treated animals had State 3 rates similar to control animals and higher than rates for animals given LPS alone (p < 0.001). State 4 rates were slightly lower for the LPS-treated group (p < 0.05) than for control, and PEG-SOD administration prevented this reduction (p < 0.02 for comparison of LPS to LPS + PEG-SOD groups).
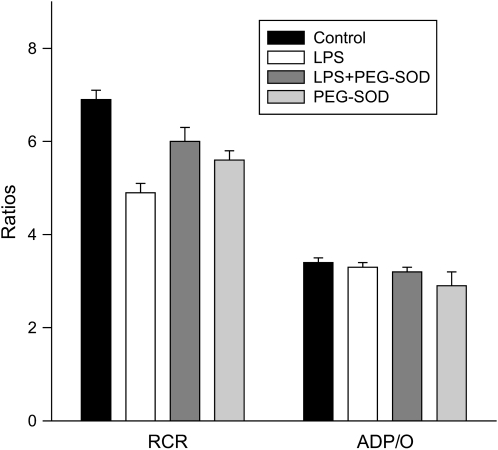
Other parameters of mitochondrial respiration measured for these four groups of experiments (i.e., control, endotoxin, endotoxin + PEG-SOD, and PEG-SOD alone) are shown in Figures 6 through 9. The ADP/O (Figure 7) was not different between control and endotoxin groups. The RCR (Figure 7) and ATP production rates (Figure 8) were lower for mitochondrial samples taken from endotoxin-treated animals as compared with control animals (p < 0.001 for comparison of RCR and ATP production rates). PEG-SOD prevented endotoxin-induced reductions in RCR and ATP production rates for mitochondrial samples (values for RCR and ATP production for the endotoxin + PEG-SOD group were significantly higher than values for the endotoxin group; p < 0.003 and p < 0.001, respectively).
Figure 9.
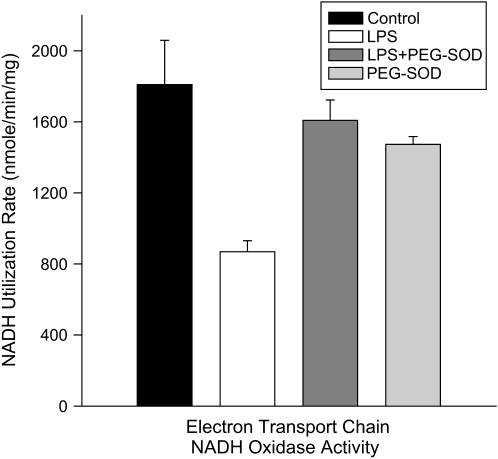
Mitochondrial NADH oxidation. NADH utilization rates for mitochondrial samples taken from control, LPS-, LPS + PEG-SOD–, and PEG-SOD–treated animals. LPS markedly reduced mitochondrial NADH utilization (p < 0.003), and concomitant administration of PEG-SOD prevented this LPS-induced effect (p < 0.02).
Figure 7.
Mitochondrial respiratory control and adenosine diphosphate/oxygen (ADP/O) ratios. Mean data for respiratory control ratios (RCR; left) and ADP/O ratios (right) for samples from control, LPS-, LPS + PEG-SOD–, and PEG-SOD-alone–treated animals. LPS administration was associated with a reduction in RCR (p < 0.001); simultaneous administration of PEG-SOD prevented this reduction (p < 0.02). ADP/O ratios did not decrease significantly in response to LPS administration.
Figure 8.
Mitochondrial ATP generation. ATP production rates are presented for samples from control, LPS-, LPS + PEG-SOD–, and PEG-SOD–treated animals. LPS markedly reduced maximal ATP generation rates (p < 0.001), and PEG-SOD administration blunted this LPS-induced effect (p < 0.001).
Endotoxin induced a significant reduction in cardiac mitochondrial electron transport chain NADH oxidase activity (Figure 9; p < 0.003), arguing that the effect of this agent to reduce State 3 respiration rates is largely due to an effect of decreasing electron transport chain utilization of NADH. PEG-SOD administration also prevented this latter endotoxin-induced effect (Figure 9; p < 0.02), with NADH oxidase activity for the PEG-SOD + endotoxin group similar to values for control animals and higher than values for the endotoxin group.
As a further control, we performed an additional group of experiments in which animals were treated both with endotoxin and heat-denatured PEG-SOD (n = 5). Other than the use of heat-denatured PEG-SOD, this group of studies used the same protocol as that used for endotoxin + PEG-SOD studies. Mitochondrial State 3 rates, ATP production, RCR, and NADH oxidase assay rates obtained for endotoxin + denatured PEG-SOD experiments were similar to levels obtained for samples from animals given endotoxin alone and significantly lower than values obtained for control or endotoxin + active PEG-SOD groups. Specifically, values for the endotoxin + denatured PEG-SOD group averaged 152 ± 12 nanoatoms O/min/mg for State 3 rates, 3.9 ± 0.2 for RCR, 411 ± 27 nmol/min/mg for ATP generation rates, and 726 ± 77 nmol/min/mg for NADH oxidase (p < 0.001, p < 0.001, p < 0.001, and p < 0.003, respectively, compared with values for the control group).
Effect of PEG-SOD on Cardiac Pressure Generation
Endotoxin significantly reduced cardiac pressure–generating capacity, as determined using a Langendorf preparation, with systolic pressure 30 to 50% lower for hearts taken from endotoxin-treated animals as compared with control animals (Figure 10). PEG-SOD administration completely prevented endotoxin-induced reductions in cardiac pressure–generating capacity, restoring systolic pressures to control levels. For example, systolic pressure generated at a diastolic pressure of 15 mm Hg averaged 110 ± 11, 66 ± 7, 129 ± 10, and 124 ± 5 mm Hg, respectively, for control, endotoxin, endotoxin + PEG-SOD, and PEG-SOD groups (p < 0.01 for comparison of endotoxin to the other three experimental groups).
Figure 10.
Cardiac systolic–diastolic pressure relationships. Cardiac systolic pressure–diastolic pressure curves determined using the Langendorf technique for hearts removed from control, LPS-, LPS + PEG-SOD–, and PEG-SOD–treated animals. Pressure generation was lower for the LPS group than for control or PEG-SOD–treated groups at diastolic pressure levels from 15 to 30 mm Hg (i.e., p < 0.01, p < 0.02, p < 0.004, and p < 0.002, respectively, for 15, 20 25, and 30 mm Hg). Pressure generation for the PEG-SOD + LPS group was higher than for the LPS-treated group (p < 0.001, p < 0.005, p < 0.002, p < 0.003, p < 0.002, respectively, for 15, 20, 25, and 30 mm Hg). Pressure differences between control and LPS-treated groups did not reach statistical significance at 10 mm Hg.
DISCUSSION
This study found that endotoxin administration elicited significant reductions in State 3 respiration rates, in mitochondrial ATP generation, and in the RCR for cardiac mitochondria isolated from endotoxin-treated animals. We also found that oxygen consumption using the NADH oxidase assay was much lower for samples from endotoxin-treated animals as compared with saline-treated control animals, paralleling endotoxin-induced reductions in State 3 respiration rates. In this latter assay, mitochondria are permeabilized and excess exogenous NADH added to deliver electrons to Complex 1. The fact that NADH oxidase assay respiration rates in response to ADP addition were lower for endotoxin samples argues, therefore, that the primary endotoxin-induced abnormality responsible for reductions in mitochondrial function is an inhibition of electron flow through Complexes I, III, and/or IV.
Endotoxin administration to intact animals also markedly reduced the systolic pressures generated by excised hearts perfused using the Langendorf technique. More importantly, all mitochondrial abnormalities and reductions in systolic pressure–generating capacity were prevented by administration of PEG-SOD, a specific superoxide scavenger, to endotoxin-treated animals. We also observed significant endotoxin-induced increases in cardiac mitochondrial generation of reactive oxygen species, substantial increases in nitrotyrosine side-group content for two mitochondrial proteins (90 and 45 kD), increased carbonyl side-group levels for a 30-kD mitochondrial protein, and increased cardiac levels of lipid aldehydes. PEG-SOD administration blocked increases in all of these free-radical reaction products in the heart in parallel with its effect to improve cardiac mitochondrial and contractile performance.
Free Radicals and Cardiac Dysfunction in Sepsis
The superoxide ion has been previously implicated as a modulator of sepsis-induced tissue dysfunction in the lung (9), diaphragm (10, 11, 25), and other tissues (26). In the lung, white cell–derived superoxide and its daughter molecules are believed to be a major cause of lung injury in sepsis and related acute inflammatory syndromes (e.g., trauma, burns). White cell–derived free radicals may also contribute to injury in other organs in sepsis, but many tissues can develop severe functional alterations with minimal, if any, evidence of tissue invasion by neutrophils or other white cell species. This has led to the concept that local production of superoxide in tissues is the likely source of free radicals in organs other than the lung in response to systemic inflammation (10, 11, 26).
In keeping with this possibility, previous studies have shown an increase in superoxide generation in the diaphragm (10, 11, 25), in liver tissue (26), and in the kidney (27) after endotoxin administration and other animal models of sepsis (e.g., cecal ligation perforation). In several of these tissues, NADPH oxidase complexes are present (e.g., in skeletal muscle, liver, endothelium), and activation of this enzyme system has been shown in several studies to be an important contributor to tissue free-radical generation in sepsis (28, 29). A second potential source of free radicals in sepsis is mitochondria. Previous studies have shown evidence for increased mitochondrial free-radical generation in both liver and skeletal muscle in sepsis (25, 26). One mechanism that has been proposed to account for excessive mitochondrial free-radical generation in sepsis is as a consequence of enhanced nitric oxide formation. Nitric oxide inhibits the distal portion of the electron transport chain with buildup of electrons in the proximal portion of the chain, with subsequent excess proximal chain electron transfer to molecular oxygen and resultant superoxide formation (30). Increased cytosolic levels of calcium have also been reported to occur in sepsis (e.g., in skeletal muscle and the heart), and some evidence suggests that calcium-induced mitochondrial free-radical generation may also occur in septic states (31).
The present study is the first, of which we are aware, demonstrating enhanced cardiac mitochondrial free-radical generation after endotoxin administration. This increase in cardiac mitochondrial free-radical generation is a reasonable explanation for the increase in cardiac mitochondrial markers of free-radical–mediated tissue modifications (e.g., protein carbonyl formation, lipid aldehyde formation) observed in the present study. The fact that administration of PEG-SOD, a superoxide scavenger, prevented both increases in tissue biomarkers of oxidative stress and mitochondrial dysfunction in concert is consistent with a link between these phenomena (i.e., a role for free radicals in mediating the development of endotoxin-induced cardiac mitochondrial dysfunction). Our data also indicate that the effects of PEG-SOD are related specifically to the ability of this agent to scavenge superoxide and not due to nonspecific effects of this protein or PEG administration because denatured PEG-SOD had no effect on mitochondrial function.
Potential Relationships between Cardiac Mitochondrial and Contractile Dysfunction in Sepsis
In addition to observing endotoxin-induced reductions in cardiac mitochondrial function, we also found that endotoxin administration elicited large reductions in cardiac pressure–generating capacity. As for mitochondrial function, this latter abnormality was also prevented by pretreatment with a superoxide scavenger. One possible explanation for this association would be that these phenomena could be downstream consequences of two different pathophysiologic processes that are both triggered by excessive superoxide free radicals. An alternative possibility is that these two phenomena, mitochondrial dysfunction and contractile dysfunction, are linked. One might suppose that reductions in mitochondrial generation of ATP could influence cardiac performance and, certainly, the magnitude of the reductions in ATP-generating capacity engendered by endotoxin administration in this study was substantial. Reductions in ATP formation by oxidative phosphorylation at times of peak energy utilization would be expected to reduce resynthesis of creatine phosphate, leading to accumulation of creatine and phosphate ions around the contractile proteins. Phosphate, in turn, is a major regulator of contractile protein cross-bridge function and cardiac myosin ATPase activity, with marked reductions in force generation in response to increasing phosphate levels (32). One recent report did observe a reduction in cardiac levels of high-energy phosphate compounds in an animal model of sepsis (33), supporting this possibility, although other reports have disputed this scenario (34).
Another possibility is that cardiac free-radical–mediated mitochondrial alterations may influence activation of the intrinsic caspase pathway. Consistent with this potential mechanism are recent observations linking cardiac contractile dysfunction after endotoxin administration to the intrinsic, caspase 9–mediated, mitochondrially dependent pathway of caspase 3 activation (35). Mitochondrially derived free radicals have been shown to modulate mitochondrial outer membrane pore formation (36) and, in turn, to activate the mitochondrially driven caspase 9 pathway. As a result, mitochondrially derived free radicals could influence caspase 9 activation after endotoxin administration, leading to caspase 3 activation and caspase 3–mediated contractile protein cleavage and/or cardiac apoptosis.
Potential Implications
Regardless of the exact mechanistic links between cardiac mitochondrial dysfunction and alterations in cardiac pressure–generating capacity, the present data suggest that both phenomena are free-radical mediated. As a result, a therapeutic strategy that scavenges free radicals at selected intracellular sites may prove an effective means of preventing sepsis-induced cardiac dysfunction. We chose to use PEG-SOD in the current study because the PEG moiety of this agent facilitates transport across membranes and penetration into intracellular spaces. Previous studies have shown that this agent can reach distant tissues (e.g., leg muscles) after intraperitoneal injection and exert significant physiologic effects on these distant organs (37). Nevertheless, newer antioxidants of greater potency that can very selectively target mitochondria are currently under investigation (38) and may prove more advantageous for clinical usage for the purpose of improving cardiac function in septic patients.
Moreover, prevention of sepsis-induced cardiac dysfunction also has the potential to improve mortality. This may depend, however, on the specific mechanism by which sepsis develops, because animal studies suggest the propensity to develop cardiac dysfunction varies tremendously depending on the manner in which systemic inflammation is induced (e.g., severe cardiac dysfunction is seen after endotoxin administration but is observed far less after injection of bacteria) (16, 17). This model-to-model variation may also explain, in part, the variable response of animal models of sepsis to administration of different free-radical scavengers (12–15). It is not surprising, therefore, that significant cardiac dysfunction is observed in some patients with sepsis, but not in all. One might anticipate that the ability of free-radical scavengers to impact patient outcome in sepsis may also be variable, with beneficial effects observed in some patient groups but not in others. More work is needed to define the patient populations most likely to develop cardiac dysfunction in response to sepsis, to determine the effect of free-radical scavengers on cardiac dysfunction in this group of patients, and to ascertain if administration of scavengers (or other cardioprotective agents, such as caspase inhibitors) can improve the survival of this patient population.
Supplementary Material
This work was supported by National Institutes of Health grants 69821 and 63698.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200410-1346OC on March 2, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Fink MP. Bench-to-bedside review: cytopathic hypoxia. Crit Care 2002;6:491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crouser ED, Julian MW, Blaho DV, Pfeiffer DR. Endotoxin-induced mitochondrial damage correlates with impaired respiratory activity. Crit Care Med 2002;30:276–284. [DOI] [PubMed] [Google Scholar]
- 3.Bernardi P, Petronelli V, DiLisa F, Forte M. A mitochondrial perspective on cell death. Trends Biochem Sci 2001;26:112–117. [DOI] [PubMed] [Google Scholar]
- 4.Adrie C, Bachlett M, Vayssier-Taussat R, Russo-Marie F, Bouchaert I, Adib-Conquy M, Cavaillon J, Pinsky MR, Dhainaut J, Polla BS. Mitochondrial membrane potential and apoptosis peripheral blood monocytes in severe human sepsis. Am J Respir Crit Care Med 2001;164:389–395. [DOI] [PubMed] [Google Scholar]
- 5.Parrillo JE, Parker MM, Natanson C, Suffredini AF, Danner RL, Cunnion RE, Ognibene FP. Septic shock in humans: advances in the understanding of pathogenesis, cardiovascular dysfunction and therapy. Ann Intern Med 1990;113:227–242. [DOI] [PubMed] [Google Scholar]
- 6.Snell RJ, Parrillo JE. Cardiovascular dysfunction in septic shock. Chest 1991;99:1000–1009. [DOI] [PubMed] [Google Scholar]
- 7.Fink MP. Cytopathic hypoxia: mitochondrial dysfunction as mechanism contributing to organ dysfunction in sepsis. Crit Care Clin 2001;17:219–237. [DOI] [PubMed] [Google Scholar]
- 8.Singer M, Brealey D. Mitochondrial dysfunction in sepsis. Biochem Soc Symp 1999;66:149–166. [DOI] [PubMed] [Google Scholar]
- 9.Bernard GR, Lucht WD, Niedermeyer ME, Snapper JR, Olgetree ML, Brigham KL. Effect of N-acetylcysteine on the pulmonary response to endotoxin in the awake sheep and upon in vitro granulocyte function. J Clin Invest 1984;73:1772–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shindoh C, DiMarco A, Nethery D, Supinski G. Effect of PEG-superoxide dismutase on the diaphragmatic response to endotoxin. Am Rev Respir Dis 1992;145:1350–1354. [DOI] [PubMed] [Google Scholar]
- 11.Boczkowski J, Lisdero CL, Lanone S, Samb A, Carreras C, Boveris A, Aubier M, Poderoso J. Endogenous peroxynitrite mediates mitochondrial dysfunction in rat diaphragm during endotoxemia. FASEB J 1999;13:1637–1647. [DOI] [PubMed] [Google Scholar]
- 12.Lancel S, Tissier S, Mordon S, Marechal X, Depontieu F, Scherpereel A, Chopin C, Neviere R. Peroxynitrite decomposition catalysts prevent myocardial dysfunction and inflammation in endotoxemic rats. J Am Coll Cardiol 2004;43:2348–2358. [DOI] [PubMed] [Google Scholar]
- 13.Matejovic M, Krouzecky A, Martinkova V, Rokyta R Jr, Radej J, Kralova H, Treska V, Radermacher P, Novak I. Effects of tempol, a free radical scavenger, on long-term hyperdynamic porcine bacteremia. Crit Care Med 2005;33:1057–1063. [DOI] [PubMed] [Google Scholar]
- 14.Broner CW, Shenep JL, Stidham GL, Stokes DC, Fairclough D, Schonbaum GR, Rehg JE, Hildner WK. Effect of antioxidants in experimental Escherichia coli septicemia. Circ Shock 1989;29:77–92. [PubMed] [Google Scholar]
- 15.Novotny MJ, Laughlin MH, Adams HR. Evidence for lack of importance of oxygen free radicals in Escherichia coli endotoxemia in dogs. Am J Physiol 1988;254:H954–H962. [DOI] [PubMed] [Google Scholar]
- 16.Neviere R, Fauvel H, Chopin C, Formstecher P, Marchetti P. Caspase inhibition prevents cardiac dysfunction and heart apoptosis in a rat model of sepsis. Am J Respir Crit Care Med 2001;163:218–225. [DOI] [PubMed] [Google Scholar]
- 17.Kumar A, Zanotti S, Bunnell G, Habet K, Anel R, Neumann A, Cheang M, Dinarello CA, Cutler D, Parrillo JE. Interleukin-10 blunts the human inflammatory response to lipopolysaccharide without affecting the cardiovascular response. Crit Care Med 2005;33:331–340. [DOI] [PubMed] [Google Scholar]
- 18.Trumbeckaite S, Poalka JR, Neuhof C, Zierz S, Gellerich FN. Different sensitivity of rabbit heart and skeletal muscle to endotoxin-induced impairment of mitochondrial function. Eur J Biochem 2001;268:1422–1429. [DOI] [PubMed] [Google Scholar]
- 19.Khadour FH, Panas D, Ferdinandy P, Schulze C, Csont T, Lalu MM, Wildhirt SM, Schulz R. Enhanced NO and superoxide generation in dysfunctional hearts from endotoxemic rats. Am J Physiol Heart Circ Physiol 2002;283:H1108–H1115. [DOI] [PubMed] [Google Scholar]
- 20.Callahan LA, White R, Supinski G. Free radicals mediate the development of cardiac mitochondrial dysfunction in sepsis [abstract]. Am J Respir Crit Care Med 2002;165:A173. [Google Scholar]
- 21.Lucas DT, Szweda LI. Cardiac reperfusion injury: aging, lipid peroxidation, and mitochondrial dysfunction. Proc Natl Acad Sci USA 1998;95:510–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernstrom M, Tonkonogi M, Sahlin K. Effects of acute and chronic endurance exercise on mitochondrial uncoupling in human skeletal muscle. J Physiol 2004;554:755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Humphries KM, Yoo Y, Szweda LI. Inhibition of NADH-linked mitochondrial respiration by 4-hydroxy-2-nonenal. Biochemistry 1998;37:552–557. [DOI] [PubMed] [Google Scholar]
- 24.Reznick AZ, Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 1994;233:357–363. [DOI] [PubMed] [Google Scholar]
- 25.Callahan LA, Stofan D, Szweda L, Nethery D, Supinski GS. Free radicals alter maximal diaphragmatic oxygen consumption in endotoxin-induced sepsis. Free Radic Biol Med 2001;30:129–138. [DOI] [PubMed] [Google Scholar]
- 26.Taylor DE, Ghio AJ, Piantadosi CA. Reactive oxygen species produced by liver mitochondria of rats in sepsis. Arch Biochem Biophys 1995;316:70–76. [DOI] [PubMed] [Google Scholar]
- 27.Leach M, Frank S, Olbrich A, Pfeilschifter J, Thiemermann C. Decline in the expression of copper/zinc superoxide dismutase in the kidney of rats with endotoxic shock: effects of the superoxide anion radical scavenger, tempol, on organ injury. Br J Pharmacol 1998;125:817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Javesghani D, Magder SA, Barreiro E, Quinn MT, Hussain SN. Molecular characterization of a superoxide-generating NAD(P)H oxidase in the ventilatory muscles. Am J Respir Crit Care Med 2002;165:412–418. [DOI] [PubMed] [Google Scholar]
- 29.Durant R, Klouche K, Delbosc S, Morena M, Amigues L, Beraud JJ, Canaud B, Cristol JP. Superoxide anion overproduction in sepsis: effects of vitamin E and simvastatin. Shock 2004;22:34–39. [DOI] [PubMed] [Google Scholar]
- 30.Radi R, Cassina A, Hodara R. Nitric oxide and peroxynitrite interactions with mitochondria. Biol Chem 2002;383:401–409. [DOI] [PubMed] [Google Scholar]
- 31.Thompson M, Kliewer A, Maass D, Becker L, White DJ, Bryant D, Arteaga G, Horton J, Giroir BP. Increased cardiomyocyte intracellular calcium during endotoxin-induced cardiac dysfunction in guinea pigs. Pediatr Res 2000;47:669–676. [DOI] [PubMed] [Google Scholar]
- 32.Nosek TM, Leal-Cardoso JH, McLaughlin M, Godt RE. Inhibitory influence of phosphate and arsenate on contraction of skinned skeletal and cardiac muscle. Am J Physiol 1990;259:C933–C939. [DOI] [PubMed] [Google Scholar]
- 33.Chen HW, Hsu C, Lu TS, Wang SJ, Yang RC. Heat shock pretreatment prevents cardiac mitochondrial dysfunction during sepsis. Shock 2003;20:274–279. [DOI] [PubMed] [Google Scholar]
- 34.Hotchkiss RS, Song SK, Neil JJ, Chen RD, Manchester JK, Karl IE, Lowry OH, Ackerman JJ. Sepsis does not impair tricarboxylic acid cycle in the heart. Am J Physiol 1991;260:C50–C57. [DOI] [PubMed] [Google Scholar]
- 35.Lancel S, Joulin O, Favory R, Goossens JF, Kluza J, Chopin C, Formstecher P, Marchetti P, Neviere R. Ventricular myocyte caspases are directly responsible for endotoxin-induced cardiac dysfunction. Circulation 2005;111:2596–2604. [DOI] [PubMed] [Google Scholar]
- 36.Kim TS, Yun BY, Kim IY. Induction of the mitochondrial permeability transition by selenium compounds mediated by oxidation of the protein thiol groups and generation of the superoxide. Biochem Pharmacol 2003;66:2301–2311. [DOI] [PubMed] [Google Scholar]
- 37.Zerba E, Komorowski TE, Faulkner JA. Free radical injury to skeletal muscles of young, adult, and old mice. Am J Physiol 1990;258:C429–C435. [DOI] [PubMed] [Google Scholar]
- 38.Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, Sammut IA. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J 2005;19:1088–1095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.