Abstract
Rationale: The IL-1 receptor–associated kinase (IRAK-1) plays a central role in TLR2- and TLR4-induced activation of nuclear factor (NF)-κB, a critical event in the transcriptional regulation of many sepsis-associated proinflammatory mediators. There are two haplotypes for the IRAK-1 gene in Caucasians, with the variant haplotype consisting of five intron single-nucleotide polymorphisms (SNPs) and three exon SNPs.
Objectives: To examine the functional significance of the IRAK-1 variant haplotype in modifying nuclear translocation of NF-κB and affecting outcomes from sepsis.
Measurements and Main Results: One hundred fifty-five Caucasian patients with sepsis were included. Twenty-one (14%) were homozygous for the IRAK-1 variant haplotype as determined by a SNP in which T is replaced with C at nucleotide 1,595 within exon 12 of the IRAK-1 gene. The IRAK-1 variant haplotype was associated with increased nuclear levels of NF-κB in LPS-stimulated peripheral blood neutrophils from patients with sepsis compared with that found in patients with wild-type IRAK-1 haplotype (p = 0.0009). There was an increased incidence of shock (p = 0.047) (odds ratio [OR], 2.9; 95% confidence interval [CI], 1.1–7.7), greater requirement for more prolonged mechanical ventilator support (p = 0.04) (OR, 2.7; 95% CI, 1.05–6.9), and higher 60-d mortality (p = 0.05) (OR, 2.7; 95% CI, 1.0–6.8) in patients with the IRAK-1 variant haplotype compared with wild type.
Conclusions: These results indicate that the IRAK-1 variant haplotype is functionally significant in patients with sepsis, being associated with increased nuclear translocation of NF-κB, more severe organ dysfunction, and higher mortality.
Keywords: acute lung injury; haplotype, inflammation; neutrophil; NF-κ; B; single nucleotide polymorphism
Sepsis is a complex syndrome that is initiated by infection and characterized by an overwhelming systemic inflammatory response. There are estimated to be ∼ 750,000 cases of sepsis per year in the United States, with more than 210,000 deaths (1). Death associated with sepsis is usually not due to progression of the infection itself, but rather is secondary to development of multiple organ dysfunction. Several studies have demonstrated that patients with sepsis and a greater and more prolonged inflammatory response are more likely to develop multiple organ failure and to die than those with less severe inflammation (2–5).
The transcriptional regulatory factor nuclear factor (NF)-κB plays a central role in modulating the expression of proinflammatory cytokines and other mediators that contribute to organ dysfunction and outcome in sepsis. Increased activation of NF-κB is found in alveolar macrophages, peripheral blood mononuclear cells, and neutrophils from patients with sepsis (6–10). In this setting, a greater or more persistent nuclear accumulation of NF-κB is associated with higher mortality and more persistent organ dysfunction, including pulmonary injury (8, 9). Recent studies have also suggested that neutrophil activation patterns, as defined by LPS-induced translocation of NF-κB to the nucleus, are able to predict subsequent clinical course in patients with sepsis. In particular, in neutrophils from patients with sepsis-induced acute lung injury, increased nuclear accumulation of NF-κB in response to LPS stimulation was associated with a more prolonged requirement for mechanical ventilatory support (10).
The IL-1 receptor–associated kinase (IRAK-1) plays an important role in transducing signals initiated by interaction of the TLR/interleukin 1 receptor (TIR) superfamily with bacterially derived products. IRAK-1 activation leads to increased transcription of NF-κB–dependent genes (11, 12). Upon binding of ligands to TIR members, such as TLR2 or TLR4, the kinases IRAK-1 and IRAK-4 and the scaffolding protein MyD88 are recruited to the intracellular domain of the receptor complex (11, 13–16). As a result, IRAK-1 becomes phosphorylated, leading to a reduction in its affinity for TOLLIP and thereby facilitating interaction with MyD88 and IRAK-4. TOLLIP negatively regulates TLR- or IL-1–induced activation of proinflammatory cytokines presumably by sequestering the activity of IRAK-1 (17, 18). Hyperphosphorylation of IRAK-1 induces activation of TRAF-6 (19) and of the IKK complex, leading to phosphorylation, ubquitination, and degradation of IκB-α by the 26S proteasome (20–28). IRAK-1 can also translocate into the nucleus upon ligand interaction with TIR, where it interacts with STAT3 and promoter elements, such as in the IL-10 gene (29, 30).
Activation of TIR pathways leads to translocation of NF-κB into the nucleus, resulting in NF-κB–dependent expression of proinflammatory mediators that contribute to sepsis-induced organ dysfunction (31–34). Macrophages deficient in IRAK-1 have an impaired response to LPS-stimulated TNF-α, and transgenic mice lacking IRAK-1 are resistant to LPS lethality (35). IRAK-1–deficient mice are also more susceptible to high-dose Staphylococcus aureus infection (36).
Genetic heterogeneity involving the immune response to infection may have potentially significant clinical consequences. There are two haplotypes for the IRAK-1 gene that have been defined among Caucasians in the NCBI database. The variant haplotype consists of five intron single-nucleotide polymorphisms (SNPs) and three exon SNPs that have a r2 near 1.0. Because these SNPs are in near complete linkage disequilibrium, we genotyped a single-nucleotide polymorphism in IRAK-1, in which a T is replaced with a C at nucleotide position 1,595 within exon 12 of the IRAK-1 gene (37), leading to a position 532 L→S substitution, to identify haplotypes in patients with sepsis.
Because NF-κB seems to occupy a critical position in modulating the proinflammatory response associated with sepsis, we speculated that genetic polymorphisms that affect crucial steps in its activation, such as those linked to IRAK-1, might be associated with outcome. We examined this possibility in the present study and found that the IRAK-1 variant haplotype is not only associated with greater LPS-induced translocation of NF-κB to the nucleus in neutrophils from septic patients but also correlates with a more severe clinical course in this setting.
METHODS
Reagents
RPMI 1640 was obtained from Life Technologies (Gaithersburg, MD). Percoll was purchased from Amersham-Pharmacia (Piscataway, NJ). Bicinchoninic acid protein assay reagent was purchased from Pierce (Rockford, IL). LPS (from Escherichia coli O111:B4) was obtained from Sigma Chemical (St. Louis, MO).
Volunteer Selection
Fifty healthy white volunteers, 18–40 yr of age, were recruited to determine the allele frequency of the IRAK-1 haplotype. The study was approved by the institutional review board, and each volunteer signed an informed consent document.
Inclusion Criteria
One hundred fifty-five Caucasian patients with sepsis were enrolled in this study. Patients were eligible for inclusion if they were in an intensive care unit (ICU), had clinical evidence of infection, and had at least one sepsis-related organ dysfunction as previously defined (38). Acute lung injury was defined by standard criteria (39) (i.e., acute onset of significantly impaired oxygenation and PaO2/FiO2 ratio < 300 (adjusted for barometric pressure [i.e., < 250 in Denver]), bilateral infiltrates consistent with pulmonary edema on a frontal chest radiograph, and no clinical evidence of left atrial hypertension, or, if a pulmonary artery catheter was in place, no clinical evidence of a pulmonary artery occlusion < 18 mm Hg. Septic shock was defined as a systolic blood pressure of < 90 mm Hg for at least 30 min despite fluid replacement or the use of inotropic support to maintain blood pressure. Patients were enrolled within 24 h of developing these criteria. The study was approved by the University of Colorado Health Sciences Center institutional review board. Consent was obtained from all patients or their surrogates before enrollment.
Exclusion Criteria
Exclusion criteria were age below 18 yr and, for patients in whom neutrophils were isolated, a neurologic condition that could impair weaning, severe chronic respiratory disease, severe chronic liver disease (defined as a Child-Pugh score of > 10), burns of ⩾ 30% of total body surface area, malignancy or other irreversible condition for which 6-mo mortality was estimated to be above 50%, use of chronic high-dose immunosuppressive therapy (i.e., corticosteroids at a dose of ⩾ 1 mg/kg/d of methylprednisolone or equivalent within 3 d before ICU admission or cytotoxic agents for immunologic or oncologic disorders), or a history of bone marrow or lung transplantation.
Isolation and Culture of Human Neutrophils
Peripheral blood for neutrophil and/or DNA isolation was obtained from patients within 24 h of admission into the ICU. Neutrophils (purity 98%) were isolated by plasma-Percoll gradients after dextran sedimentation of erythrocytes (40) and were resuspended in RPMI 1640 and 2% heat-inactivated platelet poor plasma at a final concentration of 5 × 106 cells/ml and cultured with or without 100 ng/ml LPS for 0, 30, and 60 min. A subset of 30 subjects consented to neutrophil isolation in addition to DNA isolation.
DNA Isolation
Genomic DNA was extracted from peripheral blood mononuclear cells using the QIAamp DNA Midi kit (Qiagen, Valencia, CA). In brief, cells were lysed with Buffer AL and incubated for 10 min at 70°C. After 10 min, 100% ethanol was added to the sample. The sample was mixed and transferred to a QIAamp midi column. The sample was centrifuged for 3 min. After centrifugation, DNASE-free distilled water was added onto the column, and the sample was incubated for 5 min and centrifuged for 5 min. The DNA was stored at –70°C until use.
Allelic Discrimination
Real-time polymerase chain reaction (PCR) allelic discrimination assays were developed by the assay-by-design service offered by Applied Biosystems (Foster City, CA). Probe and primer combinations were designed at the single nucleotide polymorphism 532. The probe and primer sets were optimally designed for the purpose of using universal reaction and cycling conditions. PCR reactions were performed in a final volume of 25 μl, which consisted of 1–25 ng of genomic DNA diluted in dH2O, 12.5 μl of 2× Taqman Universal PCR Master Mix (containing PCR buffer, deoxynucleotides, and AmpliTaq Gold DNA polymerase), and 1.25 μl of 20X TaqMan SNP genotyping Assay Mix (TaqMan SNP Genotyping Assays consisted of unlabeled PCR primers and TaqMan MGB probes FAM and VIC dye labeled). Thermal cycle conditions involved an initial step (10 min at 95°C) followed by 40 cycles of a denaturation step (15 s at 92°C) and an annealing/extension step (1 min at 60°C). PCR was performed using an Applied Biosystems 7300 Real-Time PCR system.
Determination of Haplotypes
Data on IRAK-1 haplotypes is available on the NCBI website (www.ncbi.nlm.nih.gov/SNP/GeneGt.cgi?rpttype=LdplotandgeneID=3654andchr=XandpopID=847). The variant haplotype block consists of five intron SNPs (rs3027898 T→G, rs731642 G→A, rs2239673 T→C, rs5945174 A→G, and rs7061789 A→G) and three exon SNPs (rs1059701 C→T [synonymous], rs1059702 C→T [nonsynonymous], and rs1059703 T→C [nonsynonymous]) and has a r2 near 1.0. This information on haplotype block structure can be used to identify the subset of SNPs that represent, or tag, each haplotype (haplotype tagging SNPs; htSNPs), thereby permitting determination of one SNP (532 L→S) to define the IRAK-1 haplotypes. Therefore, the 532 SNP was genotyped in the present population of patients with well characterized sepsis to determine if the IRAK-1 haplotype was wild type or variant (i.e., with a T→C substitution [rs1059703]).
Electrophoretic Mobility Shift Assay
Nuclear extracts were prepared as previously described (7, 41, 42). Isolated neutrophils were incubated for 15 min in buffer A (10 mM N-2-hydroxyethylpiperazine-N′-ethane sulfonic acid [pH 7.9], 1.5 mM magnesium chloride, 10 mM potassium chloride [pH 7.9]). After cytoplasm was removed from the nuclei by 15 passages through a 25-gauge needle, the nuclei were collected by centrifugation at 600 × g for 6 min at 4°C. The nuclear pellet was incubated on ice for 15 min in buffer C (20 mM N-2-hydroxyethylpiperazine-N′-ethane sulfonic acid [pH 7.9], 0.42 M sodium chloride, 1.5 mM magnesium chloride, 0.2 mM ethylenediaminetetraacetic acid, 25% glycerol), after which the extract was centrifuged at 4°C for 10 min at 12,000 × g. The supernatant was collected, divided into aliquots, and stored at −86°C. Protein concentration was determined by using a bicinchoninic acid protein assay kit (Pierce) standardized to bovine serum albumin according to the manufacturer's protocol.
Nuclear extracts (5 μg) were incubated at room temperature for 15 min in 20 μl of reaction buffer containing 10 mM tris(hydroxymethyl) aminoethane-hydrochloric acid (pH 7.5), 1 mM magnesium chloride, 0.5 mM ethylenediamine tetraacetic acid, 0.5 mM dithiothreitol, 50 mM sodium chloride, and 4% glycerol, with [32P]end-labeled, double-stranded oligonucleotide probe specific for the κB site, 5′-GCCATGG GGGGATCCCCG AAG TCC-3′ (Geneka Biotechnology Inc., Montreal, PQ, Canada) and 1 μg of poly(dI-dC)·poly(dI-dC). Specificity of binding was confirmed by incubation with a 100-fold excess of unlabeled κB and cyclic AMP response element (CRE) oligonucleotides, with confirmation of elimination of the NF-κB band with the cold κB oligonucleotide, but not the cold CRE oligonucleotide. The complexes were resolved on 5% polyacrylamide gels in tris(hydroxymethyl) aminoethane-hydrochloric acid (pH 8.0)-borate-ethylenediaminetetra-acetic acid buffer at 10 V/cm. Dried gels were exposed with Kodak Biomax MS film (Rochester, NY) for 1–24 h at −70°C. Samples from control and LPS-stimulated neutrophils from each patient were run on the same gel. Densitometry was performed using an imaging system and analysis software (Bio-Rad, Hercules, CA). A standard nuclear extract (1 μg) from pooled RAW 264.7 cells was applied to every EMSA gel to allow normalization of nuclear concentrations of NF-κB and comparison between values for each gel.
Sample Size Calculation
The primary outcome in this study was 60-d all cause mortality as a dichotomous variable. Our previous study in patients with sepsis-induced ALI examined the relationship between NF-κB translocation in neutrophils and survival and found that a LPS-induced increase in NF-κB translocation was associated with a death rate of 34%, as compared with mortality of 9% among patients in whom no increase in NF-κB activation occurred in LPS-stimulated neutrophils (10). Assuming a 25% difference in mortality rates between the two groups, the sample size (PASS Software) needed at 80% power, α of 0.05, assuming an IRAK-1 532 allele frequency of 24% in the normal Caucasian population (defined in our nonseptic healthy white population consisting of 50 volunteers) was calculated to be 150 patients.
Statistical Analysis
The primary clinical outcome variables collected were ventilator-free days (up to 28 d), presence of shock, and survival up to Day 60 after study entry admission. To calculate area under the curve for nuclear levels of NF-κB, the values at baseline and at 30 and 60 min after LPS stimulation were used. For comparison of the IRAK-1 haplotype and outcome variables, univariate and multivariate logistic regression analyses were used. Univariate chi-square analysis was performed on all clinical risk factors (chronic obstructive pulmonary disease, cancer, alcohol abuse, end-stage liver failure, immunosuppression, diabetes, congestive heart failure, and anemia), sex, and site of infection. Variables with p values < 0.05 were used in the multivariate logistic regression model. Predictor variables with 20 or fewer observations were excluded. Hardy-Weinberg equilibrium of the female population was tested by contingency table comparisons of observed versus expected genotypes. A p value < 0.05 was defined as statistically significant. Male subjects were not included in this calculation because IRAK-1 is X linked; therefore, male subjects cannot be heterozygotes at IRAK-1 loci. All tests were two-sided, and a p value of < 0.05 was considered significant. SAS statistical software (SAS Institute Inc., Cary, NC) was used for all analyses.
RESULTS
Demographics and Clinical Characteristics
Table 1 shows the clinical and demographic characteristics of the patients enrolled in this study. The lack of male subjects in the heterozygous group was expected due to the location of the IRAK-1 polymorphism on the X chromosome (37). There were no statistically significant differences associated with the haplotypes examined (i.e., homozygous/hemizygous, heterozygous, or wild-type) and preexisting demographic or clinical characteristics (Table 2). There were no statistically significant differences in allele frequencies between those found in 50 healthy volunteers (76%) and this patient population (78%). The population was in Hardy-Weinberg equilibrium for all haplotypes.
TABLE 1.
CLINICAL CHARACTERISTICS OF PATIENTS ENROLLED IN THE STUDY
| N | 155 |
| Age, yr (mean ± SD) | 52 ± 16 |
| Male, % | 58.0 |
| Apache II (mean ± SD) | 21.7 ± 7.3 |
| Shock, % | 44.1 |
| Mechanical ventilation, % | 77.5 |
| 60-d mortality, % | 31.0 |
| Source of infection, % | |
| Lung | 49.7 |
| Urinary tract | 9.7 |
| Abdomen | 9.7 |
| Blood | 5.2 |
| Other | 25.7 |
TABLE 2.
CLINICAL CHARACTERISTICS BY IRAK-1 HAPLOTYPE
| IRAK-1 Haplotype | Variant | Heterozygous | Wild Type |
|---|---|---|---|
| N (%) | 21 (14) | 12 (8) | 122 (78) |
| Age, yr (mean ± SD) | 53 ± 14.7 | 45 ± 20 | 52.5 ± 16.5 |
| Male, % | 80.0 | 0.0 | 60.0 |
| Cancer, % | 19.0 | 0.0 | 22.4 |
| End-stage liver failure, % | 14.3 | 18.0 | 10.4 |
| Chronic obstructive pulmonary disease, % | 9.5 | 9.0 | 8.0 |
| Alcohol abuse, % | 19.0 | 18.0 | 15.2 |
| Diabetes, % | 19.0 | 27.0 | 16.0 |
| Immunosuppression, % | 23.8 | 27.0 | 23.2 |
| Congestive heart failure, % | 4.8 | 0.0 | 8.8 |
| HIV, % | 0.0 | 0.0 | 0.0 |
IRAK1 Variant Haplotype Is Associated with an Increase in Nuclear Levels of NF-κB in LPS-Stimulated Peripheral Blood Neutrophils from Patients with Sepsis
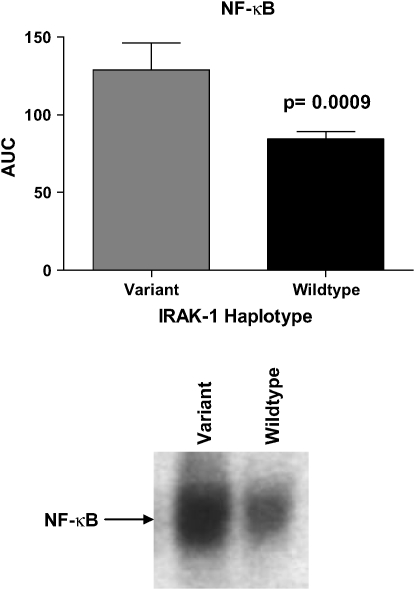
To determine if the IRAK-1 variant haplotype affects NF-κB activation, nuclear translocation of NF-κB was determined in peripheral blood neutrophils from a subset of the patients with sepsis (n = 30) after stimulation with LPS. Neutrophils from patients with the IRAK-1 variant haplotype had significantly increased nuclear accumulation of NF-κB during the 60 min after exposure to LPS (p = 0.0009 compared with wild type) (Figure 1). There were no statistically significant differences with respect to baseline levels of NF-κB between neutrophils from ALI patients with the variant IRAK-1 haplotype and those with the wild-type haplotype. Because the administration of immunosuppressive agents such as corticosteroids can affect NF-κB activation, we examined the use of corticosteroids in the wild-type or heterozygous groups. Fifty-seven percent of the patients with the variant IRAK-1 haplotype received steroid therapy, compared with 39% with the wild-type haplotype (p = 0.22).
Figure 1.
Relationship between nuclear levels of NF-κB and IRAK-1 haplotype. Peripheral blood neutrophils from patients with sepsis were stimulated with LPS, and the area under the curve (AUC) was calculated using nuclear translocation of NF-κB over the 60 min after addition of LPS to the neutrophil cultures. Nuclear levels of NF-κB were significantly increased (p = 0.0009) in patients with the variant haplotype (n = 7) when compared with those with the IRAK-1 wild type haplotype (n = 23). A representative electrophoretic mobility shift assay gel showing nuclear NF-κB translocation at 60 min after LPS stimulation is presented.
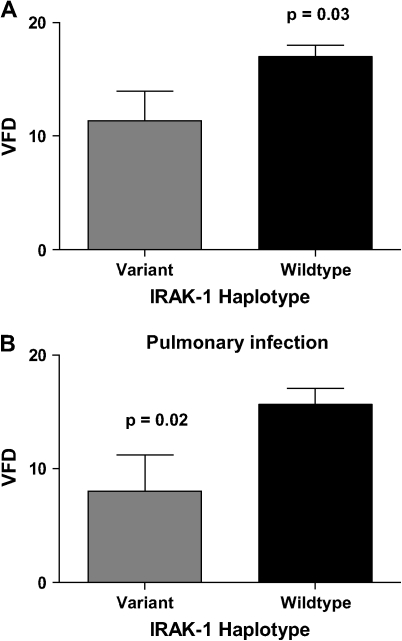
IRAK1 Variant Haplotype Is Associated with a Decrease in Ventilator-Free Days
We next examined the relationship between the IRAK-1 variant haplotype and ventilator-free days (VFD) in 155 patients with sepsis. VFD is a measure of the time that a patient requires mechanical ventilation, with fewer VFD indicating more time on the ventilator. Patients with the variant IRAK-1 haplotype had significantly fewer ventilator free days when compared with wild type (mean ± SD VFD: variant haplotype 11.4 ± 11.8, heterozygous haplotype 17 ± 12, and wild type 17 ± 10.9, p = 0.03) (Figure 2). Patients with variant IRAK-1 haplotype were 2.7 times more likely to spend 14 d or more on the ventilator compared with wild type (p = 0.04) (95% CI, 1.05–6.9). There was no significant difference in VFD between female subjects with the heterozygous haplotype as compared with wild type (p = 0.83).
Figure 2.
Relationship between ventilator free days (VFD) and IRAK-1 haplotype. (A) Patients with the variant haplotype had significantly fewer VFD (mean ± SD) when compared with wild-type patients. (B) Analysis of patients with pulmonary infections demonstrated a significant decrease in VFDs (mean ± SD) in patients with the variant haplotype when compared with wild-type patients.
In a univariate analysis considering clinical risk factors (chronic obstructive pulmonary disease, cancer, alcohol abuse, end-stage liver failure, immunosuppression, diabetes, congestive heart failure, and anemia), sex, and site of infection, no association was found between the clinical risk factors tested or sex with VFD (data not shown). Multivariate logistic regression analysis, in which haplotype and site of infection (lung: yes/no) was incorporated in the model, demonstrated a significant difference between VFD and the variant haplotype or wild type (p = 0.05) (OR, 2.6; 95% CI, 1.0–6.6). The value for the goodness-of-fit test was p = 0.75 for the model.
Subanalysis of all patients with pulmonary infections, who comprised ∼ 50% of the study population, revealed a significant difference with respect to time on the ventilator between haplotypes. VFD were significantly decreased in patients with pneumonia and variant IRAK-1 haplotype compared with wild type (p = 0.02) (Figure 2B). No association was found between the heterozygous haplotype and VFD.
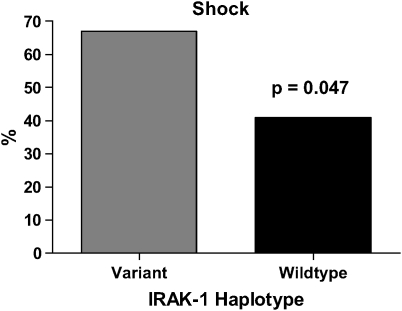
IRAK1 Variant Haplotype Is Associated with an Increased Frequency of Shock in Septic Patients
Sixty-seven percent of septic patients with the variant IRAK-1 haplotype developed fluid unresponsive hypotension (shock), compared with 41% in the wild-type group (Figure 3A). Among patients with the heterozygous haplotype, 27% became hypotensive. Patients with variant IRAK-1 haplotype were 2.9 times more likely to develop shock when compared with wild-type patients (p = 0.047) (95% CI, 1.1–7.7). A univariate chi-square analysis of clinical risk factors, sex, and site of infection found that immunosuppression was the only risk factor significantly associated with shock (p = 0.009).
Figure 3.
Relationship between IRAK-1 haplotype and septic shock. A higher proportion of patients with the variant haplotype developed septic shock as compared with those with the wild-type haplotype.
Multivariate logistic regression analysis, in which haplotype (wild-type and variant haplotype) and immunosuppression were incorporated in the model revealed that the variant haplotype (p = 0.04) (OR, 2.9; 95% CI, 1.06–7.7) and immunosuppression (p = 0.01) (OR, 2.98; 95% CI, 1.3–6.8) were risk factors for shock. The goodness-of-fit test was p = 0.53 for the model. Among patients with pulmonary infections, those with the variant haplotype were 2.2 times more likely to develop shock compared with wild type. This difference was not statistically significant (p = 0.24) (95% CI, 0.64–7.5).
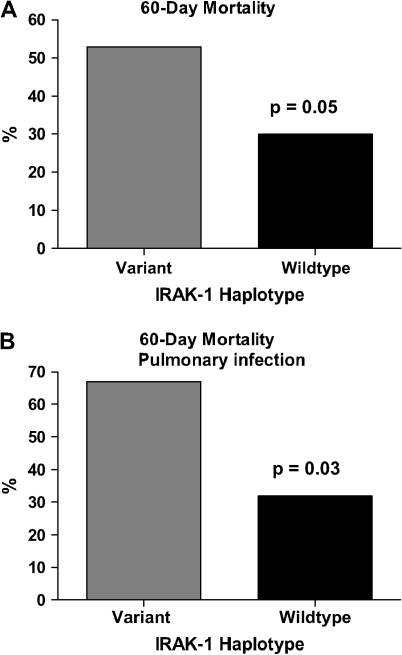
IRAK1 Variant Haplotype Is Associated with Increased Mortality in Patients with Sepsis
Patients with the variant IRAK-1 haplotype had higher 60-d mortality compared with wild-type patients (53% versus 30%; p = 0.05) (Figure 4A). Patients with variant haplotype were 2.6 times more likely to die from sepsis when compared with wild type (p = 0.05) (95% CI, 1.0–6.8). Analysis of patients with pulmonary infections revealed a significant difference between the variant haplotype versus wild type in terms of 60-d mortality (p = 0.03) (OR, 4.2; 95% CI, 1.13–15.8) (Figure 4B).
Figure 4.
Relationship between IRAK-1 haplotype and 60-d mortality. (A) Mortality was increased in patients with the variant haplotype compared with those with the wild-type haplotype. (B) Patients with the variant IRAK-1 haplotype with pulmonary infections demonstrated increased mortality compared with those with the wild-type haplotype.
There was no significant difference (p = 0.14) in 60-d mortality in female patients with the heterozygous haplotype (9%) as compared with the wild-type group. A univariate chi-square analysis on all clinical risk factors and sex found no association between any of the clinical risk factors, sex, and 60-d mortality.
DISCUSSION
In this study, the IRAK-1 variant haplotype, consisting of five intron SNPs and three exon SNPs, was found to be associated with increased nuclear levels of NF-κB in LPS-stimulated peripheral blood neutrophils from patients with sepsis. This IRAK-1 variant haplotype also was associated with more severe illness, as characterized by an increased incidence of shock, a more prolonged requirement for mechanical ventilatory support, and greater 60-d mortality. Because the IRAK-1 variant haplotype is a common X-linked genotype, occurring in 14% of the patients examined in this clinical series, its presence seems to cause a relatively frequent predisposition to a more severe clinical course in patients with sepsis-induced ALI.
IRAK-1 is a key kinase that plays a critical role in inducing cellular activation in gram-positive or gram-negative infections after engagement of TLR2 or TLR4 (35, 36). IRAK-1 is involved in signaling through both of these TLR to downstream targets, including AP-1, Stat-3, and NF-κB, which are all involved in amplifying the host inflammatory response to infection (12). In IRAK-1 knockout studies, impaired cytokine secretion occurs and results in an increased susceptibility to infection (35, 36). In contrast, our results indicate that the IRAK-1 variant haplotype is associated with a gain of function associated with increased nuclear levels of NF-κB in neutrophils stimulated through TLR4.
In experimental models of sepsis-induced ALI, large numbers of neutrophils accumulate in the lungs (43–47). These pulmonary neutrophils demonstrate increased nuclear accumulation of NF-κB and produce increased amounts of proinflammatory cytokines, such as TNF-α and IL-1β, and other proinflammatory mediators whose transcription is regulated by NF-κB. Inhibition of NF-κB diminishes the severity of ALI, indicating that activation of this transcriptional factor plays a central role in mediating the acute inflammatory response characteristic of ALI (41, 48–50). Because of the important contribution that NF-κB seems to make in the development and perpetuation of sepsis-induced ALI and in other organ dysfunctions associated with overwhelming infection, genetic alterations, such as the IRAK-1 variant haplotype, that lead to enhanced nuclear translocation of NF-κB, would be postulated to be associated with a more severe clinical course. The present results support this hypothesis.
There are several possible mechanisms through which the IRAK-1 variant haplotype could lead to an increase in NF-κB translocation to the nucleus. IRAK-1 is rapidly phosphorylated, polyubiquitinated, and degraded upon interaction of TIRs with their ligands (12). The two exon SNPs within this haplotype that lead to amino acid changes at positions 196 and 532, respectively, could individually or together result in a variant of IRAK-1 with greater kinase activity or a form of IRAK-1 that is more stable and resistant to proteosomal degradation. These nonsynomous SNPs are located in the functionally active kinase domain of IRAK-1. In addition, a splice site exists in exon 12 of IRAK-1, approximately 48 base pairs upstream of the nucleotide 1,595 T→C change that leads to the IRAK-1 532 polymorphism (51). An alternatively spliced variant of IRAK-1, IRAK-1b, although functionally inactive in terms of kinase function, is more stable than the IRAK-1a isoform and still able to enhance nuclear translocation of NF-κB induced through TIR engagement (51). It is possible that the 1,595 T→C transition examined in the present study may enhance splicing of IRAK-1 to generate higher levels of its more stable isoform, IRAK-1b, thereby leading to more sustained activation of NF-κB. Additionally, it is possible that intron SNPs within the variant IRAK-1 haplotype may also play a role in potentiating alternative splicing to the more stable IRAK-1b variant. Three intron SNPs within the variant IRAK-1 haplotype are located within the region where alternative splicing of IRAK-1 occurs. Further investigations are necessary to determine if generation of stable splice variants or other mechanisms are responsible for the potentiating effects of the IRAK-1 variant haplotype on NF-κB nuclear translocation.
In this study, patients with the variant haplotype of IRAK-1 were 2.7 times more likely to be on the ventilator for more than 14 d, as compared with individuals with the wild-type haplotype. Because the IRAK-1 haplotypes are located on the X chromosome, a greater percentage of men than women are functionally homozygous. Previous studies (52–55) have found that ALI is more common in men than women and is associated with a worse outcome in men. We therefore performed multivariate analyses to determine the relative importance of sex, as compared with the IRAK-1 variant haplotype, in contributing to clinical course. This analysis demonstrated that the effects of the IRAK-1 variant haplotype on VFDs were independent of sex and other predisposing factors, such as underlying chronic medical conditions.
Among the patients with the IRAK-1 variant haplotype, 67% developed septic shock, as compared with 47% in the wild-type group. Patients homozygous for the variant haplotype were 2.9 times more likely to develop shock when compared with wild–type patients. Several other studies have shown that genetic polymorphisms, such as −308 TNF-α promoter polymorphism or TLR4 Asp299Gly, are independent risk factors of shock (56, 57). Here we show the possibility that a variant haplotype is associated with increased nuclear translocation of NF-κB, an event likely to lead to an excessive inflammatory response, has similar deleterious effects.
Patients with the IRAK-1 variant haplotype were found to have a greater 60-d mortality rate than those lacking this variant haplotype (53% versus 30%, respectively). In addition, patients with the IRAK-1 variant haplotype and pulmonary infections as their initiating factor for ALI had an even greater risk of mortality (67% versus 32% in the wild-type group). Patients with pneumonia and the IRAK-1 variant haplotype also had a higher risk of being on the ventilator for longer periods of time, suggesting that pulmonary infection may interact with this genetic trait as a contributory risk factor for poor outcome in ALI. Preexistent comorbidities, sex, and age were not independently associated with mortality. The potentiating interaction between the IRAK-1 variant haplotype and pneumonia may reflect the high concentration of bacterial antigens that are present in the lungs in this setting, resulting in enhanced TLR2 or TLR4 associated NF-κB translocation in pulmonary neutrophils.
There has been only one previous study that evaluated associations between IRAK-1 polymorphisms and pathophysiology in humans. Ishida and colleagues (37) demonstrated that an IRAK-1 haplotype including 532Ser and 196Phe was associated with decreased radial bone density.
IRAK-1 is located on the X chromosome at position q28 (58). Therefore, men are more likely than women to demonstrate the functional effects of the IRAK-1 variant haplotype, with increased risk of developing sepsis-associated multiple organ system dysfunction and death. Homozygous women would have similar risks, although the frequency of homozygosity in woman is considerably less. In this study, we were unable to demonstrate any significantly increased adverse clinical consequences in heterozygous female patients, suggesting that the effects of the IRAK-1 haplotype are not dominant.
The present findings may have important implications in our understanding of the pathophysiology of sepsis. Although previous studies (8–10) have shown that increased nuclear translocation of NF-κB in neutrophils or other cell populations, such as peripheral blood mononuclear cells, was associated with higher mortality and worse clinical outcome in sepsis, it was unclear if these alterations were antecedent to the development of organ dysfunction and contributed to clinical outcome or whether they reflected greater severity of illness. The present study indicates that a genetic alteration affecting a crucial early component in the intracellular pathway that leads to NF-κB activation is functionally significant in modulating TLR4-induced nuclear translocation of NF-κB and is associated with a more severe clinical course and higher mortality from sepsis-induced ALI. These results therefore support the hypothesis that enhanced NF-κB activation directly contributes to the extent of organ dysfunction in ALI associated with severe infection. Early identification of the IRAK-1 variant haplotype in patients with clinical evidence of infection may not only characterize a population at increased risk for organ dysfunction and death but also may be of use in selecting patients who are likely to demonstrate greater benefit from antiinflammatory therapies directed specifically at NF-κB or at the expression of NF-κB dependent genes, such as TNF-α or IL-8.
Supplementary Material
Supported by NIH grant P01 HL 68743.
This manuscript has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.com
Originally Published in Press as DOI: 10.1164/rccm.200603-341OC on March 30, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 2003;348:138–150. [DOI] [PubMed] [Google Scholar]
- 2.Dunn DL. Gram-negative bacterial sepsis and sepsis syndrome. Surg Clin North Am 1994;74:621–635. [PubMed] [Google Scholar]
- 3.Guillou PJ. Biological variation in the development of sepsis after surgery or trauma. Lancet 1993;342:217–220. [DOI] [PubMed] [Google Scholar]
- 4.Natanson C, Hoffman WD, Suffredini AF, Eichacker PQ, Danner RL. Selected treatment strategies for septic shock based on proposed mechanisms of pathogenesis. Ann Intern Med 1994;120:771–783. [DOI] [PubMed] [Google Scholar]
- 5.Parrillo JE. Pathogenetic mechanisms of septic shock. N Engl J Med 1993;328:1471–1477. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz MD, Moore EE, Moore FA, Shenkar R, Moine P, Haenel JB, Abraham E. Nuclear factor-kappa B is activated in alveolar macrophages from patients with acute respiratory distress syndrome. Crit Care Med 1996;24:1285–1292. [DOI] [PubMed] [Google Scholar]
- 7.Moine P, McIntyre R, Schwartz MD, Kaneko D, Shenkar R, Le Tulzo Y, Moore EE, Abraham E. NF-kappaB regulatory mechanisms in alveolar macrophages from patients with acute respiratory distress syndrome. Shock 2000;13:85–91. [DOI] [PubMed] [Google Scholar]
- 8.Arnalich F, Garcia-Palomero E, Lopez J, Jimenez M, Madero R, Renart J, Vazquez JJ, Montiel C. Predictive value of nuclear factor kappaB activity and plasma cytokine levels in patients with sepsis. Infect Immun 2000;68:1942–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohrer H, Qiu F, Zimmermann T, Zhang Y, Jllmer T, Mannel D, Bottiger BW, Stern DM, Waldherr R, Saeger HD, et al. Role of NFkappaB in the mortality of sepsis. J Clin Invest 1997;100:972–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang KY, Arcaroli JJ, Abraham E. Early alterations in neutrophil activation are associated with outcome in acute lung injury. Am J Respir Crit Care Med 2003;167:1567–1574. [DOI] [PubMed] [Google Scholar]
- 11.Janssens S, Beyaert R. Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Mol Cell 2003;11:293–302. [DOI] [PubMed] [Google Scholar]
- 12.Dunne A, O'Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci STKE 2003;2003:re3. [DOI] [PubMed] [Google Scholar]
- 13.Li S, Strelow A, Fontana EJ, Wesche H. IRAK-4: a novel member of the IRAK family with the properties of an IRAK-kinase. Proc Natl Acad Sci USA 2002;99:5567–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, Modlin RL, Akira S. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol 2002;169:10–14. [DOI] [PubMed] [Google Scholar]
- 15.Horng T, Barton GM, Flavell RA, Medzhitov R. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature 2002;420:329–333. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto M, Sato S, Hemmi H, Sanjo H, Uematsu S, Kaisho T, Hoshino K, Takeuchi O, Kobayashi M, Fujita T, et al. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature 2002;420:324–329. [DOI] [PubMed] [Google Scholar]
- 17.Zhang G, Ghosh S. Negative regulation of toll-like receptor-mediated signaling by Tollip. J Biol Chem 2002;277:7059–7065. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi K, Hernandez LD, Galan JE, Janeway CA Jr, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell 2002;110:191–202. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Z, Johnson HJ, Nie H, Qin J, Bird TA, Li X. Pellino 1 is required for interleukin-1 (IL-1)-mediated signaling through its interaction with the IL-1 receptor-associated kinase 4 (IRAK4)-IRAK-tumor necrosis factor receptor-associated factor 6 (TRAF6) complex. J Biol Chem 2003;278:10952–10956. [DOI] [PubMed] [Google Scholar]
- 20.Brockman JA, Scherer DC, McKinsey TA, Hall SM, Qi X, Lee WY, Ballard DW. Coupling of a signal response domain in I kappa B alpha to multiple pathways for NF-kappa B activation. Mol Cell Biol 1995;15:2809–2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science 1995;267:1485–1488. [DOI] [PubMed] [Google Scholar]
- 22.DiDonato JA, Mercurio F, Karin M. Phosphorylation of I kappa B alpha precedes but is not sufficient for its dissociation from NF-kappa B. Mol Cell Biol 1995;15:1302–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DiDonato J, Mercurio F, Rosette C, Wu-Li J, Suyang H, Ghosh S, Karin M. Mapping of the inducible IkappaB phosphorylation sites that signal its ubiquitination and degradation. Mol Cell Biol 1996;16:1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Traenckner EB, Pahl HL, Henkel T, Schmidt KN, Wilk S, Baeuerle PA. Phosphorylation of human I kappa B-alpha on serines 32 and 36 controls I kappa B-alpha proteolysis and NF-kappa B activation in response to diverse stimuli. EMBO J 1995;14:2876–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whiteside ST, Ernst MK, LeBail O, Laurent-Winter C, Rice N, Israel A. N- and C-terminal sequences control degradation of MAD3/I kappa B alpha in response to inducers of NF-kappa B activity. Mol Cell Biol 1995;15:5339–5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Z, Hagler J, Palombella VJ, Melandri F, Scherer D, Ballard D, Maniatis T. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev 1995;9:1586–1597. [DOI] [PubMed] [Google Scholar]
- 27.Scherer DC, Brockman JA, Chen Z, Maniatis T, Ballard DW. Signal-induced degradation of I kappa B alpha requires site-specific ubiquitination. Proc Natl Acad Sci USA 1995;92:11259–11263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baldi L, Brown K, Franzoso G, Siebenlist U. Critical role for lysines 21 and 22 in signal-induced, ubiquitin-mediated proteolysis of I kappa B-alpha. J Biol Chem 1996;271:376–379. [DOI] [PubMed] [Google Scholar]
- 29.Bol G, Kreuzer OJ, Brigelius-Flohe R. Translocation of the interleukin-1 receptor-associated kinase-1 (IRAK-1) into the nucleus. FEBS Lett 2000;477:73–78. [DOI] [PubMed] [Google Scholar]
- 30.Huang Y, Li T, Sane DC, Li L. IRAK1 serves as a novel regulator essential for lipopolysaccharide-induced interleukin-10 gene expression. J Biol Chem 2004;279:51697–51703. [DOI] [PubMed] [Google Scholar]
- 31.Abraham E. NF-kappaB activation. Crit Care Med 2000;28:N100–N104. [DOI] [PubMed] [Google Scholar]
- 32.Baldwin AS Jr. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol 1996;14:649–683. [DOI] [PubMed] [Google Scholar]
- 33.Verma IM, Stevenson JK, Schwarz EM, Van Antwerp D, Miyamoto S. Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes Dev 1995;9:2723–2735. [DOI] [PubMed] [Google Scholar]
- 34.Kunsch C, Rosen CA. NF-kappa B subunit-specific regulation of the interleukin-8 promoter. Mol Cell Biol 1993;13:6137–6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swantek JL, Tsen MF, Cobb MH, Thomas JA. IL-1 receptor-associated kinase modulates host responsiveness to endotoxin. J Immunol 2000;164:4301–4306. [DOI] [PubMed] [Google Scholar]
- 36.Verdrengh M, Thomas JA, Hultgren OH. IL-1 receptor-associated kinase 1 mediates protection against Staphylococcus aureus infection. Microbes Infect 2004;6:1268–1272. [DOI] [PubMed] [Google Scholar]
- 37.Ishida R, Emi M, Ezura Y, Iwasaki H, Yoshida H, Suzuki T, Hosoi T, Inoue S, Shiraki M, Ito H, et al. Association of a haplotype (196Phe/532Ser) in the interleukin-1-receptor-associated kinase (IRAK1) gene with low radial bone mineral density in two independent populations. J Bone Miner Res 2003;18:419–423. [DOI] [PubMed] [Google Scholar]
- 38.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301–1308. [DOI] [PubMed] [Google Scholar]
- 39.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818–824. [DOI] [PubMed] [Google Scholar]
- 40.Nick JA, Avdi NJ, Young SK, Lehman LA, McDonald PP, Frasch SC, Billstrom MA, Henson PM, Johnson GL, Worthen GS. Selective activation and functional significance of p38alpha mitogen-activated protein kinase in lipopolysaccharide-stimulated neutrophils. J Clin Invest 1999;103:851–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abraham E, Arcaroli J, Shenkar R. Activation of extracellular signal-regulated kinases, NF-kappa B, and cyclic adenosine 5′-monophosphate response element-binding protein in lung neutrophils occurs by differing mechanisms after hemorrhage or endotoxemia. J Immunol 2001;166:522–530. [DOI] [PubMed] [Google Scholar]
- 42.Yum HK, Arcaroli J, Kupfner J, Shenkar R, Penninger JM, Sasaki T, Yang KY, Park JS, Abraham E. Involvement of phosphoinositide 3-kinases in neutrophil activation and the development of acute lung injury. J Immunol 2001;167:6601–6608. [DOI] [PubMed] [Google Scholar]
- 43.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334–1349. [DOI] [PubMed] [Google Scholar]
- 44.Chollet-Martin S, Jourdain B, Gibert C, Elbim C, Chastre J, Gougerot-Pocidalo MA. Interactions between neutrophils and cytokines in blood and alveolar spaces during ARDS. Am J Respir Crit Care Med 1996;154:594–601. [DOI] [PubMed] [Google Scholar]
- 45.Goodman RB, Strieter RM, Martin DP, Steinberg KP, Milberg JA, Maunder RJ, Kunkel SL, Walz A, Hudson LD, Martin TR. Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am J Respir Crit Care Med 1996;154:602–611. [DOI] [PubMed] [Google Scholar]
- 46.Suter PM, Suter S, Girardin E, Roux-Lombard P, Grau GE, Dayer JM. High bronchoalveolar levels of tumor necrosis factor and its inhibitors, interleukin-1, interferon, and elastase, in patients with adult respiratory distress syndrome after trauma, shock, or sepsis. Am Rev Respir Dis 1992;145:1016–1022. [DOI] [PubMed] [Google Scholar]
- 47.Kollef MH, Schuster DP. The acute respiratory distress syndrome. N Engl J Med 1995;332:27–37. [DOI] [PubMed] [Google Scholar]
- 48.Abraham E, Carmody A, Shenkar R, Arcaroli J. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 2000;279:L1137–L1145. [DOI] [PubMed] [Google Scholar]
- 49.Blackwell TS, Blackwell TR, Holden EP, Christman BW, Christman JW. In vivo antioxidant treatment suppresses nuclear factor-kappa B activation and neutrophilic lung inflammation. J Immunol 1996;157:1630–1637. [PubMed] [Google Scholar]
- 50.Liu SF, Ye X, Malik AB. In vivo inhibition of nuclear factor-kappa B activation prevents inducible nitric oxide synthase expression and systemic hypotension in a rat model of septic shock. J Immunol 1997;159:3976–3983. [PubMed] [Google Scholar]
- 51.Jensen LE, Whitehead AS. IRAK1b, a novel alternative splice variant of interleukin-1 receptor-associated kinase (IRAK), mediates interleukin-1 signaling and has prolonged stability. J Biol Chem 2001;276:29037–29044. [DOI] [PubMed] [Google Scholar]
- 52.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29:1303–1310. [DOI] [PubMed] [Google Scholar]
- 53.Moss M, Mannino DM. Race and gender differences in acute respiratory distress syndrome deaths in the United States: an analysis of multiple-cause mortality data (1979- 1996). Crit Care Med 2002;30:1679–1685. [DOI] [PubMed] [Google Scholar]
- 54.Schroder J, Kahlke V, Staubach KH, Zabel P, Stuber F. Gender differences in human sepsis. Arch Surg 1998;133:1200–1205. [DOI] [PubMed] [Google Scholar]
- 55.Wichmann MW, Inthorn D, Andress HJ, Schildberg FW. Incidence and mortality of severe sepsis in surgical intensive care patients: the influence of patient gender on disease process and outcome. Intensive Care Med 2000;26:167–172. [DOI] [PubMed] [Google Scholar]
- 56.Mira JP, Cariou A, Grall F, Delclaux C, Losser MR, Heshmati F, Cheval C, Monchi M, Teboul JL, Riche F, et al. Association of TNF2, a TNF-alpha promoter polymorphism, with septic shock susceptibility and mortality: a multicenter study. JAMA 1999;282:561–568. [DOI] [PubMed] [Google Scholar]
- 57.Lorenz E, Mira JP, Frees KL, Schwartz DA. Relevance of mutations in the TLR4 receptor in patients with gram-negative septic shock. Arch Intern Med 2002;162:1028–1032. [DOI] [PubMed] [Google Scholar]
- 58.Thomas JA, Allen JL, Tsen M, Dubnicoff T, Danao J, Liao XC, Cao Z, Wasserman SA. Impaired cytokine signaling in mice lacking the IL-1 receptor-associated kinase. J Immunol 1999;163:978–984. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.