Abstract
Rationale: The susceptibility of neonates to pulmonary and systemic infection has been associated with the immaturity of both lung structure and the immune system. Surfactant protein (SP) D is a member of the collectin family of innate immune molecules that plays an important role in innate host defense of the lung.
Objectives: We tested whether treatment with recombinant human SP-D influenced the response of the lung and systemic circulation to intratracheally administered Escherichia coli lipopolysaccharides.
Methods: After intratracheal lipopolysaccharide instillation, preterm newborn lambs were treated with surfactant and ventilated for 5 h.
Measurement: Survival rate, physiologic lung function, lung and systemic inflammation, and endotoxin level in plasma were evaluated.
Main Results: In control lambs, intratracheal lipopolysaccharides caused septic shock and death associated with increased endotoxin in plasma. In contrast, all lambs treated with recombinant human SP-D were physiologically stable and survived. Leakage of lipopolysaccharides from the lungs to the systemic circulation was prevented by intratracheal recombinant human SP-D. Recombinant human SP-D prevented systemic inflammation and decreased the expression of IL-1β, IL-8, and IL-6 in the spleen and liver. Likewise, recombinant human SP-D decreased IL-1β and IL-6 in the lung and IL-8 in the plasma. Recombinant human SP-D did not alter pulmonary mechanics following endotoxin exposure. Recombinant human SP-D was readily detected in the lung 5 h after intratracheal instillation.
Conclusions: Intratracheal recombinant human SP-D prevented shock caused by endotoxin released from the lung during ventilation in the premature newborn.
Keywords: cytokines, lung compliance, pulmonary surfactant, respiratory distress syndrome, sepsis
Low-birth-weight infants (< 1,500 g) frequently experience serious systemic infections (1) and septicemia-related shock that are common through exposure to chorioamnionitis in utero and pulmonary infections after birth (2, 3). Because of its immaturity, the preterm newborn lung is highly permeable, allowing the leak of proteins, organisms, toxins, and mediators from the lung into the systemic circulation (4–6). Neonatal sepsis syndrome, associated with pneumonia and chorioamnionitis, is a common cause of neonatal morbidity and mortality in both term and preterm infants (1, 7, 8). In previous studies, systemic inflammation was caused by the leak of intratracheal lipopolysaccharides (LPS) into the systemic circulation in premature newborn lambs (9). The susceptibility of neonates to pulmonary and systemic infection has been associated with the immaturity of both their lung structure and immune system. The lungs of preterm infants are deficient in pulmonary surfactant and innate host-defense proteins, including surfactant proteins (SP) A and D (10–12). Surfactant replacement preparations used for respiratory distress in neonates contain SP-B and SP-C but do not contain SP-A, SP-D, or other innate host-defense proteins. Pulmonary collectins play an important role in protection of the lung from viral, bacterial, and fungal pathogens. Both SP-A and SP-D have antimicrobial and antiinflammatory activities (10, 13). Decreased levels of SP-A and SP-D associated with lung inflammation in models of bronchopulmonary dysplasia (12) and in children with cystic fibrosis (14–16) may influence the pathogenesis of disease.
SP-D is a multimeric, collagenous lectin belonging to the collectin family of innate immune proteins. SP-D binds in a calcium-dependent manner to the complex carbohydrates and lipids that serve as pattern-recognition molecules on the surface of microbial pathogens. SP-D binds to and aggregates a wide range of microbial pathogens, including bacteria, viruses, and fungi (17–20). SP-D directly binds to bacterial components such as LPS (13). Mice lacking SP-D (Sftpd-/- mice) are highly susceptible to pulmonary infection and inflammation (21, 22). SP-D binds to the surface of Escherichia coli via its C-terminal-lectin–like domain. Because E. coli is a common pulmonary organism causing both pulmonary and systemic disease in neonates (8), we tested whether recombinant human SP-D (rhSP-D) might influence the response of the lung and systemic circulation to intratracheally administered endotoxin in the ventilated preterm newborn lamb.
METHODS
Protocols were approved by the Animal Care and Use Committee of the Cincinnati Children's Hospital Research Foundation.
rhSP-D
rhSP-D was synthesized by transfection of Chinese hamster ovary cells with a cDNA encoding full length human SP-D. For detail on rhSP-D synthesization, see online supplement.
Administration of SP-D to the Ventilated Premature Lamb after LPS Exposure
All animals were delivered by Cesarean section at 130 d gestation age from Suffolk ewes bred to Dorset rams (term 150 d GA) as previously described (9, 23). After exposure of the fetal head and neck, an endotracheal tube was tied into the trachea. The fetal lung fluid that could be easily aspirated by syringe was recovered, and the lambs were delivered and weighed. Before the first breath the lambs received 0.1 mg/kg E. coli LPS (E. coli 055:B5; Sigma, St. Louis, MO) mixed with 1 ml (25 mg) Survanta (Ross Products Division, Abbott Laboratories, Columbus, OH), followed by 10 ml air given into the airways by syringe. LPS was mixed with small amounts of surfactant and given before the first breath to facilitate uniform distribution of LPS in the lung. During and after the first breath, LPS is then distributed to the peripheral airways. Ten milliliters of air was administered via the trachea after LPS instillation to enhance the clearance of fetal lung fluid and to prevent mixing of LPS with rhSP-D before distribution of LPS to the peripheral airways. To survive, premature lambs at this gestational age require surfactant treatment soon after birth. The endotoxin was instilled with 25 mg of Survanta. The treatment dose of Survanta was adjusted to provide a total of 100 mg/kg. This later dose of Survanta was instilled approximately 30 s after LPS injection via the tracheal tube with either 12 ml of buffer containing 2 mg/kg rhSP-D (treatment group) or with 12 ml buffer only (control group). Personnel administering the rhSP-D did not maintain the animals thereafter. All animals were ventilated for 5 h with time-cycled and pressure-limited infant ventilators (Sechrist Industries, Anaheim, CA) using similar ventilation strategies. A 5F catheter was advanced into the aorta via an umbilical artery and a 10 ml/kg transfusion of filtered fetal blood collected from the placenta was administered within 10 min of delivery to correct low hematocrit associated with prematurity. Blood pressure, heart rate, tidal volume (Vt) (CP-100; Bicore Monitoring Systems, Anaheim, CA), and body temperature were monitored continuously. Blood gas, pH, base excess (BE), hematocrit, potassium, calcium, and glucose levels were analyzed by a blood gas, electrolyte, and metabolite system (Radiometer Copenhagen USA, West Lake, OH) at least every 20 min or when ventilatory status changed as indicated by changes in chest movement and tidal volumes. Rate of 40 breaths/min, inspiratory time of 0.6 s, and positive end-expiratory pressure (PEEP) of 4 cm H2O were not changed. Peak inspiratory pressure (PIP) was changed to maintain Vt at 8–9 ml/kg. Pressure was limited to PIP 35 cm H20 to avoid pneumothorax. Fraction of inspired oxygen was adjusted to keep a target Po2 of 100–150 mm Hg. Ten percent dextrose (100 ml/kg/d) was infused continuously through the arterial catheter. Dynamic compliances were calculated from Vt measured with a pneumotachometer that was normalized to body weight and divided by the ventilatory pressure (PIP-PEEP). Rectal temperature was maintained at the normal body temperature for sheep (38.5oC) with heating pads, radiant heat, and plastic body-covering wrap. Supplemental ketamine (10 mg/kg intramuscularly) and acepromzaine (0.1 mg/kg intramuscularly) were used to suppress spontaneous breathing. After 5 h, each animal was deeply anesthetized with 25 mg/kg pentobarbital intravenously and ventilated briefly with 100% oxygen. The endotracheal tube was clamped for 3 min to permit oxygen absorption to render the lung airless. For the lambs that did not survive the 5-h study period, death was determined by either systolic blood pressure lower than 10 mm Hg or the absence of a heart beat.
Methods for processing of lungs and sample analysis, including endotoxin level, rhSP-D level, protein in bronchoalveolar lavage fluid (BALF), lung inflammation, lung histology, and cytokines were done as described previously (9, 24–27). For additional details on these analyses see the online supplement.
Data Analysis
Results are given as means ± SEM. rhSP-D treatment groups and control groups were compared using two-tailed t tests or analysis of variance with Tukey's test used for post hoc analyses as appropriate. Log-rank tests were used for percentage-of-survival comparison between groups. Significance was accepted at p < 0.05.
RESULTS
rhSP-D Protected Neonatal Lambs from Systemic Effects of Intratracheal Endotoxin
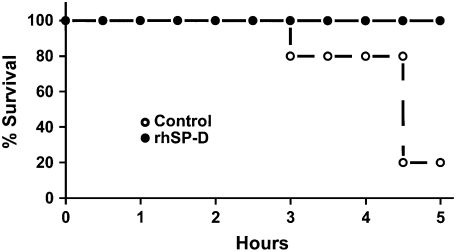
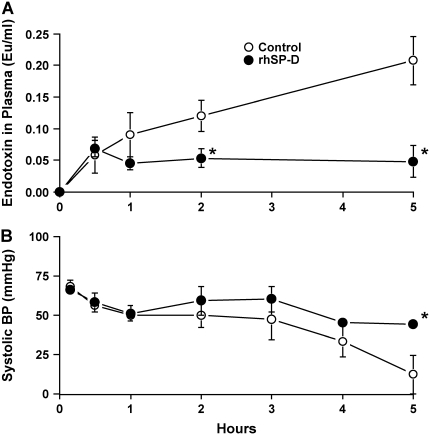
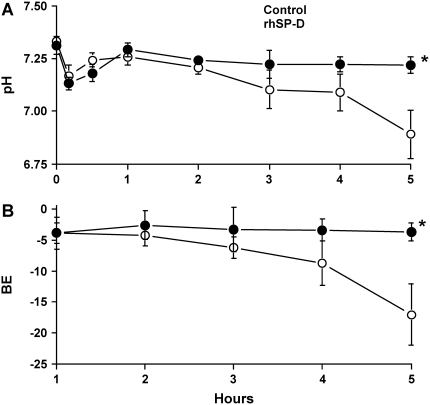
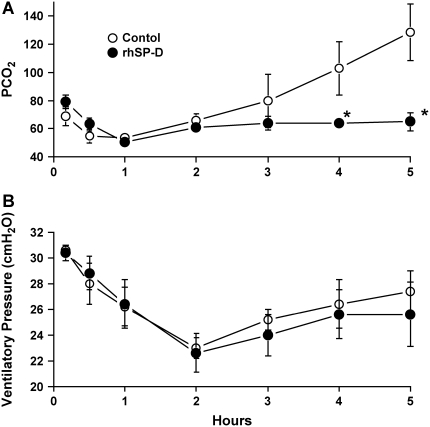
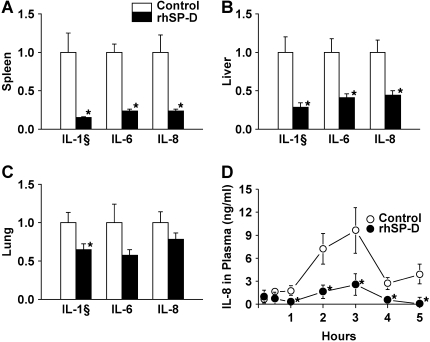
Five lambs were studied in each group. Body weight (control, 3.2 ± 0.3 kg; rhSP-D, 3.0 ± 0.2 kg), cord pH (control, 7.33 ± 0.02; rhSP-D, 7.31 ± 0.04), and sex (three females and two males in both groups) were equally distributed between treated and control groups. In the control group, four of five lambs died before the end of the 5-h study period. In contrast, all lambs treated with rhSP-D survived (Figure 1). When the animals died, the data obtained immediately before death were used for comparison among the groups. Most deaths in the control group occurred between 4 and 5 h. After intratracheal administration, endotoxin was detected in the plasma at 30 min of age in both groups of animals as assessed by Limulus lysate assay (Figure 2A). Plasma endotoxin levels continued to increase in the control lambs but did not increase over the duration of the experiment in the lambs that were treated with rhSP-D. Systolic blood pressures preceding death were similar between groups at 3 h of age and decreased thereafter in controls, but not in rhSP-D treated animals (Figure 2B). Marked systemic effects of LPS were seen after 4 h of age in the control group as indicated by decreased blood pH, BE (Figure 3), and increased Pco2 (Figure 4A). In contrast, blood pH, BE, and Pco2 remained stable throughout the 5 h of experimentation in the rhSP-D–treated animals. Hematocrit, potassium, calcium, and glucose levels were similar for both groups. Po2 was relatively unstable at this gestational age, likely related to patent ductus arteriosis, and was not different between the groups (data not shown). Relative to rhSP-D treated lambs, proinflammatory cytokine mRNAs IL-1β, IL-6, and IL-8 were increased in the spleen and liver of control animals, likely indicating leakage of LPS from the lungs to the systemic circulation in the absence of rhSP-D (Figures 5A and 5B). Splenic and hepatic levels of IL-10 and TNFα mRNAs were low in both groups of animals (data not shown). Plasma IL-8 was significantly increased in the control group after intratracheal delivery of LPS and was significantly lower in rhSP-D treated sheep (Figure 5D). Plasma IL-1β was below the levels of detectability of the assay (< 0.8 pg/ml) in both groups of animals (data not shown).
Figure 1.
Kaplan-Meier plot of recombinant human surfactant protein D (rhSP-D) treated group and control group. In the control group, only 20% of the lambs survived until the end of the 5-h study period. In contrast, all lambs treated with rhSP-D survived. p < 0.05 by log-rank test.
Figure 2.
(A) rhSP-D prevents systemic spread of lipopolysaccharides (LPS) from the lung. Intratracheal endotoxin was detected in circulation and was increased over time in the control group. rhSP-D decreased plasma endotoxin concentration during the 5-h study. (B) Treatment with rhSP-D prevented the endotoxin shock. Systolic blood pressure was maintained at normal level of premature newborn in rhSP-D–treated groups. In contrast, blood pressure gradually decreased in the control group after 3 h of age. *p < 0.05 versus control.
Figure 3.
(A) Blood pH was maintained with rhSP-D treatment. Although LPS treatment associated with decreased blood pH, treatment with rhSP-D maintained pH and prevented prenatal endotoxin-induced shock. (B) Blood base excess (BE) was altered by intratracheal LPS. Intratracheal LPS induced metabolic acidosis and rhSP-D treatment prevented the low BE and endotoxin shock.
Figure 4.
Sequential measurement of Pco2 and ventilatory pressure. (A) Endotracheal LPS caused an increase in Pco2 after 3 h of age. Pco2 was maintained in group treated with rhSP-D. (B) Ventilatory pressure (PIP-PEEP) used to maintain target tidal volume was similar for both groups. *p < 0.05 versus control.
Figure 5.
Proinflammatory cytokine expression. (A, B) IL-1β, IL-6, and IL-8 mRNAs in spleen and liver were increased in control lambs after intratracheal LPS instillation. Proinflammatory cytokine mRNAs in spleen and liver were decreased by rhSP-D. (C) Endotracheal LPS increased IL-1β, Il-6, and IL-8 mRNAs in the lung. Expression of IL-1β was decreased in the group treated with rhSP-D. (D) IL-8 concentrations in plasma were increased in the control group. Plasma IL-8 concentrations were kept low by rhSP-D treatment. *p < 0.05 versus control.
Pulmonary Inflammation and Histology
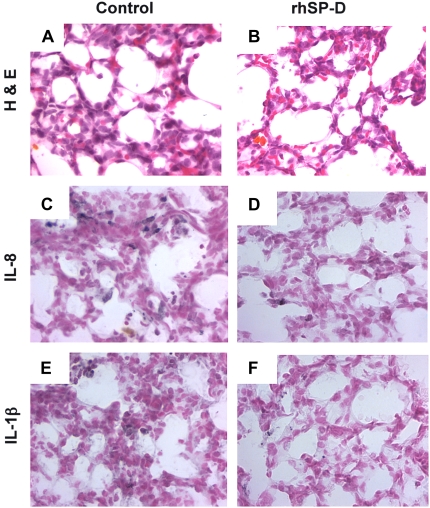
Neutrophil numbers in BALF were similar for both groups (Table 1), but were tenfold higher than previously shown for control animals that did not receive LPS (9). Hydrogen peroxide and total protein in BALF were not different between the two groups. The percent apoptotic cells and percent necrotic cells were also similar in both groups (Table 1). Consistent with the antiinflammatory effect of rhSP-D, proinflammatory cytokine IL-1β mRNA was significantly decreased in the lungs of animals treated with rhSP-D (Figure 5C). The level of IL-1β in the supernatants of lung homogenates decreased from 21.6 ± 3.6 ng/ml in controls to 12.6 ± 1.4 ng/ml after treatment with rhSP-D (p < 0.05). Likewise, rhSP-D decreased IL-6 from 7.7 ± 0.8 ng/ml to 2.3 ± 1.2 ng/ml (p < 0.05). IL-8 was not detectable by ELISA in either control or rhSP-D–treated groups. Pulmonary inflammation was observed in both rhSP-D–treated and control animals (Figures 6A and 6B). Increased immunostaining for IL-8 (Figures 6C and 6D) and IL-1β (Figures 6E and 6F) was observed in both groups of animals, but the extent and intensity of staining for both cytokines was greater in the control group.
TABLE 1.
WHITE BLOOD CELLS, INFLAMMATORY CELLS, AND TOTAL PROTEIN IN BRONCHOALVEOLAR LAVAGE FLUID
| BALF
|
||||||
|---|---|---|---|---|---|---|
| WBC/ | Cells/ | H2O2 | Apoptotic | Necrotic | Protein | |
| μl × 102 | μl × 102 | /106 Cell | (%) | (%) | (mg/kg) | |
| Control | 27 ± 4 | 66 ± 20 | 16 ± 7 | 30 ± 8 | 0.7 ± 0.1 | 67 ± 12 |
| rhSP-D | 30 ± 6 | 96 ± 21 | 8 ± 3 | 35 ± 1 | 0.7 ± 0.2 | 65 ± 12 |
Definition of abbreviations: BALF = bronchoalveolar lavage fluid; rhSP-D = recombinant human surfactant protein D; WBC = white blood cells.
Figure 6.
Lung morphology with hematoxylin and eosin staining (A, B) and immunohistochemistry of IL-8 (C, D) and IL-1β (E, F). In both control and rhSP-D groups there are increased granulocyte and positively stained inflammatory cells for IL-8 and IL-1β. The inflammatory cells immunostained for IL-8 and IL-1β were decreased by intratracheal rhSP-D treatment.
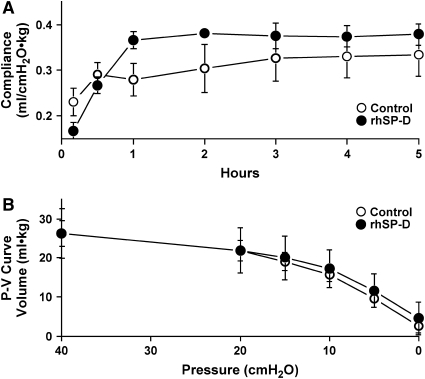
rhSP-D Did Not Alter Pulmonary Mechanics after Endotoxin Exposure
The ventilatory pressure used to maintain target tidal volume was similar in both groups (Figure 4B). Likewise dynamic lung compliance and pressure–volume curves were not altered by rhSP-D treatment (Figure 7). These lung compliances were similar to that for lambs of the same gestation age treated with Survanta without LPS (26, 28).
Figure 7.
Lung function was not affected by rhSP-D treatment. (A) Dynamic lung compliance, calculated from Vt, PIP-PEEP, and body weight during ventilation, and (B) deflation limb of static lung pressure–volume curve were similar.
Presence of rhSP-D in BALF, Lung Tissue, and Plasma
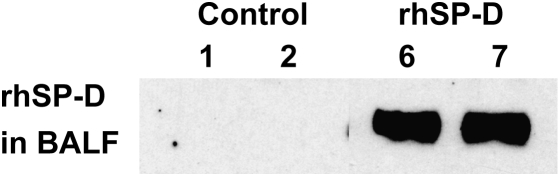
rhSP-D was detected in BALF, lung homogenate, and plasma from the rhSP-D group by ELISA (Table 2) and by immunoblot in BALF (Figure 8). rhSP-D was readily detected in the lung 5 h after intratracheal administration. rhSP-D was also detected in the plasma, indicating its leakage from the lung.
TABLE 2.
rhSP-D LEVEL AT 5 h AFTER TREATMENT*
| BALF | Lung Homogenate | Plasma | |
|---|---|---|---|
| Control | 0 | 0 | 0 |
| rhSP-D | 120 ± 33 | 91 ± 25 | 34 ± 7 |
Definition of abbreviations: BALF = bronchoalveolar lavage fluid; rhSP-D = recombinant human surfactant protein D.
rhSP-D level in ng/ml.
Figure 8.
High levels of rhSP-D were detected in bronchoalveolar lavage fluid (BALF) by Western blot 5 h after endotracheal rhSP-D instillation (animals #6 and 7). BALF from control lambs (animals #1 and 2) did not show any rhSP-D.
DISCUSSION
Infants with congenital or perinatally acquired pneumonia are at high risk of splenic sepsis and death, even when effective antibiotic treatment is given soon after birth (1–3, 7). The high incidence of congenital pneumonia in early onset sepsis suggests that infection is often acquired by aspiration of pathogens in utero or during birth. Chorioamnionitis increases the risk of premature delivery and is strongly associated with neonatal sepsis and septicemia-related shock (7). The preterm newborn lung is highly permeable (5), allowing systemic spread of proinflammatory mediators and organisms from the lung (9). Group B streptococcus and gram-negative bacteria including E. coli are organisms commonly causing congenital pneumonia (29). Systemic spread of microbial toxins and LPS, rather than the actual bacteria, can initiate the cellular and humoral responses resulting in shock (30). Septic shock is a complex pathophysiologic state that often leads to multiple organ dysfunction, multiple organ failure, and death (31). Decreases in blood pH, BE, and increases in Pco2, demonstrated in the control group in the present study, are typical of the clinical course of septic shock in premature infants. Vasoconstriction, pulmonary hypertension, deterioration of organ circulation, and metabolic acidosis frequently implicates the presence of sepsis.
The present study demonstrates that administration of intratracheal rhSP-D protected premature newborn lambs from the systemic effects of intrapulmonary E. coli LPS. Although pulmonary inflammation was not blocked by rhSP-D, the systemic effects of LPS, as indicated by levels of LPS in plasma and evidence of systemic inflammation, were ameliorated by rhSP-D. The finding that rhSP-D ameliorated systemic effects and prevented death after LPS was intratracheally administered supports the concept that rhSP-D binds to LPS and detoxifies or inhibits LPS transit from the pulmonary to the systemic compartment. Similar to findings in premature human newborns, septic shock is also a relatively frequent cause of mortality in adults (32). As in the premature lung, permeability increases after injury and ventilation of the adult lung (33, 34). Thus, rhSP-D represents a potential therapeutic strategy for prevention of the systemic inflammatory response originating from a lung with infection.
SP-D is a multimeric glycoprotein of the collectin family of innate immune molecules, and is secreted by airway epithelial cells. SP-D binds to and promotes the killing of pathogens by pulmonary phagocytes (10, 13, 17–19, 35). Findings in Sftpd-/- mice (21, 22, 36) support the importance of the role of SP-D in lung defense. Sftpd-/- mice are highly susceptible to infection by respiratory syncytial virus (21) and influenza A (22). Most microbial ligands contain mannose or glucose and SP-D is known to bind preferentially to inositol, maltose, mannose, and glucose. Unlike SP-A, SP-D does not bind to the lipid A domain (37) but binds to the contiguous core oligosaccharide of LPS (35). In this study, E. coli LPS was instilled into the lungs of prematurely delivered lambs. Binding of SP-D to E. coli LPS has been demonstrated both in vivo and in vitro (17–19, 35, 38). The rhSP-D used in the present study bound to E. coli 055:B5 LPS in the presence of 5 mM CaCl (data not shown). Premature newborns are deficient in surfactant, including SP-D (11). The commercially available surfactants for treatment of the newborn with respiratory distress syndrome contain SP-B and SP-C, but do not contain SP-A or SP-D. Increased inflammatory responses seen in the premature newborn lung may result from a deficiency in host defenses, including low levels of SP-A and SP-D and a relatively low number of macrophages (10, 12). Fetal inflammation associated with chorioamnionitis and postnatal infection of the lung are associated with the development of chronic lung injury and bronchopulmonary dysplasia (39). Treatment with surfactant containing rhSP-D given at birth represents a potential therapy for prevention or treatment of chronic lung disease in neonates.
Biologically active recombinant human and rat SP-D have been previously produced in vitro (40–42). Full-length recombinant SP-D was utilized in this study. A dose of 2 mg/kg rhSP-D was given to the premature lamb. Similar to the extremely immature newborns, the 130 d GA lamb (term 150 d) is surfactant deficient (43, 44) and requires immediate surfactant treatment and mechanical ventilation to survive. Surfactant pool sizes correlate with body weight and alveolar surface area and are similar between various mammalian species (45). Surfactant pool sizes are highest in newborn animals (46) and decrease with advancing age to adult levels (47). The clinical dose of surfactant used for treatment of the preterm lamb was similar to the surfactant pool size in the normal newborn (48). Likewise, the amount of SP-D normally present in the 2 to 3 d old term newborn lamb lung was 2.4 ± 0.7 mg/kg (n = 3) as asessed by ELISA. SP-D levels relative to Sat PC were tenfold higher in the term newborn than in the adult lung (49). Thus, the dose of rhSP-D used in the present study is similar to the content of SP-D present in the lung of term newborn lambs.
The present study demonstrated that rhSP-D can be safely administered intratracheally to prevent pathogen-induced systemic endotoxin shock in the premature newborn lamb. Such a therapy may be useful in protecting newborns from pulmonary infection and its sequelae.
Supplementary Material
Acknowledgments
The authors thank Maura Unger, Shawn Grant, Michelle Buroker, and Christy Sinclair for expert technical support.
Supported by grant HL63329 from the National Institutes of Health. Survanta was supplied by Ross Products Division, Abbott Laboratories. Genzyme Corporation has taken a short term license to explore the utility of SP-D and has provided partial research support to our laboratory ($100,000) and the license fee to the institution (∼$15,000).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200509-1485OC on March 23, 2006
Conflict of Interest Statement: M.I. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; K.C. is a Genzyme employee and has stock options. K.B. is a full time employee at Genzyme Corporation since November 1988 and has stock options; A.Y. is a full time employee at Genzyme Corporation since November 2004 and has stock options; E.M. is a full time employee at Genzyme Corporation and has stock options; W.B. is an employee at Genzyme Corporation and has stock options; R.K.S. has been a full time employee of Genzyme Corporation since 1992, and as such has received salary and Genzyme stock options as parts of his total compensation package, and, as a result of his work at Genzyme, is co-inventor on at least 12 issued patents and several pending applications; and J.A.W. received a patent using SP-D in treatment of lung disease (US# 6,838,428B2).
References
- 1.Kaufman D, Fairchild KD. Clinical microbiology of bacterial and fungal sepsis in very-low-birth-weight infants. Clin Microbiol Rev 2004;17:638–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med 2000;342:1500–1507. [DOI] [PubMed] [Google Scholar]
- 3.Wenstrom KD, Anderws WW, Hauth JC, Goldenberg RL, DuBard MB, Cliver SP. Elevated second-trimester amniotic fluid interleukin-6 levels predict preterm delivery. Am J Obstet Gynecol 1998;178:546–550. [DOI] [PubMed] [Google Scholar]
- 4.Pringle KC. Human fetal lung development and related animal models. Clin Obstet Gynecol 1986;29:502–513. [PubMed] [Google Scholar]
- 5.Jobe A, Jacobs H, Ikegami M, Berry D. Lung protein leaks in ventilated lambs: effects of gestational age. J Appl Physiol 1985;58:1246–1251. [DOI] [PubMed] [Google Scholar]
- 6.Bland RD, Carlton DP, Scheerer RG, Cummings JJ, Chapman DL. Lung fluid balance in lambs before and after premature birth. J Clin Invest 1989;84:568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dempsey E, Chen MF, Kokottis T, Vallerand D, Usher R. Outcome of neonates less than 30 weeks gestation with histologic chorioamnionitis. Am J Perinatol 2005;22:155–159. [DOI] [PubMed] [Google Scholar]
- 8.Jiang JH, Chiu NC, Huang FY, Kao HA, Hsu CH, Hung HY, Chang JH, Peng CC. Neonatal sepsis in the neonatal intensive care unit: characteristics of early versus late onset. J Microbiol Immunol Infect 2004;37:301–306. [PubMed] [Google Scholar]
- 9.Kramer BW, Ikegami M, Jobe A. Intratracheal endotoxin causes systemic inflammation in ventilated preterm lambs. Am J Respir Crit Care Med 2002;165:463–469. [DOI] [PubMed] [Google Scholar]
- 10.Mason RJ, Greene K, Voelker DR. Surfactant protein A and surfactant protein D in health and disease. Am J Physiol Lung Cell Mol Physiol 1998;275:L1–L13. [DOI] [PubMed] [Google Scholar]
- 11.Miyamura K, Malhotra R, Hoppe HJ, Reid KB, Phizackerley PJ, Macpherson P, Lopez Bernal A. Surfactant proteins A (SP-A) and D (SP-D): levels in human amniotic fluid and localization in the fetal membranes. Biochim Biophys Acta 1994;1210:303–307. [DOI] [PubMed] [Google Scholar]
- 12.Awasthi S, Coalson JJ, Crouch E, Yang F, King RJ. Surfactant proteins A and D in premature baboons with chronic lung injury: evidence for an inhibition of secretion. Am J Respir Crit Care Med 1999;160:942–949. [DOI] [PubMed] [Google Scholar]
- 13.Crouch E, Wright JR. Surfactant proteins a and d and pulmonary host defense. Annu Rev Physiol 2001;63:521–554. [DOI] [PubMed] [Google Scholar]
- 14.Noah TL, Murphy PC, Alink JJ, Leigh MW, Hull WM, Stahlman MT, Whitsett JA. Bronchoalveolar lavage fluid surfactant protein-A and surfactant protein-D are inversely related to inflammation in early cystic fibrosis. Am J Respir Crit Care Med 2003;168:685–691. [DOI] [PubMed] [Google Scholar]
- 15.Postle AD, Mander A, Reid KB, Wang JY, Wright SM, Moustaki M, Warner JO. Deficient hydrophilic lung surfactant proteins A and D with normal surfactant phospholipid molecular species in cystic fibrosis. Am J Respir Cell Mol Biol 1999;20:90–98. [DOI] [PubMed] [Google Scholar]
- 16.von Bredow C, Wiesener A, Griese M. Proteolysis of surfactant protein D by cystic fibrosis relevant proteases. Lung 2003;181:79–88. [DOI] [PubMed] [Google Scholar]
- 17.Kuan SF, Rust K, Crouch E. Interactions of surfactant protein D with bacterial lipopolysaccharides: surfactant protein D is an Escherichia coli-binding protein in bronchoalveolar lavage. J Clin Invest 1992;90:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim BL, Wang JY, Holmskov U, Hoppe HJ, Reid KB. Expression of the carbohydrate recognition domain of lung surfactant protein D and demonstration of its binding to lipopolysaccharides of gram-negative bacteria. Biochem Biophys Res Commun 1994;202:1674–1680. [DOI] [PubMed] [Google Scholar]
- 19.van Rozendaal BA, van de Lest CH, van Eijk M, van Golde LM, Voorhout WF, van Helden HP, Haagsman HP. Aerosolized endotoxin is immediately bound by pulmonary surfactant protein D in vivo. Biochim Biophys Acta 1999;1454:261–269. [DOI] [PubMed] [Google Scholar]
- 20.Hartshorn K, Chang D, Rust K, White M, Heuser J, Crouch E. Interactions of recombinant human pulmonary surfactant protein D and SP-D multimers with influenza A. Am J Physiol Lung Cell Mol Physiol 1996;271:L753–L762. [DOI] [PubMed] [Google Scholar]
- 21.LeVine AM, Elliott J, Whitsett JA, Srikiatkhachorn A, Crouch E, DeSilva N, Korfhagen T. Surfactant protein-d enhances phagocytosis and pulmonary clearance of respiratory syncytial virus. Am J Respir Cell Mol Biol 2004;31:193–199. [DOI] [PubMed] [Google Scholar]
- 22.LeVine AM, Whitsett JA, Hartshorn KL, Crouch EC, Korfhagen TR. Surfactant protein D enhances clearance of influenza A virus from the lung in vivo. J Immunol 2001;167:5868–5873. [DOI] [PubMed] [Google Scholar]
- 23.Kramer BW, Jobe AH, Bachurski CJ, Ikegami M. Surfactant protein A recruits neutrophils into the lungs of ventilated preterm lambs. Am J Respir Crit Care Med 2001;163:158–165. [DOI] [PubMed] [Google Scholar]
- 24.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Kramer BW, Jobe A, Ikegami M. Exogenous surfactant changes the phenotype of alveolar macrophages in mice. Am J Physiol Lung Cell Mol Physiol 2001;280:L689–L694. [DOI] [PubMed] [Google Scholar]
- 26.Ikegami M, Kallapur SG, Jobe AH. Initial responses to ventilation of premature lambs exposed to intra-amniotic endotoxin 4 days before delivery. Am J Physiol Lung Cell Mol Physiol 2004;286:L573–L579. [DOI] [PubMed] [Google Scholar]
- 27.Naik AS, Kallapur SG, Bachurski CJ, Jobe AH, Michna J, Kramer BW, Ikegami M. Effects of ventilation with different positive end-expiratory pressures on cytokine expression in the preterm lamb lung. Am J Respir Crit Care Med 2001;164:494–498. [DOI] [PubMed] [Google Scholar]
- 28.Ikegami M, Jobe A. Postnatal lung inflammation increased by ventilation of preterm lambs exposed antenatally to E.coli endotoxin. Pediatr Res 2002;52:356–362. [DOI] [PubMed] [Google Scholar]
- 29.Stoll BJ, Hansen NI, Higgins RD, Fanaroff AA, Duara S, Goldberg R, Laptook A, Walsh M, Oh W, Hale E. Very low birth weight preterm infants with early onset neonatal sepsis: the predominance of gram-negative infections continues in the National Institute of Child Health and Human Development Neonatal Research Network, 2002–2003. Pediatr Infect Dis J 2005;24:635–639. [DOI] [PubMed] [Google Scholar]
- 30.Grandel U, Grimminger F. Endothelial responses to bacterial toxins in sepsis. Crit Rev Immunol 2003;23:267–299. [DOI] [PubMed] [Google Scholar]
- 31.Murphy K, Haudek SB, Thompson M, Giroir BP. Molecular biology of septic shock. New Horiz 1998;6:181–193. [PubMed] [Google Scholar]
- 32.Manocha S, Feinstein D, Kumar A. Novel therapies for sepsis: antiendotoxin therapies. Expert Opin Investig Drugs 2002;11:1795–1812. [DOI] [PubMed] [Google Scholar]
- 33.Sartori C, Matthay MA. Alveolar epithelial fluid transport in acute lung injury: new insights. Eur Respir J 2002;20:1299–1313. [DOI] [PubMed] [Google Scholar]
- 34.Lecuona E, Saldias F, Comellas A, Ridge K, Guerrero C, Sznajder JI. Ventilator-associated lung injury decreases lung ability to clear edema and downregulates alveolar epithelial cell Na,K-adenosine triphosphatase function. Chest 1999;116:29S–30S. [DOI] [PubMed] [Google Scholar]
- 35.Crouch EC. Structure, biologic properties, and expression of surfactant protein D (SP-D). Biochim Biophys Acta 1998;1408:278–289. [DOI] [PubMed] [Google Scholar]
- 36.Korfhagen TR, Sheftelyevich V, Burhans MS, Bruno MD, Ross GF, Wert SE, Stahlman MT, Jobe AH, Ikegami M, Whitsett JA, et al. Surfactant protein-D regulates surfactant phospholipid homeostasis in vivo. J Biol Chem 1998;43:28438–28443. [DOI] [PubMed] [Google Scholar]
- 37.Van Iwaarden JF, Pikaar JC, Storm J, Brouwer E, Verhoef J, Oosting RS, van Golde LM, van Strijp JA. Binding of surfactant protein A to the lipid A moiety of bacterial lipopolysaccharides. Biochem J 1994;303:407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pikaar JC, Voorhout WF, van Golde LM, Verhoef J, Van Strijp JA, van Iwaarden JF. Opsonic activities of surfactant proteins A and D in phagocytosis of gram-negative bacteria by alveolar macrophages. J Infect Dis 1995;172:481–489. [DOI] [PubMed] [Google Scholar]
- 39.Li YH, Tullus K. Microbial infection and inflammation in the development of chronic lung disease of prematurity. Microbes Infect 2002;4:723–732. [DOI] [PubMed] [Google Scholar]
- 40.Erpenbeck VJ, Malherbe DC, Sommer S, Schmiedl A, Steinhilber W, Ghio AJ, Krug N, Wright JR, Hohlfeld JM. Surfactant protein D increases phagocytosis and aggregation of pollen-allergen starch granules. Am J Physiol Lung Cell Mol Physiol 2005;288:L692–L698. [DOI] [PubMed] [Google Scholar]
- 41.Clark H, Palaniyar N, Strong P, Edmondson J, Hawgood S, Reid KB. Surfactant protein D reduces alveolar macrophage apoptosis in vivo. J Immunol 2002;169:2892–2899. [DOI] [PubMed] [Google Scholar]
- 42.Clark H, Reid KB. Structural requirements for SP-D function in vitro and in vivo: therapeutic potential of recombinant SP-D. Immunobiology 2002;205:619–631. [DOI] [PubMed] [Google Scholar]
- 43.Docimo SG, Crone RK, Davies P, Reid L, Retik AB, Mandell J. Pulmonary development in the fetal lamb: morphometric study of the alveolar phase. Anat Rec 1991;229:495–498. [DOI] [PubMed] [Google Scholar]
- 44.Ikegami M, Jobe A. Phospholipid composition of fetal lung fluid and amniotic fluid during late gestation in sheep. Am J Obstet Gynecol 1981;141:227–229. [DOI] [PubMed] [Google Scholar]
- 45.Jobe AH, Ikegami M, Seidner SR, Pettenazzo A, Ruffini L. Surfactant phosphatidylcholine metabolism and surfactant function in preterm, ventilated lambs. Am Rev Respir Dis 1989;139:352–359. [DOI] [PubMed] [Google Scholar]
- 46.Ikegami M, Jobe AH. Surfactant metabolism. Semin Perinatol 1993;17:233–240. [PubMed] [Google Scholar]
- 47.Ikegami M, Whitsett JA, Jobe AH, Ross G, Fisher J, Korfhagen T. Surfactant metabolism in SP-D gene ablated mice. Am J Physiol Lung Cell Mol Physiol 2000;279:L468–L476. [DOI] [PubMed] [Google Scholar]
- 48.Ikegami M, Adams FH, Towers B, Osher AB. The quantity of natural surfactant necessary to prevent the respiratory distress syndrome in premature lambs. Pediatr Res 1980;14:1082–1085. [DOI] [PubMed] [Google Scholar]
- 49.Ikegami M, Na CL, Korfhagen TR, Whitsett JA. Surfactant protein D influences surfactant ultrastructure and uptake by alveolar type II cells. Am J Physiol Lung Cell Mol Physiol 2005;288:L552–L561. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.