Abstract
Rationale: Bombesin-like peptides promote fetal lung development. Normally, levels of mammalian bombesin (gastrin-releasing peptide [GRP]) drop postnatally, but these levels are elevated in newborns that develop bronchopulmonary dysplasia (BPD), a chronic lung disease characterized by arrested alveolarization. In premature baboons with BPD, antibombesin antibodies reduce lung injury and promote alveolarization.
Objectives: The present study tests whether exogenous bombesin or GRP given perinatally alters alveolar development in newborn mice.
Methods: Mice were given peptides intraperitoneally twice daily on Postnatal Days 1–3. On Day 14 lungs were inflation-fixed for histopathologic analyses of alveolarization.
Measurements and Main Results: Bombesin had multiple effects on Day 14 lung, when alveolarization was about half complete. First, bombesin induced alveolar myofibroblast proliferation and increased alveolar wall thickness compared with saline-treated control animals. Second, bombesin diminished alveolarization in C57BL/6 (but not Swiss-Webster) mice. We used receptor-null mice to explore which receptors might mediate these effects. Compared with wild-type littermates, bombesin-treated GRP receptor (GRPR)–null mice had increased interstitial fibrosis but reduced defects in alveolarization. Neuromedin B (NMB) receptor–null and bombesin receptor subtype 3–null mice had the same responses as their wild-type littermates. GRP had the same effects as bombesin, whereas neither NMB nor a synthetic bombesin receptor type 3 ligand had any effect. All effects of GRP were abrogated in GRPR-null mice.
Conclusions: Bombesin/GRP can induce features of BPD, including interstitial fibrosis and diminished alveolarization. GRPR appears to mediate all effects of GRP, but only part of the bombesin effect on alveolarization, suggesting that novel receptors may mediate some effects of bombesin in newborn lung.
Keywords: bronchopulmonary dysplasia, gastrin-releasing peptide, interstitial fibrosis, knock-out mice
Bombesin is a 14–amino acid peptide originally identified in 1971 by Erspamer in skin of the frog Bombina bombina (1–3). Using antibodies to amphibian bombesin, a 27–amino acid mammalian homolog was identified by McDonald and termed gastrin-releasing peptide (GRP) (4). GRP and bombesin share a highly conserved seven–amino acid C-terminus, which is required for immunogenicity and high-affinity binding to physiologic receptors. Thus, bombesin and GRP are referred to collectively as “bombesin-like peptides” (BLPs), because both peptides bind to and elicit the same physiologic effects at mammalian bombesin/GRP-preferring receptors. BLP immunoreactivity is widely distributed in the central nervous system, the lung, and the gut, but the highest levels occur in mid-gestation human fetal lung (2, 5).
During lung development, the first epithelial cells to differentiate are the pulmonary neuroendocrine cells (6, 7), which contain high levels of BLP immunoreactivity. BLP can promote growth and maturation of developing fetal lung in humans, nonhuman primates, rats, and mice (8–10). Levels of GRP receptor (GRPR) and GRP mRNAs peak during the canalicular phase, then fall to much lower levels during normal alveolarization after birth (8, 11).
Bronchopulmonary dysplasia (BPD), also known as chronic lung disease of newborns, is the most prevalent of the long-term sequelae that affect surviving preterm infants. The pathophysiology of BPD is multifactorial, with contributing factors including barotrauma, oxygen toxicity, and pulmonary immaturity (12, 13). Increased numbers of BLP-positive pulmonary neuroendocrine cells have been observed in infants dying with BPD (14). Elevated urine BLP during the first week after birth is a strong risk factor for premature human infants, associated with a 10-fold increased risk of developing BPD, occurring shortly after birth in a majority of premature human infants who later develop BPD (15). Similarly, elevated urine BLP levels occur in premature newborns from two different baboon models of BPD (16). As a potent growth factor for normal bronchial epithelial cells and embryonic fibroblasts, and a direct bronchoconstrictor, BLP could promote peribronchiolar fibrosis, obliterative bronchiolitis, and reactive airways disease, which are characteristic of BPD (2, 3, 17, 18). Consistent with this hypothesis, the blocking anti-BLP antibody 2A11 given postnatally abrogates clinical and pathologic parameters of lung injury in baboons at risk for BPD (16).
The present study addresses two novel hypotheses: first, that BLP given to developing mouse pups can directly induce histopathologic features of BPD; and second, that these changes are mediated by one or more mammalian BLP receptors. The three cloned mammalian bombesin receptor subtypes have distinct distributions in the central nervous system and peripheral organs (19), suggesting that they may transduce different biological effects. These heptahelical G protein–coupled receptors are: GRPR (20), neuromedin B receptor (NMBR) (21), and the orphan bombesin receptor subtype 3 (BRS3) (22). GRPR-null mice manifest increased locomotor activity, whereas analyses of NMBR-null mice support a role for NMBR in thermoregulation (20, 21). Finally, mice lacking BRS3 develop hyperphagia, obesity with associated hypertension, reduced metabolic rate, and impaired glucose metabolism (22).
It is known that both GRP and bombesin bind to the same high-affinity bombesin/GRP–preferring receptor, leading to essentially identical effects in mammalian systems, which is due to their shared bioactive amidated carboxy terminal sequence (2). In fact, amphibian bombesin binds and functions approximately fourfold better than GRP itself at the mammalian bombesin/GRP receptor, apparently due to conformational differences related to its lower molecular weight that permit greater access to GRPR cell-surface receptors in tissues (23–25). Cumulatively, these observations provided our rationale for choosing bombesin as well as GRP for the present studies. To evaluate the possible contribution of other bombesin-related peptides, we also tested NMB and a synthetic BRS3 ligand (26). Subsequent studies in GRPR, NMBR, and BRS3 knock-out (KO) mice were performed to determine which receptor might be mediating alveolar changes in response to BLP.
Finally, the experimental design was chosen in an attempt to recapitulate the molecular events occurring in infants that develop BPD (27). In human infants with BPD, an elevated BLP level in the first 5 d after birth is a strong risk factor for developing BPD 2–3 mo later. In 2 baboon models of BPD, elevated BLP in the first 3 d after birth was closely correlated with impaired pulmonary function 10–21 d later.
This work was presented in part at the meeting of the Pediatric Research Society in 2002 (28).
METHODS
Animals
The National Institutes of Health Guide for the Care and Use of Laboratory Animals was strictly adhered to throughout this study.
Timed-pregnant Swiss Webster mice were from Charles River Laboratories (Wilmington, MA). GRPR-, NMBR-, and BRS3-KO mice were inbred into a C57BL/6 background for 10 generations or more. Tail DNA was prepared as previously described (20, 22). Mice were genotyped using polymerase chain reaction for GRPR, NMBR, and BRS3 as previously described (20, 22). Wild-type (WT) littermates were the matched control animals for the KO mice in all experiments. Mice were killed on Postnatal Day 14 using CO2, lungs were inflated in situ (details given in online supplement) and fixed overnight in 4% paraformaldehyde, then processed into paraffin.
Experimental mice were divided into four groups. Groups 1 and 2 are referred to as “prenatal,” and Groups 3 and 4 as “postnatal”:
Group 1: bombesin at 200 μg/kg in phosphate-buffered saline (PBS) was administered intraperitoneally twice daily to pregnant mice at Embryonic Days 14–16 (8).
Group 2: an equal volume of PBS was given intraperitoneally in parallel with Group 1.
Group 3: Bombesin was given intraperitoneally twice daily for 3 d to pups beginning at 24 h of age (P1–P3). In some experiments, GRP, NMB, or BRS3 ligand were tested using doses at equivalent molarity to 200 μg/kg bombesin. Bombesin, GRP, and NMB were from Peninsula Laboratories (San Carlos, CA). The BRS3 ligand was provided by Dr. David Coy at Tulane University, New Orleans, LA (26).
Group 4: an equal volume of PBS was given intraperitoneally twice daily at P1–P3. Mice were weighed daily to determine the proper volume for 200 μg/kg/injection. This dose was previously determined to elicit significant responses in fetal mouse lung (8).
Immunostaining
A variation of the avidin-biotin complex technique was used (16). Detailed methods are given in the online supplement. The primary antibodies are mouse monoclonals: anti–α-smooth muscle actin (SMA): clone 1A4 (Sigma, St. Louis, MO); and anti–proliferating cell nuclear antigen (PCNA): clone PC10 (Dako, Carpinteria, CA).
Morphometric Analyses
General.
Digitized images were captured at 20× magnification using a Nikon Labphot camera (Nikon, Tokyo, Japan). A total of 8–20 20× images were taken randomly from each lung lobe per mouse, using nonoverlapping fields at 3, 6, 9, and 12 o'clock.
PCNA.
The percentage of nuclei with PCNA staining was calculated as follows: (no. PCNA-positive cells)/(total no. nucleated cells, both positive and negative) × 100%.
SMA.
A 365-point grid was superimposed on each captured field. The volume–percent of SMA-positive lung tissue was calculated as follows: (total number of SMA-positive points)/(365 − [positive points over airspaces]) × 100%.
Alveolar wall thickness.
Using the same images, approximately 60–80 lines per field were drawn perpendicular to the narrowest segment of primary and secondary alveolar septa. The mean length of lines crossing the septa was determined using Scion Image (Scion Corp., Frederick, MD) (29).
Mean linear intercept.
Using a predetermined grid with randomly distributed lines totaling 1-mm in length, mean linear intercept (MLI) was calculated as follows: 1/(no. air–tissue interfaces) × 1000, yielding the average distance between two air–tissue interfaces in microns.
Statistical Analysis
Data are given as mean ± SEM, determined using one-way analysis of variance. Statistical significance was defined as p < 0.05.
RESULTS
PCNA Immunostaining
The first approach of the current investigation was to determine whether bombesin treatment alters cell proliferation in developing postnatal lung, because BLP was previously demonstrated to be to be a potent growth factor for normal bronchial epithelial cells, lung cancer cell lines, and fetal lung (2, 8, 30). We performed immunostaining for the cell cycle–specific marker, PCNA, which is expressed only by actively dividing cells with a half-life of 20 h. We chose the experimental design described here in an attempt to recapitulate the molecular events occurring in infants that develop BPD, who have elevated BLP levels shortly after birth followed by lung disease weeks to months later (16, 27).
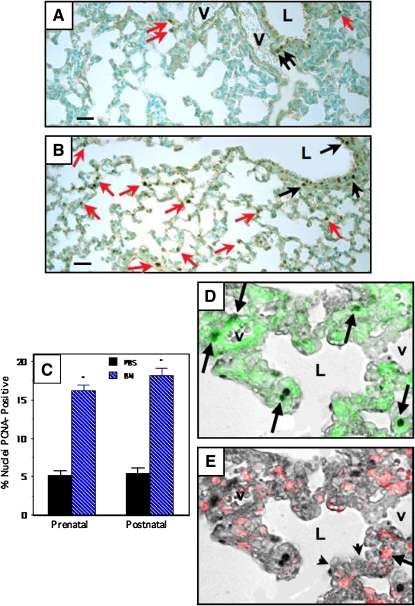
Representative photomicrographs are given in Figure 1. Compared with PBS-treated control mice (n = 6) (Figure 1A), pups treated with bombesin either prenatally or postnatally (n = 8–9 in each group) (Figure 1B) had increased relative numbers of PCNA-positive lung cells. The increased PCNA labeling was localized predominantly to alveoli (Figure 1B, red arrows), but was also evident in airway epithelium (Figure 1B, black arrows), as compared with PBS-treated control animals (Figure 1A). Morphometric analyses demonstrated a threefold increase in the percentage of nuclei that were PCNA-positive, both in the developing alveoli (Figure 1C) and in airway epithelium (data not shown). Mice treated either prenatally or postnatally had essentially identical bombesin-induced PCNA responses.
Figure 1.
Bombesin (BN) increases alveolar cell proliferation in newborn Swiss-Webster mice as assessed by proliferating cell nuclear antigen (PCNA) immunostaining. Swiss-Webster mice were treated with BN or phosphate-buffered saline (PBS) prenatally (E16–E18) or postnatally (P1–P3), as detailed in Methods. All of the lungs analyzed in this study were from Postnatal Day 14 animals. Immunostaining for PCNA was performed to evaluate the prevalence of alveolar cell proliferation in lung tissue sections. Details of computerized image analysis are given in Methods. (A) Representative section of lung from mouse given PBS postnatally. Note several PCNA-positive nuclei lining airspaces (red arrows). There are also scattered PCNA-positive cells in the airway epithelium (black arrows). (B) Lung from mouse given BN (200 μg/kg) postnatally. There are numerous PCNA-positive nuclei in the alveolar walls (many are indicated by red arrows). There are also multiple PCNA-positive cells in the airway epithelium (some indicated by black arrows). (C) Results of morphometric analyses determining the percentage of nuclei in the alveolar wall that are PCNA-positive. Mice treated with BN either prenatally or postnatally have over a threefold increase in the percentage of PCNA-positive cells (*p < 0.0001 compared with corresponding PBS-treated control group). (D) Immunohistochemistry using lung slides from mice treated with BN demonstrated PCNA labeling by bright-field microscopy merged with SMA immunofluorescence (arrows indicate cells with green cytoplasm and PCNA-positive nuclei). (E) In contrast, PCNA positivity only infrequently colocalized with surfactant protein C (SPC), a marker of alveolar type II cells. Note red cytoplasm indicating SPC immunoreactivity in a cell with PCNA positivity (arrow). Scale bar in lower left corner of (A) and (B) = 50 μm. L = airway lumen; V = vessel.
To identify which cells are proliferating in the developing alveoli, we performed immunofluorescence analysis for SMA (green) and surfactant protein C (SPC; red) in slides that had been previously immunostained for PCNA. Representative results from a mouse treated postnatally with bombesin are given in Figure 1D. Over 80% of the PCNA-positive cells (left panel) were also SMA-positive (Figure 1D, arrows indicate cells with cytoplasmic SMA green fluorescence superimposed on PCNA-immunopositive nuclei). Less than 5% of the PCNA-positive cells were SPC-positive (Figure 1E, long arrow). About 10–20% of the PCNA-positive cells did not label with either SMA or SPC, and most of these appeared to be airway epithelial cells, endothelial cells, or inflammatory cells (data not shown).
PCNA labeling was then examined in untreated KO mice on a C57BL/6 background. The untreated KO mice were age-matched with the bombesin-treated KO mice (i.e., 14 d of age), when alveolarization was about half complete. For all three BLP receptors (GRPR, NMBR, and BRS3), the same observation was made: at baseline, there was no significant difference in PCNA labeling between KOs (n = 7) and WT littermates (n = 7) (Figure 2A). Also, there was no difference in PCNA labeling between untreated mice and mice treated with PBS postnatally, in either KO or WT mice (data not shown).
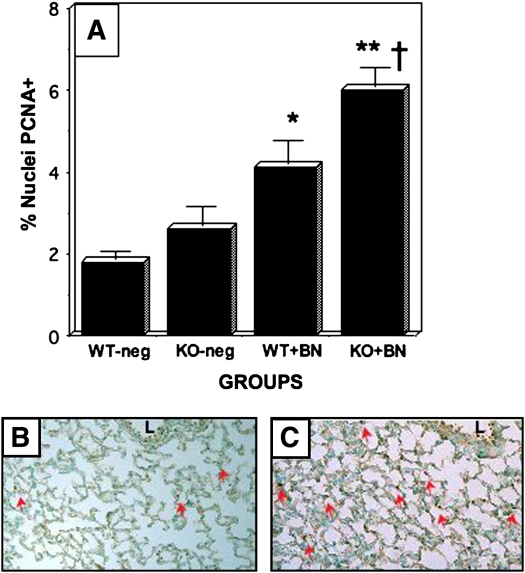
Figure 2.
Bombesin-induced alveolar cell proliferation in GRPR-WT and GRPR-KO mice. GRPR KO and WT mice on a C57BL/6 background were treated with BN or PBS postnatally (P1–P3), and immunostaining for PCNA was performed as described in Figure 1. (A) Results of morphometric analyses determining the percentage of nuclei in the alveolar wall that are PCNA-positive. There was no significant difference in PCNA labeling between untreated WT and untreated KO mice (KO is 1.4-fold greater than WT; p = 0.30). KO mice treated with BN, however, had significantly more PCNA-labeling than BN-treated WT littermates (†p < 0.02). Both WT and KO mice had significant BN-induced responses compared with the corresponding untreated control animals (**p < 0.0001 for KO mouse responses, and *p < 0.005 for WT mouse responses). (B) Representative section of lung from GRPR-WT mouse given BN. Note several PCNA-positive nuclei in developing alveoli (red arrows). (C) Representative section showing PCNA immunostaining of lung from BN-treated GRPR-KO mouse. There are numerous PCNA-positive nuclei in the alveolar walls (many are indicated by red arrows). L = airway lumen.
Next, we evaluated PCNA labeling in KO mice treated postnatally with bombesin. We used only postnatal treatment because the KO mice have reduced fertility, which limited the number of feasible experiments. The results of three experiments are given in Figure 2, with pooled results of quantitative morphometry summarized in Figure 2A. Compared with untreated WT C57BL/6 mice (Figure 2A), bombesin-treated WT C57BL/6 pups had a doubling in the percentage of alveolar cells with PCNA-positive nuclei (Figures 2A and 2B, red arrows). In the same animals, there was no difference in PCNA labeling of airway epithelial cells. Paradoxically, bombesin-treated GRPR KO mice demonstrated significantly more PCNA labeling of cells in the distal lung (Figures 2A and 2C, red arrows). In contrast, there was no difference in relative numbers of PCNA-labeled nuclei between NMBR or BRS3 KO mice and their WT littermates (data not shown).
α-SMA Immunostaining
We next tested the hypothesis that bombesin treatment regulates interstitial fibrosis by altering the relative numbers of myofibroblasts, which are the proliferating subtype of fibroblasts involved in fibrotic reactions, and are SMA-positive.
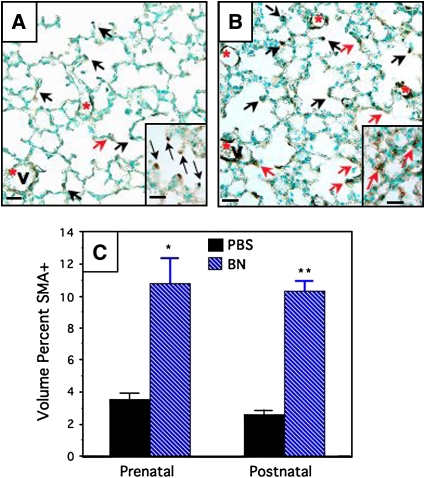
In the first series of experiments, bombesin was given to Swiss-Webster mice either prenatally or postnatally, as described previously here, and lungs were harvested at P14. Representative photomicrographs of SMA-immunostained slides from mice treated postnatally are presented in Figures 3A and 3B (PBS- and bombesin-treated, respectively). By morphometric analysis (Figure 3C), there was a three-to fourfold increase in the volume percent of SMA immunostaining in mice given bombesin prenatally or postnatally compared with PBS-treated control mice. Much of this increase was in the form of interstitial SMA positivity (Figures 3A and 3B, red arrows), rather than the normal SMA positivity that occurs at the tips of developing septa (Figures 3A and 3B, black arrows). It should be noted that SMA positivity associated with airways (data not shown) and blood vessels (Figures 3A and 3B, V, red asterisk) was excluded from the analysis. The magnitude of these data are similar to results with PCNA immunostaining (described previously here).
Figure 3.
Bombesin increases SMA-positive cells in alveoli of newborn mice. Swiss-Webster mice were treated with BN or PBS prenatally (E16–E18) or postnatally (P1–P3), as described in Methods. Immunostaining for SMA was performed to determine the prevalence of myofibroblasts in developing alveoli. Black arrows indicate SMA-positive cells along the surface of alveolar spaces, consistent with developing septa. Red arrows indicate SMA-positive cell(s) within the interstitium. (A) Representative section of lung from mouse given PBS postnatally. Inset: note SMA-positive cells predominantly at the tips of developing septa (a few are indicated by black arrows). (B) Representative section of lung from mouse given BN postnatally. Compared with (A), there is an increased volume percent of SMA-positive cells, most of which occurs in the alveolar interstitum (red arrows), as shown at higher magnification in the inset. V = vessel, also indicated by red asterisk. Scale bars in lower left corners of (A) and (B) = 50 μm. (C) Results of morphometry assessing SMA-positive cells in developing alveoli. Mice treated with BN either prenatally or postnatally had ∼ threefold increased volume percent of myofibroblasts (*p < 0.0001 and **p < 0.01 compared with corresponding PBS-treated control group). There was no significant difference between mice given PBS prenatally versus postnatally.
In the second series of experiments, SMA labeling was analyzed in untreated KO mice (on a C57BL/6 background) and their WT littermates. GRPR-null mice (n = 6) had a 1.5-fold increase in the volume percent of SMA staining when compared with control littermates (n = 7) (p < 0.02). However, in untreated NMBR and BRS3 mice, there was no significant difference in SMA labeling between KOs (n = 7) and WT littermates (n = 7) (data not shown). Also, there was no difference in SMA labeling between PBS-treated and untreated mice, in either KO or WT animals (data not shown).
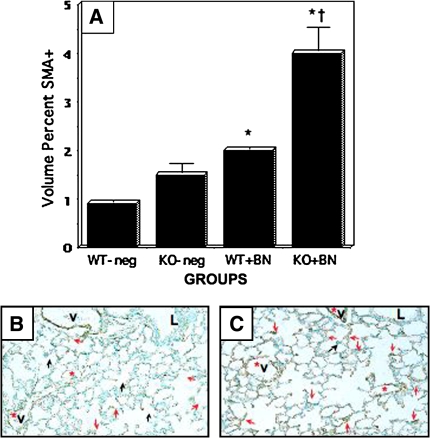
In the third series of experiments, GRPR-null, NMBR-null, and BRS3-null pups were treated with bombesin intraperitoneally from P1–P3. Again, we chose to treat postnatally because the number of KO mice was limited and postnatal bombesin is most similar to the elevated BLP levels occurring postnatally in premature infants with elevated BLP during the period of alveolarization. The results of these experiments are given in Figure 4. Bombesin-treated GRPR WT pups had a twofold increase in interstitial SMA positivity (p < 0.001 compared with untreated WT control animals), which was predominantly interstitial (Figure 4B, red arrows) rather than at developing septal tips (Figure 4B, black arrows). In contrast, bombesin-treated GRPR KO pups had increased SMA-positivity representing about a threefold increase over baseline values for the untreated KO mice (p < 0.001). Most of this SMA immunoreactivity was interstitial (Figure 4C). Bombesin-treated KO mice had significantly more SMA positivity compared with bombesin-treated WT littermates (p < 0.001).
Figure 4.
Bombesin-induced SMA-positivity is enhanced in GRPR-KO mice. GRPR-WT and GRPR-KO mice were treated with BN postnatally (P1–P3), as described in the legend to Figure 2. Immunostaining for SMA was performed as in Figure 3. Black arrows indicate SMA positivity consistent with developing septa. Red arrows indicate SMA-positivity within the interstitium. (A) Results of morphometry assessing the volume percent of SMA-positive cells in developing alveoli. Mice treated with BN have ∼2 to 3-fold increased volume percent of myofibroblasts (*p < 0.0001 compared with the corresponding untreated control group). Bombesin-treated KO mice have significantly more SMA staining than BN-treated WT mice (twofold greater volume percent of SMA; †p < 0.001). (B) Representative section of lung from GRPR-WT mouse given BN. (C) Representative section of lung from GRPR-KO mouse given BN. Compared with Figure 2B, there is an increased prevalence of SMA-positive cells, especially in the alveolar interstitum (red arrows). V = vessel, also indicated by red asterisk.
There was no significant difference in SMA staining for either NMBR-null or BRS3-null mice (n = 3 each) compared with their WT littermates (n = 5) (data not shown). These data are consistent with the increased PCNA immunostaining in lung parenchyma of GRPR-null mice compared with their WT littermates.
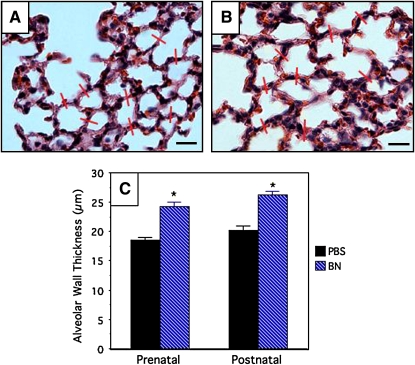
Alveolar Wall Thickness
During the initial slide review, we observed increased interstitial thickness as a prominent and consistently noticeable feature of the bombesin-treated lungs. Alveolar wall thickness was measured in a rigorous and exhaustive fashion, restricting the analysis to lobes that were well inflated. Using the hematoxylin and eosin–stained slides, we measured alveolar wall thickness in PBS-treated Swiss Webster mice (n = 6–8; Figure 5A) and pups from the same litters treated with bombesin (n = 8–10; Figure 5B). The pooled results of these quantitative analyses are given in Figure 5C. There is a significant increase (∼ 24–27%) in alveolar wall thickness in bombesin-treated mice as compared with control mice (p < 0.0001), whether mice were treated prenatally or postnatally. These data are highly significant and closely correlate with increased numbers of proliferating SMA-positive cells in the interstitium. The same results of increased alveolar wall thickness were obtained with GRP treatment of C57BL/6 mice. In contrast, GRP-treated GRPR KO mice did not demonstrate any increase in alveolar wall thickness compared with untreated GRPR KO littermates, whereas GRPR WT littermates did have increased alveolar wall thickness with GRP treatment (data not shown).
Figure 5.
Bombesin increases alveolar wall thickness in alveoli of newborn mice. Swiss-Webster mice were treated with BN or PBS, as described in the legend to Figure 1. Lung tissue sections were stained with hematoxylin and eosin (H&E) to demonstrate alveolar architecture. Representative red lines are drawn at 90° across alveolar septa. (A) Representative section of lung from a mouse given PBS postnatally. (B) Representative section of lung from a mouse given BN postnatally. Scale bar in lower right hand corners = 30 μm. (C) Results of morphometry assessing alveolar wall thickness. Mice treated with BN either prenatally or postnatally have a ∼ 20–25% increase in alveolar wall thickness (*p < 0.0001compared with the corresponding PBS-treated control group).
Alveolarization
As a general index of lung growth and alveolar development, we measured lung weights and volumes, which were then normalized for the total body weight of each pup. There were no significant differences between the groups (data not shown), but there was considerable variability within each group. Some of this variability could be related to the difficulty in measuring accurate lung volumes on the small P14 lungs.
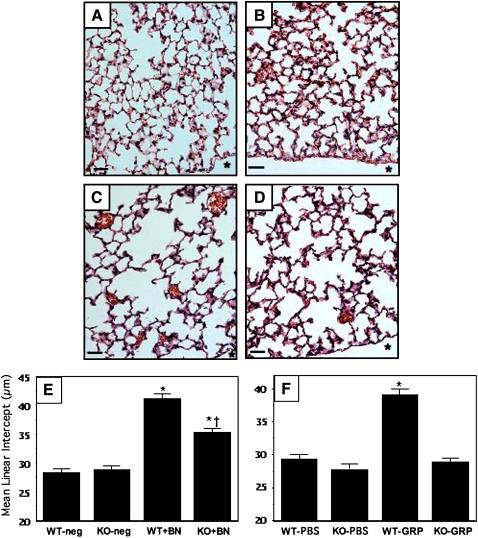
We turned to a more specific measurement of alveolar septal development: MLI, measured as described in Methods. In outbred Swiss-Webster mice, there was no significant difference in MLI between bombesin-treated and PBS-treated pups (data not shown). However, there was a significant bombesin-induced decrease in alveolarization in GRPR-WT mice, which are inbred onto a C57BL/6 background for over 12 generations. As shown in Figure 6A, untreated GRPR-WT mice have well developed alveoli at Postnatal Day 14; PBS-treated GRPR-WT mice appeared identical (data not shown). At baseline, GRPR-KO mice had the same MLI (Figure 6B) as the WT littermates (Figure 6A). Bombesin-treated GRPR-WT mice had a marked increase in the MLI (1.5-fold), reflecting fewer alveolar septa and larger alveoli (Figure 6C). Bombesin-treated GRPR-KO mice also had increased MLI (Figure 6D), but this increase was smaller in magnitude (1.25-fold) than that in the WT littermates. The average MLI for each group is given in Figure 6E. There was a significant difference in MLI between bombesin-treated GRPR-KO and bombesin-treated WT mice (p < 0.001).
Figure 6.
Bombesin and GRP arrest alveolarization. GRPR-WT and GRPR-KO mice were treated with BN or left untreated, as described in Methods and in the legend to Figure 2. Lung tissue sections were stained with H&E to demonstrate alveolar architecture. Representative sections are given in (A)–(D): (A) GRPR-WT, untreated. (B) GRPR-KO, untreated. (C) GRPR-WT treated with BN. (D) GRPR-KO treated with BN. * Pleural surface. Scale bar in lower left hand corner = 50 μm. (E) Results of morphometry for mean linear intercept (MLI) in the above BN-treated groups (n = 8–10 animals per group; 10–12 20× fields per animal) (*p < 0.0001 compared with corresponding untreated group; †p < 0.001 compared with BN-treated WT pups). (F) Results of morphometry for MLI in similar groups, but treated with GRP rather than BN (n = 6–10 animals per group) (*p < 0.0001 compared with either the GRP-treated KO group or the corresponding PBS-treated WT group).
We performed additional experiments treating newborn mice with GRP, NMB, and a synthetic BRS3 ligand, (DTyr6,[R-Apa11, Phe13, Nle14]Bombesin-[6–14]) (31), using doses that are equimolar to 200 μg/kg bombesin (detailed in the online supplement). GRP has the same effects on alveolarization as bombesin (Figure 6F). The effect of GRP on alveolarization was completely abrogated in GRPR KO mice (Figure 6F), indicating that this effect is entirely mediated by the bombesin/GRP–preferring receptor. In contrast, NMB had no effect on lung histopathology (data not shown). We also tested the synthetic BRS3 ligand (31) according to the same protocol, but no effects on lung histopathology were observed (data not shown). In summary, it appears that GRP is the mammalian peptide relevant to decreased alveolarization because GRP elicits the same responses as bombesin, whereas equivalent doses of NMB or a BRS3 ligand have no effect. GRPR mediates all of the GRP effects and much of the bombesin effect on alveolarization, but we do not know which receptor is mediating the remainder of the decreased alveolarization or the interstitial fibrosis in response to bombesin.
DISCUSSION
The objective of the present study was to test two hypotheses: (1) elevated BLP levels during perinatal development can lead to changes in lung histopathology that are characteristic of BPD; and (2) effects of BLP on developing lung are mediated via one or more of the three cloned mammalian BLP receptors. The major histopathologic changes that we observed were: (1) a significant increase in alveolar wall thickness that closely correlates with increased numbers of proliferating SMA-positive cells in the interstitium; and (2) a significant decrease in alveolarization in C57BL/6 (but not Swiss-Webster) mice.
Our observations support the hypothesis that GRP contributes to the interstitial fibrosis and decreased alveolarization that are characteristic of BPD (12). It is intriguing that an early elevation in bombesin or GRP levels in newborn mice could result in altered alveolar structure weeks later. We chose the described experimental design in an attempt to recapitulate the sequence of events occurring in infants that develop BPD. In human infants with BPD, an elevated BLP level in the first 5 d after birth confers a 10-fold increased risk for developing BPD 2–3 mo later (15). In two baboon models of BPD, elevated BLP in the first 3 d after birth is closely correlated with impaired pulmonary function 10–21 d later (16). There is no correlation between early BLP elevation and early parameters of lung function, nor is there any correlation between late BLP elevation (at 10–14 d) and late impairment of pulmonary function (16) (M. Sunday, unpublished data). These observations are reminiscent of rat studies in which prenatal dexamethasone resulted in altered alveolar structure at Postnatal Day 14, but not Postnatal Day 2 (32). Although we do not yet know the mechanism for the observed late effects of early bombesin treatment, it should be kept in mind that bombesin can function as a neuroregulatory hormone, altering the production of other regulatory peptides, including insulin and adrenocorticotropic hormone, and modulating expression of growth factor receptors, such as EGFR and GRPR (33). In previous work, we demonstrated that the same dose of bombesin is locally active over a short time course in mice in utero or in fetal lung organ cultures (8). The present study extends those earlier experiments by addressing late consequences of early BLP elevation. The late PCNA labeling is consistently elevated in pups treated early with bombesin, consistent with a late effect that is likely downstream from the initial BLP binding to its cognate receptor, via a mechanism that is secondary or indirect.
In newborn mice, GRP has the same effects on alveolarization as bombesin, and these effects are completely abrogated in GRPR KO mice, indicating that GRPR mediates this effect of GRP. In contrast, neither NMB nor a BRS3 ligand has any effect on lung histopathology. Our observations are consistent with numerous pharmacokinetic studies indicating that GRP and bombesin are nearly identical in mammalian systems, due to their shared bioactive amidated carboxyterminal sequence, both binding to the same high-affinity bombesin/GRP–preferring receptor. In fact, amphibian bombesin binds to and functions better at the mammalian bombesin/GRP receptor than does GRP itself, probably due to conformational differences related to its smaller molecular weight that permit greater access to the cell-surface GRPR in tissues (23–25). Three of the four amino acids required for high-affinity bombesin binding to GRPR are also required for high-affinity GRP binding (34, 35). The peptide-specific binding site for GRP has been mapped to the third extracellular domain (35), whereas NMB selectivity has been mapped to the fifth transmembrane region (36). Four times as much GRP or 300 times as much NMB is required to elicit the same responses as bombesin (on a molar basis) at the bombesin/GRP–preferring receptor (23). It is also known that bombesin does not appreciably bind to the BRS3 receptor (26, 34). In some experimentally mutated receptors, however, bombesin has markedly higher affinity than does GRP (35), suggesting that a novel bombesin receptor could, in fact, mediate effects of bombesin that might persist in GRPR KO mice, as observed with bombesin-induced interstitial fibrosis and decreased alveolarization in the present study.
Normally, both GRP and the GRPR are expressed at low or undetectable levels after birth, when alveolarization normally takes place (33). Previously, we demonstrated that BLP promotes growth and maturation of fetal murine lung (8, 37). In the present study of bombesin-treated outbred mice, we demonstrate a threefold increase in both cell proliferation and the prevalence of myofibroblasts in the distal lung parenchyma, consistent with early signs of pulmonary interstitial fibrosis, such as occurs with BPD. Bombesin-treated GRPR KO mice had increased interstitial fibrosis (distal parenchymal cell proliferation and increased myofibroblasts) that was significantly greater than that in bombesin-treated GRPR-WT littermates. These data suggest that GRPR does not mediate this effect of bombesin, and could even protect against pulmonary interstitial fibrosis in newborn mice, or simply that a novel BLP receptor mediating the fibrosis is upregulated in a compensatory fashion in the absence of GRPR. Interestingly, GRP-induced interstitial thickening was completely abrogated in GRPR KO mice, further supporting the concept that a novel receptor may be mediating the bombesin effect on interstitial fibrosis.
It is unlikely that upregulation of NMBR and/or BRS3 would account for the above observations in bombesin-treated GRPR KO mice. First, neither NMB nor a synthetic BRS3 ligand had any effect on lung histopathology in the described protocol. Also, we did not observe any differences in mRNA levels for NMBR or BRS3 in newborn GRPR KO mice. Finally, NMBR and BRS3 KO mice had the same responses to bombesin as their normal littermates, further arguing against the involvement of NMBR and BRS3 in these responses.
Cumulatively, these observations suggest that a novel BLP receptor may mediate the effects of bombesin on alveolarization (in part) and interstitial thickening in developing lung. One possibility might be a mammalian phyllolitorin receptor. Bronchoalveolar lavage fluid from smokers has been found to have high levels of bombesin-like immunoreactivity most consistent with a mammalian phyllolitorin (38). Phyllolitorins can stimulate cell proliferation and branching morphogenesis in murine embryonic lung buds (11). An amphibian phyllolitorin has been cloned (39), but its mammalian homolog and a phyllolitorin receptor have yet to be identified. However, phyllolitorins have only very low affinity for GRPR (23), so it seems unlikely that a mammalian phyllolitorin receptor reciprocally would mediate the bombesin-induced effects observed in the present study.
Our analyses of alveolar development after bombesin treatment have yielded additional novel observations. First, bombesin diminished alveolarization in C57BL/6 mice, but not in outbred Swiss-Webster mice, suggesting that this response depends on background genes that are as yet unidentified. Second, GRPR-KO mice are protected in part against this inhibitory effect of bombesin on alveolarization, indicating that GRPR mediates a significant part of the reduced alveolarization. These observations are of particular interest because only a subset (∼ 30%) of premature infants develop BPD (40), and twin studies indicate that genetic factors play an important role in human BPD (41). Similarly, it has been suggested that elevated BLP levels in humans might predict which adult patients will develop smoking-related lung diseases, including emphysema (42). Analogous to BPD developing in less than a third of oxygen-treated preterm infants, only about 20% of smokers develop chronic lung disease, and these appear to be the same patients that have elevated BLP levels (43). Thus, increased BLP receptor signaling due to increased BLP levels could be a common denominator for lung diseases with decreased alveolarization, including both BPD and emphysema.
The observed dissociation between interstitial fibrosis and diminished alveolarization supports the concept that BPD can result from the activation of multiple signaling pathways, even when a single peptide (bombesin or GRP) is used to mimic the effect of hyperoxia in a premature infant. We have demonstrated that increased BLP levels in baboons with BPD are directly linked to oxidant exposure, because treatment of animals with a novel antioxidant abrogates these increased BLP levels (18, 44). Similarly, GRP may have a role in acute lung injury (16, 45). In view of the fact that BPD is a multifactorial disorder, it remains surprising that several characteristic histopathologic features of BPD can be induced by a single peptide in a simple newborn mouse model. The present study suggests that early overproduction of BLP by pulmonary neuroendocrine cells might be sufficient to trigger the cascade of events leading to arrested alveolarization and interstitial fibrosis. In conclusion, it appears that GRP is the relevant mammalian peptide because it elicits the same responses as bombesin, whereas neither NMB nor a BRS3 ligand has any effect. Detailed mechanisms underlying these complex developmental pathobiological effects remain to be explored.
Supplementary Material
Supported by National Institutes of Health grants RO1-HL50045, UO1-HL52638, and P50-HL67669, and a Children's Hospital Training Grant.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200507-1014OC on April 7, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Sunday ME, Wolfe HJ, Roos BA, Chin WW, Spindel ER. Gastrin-releasing peptide gene expression in developing, hyperplastic, and neoplastic human thyroidal C-cells. Endocrinology 1988;122:1551–1558. [DOI] [PubMed] [Google Scholar]
- 2.Sunday ME. Neuropeptides and lung development. In: McDonald JA, editor. Lung growth and development, 1st ed. New York: Marcel Dekker; 1997. pp. 401–494.
- 3.Erspamer V. Amphibian skin peptides in mammals: looking ahead. Trends Neurosci 1983;6:200–201. [Google Scholar]
- 4.McDonald TJ, Jornvall H, Nilsson G, Vagne M, Ghatei M, Bloom SR, Mutt V. Characterization of a gastrin-releasing peptide from porcine non-antral gastric tissue. Biochem Biophys Res Commun 1979;90:227–233. [DOI] [PubMed] [Google Scholar]
- 5.Wharton J, Polak JM, Bloom SR, Ghatei MA, Solcia E, Brown MR, Pearse AGE. Bombesin-like immunoreactivity in the lung. Nature 1978;273:769–770. [DOI] [PubMed] [Google Scholar]
- 6.Cutz E. Cytomorphology and differentiation of airway epithelium in developing human lung. In: McDowell EM, editor. Lung carcinomas: current problems in tumor pathology, Edinburgh: Churchill Livingstone; 1987. pp. 1–41.
- 7.Ten Have-Opbroek AAW. Lung development in the mouse embryo. Exp Lung Res 1991;17:111–130. [DOI] [PubMed] [Google Scholar]
- 8.Sunday ME, Hua J, Dai HB, Nusrat A, Torday JS. Bombesin increases fetal lung growth and maturation in utero and in organ culture. Am J Respir Cell Mol Biol 1990;3:199–205. [DOI] [PubMed] [Google Scholar]
- 9.Sunday ME, Hua J, Reyes B, Masui H, Torday JS. Anti-bombesin antibodies modulate fetal mouse lung growth and maturation in utero and in organ cultures. Anat Rec 1993;236:25–32. [DOI] [PubMed] [Google Scholar]
- 10.Sunday ME, Hua J, Torday J, Reyes B, Shipp MA. CD10/neutral endopeptidase 24.11 in developing human fetal lung: patterns of expression and modulation of peptide-mediated proliferation. J Clin Invest 1992;90:2517–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King KA, Torday JS, Sunday ME. Bombesin and [leu8]phyllolitorin promote fetal mouse lung branching morphogenesis via a specific receptor-mediated mechanism. Proc Natl Acad Sci USA 1995;92:4357–4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Northway WH. Bronchopulmonary dysplasia: twenty-five years later. Pediatrics 1992;89:969–973. [PubMed] [Google Scholar]
- 13.Abman SH, Groothius JR. Pathophysiology and treatment of bronchopulmonary dysplasia: current issues. Pediatr Clin North Am 1994;41:277–315. [DOI] [PubMed] [Google Scholar]
- 14.Johnson DE, Lock JE, Elde RP, Thompson TR. Pulmonary neuroendocrine cells in hyaline membrane disease and bronchopulmonary dysplasia. Pediatr Res 1982;16:446–454. [DOI] [PubMed] [Google Scholar]
- 15.Cullen A, Van Marter LJ, Moore M, Parad R, Sunday ME. Urine bombesin-like peptide elevation precedes clinical evidence of bronchopulmonary dysplasia. Am J Respir Crit Care Med 2002;165:1093–1097. [DOI] [PubMed] [Google Scholar]
- 16.Sunday ME, Yoder BA, Cuttitta F, Haley KJ, Emanuel RL. Bombesin-like peptide mediates lung injury in a baboon model of bronchopulmonary dysplasia. J Clin Invest 1998;102:584–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Impicciatore M, Bertaccini G. The bronchoconstrictor action of the tetradecapeptide bombesin in the guinea-pig. J Pharm Pharmacol 1973;25:872–875. [DOI] [PubMed] [Google Scholar]
- 18.Ganter MT, Pittet JF. Bombesin-like peptides: modulators of inflammation in acute lung injury? Am J Respir Crit Care Med 2006;173:1–2. [DOI] [PubMed] [Google Scholar]
- 19.Ohki-Hamazaki H, Wada E, Matsui K, Wada K. Cloning and expression of the neuromedin B receptor and the third subtype of bombesin receptor genes in the mouse. Brain Res 1997;762:165–172. [DOI] [PubMed] [Google Scholar]
- 20.Wada E, Watase K, Yamada K, Ogura H, Yamano M, Inomata Y, Eguchi J, Yamamoto K, Sunday ME, Maeno H, et al. Generation and characterization of mice lacking gastrin-releasing peptide receptor. Biochem Biophys Res Commun 1997;239:28–33. [DOI] [PubMed] [Google Scholar]
- 21.Ohki-Hamazaki H, Sakai Y, Kamata K, Ogura H, Okuyama S, Watase K, Yamada K, Wada K. Functional properties of two bombesin-like peptide receptors revealed by the analysis of mice lacking neuromedin B receptor. J Neurosci 1999;19:948–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohki-Hamazaki H, Watase K, Yamamoto K, Ogura H, Yamano M, Yamada K, Maeno H, Imaki J, Kikujama S, Wada E, et al. Mice lacking bombesin receptor subtype-3 develop metabolic defects and obesity. Nature 1997;390:165–169. [DOI] [PubMed] [Google Scholar]
- 23.Lin JT, Coy DH, Mantey SA, Jensen RT. Comparison of the peptide structural requirements for high affinity interaction with bombesin receptors. Eur J Pharmacol 1995;294:55–69. [DOI] [PubMed] [Google Scholar]
- 24.Benya RV, Kusui T, Pradhan TK, Battey JF, Jensen RT. Expression and characterization of cloned human bombesin receptors. Mol Pharmacol 1995;47:10–20. [PubMed] [Google Scholar]
- 25.Lach E, Haddad EB, Gies JP. Contractile effect of bombesin on guinea pig lung in vitro: involvement of gastrin-releasing peptide–preferring receptors. Am J Physiol 1993;264:L80–L86. [DOI] [PubMed] [Google Scholar]
- 26.Mantey SA, Coy DH, Entsuah LK, Jensen RT. Development of bombesin analogs with conformationally restricted amino acid substitutions with enhanced selectivity for the orphan receptor human bombesin receptor subtype 3. J Pharmacol Exp Ther 2004;310:1161–1170. Epub 2004 Apr 21. [DOI] [PubMed] [Google Scholar]
- 27.Sunday ME, Shan L, Subramaniam M. Immunomodulatory functions of the diffuse neuroendocrine system: implications for bronchopulmonary dysplasia. Endocr Pathol 2004;15:91–106. [DOI] [PubMed] [Google Scholar]
- 28.Ashour KMN, Sunday ME. Bombesin given perinatally diminished alveolar development in mice [abstract]. Pediatr Res 2002;51:61A(abstract 353). [Google Scholar]
- 29.Pua ZJ, Stonestreet BS, Cullen A, Shahsafaei A, Sadowska GB, Sunday ME. Histochemical analyses of altered fetal lung development following single versus multiple courses of antenatal steroids. J Histochem Cytochem 2005;53:1459–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sunday ME, Kaplan LM, Motoyama E, Chin WW, Spindel ER. Biology of disease: gastrin-releasing peptide (mammalian bombesin) gene expression in health and disease. Lab Invest 1988;59:5–24. [PubMed] [Google Scholar]
- 31.Mantey SA, Coy DH, Pradhan TK, Igarashi H, Rizo IM, Shen L, Hou W, Hocart SJ, Jensen RT. Rational design of a peptide agonist that interacts selectively with the orphan receptor, bombesin receptor subtype 3. J Biol Chem 2001;276:9219–9229. Epub 2000 Dec 8. [DOI] [PubMed] [Google Scholar]
- 32.Massaro GD, Massaro D. Formation of alveoli in rats: postnatal effect of prenatal dexamethasone. Am J Physiol 1992;263:L37–L41. [Published erratum appears in Am J Physiol 1992;263.] [DOI] [PubMed] [Google Scholar]
- 33.Sunday ME. Bioactive peptides and lung development. In: Gaultier C, Bourbon JR, Post M, editors. Lung development, 1st ed. New York, Oxford: Oxford University Press; 1999. pp. 304–326.
- 34.Nakagawa T, Hocart SJ, Schumann M, Tapia JA, Mantey SA, Coy DH, Tokita K, Katsuno T, Jensen RT. Identification of key amino acids in the gastrin-releasing peptide receptor (GRPR) responsible for high affinity binding of gastrin-releasing peptide (GRP). Biochem Pharmacol 2005;69:579–593. Epub 2004 Dec 23. [DOI] [PubMed] [Google Scholar]
- 35.Tokita K, Hocart SJ, Coy DH, Jensen RT. Molecular basis of the selectivity of gastrin-releasing peptide receptor for gastrin-releasing peptide. Mol Pharmacol 2002;61:1435–1443. [DOI] [PubMed] [Google Scholar]
- 36.Fathi Z, Benya RV, Shapira H, Jensen RT, Battey JF. The fifth transmembrane segment of the neuromedin B receptor is critical for high affinity neuromedin B binding. J Biol Chem 1993;268:14622–14626. [PubMed] [Google Scholar]
- 37.King KA, Hua J, Torday JS, Drazen JM, Graham SA, Shipp MA, Sunday ME. CD10/neutral endopeptidase regulates fetal lung growth and maturation in utero by potentiating endogenous bombesin-like peptides. J Clin Invest 1993;91:1969–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aguayo SM, Kane MA, King TE, Schwarz MI, Grauer L, Miller YE. Increased levels of bombesin-like peptides in the lower respiratory tract of asymptomatic cigarette smokers. J Clin Invest 1989;84:1105–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagalla SR, Barry BJ, Spindel ER. Cloning of complementary DNAs encoding the amphibian bombesin-like peptides Phe8 and Leu8 phyllolitorin from Phyllomedusa sauvagei: potential role of U to C RNA editing in generating neuropeptide diversity. Mol Endocrinol 1994;8:943–951. [DOI] [PubMed] [Google Scholar]
- 40.Farstad T, Bratlid D. Incidence and prediction of bronchopulmonary dysplasia in a cohort of premature infants. Acta Paediatr 1994;83:19–24. [DOI] [PubMed] [Google Scholar]
- 41.Parker RA, Lindstrom DP, Cotton RB. Evidence from twin study implies possible genetic susceptibility to bronchopulmonary dysplasia. Semin Perinatol 1996;20:206–209. [DOI] [PubMed] [Google Scholar]
- 42.Aguayo S, King T, Kane M, Sherritt K, Silvers W, Nett L, Petty T, Miller Y. Urinary levels of bombesin-like peptides in asymptomatic cigarette smokers: a potential risk marker for smoking-related diseases. Cancer Res 1992;52(Suppl. 9):2727s–2731s. [PubMed] [Google Scholar]
- 43.Aguayo SM. Determinants of susceptibility to cigarette smoke. Am J Respir Crit Care Med 1994;149:1692–1698. [DOI] [PubMed] [Google Scholar]
- 44.Chang L, Subramaniam M, Yoder BA, Day BJ, Coalson JJ, Sunday M, Crapo JD. A catalytic antioxidant attenuates alveolar structural remodeling in bronchopulmonary dysplasia. Am J Respir Cell Mol Biol 2003;167:57–64. [DOI] [PubMed] [Google Scholar]
- 45.Dal-Pizzol F, Di Leone LP, Ritter C, Martins MR, Reinke A, Pens Gelain D, Zanotto-Filho A, de Souza LF, Andrades M, Barbeiro DF, et al. Gastrin-releasing peptide receptor antagonist effects on an animal model of sepsis. Am J Respir Crit Care Med 2006;173:84–90. Epub 2005 Sep 28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.