Abstract
Patients with spinal cord injury have an increased risk of developing respiratory tract infections as the result of expiratory muscle paralysis and consequent inability to cough. We have developed a method by which the expiratory muscles can be activated via lower thoracic and upper lumbar spinal cord stimulation to produce an effective cough mechanism. In a tetraplegic patient who required frequent (8.57 ± 2.3 times per week [mean ± SEM]) caregiver assistance to facilitate airway clearance and expectoration of secretions, three epidural electrodes were applied in the T9, T11, and L1 spinal cord regions. During stimulation at the T9 and L1 levels, airway pressures were 90 and 82 cm H2O, respectively. Peak expiratory flow rates were 6.4 L/s and 5.0 L/s; respectively. During combined (T9+L1) stimulation, airway pressure and expiratory flow rate increased to 132 cm H2O and 7.4 L/s, respectively. Addition of the third lead did not result in further increases in pressure generation. These values are characteristic of those observed with a normal subject. Because the patient is able to trigger the device independently, he no longer requires caregiver support for airway management. If confirmed in additional patients, spinal cord stimulation may be a useful method to restore an effective cough mechanism in patients with spinal cord injury.
Keywords: spinal cord injuries, cough
Patients with cervical and thoracic spinal cord injuries suffer from paralysis of a major portion of their expiratory muscles and therefore lack a normal cough mechanism (1–3). Consequently, most of these patients have a markedly reduced ability to clear airway secretions and are dependent upon caregiver support to apply suctioning, manually assisted coughing, or other techniques for airway management. Moreover, lack of an adequate cough contributes to their development of recurrent respiratory tract infections and atelectasis (4–6) and attendant high morbidity and mortality. Respiratory tract infections remain a major cause of death in this patient population (7–10).
The network of neurons in the spinal cord and peripheral neuromuscular system below the level of injury remains intact in most patients with spinal cord injury (3). Therefore, the expiratory muscles are amenable to various stimulation techniques to generate large positive airway pressures and peak expiratory flow rates with the potential to produce a functionally effective cough mechanism (11–13).
METHODS
This investigation was approved by the Investigational Review Boards at MetroHealth Medical Center and the National Institute of Neurological Disorders and Stroke. Informed consent was obtained from each patient before enrollment in the study.
A 52-yr-old man developed incomplete tetraplegia (C5-C6 level, ASIA C) after a motor vehicle accident 7 yr ago. Before his injury, he had smoked 1.5 packs of cigarettes per day for 30 yr. He had no history of chronic lung, heart, or cerebrovascular disease. Since his injury, he had experienced difficulty clearing his throat and expectorating secretions and relied on frequent assisted cough maneuvers each week (8.57 ± 2.3 times per week [mean ± SEM]). On several occasions, he nearly asphyxiated secondary to aspiration of food particles. He had been treated for upper respiratory tract infections on a regular basis–two to three times per year and had required antibiotics and aggressive respiratory management, including frequent manually assisted coughing and aerosolized bronchodilators. He was hospitalized for management of pneumonia, which necessitated tracheostomy and mechanical ventilation. He had a history of autonomic dysreflexia, which occurred in association with infections. On physical examination, his height and weight were 183 cm and 99 kg, respectively. His lung fields were clear to auscultation. His upper rib cage retracted slightly inward with inspiration. His abdomen was protuberant. A Foley catheter was present. Neurologically, he had complete paralysis of his lower extremities and partial paralysis of both upper extremities. Chest X-ray was clear. His FVC, FEV1, and inspiratory capacity were 1.96 L (38.8% predicted), 1.64 L (41.1% predicted), and 1.33 L (38.6% predicted), respectively. Maximal expiratory and inspiratory pressures and peak expiratory flow rates were also reduced (22 cm H2O, 72 cm H2O, and 2.4 L/s, respectively).
Based upon previous animal studies (14–16), an electrical stimulation system (ESS) was surgically implanted in a single procedure in an attempt to produce an effective cough system. After partial hemilaminectomies at the T9, T11, and L1 spinal cord levels, three 4-mm, single-lead, platinum-iridium disc electrodes (Freehand Epimysial Electrode; NeuroControl Corp., Valley View, OH) were positioned midline in the epidural space on the thecal sac at the T9, T11, and L1 levels with fluoroscopic guidance. A single-disc, ground electrode (30 mm) was positioned on the surface of the thoracic muscle fascial plane. A radiofrequency receiver (7.6 × 4.6 × 0.85 cm; 12 g) (Finetech Medical Ltd., Welwyn Garden City, Hertfordshire, UK) was placed in a subcutaneous pocket just above the left costal margin. The electrode wires were tunneled subcutaneously and connected to the receiver. Contraction of the abdominal muscles was confirmed by visual inspection and palpation during electrical stimulation applied in the operating room.
Postoperatively, stimulation was applied by activating a small, portable external transmitter (9.5 × 6 × 2.5 cm) connected to a rubberized antenna, which was secured on the skin with tape over the implanted receiver. The transmitter, powered by a rechargeable battery, delivers a radiofrequency signal to the implanted receiver, which converts this to an electrical signal that is transmitted to the electrodes. The patient underwent a conditioning period consisting of supramaximal stimulation for 6 wk. The patient also used the device as needed for clearing his throat or evacuating secretions. Because the patient had the ability to move his right hand, he was able to trigger the device independently by applying pressure to one of several buttons on the transmitter. While using the device, the patient mimicked a normal cough: He initiated an inspiratory effort just before stimulation, briefly maintained a closed glottis during stimulation, and opened his glottis while attempting to forcefully exhale.
Measurements of airway pressure, expired volume, and expiratory flow rate during stimulation were recorded using previously described conventional techniques (17, 18) through a full-face mask to assess the force of expiratory muscle contraction and cough efficacy.
The patient's requirement for caregiver assistance and need for assisted coughing were assessed over a 6-wk period before institution of the ESS. The volume of respiratory secretions evacuated was quantitated.
RESULTS
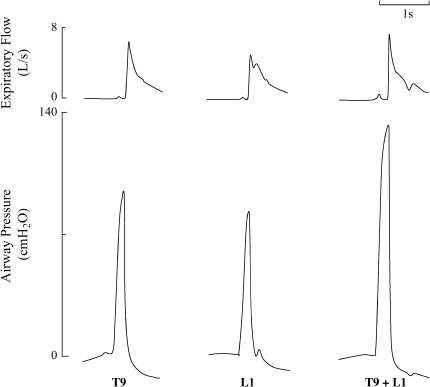
Representative tracings of airway pressures and expiratory flow rates during supramaximal stimulation at the T9 and L1 spinal cord levels and during combined stimulation at total lung capacity (TLC) after the reconditioning period are shown in Figure 1. During stimulation at the T9 and L1 levels, airway pressures were 90 and 82 cm H2O, respectively, and peak expiratory flow rates were 6.4 L/s and 5.0 L/s, respectively. During combined stimulation, airway pressure and expiratory flow rate increased to 132 cm H2O and 7.4 L/s, respectively.
Figure 1.
Representative tracings of expiratory flow and airway pressure during stimulation at T9 (40 V, 53 Hz, 150 μs), L1 (40 V, 53 Hz, 200 μs), and combined stimulation (T9+L1) at TLC.
Mean changes in airway pressure generation during supramaximal stimulation at each spinal cord level alone and in combination at FRC and TLC are shown in Figure 2. Stimulation at any one of the three sites alone resulted in similar maximal pressure changes at FRC and TLC. Stimulation of any combination of two or more electrodes resulted in significantly greater pressure generation than any single electrode alone. However, combined stimulation of the T9 and L1 levels resulted in the largest pressure development for two leads. Addition of the third lead did not result in any further increases in pressure generation. Similar results were obtained with peak flow rate generation.
Figure 2.
Mean changes ± SEM in airway pressure generation during stimulation at T9, T11 (40 V, 53 Hz, 200 μs), L1 and combined stimulation (T9+T11+L1) at FRC and TLC (stimulus parameters same as Figure 1).
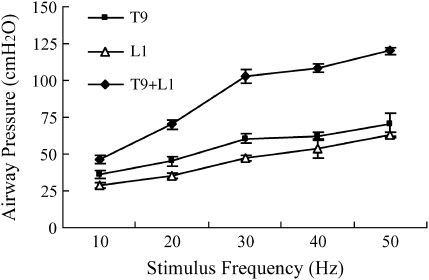
The relationship between stimulus frequency and airway pressure generation during stimulation at T9 and L1 alone and with both electrodes in combination are shown in Figure 3. With increasing stimulus frequency, there were increases in pressure generation. Beyond 30 Hz, there were only small increases in pressure generation.
Figure 3.
Relationship between stimulus frequency and mean airway pressure generation ± SEM during stimulation at T9, L1, and combined stimulation (T9+L1) at FRC (stimulus parameters same as Figure 1).
During chronic use of stimulation for perceived clinical need for cough, combined stimulation of T9 and L1 was used at supramaximal stimulus parameters. The stimulus transmitter allowed for several different combinations of stimulus parameters. The patient initially had access to a selection of a single maximal cough effort or a series of three cough efforts.
After the conditioning period, caregiver support was not required because the patient was able to manage his secretions independently on an as-needed basis. The volume of secretions remained approximately the same. The patient stated that the ESS resulted in a much more effective and comfortable method of secretion removal. Moreover, the variable waiting time of several minutes for the arrival of caregivers for airway management was eliminated.
DISCUSSION
Due to expiratory muscle paralysis, patients with spinal cord injury are dependent upon one of several methods of airway clearance (5). These include gravity, active suctioning with a catheter connected to a mechanical pump, a manually assisted cough method whereby external force is applied to the abdominal wall (19), and a mechanical insufflation–exsufflation device that applies a large positive followed by a large negative pressure to the airway (20). Each of these methods has significant limitations that restrict their efficacy, including patient discomfort, requirement of provider–patient coordination, and lack of uniform distribution of pressure within the intrathoracic cavity. Additionally, these methods are costly and labor intensive and require the presence of trained personnel. Despite the use of these various modalities, respiratory complications continue to be a major cause of morbidity and mortality in this patient population.
This report represents the first demonstration of a portable stimulation system in a tetraplegic patient resulting in airway pressures and airflows comparable to a normal cough (21–24). This patient can generate a cough on demand and effectively evacuate his secretions and clear his throat without caregiver assistance. This patient had limited movement, which facilitated use of the device. However, the transmitter could also be triggered by an object held in the mouth. Although the peak pressures achieved by our patient are somewhat lower than those described in normal subjects (21), he was unable to achieve a normal TLC. Because expiratory muscle force development is related to muscle length, which is a function of lung volume, it is likely that his reduced TLC restricted maximum force development.
Because this technique results in generalized stimulation of the lower thoracic and upper lumbar motor roots, the paraspinal muscles were also stimulated, resulting in some trunk motion. Although our patient was aware of the sensation of lower-body movement during stimulation, this was tolerated without pain or discomfort. No leg movement, bowel leakage, or symptoms or signs of autonomic dysfunction were observed.
The mechanism by which the expiratory muscles are activated through the application of electrical current over the dorsal surface of the spinal cord has been studied extensively in animals (14–16). This work was instrumental in the implementation of these techniques in humans. Based upon studies in dogs, stimulation applied in the T9 region with a single electrode results in large positive airway pressures as a consequence of direct activation of motor roots in the vicinity of the electrode and more caudal motor roots via activation of spinal cord pathways (16). Stimulation at T9 alone does not result in complete abdominal muscle activation since stimulation with a second electrode in the T13/L1 spinal cord region of the canine model results in significantly greater changes in airway pressure generation (15). Stimulation with three or more electrodes does not result in significantly greater pressure generation. Although a three-electrode system was implanted in this subject due to size differences between humans and dogs, the results in this subject suggest that a two-electrode system is adequate, as in the dog model.
Other methods of stimulating the expiratory muscles have been proposed to generate an effective cough mechanism in patients with spinal cord injury. Stimulation applied to the surface of the abdominal wall results in only modest increases in expiratory pressure generation and no significant abdominal muscle contraction in a significant number of subjects (11–13). High-frequency magnetic stimulation applied to the lower back, although a successful method of stimulating the expiratory muscles (25–27), requires precise positioning of an external coil over the back and an external power source. Because this device generates substantial heat at the stimulating coil, it also carries the risk of thermal injury. Moreover, this device is large, expensive, and not easily portable.
Because this is the first demonstration of spinal cord stimulation for these purposes, the long-term tolerance and effects are unknown. However, spinal cord stimulation has been used for over 35 yr in the treatment of chronic back pain using similar techniques as those reported here. The potential complications associated with these procedures, as with any surgical procedure, include a low incidence of infection and bleeding (28).
The ESS technique has provided this patient with significant short-term benefits in terms of airway management. Overall quality of life is also improved. For example, our patient no longer must wait for caregiver assistance to clear his throat or provide assisted cough maneuvers. In addition, his need for caregiver assistance while traveling with family or for recreational activities has been eliminated. Longer-term, this technique has the potential to reduce the morbidity and mortality associated with respiratory complications prevalent in this patient population.
Supported by National Institute of Neurological Disorders and Stroke grant R01 NS-049516 and by National Center for Research Resources grant M01 RR-000080 granted to the General Clinical Research Center of MetroHealth Medical Center.
Originally Published in Press as DOI: 10.1164/rccm.200601-097CR on March 16, 2006
Conflict of Interest Statement: A.F.D. holds a patent (5,999,855), not assigned to any commercial entity, for the technology discussed in this manuscript. K.E.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.T.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.R.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.DeTroyer A, Estenne M, Heilporn A. Mechanism of active expiration in tetraplegic subjects. N Engl J Med 1986;314:740–744. [DOI] [PubMed] [Google Scholar]
- 2.Estenne M, DeTroyer A. Cough in tetraplegic subjects: an active process. Ann Intern Med 1990;112:22–28. [DOI] [PubMed] [Google Scholar]
- 3.Kelly BJ, Luce JM. The diagnosis and management of neuromuscular diseases causing respiratory failure. Chest 1991;99:1485–1494. [DOI] [PubMed] [Google Scholar]
- 4.McMichan JC, Michel L, Westbrook PR. Pulmonary dysfunction following traumatic quadriplegia: recognition, prevention and treatment. JAMA 1980;243:528–531. [PubMed] [Google Scholar]
- 5.Carter RE. Respiratory aspects of spinal cord injury management. Paraplegia 1987;25:262–266. [DOI] [PubMed] [Google Scholar]
- 6.Jackson AB, Groomes TE. Incidence of respiratory complications following spinal cord injury. Arch Phys Med Rehabil 1994;75:270–275. [DOI] [PubMed] [Google Scholar]
- 7.Kiwerski JE. Factors contributing to the increased threat of life following spinal cord injury. Paraplegia 1993;31:793–799. [DOI] [PubMed] [Google Scholar]
- 8.Hartkopp A, Bronnum-Hansen H, Seidenschnur AM, Biering-Sorensen F. Survival and cause of death after traumatic spinal cord injury: a long-term epidemiological survey from Denmark. Spinal Cord 1997;35:76–85. [DOI] [PubMed] [Google Scholar]
- 9.Frankel HL, Coll JR, Charlifue SW, Whiteneck GG, Gardner BP, Jamous MA, Krishan KR, Nuseibeh I, Savic G, Sett P. Long-term survival in spinal cord injury: a fifty year investigation. Spinal Cord 1998;36:266–274. [DOI] [PubMed] [Google Scholar]
- 10.DeVivo MJ, Krause JS, Lammertse DP. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil 1999;80:1411–1419. [DOI] [PubMed] [Google Scholar]
- 11.Linder SH. Functional electrical stimulation to enhance cough in quadriplegia. Chest 1993;103:166–169. [DOI] [PubMed] [Google Scholar]
- 12.Jaeger RJ, Turba RM, Yarkony GM, Roth EJ. Cough in spinal cord injured patients: comparison of three methods to produce cough. Arch Phys Med Rehabil 1993;74:1358–1361. [DOI] [PubMed] [Google Scholar]
- 13.Jaeger RJ, Langbein EW, Kralj AR. Augmenting cough by FES in tetraplegia: a comparison of results at three clinical centers. Basic Appl Myol 1994;4:195–200. [Google Scholar]
- 14.DiMarco AF, Romaniuk JR, Supinski GS. Electrical activation of the expiratory muscles to restore cough. Am J Respir Crit Care Med 1995;151:1466–1471. [DOI] [PubMed] [Google Scholar]
- 15.DiMarco AF, Romaniuk JR, Kowalski KE, Supinski G. Pattern of expiratory muscle activation during lower thoracic spinal cord stimulation. J Appl Physiol 1999;86:1881–1889. [DOI] [PubMed] [Google Scholar]
- 16.DiMarco AF, Kowalski KE, Supinski G, Romaniuk JR. Mechanism of expiratory muscle activation during lower thoracic spinal cord stimulation. J Appl Physiol 2002;92:2341–2346. [DOI] [PubMed] [Google Scholar]
- 17.DiMarco AF, Onders RP, Kowalski KE, Miller ME, Ferek S, Mortimer JT. Phrenic nerve pacing in a tetraplegic patient via intramuscular diaphragm electrodes. Am J Respir Crit Care Med 2002;166:1604–1606. [DOI] [PubMed] [Google Scholar]
- 18.DiMarco AF, Takaoka Y, Kowalski KE. Combined intercostal and diaphragm pacing to provide artificial ventilation in patients with tetraplegia. Arch Phys Med Rehabil 2005;86:1200–1207. [DOI] [PubMed] [Google Scholar]
- 19.Braun SR, Giovannoni R, O'Connor M. Improving the cough in patients with spinal cord injury. Am J Phys Med 1984;63:1–10. [PubMed] [Google Scholar]
- 20.Bach JR. Mechanical insufflation-exsufflation: comparison of peak expiratory flows with manually assisted and unassisted coughing techniques. Chest 1993;104:1553–1562. [DOI] [PubMed] [Google Scholar]
- 21.Loudon RG, Shaw GB. Mechanics of cough in normal subjects and in patients with obstructive respiratory disease. Am Rev Respir Dis 1967;96:666–677. [DOI] [PubMed] [Google Scholar]
- 22.Langlands J. The dynamics of cough in health and in chronic bronchitis. Thorax 1967;22:88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arora NS, Gal TJ. Cough dynamics during progressive expiratory muscle weakness in healthy curarized subjects. J Appl Physiol 1981;51:494–498. [DOI] [PubMed] [Google Scholar]
- 24.Siebens AA, Kirby NA, Poulos DA. Cough following transection of spinal cord at C-6. Arch Phys Med Rehabil 1964;45:1–8. [PubMed] [Google Scholar]
- 25.Kyroussis D, Polkey MI, Mills GH, Hughes PD, Moxham J, Green M. Stimulation of cough in man by magnetic stimulation of the thoracic nerve roots. Am J Respir Crit Care Med 1997;156:1696–1699. [DOI] [PubMed] [Google Scholar]
- 26.Lin VWH, Singh H, Chitkara RK, Perkash I. Functional magnetic stimulation for restoring cough in patients with tetraplegia. Arch Phys Med Rehabil 1998;79:517–522. [DOI] [PubMed] [Google Scholar]
- 27.Lin VWH, Hsieh C, Hsiao IN, Canfield J. Functional magnetic stimulation of expiratory muscles: a noninvasive and new method for restoring cough. J Appl Physiol 1998;84:1144–1150. [DOI] [PubMed] [Google Scholar]
- 28.Turner JA, Loeser JD, Deyo RA, Sanders SB. Spinal cord stimulation for patients with failed back surgery syndrome or complex regional pain syndrome: a systematic review of effectiveness and complications. Pain 2004;108:137–147. [DOI] [PubMed] [Google Scholar]