Abstract
Micronemal proteins (MICs) are key mediators of cytoadherence and invasion for Toxoplasma gondii. Emerging evidence indicates that carbohydrate binding facilitates Toxoplasma entry into host cells. TgMIC1s recently solved structure reveals the presence of novel specialized domains able to discriminate between glycan residues. Comparison with Plasmodium erythrocyte-binding antigen 175 reveals that terminal sialic acid residues may represent a shared but tailored invasion pathway among apicomplexan parasites.
Keywords: Apicomplexa, Toxoplasma, microneme, structure, invasion
The secrets of a successful pathogen
Toxoplasma gondii is an obligate intracellular parasite with a broad host range 1. A member of the phylum Apicomplexa that includes the medically virulent pathogens Plasmodium falciparum (human malaria) and Eimeria tenella (poultry coccidiosis), T. gondii is capable of causing severe opportunistic disease in neonates and immunocompromised individuals 2. Compared to viral and bacterial infections that rely on the host for entry, invasion is a highly active process for Toxoplasma and other apicomplexans.
The success of T. gondii as a widely distributed pathogen centers on its ability to invade virtually any nucleated cell using secreted parasite proteins. Among this arsenal of proteins are the micronemal proteins (MICs) 3. MICs function in cellular adhesion and link the parasite actin/myosin system to the host surface, leveraging parasite entry into the host cell 4. TgMIC1, one of the first micronemal proteins to be characterized, contributes to both cell invasion and parasite virulence 5. Although the importance of receptor-ligand interactions during the first step of invasion of Apicomplexa has been established 4, 6–9., identification of host-cell receptors and the structure of parasite ligands have been largely elusive. In an elegant study recently published in EMBO J. 10, Blumenschein et al., reveal by X-ray crystallography and carbohydrate microarrays that TgMIC1 binds sialylated oligosaccharides using a micronemal adhesive repeat (MAR) 10. MAR bears no resemblance to other sialic acid lectins, including a key plasmodial ligand involved in host recognition: P. falciparum erythrocyte-binding antigen 175 (PfEBA175). This study represents a major advance in our understanding of invasion by delineating the structure of an important parasite ligand and the identification of its host receptor.
Mysteries of Toxoplasma adhesion
TgMIC1 is tightly associated with two other MICs called TgMIC4 and TgMIC6. As the ’meat‘ of the complex, TgMIC1 is sandwiched between its partners, it promotes folding of TgMIC6, and it efficiently binds host receptors using its two MAR domains even in the absence of TgMIC4 and TgMIC6 11. In this multifunctional capacity, TgMIC1 contributes to microneme targeting 12, attachment to the host cell surface 6, and parasite virulence 5. Although many pieces seemed to be falling into place regarding its function, several unknowns remained. For example, lactose was implicated as a receptor for TgMIC1 based on affinity chromatography, yet little evidence exists regarding the specificity of this interaction or its significance for Toxoplasma attachment to host cells 13. Also, structural analysis of TgMIC1’s C-terminal domain revealed a galectin-like fold with the potential to bind carbohydrates; however, key sugar-binding residues were absent and in their place stood hydrophobic amino acids that form the protein binding interface with TgMIC6 14. Additionally, based on primary sequence the TgMIC1 tandem MAR domains loosely resemble thrombospondin type 1 (TSP1) repeats that possess carbohydrate binding activity in thrombospondin and other proteins, but TSP1 repeats typically recognizes sulfated glycosaminoglycans (GAGs) such as heparin and have not been associated with binding lactose or other sugars. Finally, although negatively charged carbohydrates such as GAGs and sialic acid have been implicated as receptors for Toxoplasma attachment, the parasite ligands responsible for binding such carbohydrates remain largely obscure.
Atomic resolutions
Peering into the molecular basis of Toxoplasma adhesion, Blumenschein et al. 10 used X-ray crystallography to solve the structure of the TgMIC1 tandem MAR domains, which together comprise the TgMIC1 N-terminus (TgMIC1-NT). Surprisingly, these domains adopt a novel fold unrelated to any other including TSP1. Each MAR domain consists of a small, distorted barrel of five β-strands flanked on one side by an antiparallel helical bundle. Although MAR1 and MAR2 share only 27% sequence identity, their structures are highly alike except for a short extension on MAR2 that forms a β-finger possibly involved in binding TgMIC4. Native TgMIC1 and recombinant TgMIC1-NT were shown to bind host cells but, unexpectedly, these interactions were not disrupted by lactose, galactose, or heparin. The study used a carbohydrate microarray encompassing more than 200 diverse oligosaccharides to interrogate the sugar binding specificity of TgMIC1, the first application of such technology for a protozoan. Strikingly, TgMIC1-NT bound exclusively to oligosaccharides containing terminal sialic acids. This specificity was elegantly confirmed by showing that: (i) Sialic acids dose-dependently obstruct both binding of TgMIC1 to host cells and parasite invasion; (ii) Sialidase treatment of host cells impairs TgMIC1 binding and parasite invasion; and (iii) Each MAR domain is capable of binding sialic acids according to nuclear magnetic resonance (NMR) measurements.
TgMIC1-NT crystals soaked with α-2,3-sialyl-N-acetyllactosamine or α-2,6-sialyl-N-acetyllactosamine revealed binding in a shallow pocket that comprised six contiguous residues (amino acids 216–221) of MAR2. Although neither sugar occupied the MAR1 binding pocket, this is likely because crystal contacts in this region provided insufficient space for binding. MAR1 may also have a lower binding affinity than MAR2. Also, the affinity of individual MAR domains is low and binding may be cooperative, requiring use of both sites. This notion is further supported by the strong propensity of TgMIC1-NT to recognize branched, multisialylated glycans. Binding was particularly robust with sialyl residues separated by five to eight carbohydrate units, presumably because this spacing allows occupation of both binding pockets.
A tail of two (or more) sugars
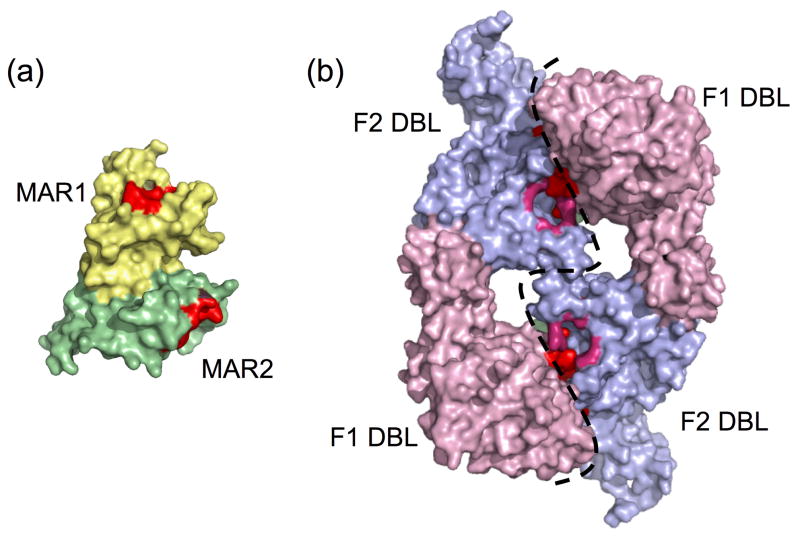
Whereas Toxoplasma tachyzoites invade and replicate in virtually any animal cell except erythrocytes, P. falciparum merozoites only infect erythrocytes. This specificity is dictated largely by apical secretory proteins including PfEBA175, a micronemal protein that recognizes the major erythrocyte surface protein glycophorin A 15. Like the MAR domains of TgMIC1, PfEBA175 binds sialic acid residues through tandem N-terminal adhesive domains called Duffy Binding Ligands (DBLs) 16. While TgMIC1 and PfEBA175 share these characteristics, they are fundamentally different on the structural level (Figure 1, Table 1). TgMIC1-NT is monomeric, whereas PfEBA175 is a dimer. The features they recognize on sialic acid are distinct and the adhesive domains differ in sequence, fold, and spacing (Figure 1, Table 1) 10, 16. Most notably, each TgMIC1 MAR domain contains a central sialic acid binding pocket 10 whereas PfEBA175 displays three sialic acid binding pockets at each of two dimer interfaces 16. Thus, while these pathogens employ MICs that recognize a similar receptor for invasion the basic mechanisms involved differ dramatically. Perhaps given the broad distribution of sialic acids among animal tissues it is not surprising that they make attractive receptors. More remarkable is how the differences in sialic acids may be selectively exploited during parasite recognition of its host 17. For example, the Plasmodium species that infects chimpanzees, P. reichenowei, preferentially binds to N-glycolylneuraminic acid (Neu5Gc), the predominant sugar on chimp erythrocytes 15, 18, 19. By contrast, the human pathogen, P. falciparum, displays a marked predilection for the metabolic precursor of Neu5Gc, Neu5Ac 19. This sugar is found on human erythrocytes. Intriguingly, humans are the only primates unable to synthesize Neu5Gc 17. The authors of the recent structural study of TgMIC1 speculate that the high binding affinity of TgMIC1 for polyvalent carbohydrates possessing two or more sialic acid resides such as gangliosides (enriched in neuronal tissue), may be a reflection of the asexual life cycle of Toxoplasma where cysts are formed within the brain 10, 20.
Figure 1.
Structural comparison of sialic acid binding domains of TgMIC1 (a) and PfEBA175 (b). The TgMIC1-NT (PDB 2JH1) is monomeric and comprised of two domains, MAR1 23 and MAR2 (green) each with one shallow SA binding pocket (red). The N-terminal region of PfEBA175 (PDB 1ZRL) consists of two domains, F1 DBL (light pink) and F2 DBL (blue) that form a homodimer with approximate two-fold symmetry. SA binding sites are positioned at the dimer interface (dashed line) and are colored pink (sites 1 and 2), red (sites 3 and 4) and green (sites 5 and 6, only partially visible from this angle).
Table 1.
Comparison of Parasite Ligandsa
| Properties | TgMIC1 | PfEBA175b |
|---|---|---|
| Target cell | Nucleated Cellc | Erythrocyte |
| Target receptor | Branched carbohydrates with two or more terminal sialic acidsd | N-acetylneuraminic acid (Neu5Ac)(α2, 3)-Gal on glycophorin A (GpA) |
| No. of ligand subunits | Monomer | Dimer |
| Adhesive domain nomenclature | MAR | DBL |
| Secondary structure composition | β-sheet enriched | α-helices enriched |
| No. of disulfide bonds formed within parasite protein | 6–8 | 13 |
| Receptor-ligand biochemistry | Novel hydrogen bonding | Salt-bridge bonding |
| Contact residues involved in glycan bindinge, f | YY219g, R217g, T126g, K216g, H218g, T220g. | N417h, R422h, N429 h, K439h, D422h, K28 h; N33i, N550i, N551i, Y552ii, K553i, M554i; T340j, K341j, D342j V343j, Y415j, Q542j, Y546j, K28j, N29j, R31j, S32j |
| No. of glycan binding sites | 2 | 6 |
| Location of glycan binding sites | Occurs centrally with each MAR domain | Occurs at dimer interface |
| Spacing between glycan binding sites | 32.3 Åk | 40.4 to 44.8 A depending on the pair |
Parasite protein is referred to as the ligand in Blumschein et al. 10
EBA175 structural components were obtained from Tolia et al. 16
Tissue cyst tropisms are observed in the central nervous system, eye, and muscle tissue 20, 24, 25.
Optimal binding for the MAR domains occurs when five to eight carbohydrates units separate the sialic acid termini.
Bold residues: Mutagenesis of these residues greatly decreased glycan binding.
Residues between K216 and E221 form a shallow binding pocket in the MAR2 domain and most of these residues make specific, direct contacts with the sialy moiety10.
Key binding residues for TgMIC1. Note: double mutant T126/T220 completely abrogates glycan binding.
Contact sites for binding of glycans 1 and 2.
Contact sites for binding of glycans 3 and 4
Contact sites for binding of glycans 5 and 6
Å=Angstrom
Correct spacing and configuration of sialic acid binding sites on the individual parasite ligands might dictate another level of host cell specificity. The sialic acid binding sites for TgMIC1 are closer together than those for PfEBA175 (Table 1). These ligands may have evolved to be highly complementary to their respective receptors. This may be especially true for Eimeria EtMIC3, a protein that harbors seven sequential MAR domains. Molecular modeling of EtMIC3 predicts a regular arrangement of binding pockets laterally positioned along its length 10. Perhaps this multivalent configuration is specifically tuned for complementarity with heavily sialylated glycoproteins in the intestinal mucosa and epithelium where Eimeria thrives.
Concluding remarks and future perspectives
Like most major advances, the important insight provided by Blumenschein et al. inspires several new questions. Does Toxoplasma rely on sialic acid binding for invasion of certain cell types more than others? This is hinted at by the finding that tachyzoite invasion is inhibited by only 30% in sialic acid deficient Chinese hamster ovary cells 21 whereas a >85% invasion block was seen in human fibroblasts exhaustively treated with sialidase or preincubated with excess sialic acid 10. Such differences might reflect the parasite’s ability to opt for the most appropriate invasion pathway for each cell type, depending on receptor availability. Accordingly, genetic evidence suggests that other micronemal proteins also contribute significantly to tachyzoite adhesion 9, 22, although in these cases the cognate receptors are less well defined. Do MAR domains contribute to T. gondii’s broad host range? Other MAR domain-expressing parasites such as Eimeria and Neospora have a much more limited host range. Therefore any contributions MAR domains make in this respect must be through recognition of an appropriately narrow or wide array of sialic acid structures. Do MAR domains recognize sialic acids in the context of particular glycoproteins? Perhaps MAR domains discriminate receptors by recognizing features of the polypeptide in addition sialylated moieties, thereby providing yet another layer of specificity. With these and other radiating lines of investigation the new molecular picture of MAR domain structure and function will likely ripple through the field for some time to come.
Acknowledgments
We gratefully acknowledge Jeanne Stuckey (The University of Michigan) for her expert help with structural modeling. We thank Rusty Bishop and Jeff Esko for helpful conversations regarding this work. Work in our laboratories is supported by grants from Ellison Medical Foundation (ID-NS-0058-02) to K.M.H. and The National Institutes of Health (R01AI046675) to V.B.C.
References
- 1.Hill DE, et al. Biology and epidemiology of Toxoplasma gondii in man and animals. Animal health research reviews/Conference of Research Workers in Animal Diseases. 2005;6:41–61. doi: 10.1079/ahr2005100. [DOI] [PubMed] [Google Scholar]
- 2.Samuel R, et al. AIDS related opportunistic infections, going but not gone. Arch Pharm Res. 2002;25:215–228. doi: 10.1007/BF02976619. [DOI] [PubMed] [Google Scholar]
- 3.Facility EQPC. Advantages of MALDI-TOF. Eastern Quebec Proteomics Core Facility. 2001 http://www.crchul.ulaval.ca/crchul/en/serv/sspeq.asp#Spectrometry.
- 4.Carruthers V, Boothroyd JC. Pulling together: an integrated model of Toxoplasma cell invasion. Curr Opin Microbiol. 2007;10:83–89. doi: 10.1016/j.mib.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 5.Cerede O, et al. Synergistic role of micronemal proteins in Toxoplasma gondii virulence. J Exp Med. 2005;201:453–463. doi: 10.1084/jem.20041672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fourmaux MN, et al. The MIC1 microneme protein of Toxoplasma gondii contains a duplicated receptor-like domain and binds to host cell surface. Mol Biochem Parasitol. 1996;83:201–210. doi: 10.1016/s0166-6851(96)02773-9. [DOI] [PubMed] [Google Scholar]
- 7.Brecht S, et al. The toxoplasma micronemal protein MIC4 is an adhesin composed of six conserved apple domains. J Biol Chem. 2001;276:4119–4127. doi: 10.1074/jbc.M008294200. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Reguet N, et al. The microneme protein MIC3 of Toxoplasma gondii is a secretory adhesin that binds to both the surface of the host cells and the surface of the parasite. Cell Microbiol. 2000;2:353–364. doi: 10.1046/j.1462-5822.2000.00064.x. [DOI] [PubMed] [Google Scholar]
- 9.Mital J, et al. Conditional Expression of Toxoplasma gondii Apical Membrane Antigen-1 (TgAMA1) Demonstrates That TgAMA1 Plays a Critical Role in Host Cell Invasion. Mol Biol Cell. 2005 doi: 10.1091/mbc.E05-04-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blumenschein TM, et al. Atomic resolution insight into host cell recognition by Toxoplasma gondii. Embo J. 2007;26:2808–2820. doi: 10.1038/sj.emboj.7601704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saouros S, et al. Complete resonance assignments of the C-terminal domain from MIC1: a micronemal protein from Toxoplasma gondii. Journal of biomolecular NMR. 2005;31:177–178. doi: 10.1007/s10858-004-8237-1. [DOI] [PubMed] [Google Scholar]
- 12.Reiss M, et al. Identification and characterization of an escorter for two secretory adhesins in Toxoplasma gondii. J Cell Biol. 2001;152:563–578. doi: 10.1083/jcb.152.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lourenco EV, et al. Toxoplasma gondii micronemal protein MIC1 is a lactose-binding lectin. Glycobiology. 2001;11:541–547. doi: 10.1093/glycob/11.7.541. [DOI] [PubMed] [Google Scholar]
- 14.Saouros S, et al. A novel galectin-like domain from Toxoplasma gondii micronemal protein 1 assists the folding, assembly, and transport of a cell adhesion complex. J Biol Chem. 2005;280:38583–38591. doi: 10.1074/jbc.C500365200. [DOI] [PubMed] [Google Scholar]
- 15.Chattopadhyay D, et al. The structure of the Plasmodium falciparum EBA175 ligand domain and the molecular basis of host specificity. Trends Parasitol. 2006;22:143–145. doi: 10.1016/j.pt.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tolia NH, et al. Structural basis for the EBA-175 erythrocyte invasion pathway of the malaria parasite Plasmodium falciparum. Cell. 2005;122:183–193. doi: 10.1016/j.cell.2005.05.033. [DOI] [PubMed] [Google Scholar]
- 17.Bishop JR, Gagneux P. Evolution of carbohydrate antigens--microbial forces shaping host glycomes? Glycobiology. 2007;17:23R–34R. doi: 10.1093/glycob/cwm005. [DOI] [PubMed] [Google Scholar]
- 18.Chou HH, et al. Inactivation of CMP-N-acetylneuraminic acid hydroxylase occurred prior to brain expansion during human evolution. Proc Natl Acad Sci U S A. 2002;99:11736–11741. doi: 10.1073/pnas.182257399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin MJ, et al. Evolution of human-chimpanzee differences in malaria susceptibility: relationship to human genetic loss of N-glycolylneuraminic acid. Proc Natl Acad Sci U S A. 2005;102:12819–12824. doi: 10.1073/pnas.0503819102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubey JP. Tissue cyst tropism in Toxoplasma gondii: a comparison of tissue cyst formation in organs of cats, and rodents fed oocysts. Parasitology. 1997;115( Pt 1):15–20. doi: 10.1017/s0031182097008949. [DOI] [PubMed] [Google Scholar]
- 21.Monteiro VG, et al. Host cell surface sialic acid residues are involved on the process of penetration of Toxoplasma gondii into mammalian cells. FEMS Microbiol Lett. 1998;164:323–327. doi: 10.1111/j.1574-6968.1998.tb13105.x. [DOI] [PubMed] [Google Scholar]
- 22.Huynh MH, Carruthers VB. Toxoplasma MIC2 is a major determinant of invasion and virulence. PLoS pathogens. 2006;2:e84. doi: 10.1371/journal.ppat.0020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sam-Yellowe TY. Rhoptry organelles of the apicomplexa: Their role in host cell invasion and intracellular survival. Parasitol Today. 1996;12:308–316. doi: 10.1016/0169-4758(96)10030-2. [DOI] [PubMed] [Google Scholar]
- 24.Boothroyd JC, Grigg ME. Population biology of Toxoplasma gondii and its relevance to human infection: do different strains cause different disease? Curr Opin Microbiol. 2002;5:438–442. doi: 10.1016/s1369-5274(02)00349-1. [DOI] [PubMed] [Google Scholar]
- 25.Kodjikian L, et al. Ocular manifestations in congenital toxoplasmosis. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle. Ophthalmologie. 2006;244:14–21. doi: 10.1007/s00417-005-1164-3. [DOI] [PubMed] [Google Scholar]