Abstract
Much of the permanent damage that occurs in response to nervous system damage (trauma, infection, ischemia, etc.) is mediated by endogenous secondary processes that can contribute to cell death and tissue damage (excitotoxicity, oxidative damage and inflammation). For humans to evolve mechanisms to minimize secondary pathophysiological events following CNS injuries, selection must occur for individuals who survive such insults. Two major factors limit the selection for beneficial responses to CNS insults: for many CNS disease states the principal risk factor is advanced, post-reproductive age and virtually all severe CNS traumas are fatal in the absence of modern medical intervention. An alternative hypothesis for the persistence of apparently maladaptive responses to CNS damage is that the secondary exacerbation of damage is the result of unavoidable evolutionary constraints. That is, the nervous system could not function under normal conditions if the mechanisms that caused secondary damage (e.g., excitotoxicity) in response to injury were decreased or eliminated. However, some vertebrate species normally inhabit environments (e.g. hypoxia in underground burrows) that could potentially damage their nervous systems. Yet, profound neuroprotective mechanisms have evolved in these animals indicating that natural selection can occur for traits that protect animals from nervous system damage. Many of the secondary processes and regeneration-inhibitory factors that exacerbate injuries likely persist because they have been adaptive over evolutionary time in the healthy nervous system. Therefore, it remains important that researchers consider the role of the processes in the healthy or developing nervous system to understand how they become dysregulated following injury.
“I was taught that the human brain was the crowning glory of evolution so far, but I think it's a very poor scheme for survival.” –Kurt Vonnegut. London Observer (27 December 1987)
1. Introduction
The vertebrate central nervous system (CNS) is a remarkably complex organ system consisting of hundreds of billions of neurons and trillions of connections among them. Neuroscientists are continually fascinated by the immense capacity of the nervous system and its remarkable ability to remodel itself in response to experience and the environment. Evolutionary biologists view the vertebrate nervous system as the result of countless generations of fine-tuning through natural selection. This has produced an organ system that has been called the most complicated machine ` in the known universe. The ability of the nervous system to perceive, store, recall, and analyze complex information is immense.
In contrast, the responses of an injured nervous system are not nearly so elegant. Indeed, much of the permanent damage that occurs in response to nervous system damage (trauma, infection, ischemia, etc.) is mediated by endogenous secondary processes that can contribute to cell death and tissue damage (Bramlett and Dietrich, 2004). Thus, considered in the context of the rest of the body’s responses to injury, the vertebrate nervous system response to injury is fair at best. This limited ability to repair or sustain neural tissue after injury suggests that selection has not acted (or at least not to the same extent) on the processes that occur following severe nervous system damage as it has on processes that isolate the nervous system from potential injuries. For example, the brain and spinal cord are protected from physical trauma by encasement in bony structures, and from peripheral infection and some chemical influences by the blood brain barrier. Also, sophisticated cognitive abilities, including fear, risk assessment, and impediments to impulsivity, have evolved to minimize risky behaviors that might lead to CNS damage (Nesse and Williams, 1996).
Cerebrovascular and cardiovascular diseases are currently among the most common causes of serious brain injury. It is unlikely, however, that the evolution of CNS responses to trauma has been shaped by exposure to these so-called diseases of modernity. First, they likely were not experienced by many ancestral humans because individuals tended to live much shorter, less sedentary, lives than humans today. Second, variation is the grist upon which natural selection acts; thus, even among relatively young individuals who suffered strokes or other brain trauma, natural selection may not have been able to act because variance in outcome was presumably nonexistent—all stricken individuals likely died prior to the advent of modern medical technology (Strasser et al., 1996). Greatly impaired survival following damage to the nervous system impedes the evolution of mechanisms that would tend to promote adaptive responses to injury. This hypothesis has important implications for the study and clinical treatment of humans who suffer CNS damage today.
For humans and nonhuman animals to evolve mechanisms to minimize secondary pathophysiological events following CNS injuries, selection must occur for individuals who survive such insults or related processes. Two major factors limit the selection pressure for beneficial responses to CNS insults: (1) for many CNS disease states the principal risk factor is advanced, post-reproductive age and (2) virtually all severe CNS traumas are fatal in the absence of modern medical intervention. In terms of age, among modern humans 95–97% of all ischemic strokes occur in patients 45 years of age or older. However, the average lifespan in hunter-gatherer societies can be conservatively estimated at approximately 28 years, even after adjusting for elevated infant mortality (Holliday, 2005). For example, members of the Yanomami Indians of South America, an extant hunter-gatherer society, have an average lifespan of only 20–22 years (Meindl, 1992). Although there are inherent limitations to inferring past human traits based on extant aboriginal cultures (Bettinger, 1991), general patterns emerge that may be useful in understanding past selection pressures (Lee and Devore, 1968). Even if survivable strokes or other types of injuries occur in these traditional settings, successful reproduction following injury would be highly unlikely. In modern medically-advanced countries, sexual motivation and performance decline following CNS injury (Anderson et al., 2005; Dahlberg et al., 2007; Giaquinto et al., 2003; Rees et al., 2007); the effect persists even after controlling for the economic burden of having children with an injured- partner and potentially sexually-unattractive traits associated with CNS damage (Giaquinto et al., 2003). Thus, the decline in reproductive motivation among individuals with CNS injury further limits selection.
Secondly, almost all types of serious CNS injuries are fatal in the absence of immediate and/or long-term medical care. To illustrate the argument that ancestral humans or non-human animals could not have survived nervous system injury, we will briefly consider some of the medical interventions that are necessary to keep patients with spinal cord injuries (SCI) alive. For example, one medical intervention that was unavailable to our ancestors in cases of traumatic SCI is surgical decompression of the cord; this procedure, when conducted within 24 hours of injury, is typically associated with shorter inpatient hospitalization and greater neurological recovery (Fehlings and Perrin, 2005; McKinley et al., 2004). In the hours and days following traumatic or ischemic SCI, most patients are in a critical condition. Shock (spinal, neurogenic, hypovolemic, or cardiogenic) can occur and often requires both fluid and vasopressor administration to normalize blood pressure (Wuermser et al., 2007). Sepsis is also a concern and often requires treatment with high doses of powerful antibiotics. Another potentially lethal side-effect of SCI is deep vein thrombosis and pulmonary embolism, a condition that is treated prophylactically with anticoagulant drugs such as low-molecular weight heparin (Saito et al., 2005). Even if patients can be stabilized following SCI, they then require costly, time-consuming and in some cases potentially risky interventions for extended periods after the injury (Dryden et al., 2005; Payne et al., 2002). These interventions can include (but are not limited to) repeated catheterizations, bowel treatments, turning to avoid pressure sores, and monitoring to prevent infections (Wuermser et al., 2007).
Such sophisticated medical care could not have been provided by pre-industrial humans let alone hunter-gatherer societies. In industrialized nations SCI-related mortality has been reduced to less than half of the approximately 90% mortality that occurred prior to the First World War (DeVivo et al., 1999; Guttman, 1976; Yeo et al., 1998). Similarly, in developing countries with less advanced health care infrastructures, such as Nigeria and Zimbabwe, mortality rates for CNS damage were still approximately 90% as late as the 1970s (Avivi et al., 1999; Nwuga, 1979). Finally, the potential argument that non-human animals might be able to survive spinal injuries with less care is belied by the extensive human intervention needed to keep both laboratory rats and monkeys viable following experimental SCI (Santos-Benito et al., 2006).
It should be noted that both less severe injuries and also neonatal hypoxia ischemia likely were survived by both ancestral humans and non-human animals. In considering the evidence for evolutionarily-shaped responses to injury it will be beneficial to consider what selection pressures have existed over evolutionary time and how natural selection may have shaped the responses to mild and more severe injuries. If such mechanisms exist to counteract minor CNS damage, clinical interventions might be able to expand and improve these mechanisms to treat more severe CNS trauma. Additionally, natural selection can operate when survival rates are low. For example, although rare, survival of serious CNS insults occurs.
2. Common pathways underlying secondary nervous system damage
This review focuses on three types of clinically-relevant CNS insults: infection, physical trauma, and ischemia. Importantly, these three classes of injuries have the potential to kill neurons and other CNS cell-types directly and initiate a pathological cascade that can exacerbate tissue damage. However, all three types of CNS damage share pathophysiological features common to virtually all other types of CNS injuries. The majority of nervous system injuries are characterized by a two-phase process of cell death and tissue damage. Primary injuries are the direct result of the precipitating insult. For example, in traumatic CNS injury many cells are killed immediately by injury from mechanical membrane disruption, hemorrhage, and ischemia (Bramlett and Dietrich, 2004; Pettus et al., 1994). Similarly, following stroke, many neurons die from acute energetic failure and the resulting inability of affected cells to maintain membrane potentials (Hertz et al., 1992; Sapolsky, 1985; Sapolsky and Pulsinelli, 1985). It is difficult to spare CNS tissue injured by these primary processes. However, following traumatic brain injury and ischemia, as well as in most other CNS injuries, a significant proportion of cell death occurs during the hours and days after the initial insult. Secondary cell death presents an attractive target for clinical intervention because the temporal lag between injury and cell loss provides a potential treatment window (Kermer et al., 1999). These secondary processes are mediated by endogenous factors and are not the direct result of injury. Here, we focus on three interactive and overlapping secondary processes that can serve to exacerbate the initial injury: (1) excitotoxicity, (2) oxidative damage, and (3) inflammation.
Although a complete overview of the mechanisms of secondary tissue damage following CNS injuries is beyond the scope of this review, a brief overview of key secondary pathophysiological processes that exacerbates primary injuries including excitotoxicity, oxidative damage, and inflammation are included below.
2.1 Immune and inflammatory responses in CNS injuries
The CNS, once considered independent from interactions with the immune system (immune privileged), is now recognized to be in constant communication with the immune system via neural (reviewed in (Nance and Sanders, 2007; Tracey, 2002)) and endocrine (reviewed in (Webster et al., 2002)) systems. The CNS has intrinsic immune cells, such as microglia and immunologically-active astrocytes, which are potent regulators of immune responses. The CNS actively regulates neuroimmune interactions under normal physiological conditions via the blood brain barrier and anti-inflammatory cytokines such as interleukin-10 and interleukin-1 receptor antagonist (IL-1 and IL1-ra respectively) (Carson et al., 2006; Ransohoff et al., 2003). Within minutes of trauma, an immune response ensues and the surrounding tissue becomes immersed in a pro-inflammatory and pro-thrombotic environment (Bramlett and Dietrich, 2004; Wang et al., 2007). This inflammatory cascade includes activation of microglia and astrocytes, release of soluble factors such as cytokines and chemokines, infiltration of blood-leukocytes such as monocytes/macrophages, neutrophils, and T-cells, as well as upregulation of adhesion molecules to facilitate cell adhesion, migration, and extravasation to the site of injury (Bramlett and Dietrich, 2004; Danton and Dietrich, 2003; Ransohoff et al., 2003; Siesjo and Siesjo, 1996; Wang et al., 2007). The inflammatory response is an attempt to restore homeostasis via elimination of pathogens and removal of damaged cells. This response typically leads to apoptosis and deleterious effects on surrounding healthy tissue due to the release of toxic substances (Beattie et al., 2000; Casha et al., 2001). Typically, in non-CNS tissue, such as skin, this damage is an acceptable cost of inflammation because the tissue can be repaired or regenerated over time. The limited ability of CNS tissue to regenerate accounts for its strong predisposition to permanent damage from inflammatory-related damage. Although many cytokines are involved in the inflammatory cascade, interleukin-1β (IL-1β) has been identified as the primary regulator of the CNS pro-inflammatory cascade associated with secondary cell death (Allan et al., 2005; Basu et al., 2005; Touzani et al., 2002). After CNS injury, IL-1β expression is associated with increased neuronal damage (Friedman, 2001). Likewise, administration of exogenous IL-1β increases tissue damage and inflammation (Loddick and Rothwell, 1996), whereas administration of exogenous IL-1ra significantly reduces neural injury (Relton and Rothwell, 1992). Notably, not all inflammation is pathogenic; IL-1 also has protective functions (Mason et al., 2001) (See section on Immune Privilege). Mice that have been genetically engineered to lack specific inflammatory gene receptors, such as TNF-α p55/p75, display increased cell death in animal models of CNS pathology (Gary et al., 1998). To focus solely on pro-inflammatory cytokines would be an overly simplified portrayal of the inflammatory response as it has been recognized that the anti-inflammatory cytokines play a regulatory role in CNS damage. IL-10 and IL-1ra knockout mice have increased neuronal damage following trauma (Boutin and Rothwell, 2002; Grilli et al., 2000). Additionally, infusions of IL-1ra & IL-10 into trauma sites are neuroprotective (Bethea et al., 1999; Knoblach and Faden, 1998; Nesic et al., 2001). Inflammation may play a neuroprotective role in some forms of trauma (Correale and Villa, 2004) and although acute inflammatory events may be devastating to CNS tissue, some delayed inflammatory events can potentially be reparative and beneficial (reviewed in (Hohlfeld et al., 2000; Kerschensteiner et al., 1999) and see below).
2.2 Excitotoxicity
One of the striking features of the evolution of vertebrate nervous systems is that the amino acid glutamate is both the most prevalent amino acid and neurotransmitter within the CNS (Fonnum, 1984), and one of the key instigators of secondary injury. Within minutes after CNS trauma, toxic elevations of extracellular glutamate can be detected at the site of injury (Farooque et al., 1996; Liu et al., 1991; McAdoo et al., 1999). Importantly, a key early pathophysiological event in most types of CNS injury is energetic failure (associated with ischemia or disruption of the vasculature or other processes that interfere with normal cellular metabolism) that quickly exhausts limited energy stores. One of the most energy-intensive cellular processes in neurons is the maintenance of membrane polarization through ionic transporters. When these transporters fail, neurons (and other CNS cell types) are unable to maintain their membrane polarization and are thus more easily excitable and more likely to release excitatory transmitters such as glutamate. This quickly leads to a maladaptive cycle wherein cells become more depolarized (and unable to repolarize) and more likely to release additional neurotransmitters including glutamate (Dirnagl et al., 1999; Katsura et al., 1994). In other words, increased excitatory signals are present in the extracellular environment and less input is required to drive synaptic activity.
Increased glutamate is particularly toxic to cells that have been weakened by other processes (Arundine et al., 2003; Kass and Lipton, 1982; Lucas and Newhouse, 1957). Glutamate induces synaptic responses via metabotropic and ionotropic receptor systems leading to increased cell excitability and increases in intracellular calcium (Choi, 1988). Metabotropic glutamate receptors activate G proteins that induce the release of calcium from intracellular stores. On the other hand, activation of ionotropic receptors increases membrane permeability to sodium, potassium, and calcium ions (Choi, 1988).
After trauma, excess glutamate binds to extracellular receptors, such as NMDA (N-methyl-D-aspartate) and AMPA (α-amino-3hydroxy-5methyl-4isoxazole propionic acid) receptors, resulting in excessive intracellular calcium levels via increases in both ion influx and phospholipase C mediated release from intercellular stores (Agrawal et al., 1998). Calcium ions are critical mediators of multiple intracellular functions such as enzyme phosphorylation, exocytosis, and cell excitability, and therefore are under tight cellular regulatory control via ion transporters and reservoirs (Carafoli, 1987). The inability of cells to maintain a homeostatic balance of intracellular calcium levels following CNS injury leads to inappropriate activation of numerous downstream signals resulting in over-activation of mitochondria, phospholipase, and protein kinases (Lipton and Rosenberg, 1994; Park et al., 2004). The calcium-induced activation of these enzymes leads to degradation of proteins and membranes and impairs cellular survival (Casha et al., 2001; Liu et al., 1997). Additionally, the toxic levels of intracellular calcium and downstream signaling molecules can lead to mitochondrial dysfunction, cytochrome C release, and apoptosis (Rego and Oliveira, 2003). Further evidence for the role of excitotoxicity in CNS injuries is evident from studies demonstrating the efficacy of NMDA antagonists to attenuate nervous tissue injury in both ischemia (Bao et al., 2001) and traumatic brain injury (Faden et al., 2001). Similarly, the infusion of high doses of excitatory neurotransmitters leads to excitotoxic damage similar to that seen in CNS trauma models (Canudas et al., 2003; Micu et al., 2006). Neurons are not unique in their vulnerability to excitotoxic damage as multiple CNS cells types such as oligodendrocytes (Matute et al., 1997; McDonald et al., 1998) and astrocytes (Haas and Erdo, 1991) are susceptible to similar damage. The dysregulation of astrocytes can lead to an inability to buffer extracellular glutamate levels and a subsequent increase in glutamate levels (Haas and Erdo, 1991; Rothstein et al., 1996) resulting in a vicious cycle of excitotoxicity and subsequent cell death.
2.3 Oxidative damage
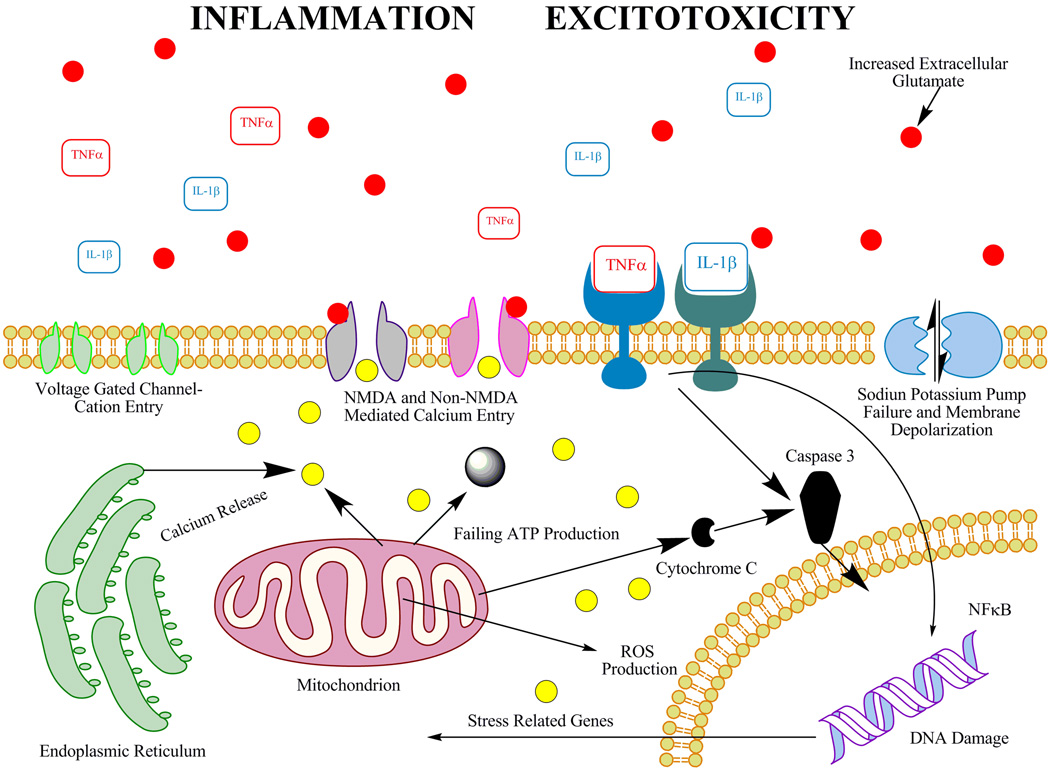
The CNS consumes a disproportionate amount of the body's oxygen, as it derives its energy almost exclusively from oxidative metabolism of the mitochondrial respiratory chain (Coyle and Puttfarcken, 1993). Nervous tissue utilizes oxygen for vital oxygenase and oxidase activities such as lipoxygenase, heme oxygenase, and tyrosine hydroxylase (Kim et al., 1999; Tyurin et al., 2000). The asymmetrical consumption of oxygen molecules by the CNS leaves it exceedingly susceptible to damaging effects of oxidative stress. Reactive oxygen species (ROS) have been implicated as a source of pathogenesis in multiple CNS disorders including neurodegenerative diseases (Barnham et al., 2004; Charvin et al., 2005), ischemia (Chan, 2001; Piantadosi and Zhang, 1996b) and traumatic brain and spinal cord injury (Robertson et al., 2007; Xu et al., 2005). Treatment with ROS synthesis inhibitors, such as diphenyleneiodonium, attenuates nervous tissue damage following CNS damage (Kajihara et al., 1973; Nagel et al., 2007). ROS, such as superoxide, nitric oxide, and hydroxyl radicals, are normally produced as a byproduct of the mitochondrial respiratory chain and account for 1–2% of all molecular oxygen metabolism under normal physiological conditions (Orrenius et al., 2007). Organisms have evolved mechanisms for abrogating the damaging effects of ROS by oxidative scavenging via low molecular weight anti-oxidant molecules such as such vitamin E and glutathione, as well as anti-oxidant enzymes such as glutathione peroxidase and superoxide dismutase. Under normal physiological conditions, ROS are kept under tight control by these anti-oxidant compounds and do not lead to pathology. Following nervous system trauma, acute ischemia is common (Hlatky et al., 2003) and reoxygenation during reperfusion induces a marked increase in ROS via mitochondrial dysfunction (Piantadosi and Zhang, 1996a). Additionally, ROS compounds are released in excitotoxic neurons following trauma (Singh et al., 2003). The subsequent calcium influx due to excitotoxicity increases ROS production, leading to inhibited ATP synthesis, release of cytochrome c, as well as mitochondrial permeability transitions (Sullivan et al., 2005). Elevation in ROS levels leads to the inability of the cellular environment to scavenge the nocent molecules that lead to oxidative damage (Shohami et al., 1997). The injury-induced imbalance of antioxidant and ROS induces a runaway reaction in which deleterious ROS molecules are released in increasing amounts resulting in an array of pathological effects (Saito et al., 2005). ROS mediated pathological cascades include altered cellular protein activity (Chan, 1996), DNA damage (Slupphaug et al., 2003), and lipid degradation (Azbill et al., 1997). ROS also lead to excitotoxicity via calcium- induced mitochondrial dysfunction (Mattson and Chan, 2003) and axon demyelination (Smith et al., 1999). An additional mode of ROS-mediated CNS damage is via alterations in blood brain barrier permeability which mediates increased recruitment in inflammatory cells further potentiating inflammatory damage (van der Goes et al., 1998). Figure 1 summarizes some of the secondary mechanisms of cell death following ischemia or trauma.
Figure 1.
Schematic of some of the secondary mechanisms of neuronal cell death following traumatic or ischemic injury (Barnham et al., 2004; Basu et al., 2005; Bramlett and Dietrich, 2004; Dirnagl et al., 1999; Katsura et al., 1994; Lipton and Rosenberg, 1994; Liu et al., 1991; Park et al., 2004; Singh et al., 2003; Wang et al., 2007) and references in the text.
2.4 Immune privilege
The CNS has decidedly limited ability to regenerate damaged or dead cells. Vertebrate evolution has favored a strategy of protecting the CNS from potential injuries rather than evolving mechanisms to regenerate damaged CNS tissue. Immune responses in the CNS are greatly restricted compared to other regions of the body, a phenomenon termed ‘immune privilege’. Because immune mediated inflammatory responses can have catastrophic and irreversible effects on CNS cells, this restriction makes adaptive and intuitive sense. The general principle of immune privilege and the consequences of this evolutionary strategy for recovery and regeneration following CNS injury are discussed below.
The concept of immune privilege was based on work from the 19th and 20th centuries which demonstrated that foreign tissue grafts persisted in the eye and brain, but were readily rejected when grafted into the periphery (Medawar, 1948; van Dooremal, 1873). Peter Medawar interpreted this phenomenon, along with the lack of readily apparent afferent lymphatic drainage, as evidence that antigens were sequestered in the CNS and thus the immune system was ‘ignorant’ of the antigenic milieu of the CNS (Medawar, 1948). Indeed, an additional allogeneic graft from the same donor in the periphery could initiate rejection of the graft in the CNS (Medawar, 1948) suggesting that the efferent arm was intact (Medawar, 1948). Over time and with the advent of sensitive immunological techniques this idea that the afferent arm of the immune system was deficient in the CNS has been greatly revised because cells or soluble antigens injected into the CNS or the anterior chamber of the eye (a tissue with highly similar immunological properties and developmental origins as the brain) both enter the general circulation and induce antibody production (Gordon et al., 1992; Kaplan and Streilein, 1977). Indeed, it is now well-established that the CNS hardly ‘ignores’ immunological stimuli; rather, the restriction of immunological activity in the CNS is a highly regulated, active, and adaptive process (Niederkorn, 2006).
Various processes in the CNS restrict immune-mediated damage. In general, adaptive immune responses in the CNS rely on humoral (antibody-mediated; Th2) rather than notoriously destructive cell-mediated (Th1) mechanisms, such as delayed-type hypersensitivity (DTH) (Niederkorn, 2006). This pattern is particularly salient when an animal is immunized with a soluble antigen behind the blood brain barrier resulting in systemic suppression of DTH and cytotoxic T-lymphocytes activity while sparing (or even enhancing) antibody responses, thereby reducing the possibility of T cell priming and entry into the CNS (Gordon et al., 1992; Harling-Berg et al., 1991; Wenkel et al., 2000). Although nearly all nucleated vertebrate cells constitutively express the major histocompatibility complex type 1 antigen, neuronal cells and cells from other critical tissues that cannot regenerate express MHC-1 weakly or not at all (Lampson and Fisher, 1984). The lack of MHC-1 expression putatively protects virally infected neuronal cell types from cytotoxic T-cell mediated lysis. In the CNS both soluble and membrane-bound proteins participate in the suppression of immune responses. For instance, most CNS cell types (i.e., neurons, glia, and endothelial cells) constitutively express the cytokine Fas-ligand (Fas-L), the receptor for which is on most leukocytes (Choi and Benveniste, 2004; Niederkorn, 2006). The ligand and receptor are part of the TNF and TNF receptor superfamilies, respectively, and are both upregulated by inflammation (Choi and Benveniste, 2004; Locksley et al., 2001). Fas-Fas-L interactions are involved in a variety of immunomodulatory actions, but also can induce apoptosis in infiltrating leukocytes and thus tonically suppress CNS inflammation (Choi and Benveniste, 2004). Further, cerebrospinal fluid (CSF) and extracellular fluid in the CNS contains relatively high concentrations of immunomodulatory proteins such as transforming growth factor-β (TGFβ), α-melanocyte stimulating hormone (α-MSH), calcitonin gene-related peptide (CGRP), and vasoactive intestinal peptide (VIP) with strong anti-inflammatory and immunosuppressive properties (Taylor, 1996; Taylor and Streilein, 1996; Taylor et al., 1994).
It has been proposed that the relative restriction of immunological activity within the CNS could potentially limit neuroregeneration and exacerbate damage following injury (Bechmann, 2005). Although there is no question that inflammatory responses are major components of many types of CNS pathology, evidence exists that immune cells and soluble mediators can participate in regenerative processes. For instance, T cells appear to be capable of buffering glutamate and secreting neuroprotective factors including neurotrophins. Additionally, one of the major inhibitors of axonal regeneration in the CNS is myelin; T-cell responses can direct myelin debris phagocytosis. So-called ‘protective autoimmunity’ may occur when myelin reactive T cells abrogate CNS injury (Yoles et al., 2001). However, in some cases, the presence of myelin reactive T cells can exacerbate injury following spinal cord trauma (Jones et al., 2002; Popovich et al., 1997).
Considered together, the concept of immune privilege is interesting from an evolutionary perspective for several reasons. It is a testament to both the critical role of the CNS and the potential for immune-mediated pathology that such a system of relative immunological tolerance has evolved, especially because this renders the CNS susceptible to infection. Presumably, the costs of allowing viruses to persist in CNS tissues is outweighed by the heavy burden of killing all virally-infected neurons (and other CNS cell-types) (Kwidzinski et al., 2003). The remarkable susceptibility to immune-mediated tissue damage following CNS insult in concert with the suppression of most types of inflammatory responses in the healthy CNS strongly supports the hypothesis that selection has not occurred for responses to CNS injury. If vertebrate evolution had been faced with selection pressure to minimize secondary damage to the CNS following an insult rather than preventing insults, then such a system would likely have evolved very differently. Although this could merely reflect constraints in the underlying substrates upon which evolution through natural selection had to act. In other words, selection has responded to the negative consequences of inflammation in the CNS and instead of selection favoring individuals whose neurons do not die from inflammation-associated mechanisms, it has favored the strategy of isolation of the CNS from those mechanisms. It is critical to emphasize that we are not suggesting that designing experimental or clinical interventions to break or modulate CNS immune privilege would be beneficial in CNS injury (although some labs have reported beneficial effects while others have reported exacerbation of injury with this type of manipulation); rather, it is important to be aware of how CNS immune responses function under basal conditions and the nature of the selection pressures that formed these patterns when considering how to design interventions.
3. Neuroregeneration
The postnatal mammalian nervous system has relatively limited capacity to regenerate following injury. Although there are specific populations of cells that remain mitotic into adulthood, the vast majority of neurons that are present in adult nervous systems cannot be replaced if they die. Additionally, traumatic and ischemic injuries often result in loss of axons without the loss of the neuron itself (Edgerton et al., 2004; Yiu and He, 2006), but a variety of factors inhibit the regeneration of axons through the site of injury. Importantly, peripheral axons are capable of regeneration. However, no fundamental differences exist between peripheral and central neurons; indeed dorsal root ganglia neurons send axons back into the spinal cord and out into the periphery. The peripheral axons regenerate after transection, but the central branches do not (Ramon y Cajal, 1928; Schnell and Schwab, 1990). Peripheral neurons implanted into the CNS also fail to regenerate, suggesting that the CNS has inhibitory signals that prevent axonal outgrowth (Busch and Silver, 2007). Two large classes of molecules inhibit axonal regeneration in the CNS: (1) the myelin associated molecules and those associated with extracellular matrix and (2) glial scars which are often formed after CNS injuries.(Rhodes and Fawcett, 2004). The scar is formed largely by reactive astrocytes that proliferate and secrete a variety of extracellular matrix proteins. Axons are not able to extend through areas of gliosis. Ramón y Cajal originally described the axonal response which he termed as ‘dystrophic endbulbs’ and interpreted this morphological state as evidence of permanent quiescence (Ramon y Cajal, 1928). It is now apparent that within the glial scar and healthy myelin there are a variety of proteins that inhibit axonal regeneration. These proteins, such as the chondroitin sulfate proteoglycans, aggregan and versican, as well as neuronal-glial antigen 2 are part of the physiological mediators that prevent aberrant axonal connections in the adult brain (Matthews et al., 2002; Rhodes and Fawcett, 2004), but are also upregulated following injury. That is not to say that the gliotic scar is not an important part of the response to mild CNS injury. Faulkner and colleagues (Faulkner et al., 2004) created transgenic mice carrying a viral gene under the control of the glial fibrillary acidic protein promoter. Treatment with the antiviral drug ganciclovir ablated proliferating astrocytes and thus prevented the formation of the glial scar. Mechanical lesions in the wild-type animals produced small circumscribed lesions, whereas transgenic mice treated with ganciclovir exhibited dramatically exacerbated inflammatory responses, blood-brain barrier disruption, demyelination, and cell loss (Faulkner et al., 2004). Taken together, these studies suggest that the injured CNS does not favor a strategy of regeneration, but rather one of minimizing further damage. Several proximate reasons can explain why regeneration occurs in the periphery, but not in the CNS (e.g., oligodendrocytes-derived myelin inhibitory proteins, inhibitory extracellular matrix, etc.). From a proximate perspective, however, it seems likely that injuries to peripheral nerves occurred and were associated with injuries that were sufficiently mild to survive. Presumably, the evolutionary pressure for preventing aberrant axonal connectivity outweighed the potential benefits of axonal regeneration.
Notably, regenerative capacity of the nervous system is not consistent across development. Young organisms can suffer relatively large lesions of the nervous system and suffer only mild functional impairments (Vargha-Khadem et al., 1994) (but see (Anderson et al., 2005)) depending on the type of injury sustained. At the cellular level there is evidence for spontaneous regeneration of CNS cells and axons (Bregman and Goldberger, 1982; Gu et al., 2000; Liu et al., 1998). Importantly, even later in life human brains retain remarkable capacity for recovery of function that may be mediated by the remaining components of distributed circuitry or the assumption of new tasks by spared tissue (reviewed in Stein and Hoffman, 2003). Two key points are (1) the nervous system may have evolved some redundancy in order to prevent mild CNS injuries from inducing devastating functional consequences and (2) many endogenous repair processes work ‘better’ during early development and some of the repair processes that occur in adulthood resemble those that occur during normal development (Filbin, 2006; Teitelbaum et al., 1969; Wolgin et al., 1980). Taken together, these data provide further evidence that plasticity is limited but possible in the injured nervous system and that it is much better able to restore function following mild than severe injuries.
4. Evolution of Neuroprotective Traits
The strategy to identify and describe the physiological and pathophysiological processes that occur in injured nervous system tissue and then design compounds that can influence those pathways to provide neuroprotection has been slow. An alternative approach would be to examine natural phenomena, such as hibernation and aestivation, to discover naturally-selected solutions to ischemia that might serve as starting points for understanding, which in turn may lead to interventions for limiting human brain damage. The thesis of this review is that natural selection has not optimized the responses to nervous system damage because in the majority of cases CNS damage is fatal and thus there has been little variation upon which natural selection could act. An alternative hypothesis for the myriad of apparently maladaptive responses to CNS damage is that evolutionary processes have shaped the responses to injury and the secondary exacerbation of damage is the result of unavoidable evolutionary constraints. That is, the nervous system could not function under normal conditions if the mechanisms that caused secondary damage (e.g., excitotoxicity) in response to injury were decreased or eliminated. Presumably, evolution has selected for trade-offs between beneficial responses to injury to the extent that it can without selecting for adaptations that interfere with normal nervous system function.
A comparative approach to test this hypothesis would identify species that experience (and that survive) an insult to the nervous system in its natural habitat. If those species have evolved (1) mechanisms to reduce or prevent damage from that insult and (2) these mechanisms are distinct, qualitatively and/or quantitatively, from similar mechanism in animals that do not face similar types of challenges, then we can conclude that evolution of traits that are protective against neurological damage is possible.
There are several examples of animal species that appear to satisfy the criteria that mechanisms have evolved to combat neural insult. For instance, in the wild, heterothermic (hibernating) mammals undergo physiological events that closely resemble ischemia-reperfusion. Hibernation is characterized by a marked reduction in metabolic, respiratory, and heart rates (Tan et al., 2005). In some species, cerebral blood flow is reduced by <90% during torpor (Dave et al., 2006). Return to the euthermic (non-hibernating) state is an energetically demanding event that induces transient, but severe hypoxia. Additionally, as in clinical ischemia-reperfusion injury, the return of oxygenated blood following prolonged oxygen reduction has the potential to exacerbate ischemic damage. However, the return to the euthermic state is not associated with CNS damage in hibernating animals. Even in the euthermic state, the arctic ground squirrel brain (Spermophilus parryi) is remarkably resistant to ischemia-reperfusion injury. Hippocampal slices from hibernating ground squirrels survive oxygen glucose deprivation (OGD; an in vitro model of ischemia), as well as NMDA-induced cell death when compared to both interbout euthermic arctic ground squirrels and laboratory rats (Ross et al., 2006). Similarly, an in vivo cardiac arrest procedure that produces severe neuronal cell loss in laboratory rats induced virtually no histological evidence of cell death in euthermic arctic ground squirrels (Dave et al., 2006). There has been great interest in identifying the mechanisms that heterothermic animals use to protect their nervous systems from IR injury during hibernation. Arctic ground squirrels appear to utilize a number of neuroprotective mechanisms in addition to hypothermia, including increased expression of antioxidant enzymes (Drew et al., 1999), alterations in NMDA receptor dynamics (Zhao et al., 2006), ion channel arrest, hypocoagulability (Svihla et al., 1951), and suppression of inflammatory processes (Zhou et al., 2001; Zhu et al., 2005). However, extracellular striatal GABA concentrations fall during torpor and glutamate remains relatively constant between torpor and euthermy.
The brains of arctic ground squirrels appear to be chronically, but mildly, hypoxic during the euthermic state as measured by enhanced expression of hypoxia-inducible factor 1 alpha (HIF-1α)(Ma et al., 2005). It is possible that alterations in metabolic processes that allow arctic ground squirrels to survive torpor and cardiac arrest have deleterious consequences for cerebral metabolism in the euthermic state. It would be useful to determine whether this chronic mild hypoxia has functional costs for arctic ground squirrels during euthermia, suggesting that an evolutionary trade-off has occurred.
Other vertebrates with specialized life-history traits also utilize a variety of neuroprotective strategies (Lutz, 1992; Lutz et al., 1996). For instance, many aquatic turtles exhibit dramatic resistance to anoxia. In nature, these animals spend months continuously submerged under frozen water (Carr, 1952). In the laboratory, freshwater painted turtles (Chrysemys picta) can survive for months in severely hypoxic 3° C water (Jackson and Heisler, 1983). These turtles are both more resistant to anoxia in general, and able to enter a physiological state of profound anoxia-resistance in response to reduced oxygen availability (Lutz, 1992). Although not identical, the mechanisms display an overall similarity between the adaptations associated with anoxia-tolerance in mammals (Lutz, 1992). In general, turtles enter a state of profound hypometabolism and hypothermia in response to hypoxic conditions. Additionally, the glutamate:GABA ratio decreases, neurotransmitter dynamics change (Lutz and Milton, 2004), and ion channels are blocked (Lutz and Milton, 2004; Nilsson and Lutz, 1991). Turtle brain slices are resistant to oxygen deprivation, as well as glutamate toxicity (Wilson and Kriegstein, 1991). Other vertebrate species including fossorial mammals, frogs, and cold-water fish also exhibit anoxia-tolerance (Ultsch, 1989). For instance, blind mole rats (Spalax ehrenbergi) tolerate hypoxia far better than other rodent species, and do so in large part by increasing the density of blood vessels and angiogenic signals like vascular endothelial growth factor in the brain and in the periphery (Avivi et al., 1999; Band et al., 2007).
An additional ‘natural’ example of IR occurs during parturition in mammals. Low in utero oxygen pressure and the temporal gap between delivery and the onset of spontaneous breathing can be conceptualized as an example of physiological IR. Not surprisingly, neonatal mammalian brains are much more resistant to ischemia reperfusion injury than conspecific adult brains (Duffy et al., 1975; Singer, 1999). There is now strong cellular evidence that neonatal brains possess several strategies that aid in preventing ischemic damage. Elevated melatonin concentration, resistance to energetic depletion, and altered NMDA receptor composition, distribution and attenuated receptor currents have all been described in response to hypoxia in neonatal mammal brain (Hansen, 1977; Singer, 1999; Tan et al., 2005). Few researchers interested in perinatal hypoxia have addressed the question of why (from the ultimate, adaptive functional perspective) mammals lose this relative resistance to hypoxia as they mature. What do adult mammals gain by giving up this relative hypoxia resistance with increasing age? Has natural selection favored the loss of hypoxia tolerance in order to allow the full capacity of the CNS to be reached in adulthood? These ‘why questions’ are important to address in order to achieve a complete understanding of the ‘how questions’ including the mechanisms underlying the constraints and possibilities of brain injury recovery.
The neurohormone melatonin also has been implicated as a neuroprotectant agent for individuals that experience physiological ischemia-reperfusion. Melatonin is the principal secretory product of the pineal gland although it is also produced by a variety of other tissues. In addition to its role in biological timing, melatonin is also a potent antioxidant with the capacity to scavenge free radicals directly and also upregulate other antioxidant enzymes. Elevated melatonin concentrations may be an adaptation that aids animals in surviving ischemia-reperfusion that they experience in their natural habitat (Tan et al., 2005). For instance, in a variety of hibernating animals including hamsters and snakes melatonin concentrations increase rapidly during arousal from torpid states (Mendonca et al., 1995;, 1996; Vanecek et al., 1984) Diving marine mammals generally have extremely large pineal glands, as well as high basal melatonin concentrations during the time of the year when diving most often occurs (Aarseth and Stokkan, 2003; Ralph, 1975)
Estrogens represent an additional class of endogenous hormones with potent neuroprotective effects (McCullough and Hurn, 2003); through middle age, men are more likely to suffer from a stroke then women (Barrett-Connor and Bush, 1991). This relative advantage declines at menopause suggesting a role for ovarian steroids generally and estrogens specifically. In several animal models of global and focal cerebral ischemia, males display more tissue damage than females despite similar insults (Barrett-Connor and Bush, 1991; Jover et al., 2002; Shughrue and Merchenthaler, 2003; Touzani et al., 2002). Surgical, genetic, or pharmacological interruption of estrogen signaling eliminates this female advantage (McCullough and Hurn, 2003). Exogenous estrogen administration reduces tissue damage when administered to either males or females (Toung et al., 1998). Estrogenic effects on ischemic tissue are mediated through both receptor-dependent and -independent mechanisms. Estrogens have neuroprotective effects during acute ischemia/reperfusion largely via its effects on cerebral vasculature (e.g. increasing cerebral blood flow) (Farhat et al., 1996; Krause et al., 2006), through its antioxidant properties (Moosmann and Behl, 1999), and by altering inflammatory responses (Santizo et al., 2000; Vegeto et al., 2006). Estrogens also can aid in functional recovery during the period after reperfusion by promoting angiogenesis (Ardelt et al., 2005) and synaptogenesis (McEwen and Woolley, 1994), and by preventing delayed apoptosis (Dubal et al., 2006; Jover et al., 2002).
Progestins also appear to be protective in experimental stroke traumatic brain injury (Labombarda et al., 2006; Sayeed et al., 2007; Stein et al., 2008). Specifically, high endogenous (related to sex or estrous cyclicity) or exogenous concentrations of progesterone were associated with significant neuroprotection in rats with either experimental strokes or traumatic forebrain injuries (Jiang et al., 1996; Roof et al., 1993) Progesterone also appears to be efficacious across a relatively wide therapeutic window and in both males and females (Galani et al., 2001). Progesterone also has been successful in a recent clinical trial with traumatic brain injury patients(Wright et al., 2007). The mechanisms of action of progesterone include increasing inhibitory neurotransmission, regulation of inflammatory and oxidative responses and antagonizing cell death cascades as well has having significant effects on tissue regeneration (Belelli et al., 2006; Galani et al., 2001; Grossman et al., 2004; McEwen and Woolley, 1994; Roof et al., 1997; Shear et al., 2002) Although, there is little evidence that damaged tissue produces de novo steroids or increases gonadal or adrenal estrogen production to take advantage of these neuroprotective effects progesterone and pregnenolone (direct precursor of progesterone) increase after spinal cord injury and also nonspecifically after psychological stressors (Labombarda et al., 2006; Persengiev et al., 1991). Taken together these data suggest that progesterone modification of CNS pathophysiology may be part of an extant endogenous neuroprotective system.
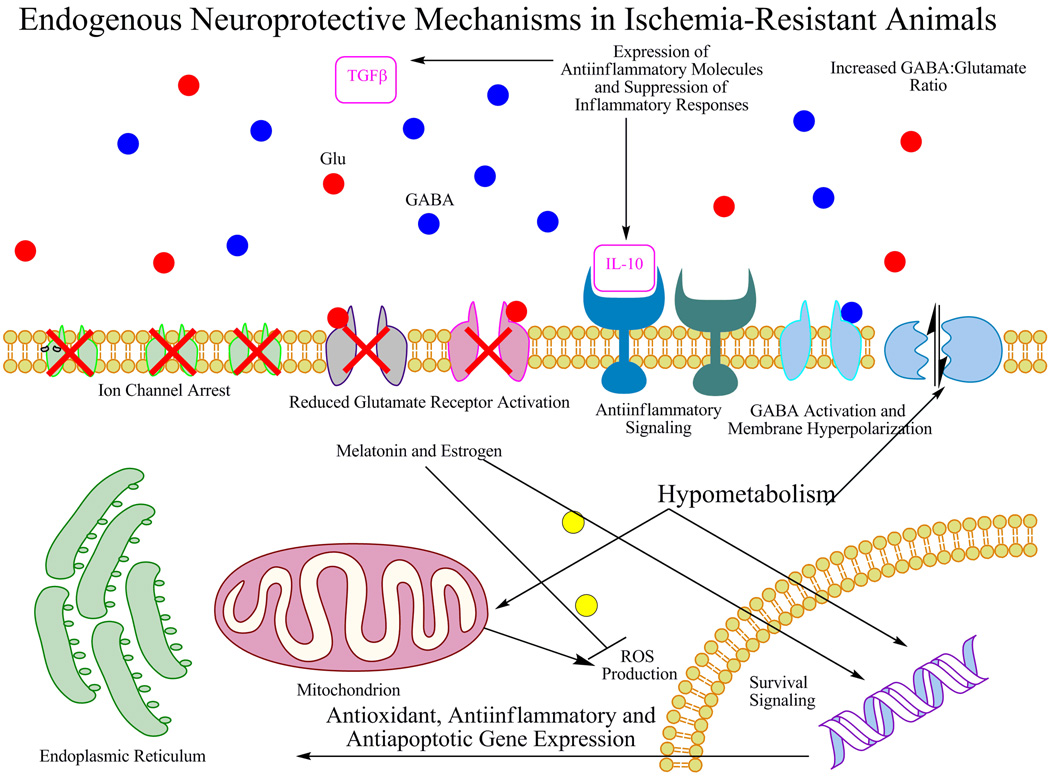
Another important neuroprotective mechanism, ischemic preconditioning, occurs when tissues are transiently exposed to a non-injurious stimulus prior to a more severe ischemic event (Gidday, 2006). Animals that undergo brief anoxia are better able to survive a later, more severe, and prolonged period of anoxia (Dahl and Balfour, 1964). These brief preconditioning events induce a series of processes that do not typically occur in the ischemic CNS and require de novo protein synthesis and the expression of many neuroprotective genes (Kawahara et al., 2004; Stenzel-Poore et al., 2003). Overall, preconditioning establishes, at least temporarily, a relative ischemia-resistant brain through the attenuation of many classes of injury including oxidative and excitotoxic injury, and ultimately the prevention of apoptotic and necrotic cell death (Dhodda et al., 2004). Many stimuli, such as proinflammatory cytokines (Nawashiro et al., 1997), forced exercise (Stummer et al., 1995), and metabolic inhibitors (Pera et al., 2004) (among others) in addition to hypoxia and ischemia (Stagliano et al., 1999; Tang et al., 2006; Ueda and Nowak, 2005) provide effective pre-conditioning and reduce damage both in vivo and in vitro from different models of cerebral ischemia (reviewed in (Gidday, 2006)). Many of the same neuroprotective mechanisms that exist in heterothermic mammals can be induced by these preconditioning stimuli including an increase in inhibitory neurotransmission, ion channel arrest and hypometabolism within the CNS (Dhodda et al., 2004; Stenzel-Poore et al., 2003). It is conceivable that ischemic preconditioning represents an unmasking of latent neuroprotective mechanisms that are vestigial under physiological (non-heterothermic) conditions. What is apparent, however, is that the CNS can be coaxed into a relatively protected state with preconditioning providing the best evidence that physiological constraints do not prevent the evolution of endogenous neuroprotection. These mechanisms are extant in modern humans and other mammals, but are largely not utilized in cases of cerebral ischemia without preconditioning. Thus, it seems possible in theory that natural selection could have favored the initiation of many of these underutilized processes if given the opportunity. Figure 2 summarizes some of the extant neuroprotectant strategies that exist in animals discussed above.
Figure 2.
Summary of neuroprotective mechanisms that exist in animals that experience hypoxia in their natural environments. Including, ion channel arrest, increased GABA:glutamate ratio, anti-inflammatory, anti-apoptotic and anti-oxidant defenses, reduced glutamate-mediated depolarization and metabolic suppression (Dave et al., 2006; Drew et al., 1999; Gidday, 2006; Lutz et al., 1996; Ross et al., 2006; Stenzel-Poore et al., 2003; Tang et al., 2006; Zhao et al., 2006) and references in the text.
Taken together, these data suggest that mechanisms can (and have) evolved to subserve tolerance to ischemia-reperfusion injury. The specific mechanisms that have evolved to render neuronal tissue resistant to IR injury are varied and often tailored to the specific challenges facing these animals. These examples offer ‘existence proof’ that it is possible to evolve mechanisms that protect the nervous system from damage. Therefore, it seems unlikely that the responses of non-heterothermic mammalian brains to injury have been directly selected for over time. In other words, if ancestral humans or nonhuman animals had survived neuronal insult caused by trauma, stroke, or cardiovascular disease over evolutionary history, then we can presume that neuronal responses to injury in laboratory animals and modern humans might be very different.
5. Conclusions
Despite intensive research, the numbers of effective clinical treatments to minimize or reverse damage to the nervous system remain limited. The general strategy of basic neuroscientists and clinical researchers has been to identify and describe the physiological and pathophysiological processes that occur in injured nervous system tissue and then design compounds that can manipulate those pathways to provide neuroprotection. Although much has been learned about the physiology of CNS injury, this approach has produced few effective clinical interventions. However, there have been some successes particularly in the use of therapeutic hypothermia and steroid hormones in clinical trials (Wright et al., 2007; Zeiner et al., 2000).
Although perhaps no descriptor is more damning in current grant reviews than ‘phenomenological’ (except perhaps ‘descriptive’), clinical and translational research would be well-served to address both the basic phenomena in neurobiology and also return to a strategy of using natural phenomena such as species that exhibit ischemic tolerance or regenerative abilities to start to address clinical issues. There are few medical problems that evolution failed to address and constructing an injury-resistant brain is certainly not one of them. Researchers who are able to capitalize on the ‘work’ of natural selection to solve problems associated with ischemic CNS tissue will likely gain important insights. For two key reasons, researchers must move past the dichotomous thinking that certain processes that occur after an injury are adaptive or detrimental. First, rarely is anything so simple as “good” or “bad”; indeed, in most cases (e.g. inflammation) nearly every process has both detrimental and beneficial aspects, and it is probably best to view adaptations in terms of costs versus benefits. Second, many of the secondary processes and regeneration-inhibitory factors persist because they have been adaptive over evolutionary time. Therefore, it remains important that researchers consider the role of the processes in the healthy or developing CNS in order to understand how they become dysregulated following injury.
Acknowledgments
The authors thank Gary Berntson, Kate Karelina, Lynn Martin and Kristen Navara for helpful discussion. Preparation of this manuscript was supported by NSF grant IOS 04-16897 (RJN), NIH grants NS40267 and HL080249 (ACD), The American Heart Association (EIA award to ACD) and The Ohio State University Presidential Fellowship (ZMW).
Abbreviations
- α-MSH
alpha-melanocyte stimulating hormone
- AMPA
α-amino-3hydroxy-5methyl-4isoxazole propionic acid
- ATP
Adenosine Triphosphate
- CGRP
Calcitonin generelated peptide
- CNS
Central Nervous System
- CSF
Cerebrospinal Fluid
- DTH
Delayed-type Hypersensitivity
- GABA
gamma-Amino Butyric acid
- HIF-1α
Hypoxia inducible factor 1 alpha
- IL-1β
Interleukin-1 beta
- IL-1ra
Interleukin-1ra
- IL-10
Interleukin 10
- IR
Ischemia-reperfusion
- MHC
Major Histocompatibility Complex
- NMDA
N-methyl-D-aspartate
- OGD
Oxygen Glucose Deprivation
- ROS
Reactive Oxygen Species
- SCI
Spinal Cord Injury
- TGFα
Transforming Growth Factor alpha
- TNFα
Tumor Necrosis Factor Alpha
- VIP
Vasoactive Intestinal Peptide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarseth JJ, Stokkan KA. Quantitative differences in the pineal ultrastructure of perinatal and adult harp (Phoca groenlandica) and hooded seals (Cystophora cristata) J Pineal Res. 2003;35:188–195. doi: 10.1034/j.1600-079x.2003.00076.x. [DOI] [PubMed] [Google Scholar]
- Agrawal SK, Theriault E, Fehlings MG. Role of group I metabotropic glutamate receptors in traumatic spinal cord white matter injury. J Neurotrauma. 1998;15:929–941. doi: 10.1089/neu.1998.15.929. [DOI] [PubMed] [Google Scholar]
- Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- Anderson V, Catroppa C, Morse S, Haritou F, Rosenfeld J. Functional plasticity or vulnerability after early brain injury? Pediatrics. 2005;116:1374–1382. doi: 10.1542/peds.2004-1728. [DOI] [PubMed] [Google Scholar]
- Ardelt AA, McCullough LD, Korach KS, Wang MM, Munzenmaier DH, Hurn PD. Estradiol regulates angiopoietin-1 mRNA expression through estrogen receptor-alpha in a rodent experimental stroke model. Stroke. 2005;36:337–341. doi: 10.1161/01.STR.0000153795.38388.72. [DOI] [PubMed] [Google Scholar]
- Arundine M, Chopra GK, Wrong A, Lei S, Aarts MM, MacDonald JF, Tymianski M. Enhanced vulnerability to NMDA toxicity in sublethal traumatic neuronal injury in vitro. J Neurotrauma. 2003;20:1377–1395. doi: 10.1089/089771503322686166. [DOI] [PubMed] [Google Scholar]
- Avivi A, Resnick MB, Nevo E, Joel A, Levy AP. Adaptive hypoxic tolerance in the subterranean mole rat Spalax ehrenbergi: the role of vascular endothelial growth factor. FEBS Lett. 1999;452:133–140. doi: 10.1016/s0014-5793(99)00584-0. [DOI] [PubMed] [Google Scholar]
- Azbill RD, Mu X, Bruce-Keller AJ, Mattson MP, Springer JE. Impaired mitochondrial function, oxidative stress and altered antioxidant enzyme activities following traumatic spinal cord injury. Brain Res. 1997;765:283–290. doi: 10.1016/s0006-8993(97)00573-8. [DOI] [PubMed] [Google Scholar]
- Band M, Shams I, Joel A, Avivi A. Cloning and in vivo expression of vascular endothelial growth factor receptor 2 (Flk1) in the naturally hypoxia-tolerant subterranean mole rat. Faseb J. 2007 doi: 10.1096/fj.07-8892com. [DOI] [PubMed] [Google Scholar]
- Bao WL, Williams AJ, Faden AI, Tortella FC. Selective mGluR5 receptor antagonist or agonist provides neuroprotection in a rat model of focal cerebral ischemia. Brain Res. 2001;922:173–179. doi: 10.1016/s0006-8993(01)03062-1. [DOI] [PubMed] [Google Scholar]
- Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Bush TL. Estrogen and coronary heart disease in women. Jama. 1991;265:1861–1867. [PubMed] [Google Scholar]
- Basu A, Lazovic J, Krady JK, Mauger DT, Rothstein RP, Smith MB, Levison SW. Interleukin-1 and the interleukin-1 type 1 receptor are essential for the progressive neurodegeneration that ensues subsequent to a mild hypoxic/ischemic injury. J Cereb Blood Flow Metab. 2005;25:17–29. doi: 10.1038/sj.jcbfm.9600002. [DOI] [PubMed] [Google Scholar]
- Beattie MS, Farooqui AA, Bresnahan JC. Review of current evidence for apoptosis after spinal cord injury. J Neurotrauma. 2000;17:915–925. doi: 10.1089/neu.2000.17.915. [DOI] [PubMed] [Google Scholar]
- Bechmann I. Failed central nervous system regeneration: a downside of immune privilege? Neuromolecular Med. 2005;7:217–228. doi: 10.1385/NMM:7:3:217. [DOI] [PubMed] [Google Scholar]
- Belelli D, Herd MB, Mitchell EA, Peden DR, Vardy AW, Gentet L, Lambert JJ. Neuroactive steroids and inhibitory neurotransmission: mechanisms of action and physiological relevance. Neuroscience. 2006;138:821–829. doi: 10.1016/j.neuroscience.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Bethea JR, Nagashima H, Acosta MC, Briceno C, Gomez F, Marcillo AE, Loor K, Green J, Dietrich WD. Systemically administered interleukin-10 reduces tumor necrosis factor-alpha production and significantly improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma. 1999;16:851–863. doi: 10.1089/neu.1999.16.851. [DOI] [PubMed] [Google Scholar]
- Bettinger RL. Hunter-Gatherers: Archaeological and Evolutionary Theory. New York: Springer; 1991. [Google Scholar]
- Boutin H, Rothwell NJ. Cerebral ischaemic processes and cytokines: can transgenic mice help? In: Krieglstein J, Klumpp S, editors. Pharmacology of cerebral ischemia. Stuttgart: Medpharm; 2002. pp. 183–190. [Google Scholar]
- Bramlett HM, Dietrich WD. Pathophysiology of cerebral ischemia and brain trauma: similarities and differences. J Cereb Blood Flow Metab. 2004;24:133–150. doi: 10.1097/01.WCB.0000111614.19196.04. [DOI] [PubMed] [Google Scholar]
- Bregman BS, Goldberger ME. Anatomical plasticity and sparing of function after spinal cord damage in neonatal cats. Science. 1982;217:553–555. doi: 10.1126/science.7089581. [DOI] [PubMed] [Google Scholar]
- Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007;17:120–127. doi: 10.1016/j.conb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Canudas AM, Pubill D, Sureda FX, Verdaguer E, Camps P, Munoz-Torrero D, Jimenez A, Camins A, Pallas M. Neuroprotective effects of (+/−)-huprine Y on in vitro and in vivo models of excitoxicity damage. Exp Neurol. 2003;180:123–130. doi: 10.1016/s0014-4886(02)00029-8. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Carr A. Handbook of Turtles. Ithaca, NY: Cornell University Press; 1952. [Google Scholar]
- Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC. CNS immune privilege: hiding in plain sight. Immunol Rev. 2006;213:48–65. doi: 10.1111/j.1600-065X.2006.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casha S, Yu WR, Fehlings MG. Oligodendroglial apoptosis occurs along degenerating axons and is associated with FAS and p75 expression following spinal cord injury in the rat. Neuroscience. 2001;103:203–218. doi: 10.1016/s0306-4522(00)00538-8. [DOI] [PubMed] [Google Scholar]
- Chan PH. Role of oxidants in ischemic brain damage. Stroke. 1996;27:1124–1129. doi: 10.1161/01.str.27.6.1124. [DOI] [PubMed] [Google Scholar]
- Chan PH. Reactive Oxygen Radicals in Signaling and Damage in the Ischemic Brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Charvin D, Vanhoutte P, Pages C, Borrelli E, Caboche J. Unraveling a role for dopamine in Huntington's disease: the dual role of reactive oxygen species and D2 receptor stimulation. Proc Natl Acad Sci U S A. 2005;102:12218–12223. doi: 10.1073/pnas.0502698102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C, Benveniste EN. Fas ligand/Fas system in the brain: regulator of immune and apoptotic responses. Brain Res Brain Res Rev. 2004;44:65–81. doi: 10.1016/j.brainresrev.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Choi DW. Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci. 1988;11:465–469. doi: 10.1016/0166-2236(88)90200-7. [DOI] [PubMed] [Google Scholar]
- Correale J, Villa A. The neuroprotective role of inflammation in nervous system injuries. J Neurol. 2004;251:1304–1316. doi: 10.1007/s00415-004-0649-z. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Dahl NA, Balfour WM. Prolonged Anoxic Survival Due to Anoxia Pre-Exposure: Brain Atp, Lactate, and Pyruvate. Am J Physiol. 1964;207:452–456. doi: 10.1152/ajplegacy.1964.207.2.452. [DOI] [PubMed] [Google Scholar]
- Dahlberg A, Alaranta HT, Kautiainen H, Kotila M. Sexual activity and satisfaction in men with traumatic spinal cord lesion. J Rehabil Med. 2007;39:152–155. doi: 10.2340/16501977-0029. [DOI] [PubMed] [Google Scholar]
- Danton GH, Dietrich WD. Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol. 2003;62:127–136. doi: 10.1093/jnen/62.2.127. [DOI] [PubMed] [Google Scholar]
- Dave KR, Prado R, Raval AP, Drew KL, Perez-Pinzon MA. The arctic ground squirrel brain is resistant to injury from cardiac arrest during euthermia. Stroke. 2006;37:1261–1265. doi: 10.1161/01.STR.0000217409.60731.38. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Krause JS, Lammertse DP. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil. 1999;80:1411–1419. doi: 10.1016/s0003-9993(99)90252-6. [DOI] [PubMed] [Google Scholar]
- Dhodda VK, Sailor KA, Bowen KK, Vemuganti R. Putative endogenous mediators of preconditioning-induced ischemic tolerance in rat brain identified by genomic and proteomic analysis. J Neurochem. 2004;89:73–89. doi: 10.1111/j.1471-4159.2004.02316.x. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Drew KL, Osborne PG, Frerichs KU, Hu Y, Koren RE, Hallenbeck JM, Rice ME. Ascorbate and glutathione regulation in hibernating ground squirrels. Brain Res. 1999;851:1–8. doi: 10.1016/s0006-8993(99)01969-1. [DOI] [PubMed] [Google Scholar]
- Dryden DM, Saunders LD, Jacobs P, Schopflocher DP, Rowe BH, May LA, Yiannakoulias N, Svenson LW, Voaklander DC. Direct health care costs after traumatic spinal cord injury. J Trauma. 2005;59:443–449. doi: 10.1097/01.ta.0000174732.90517.df. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Rau SW, Shughrue PJ, Zhu H, Yu J, Cashion AB, Suzuki S, Gerhold LM, Bottner MB, Dubal SB, Merchanthaler I, Kindy MS, Wise PM. Differential modulation of estrogen receptors (ERs) in ischemic brain injury: a role for ERalpha in estradiol-mediated protection against delayed cell death. Endocrinology. 2006;147:3076–3084. doi: 10.1210/en.2005-1177. [DOI] [PubMed] [Google Scholar]
- Duffy TE, Kohle SJ, Vannucci RC. Carbohydrate and energy metabolism in perinatal rat brain: relation to survival in anoxia. J Neurochem. 1975;24:271–276. doi: 10.1111/j.1471-4159.1975.tb11875.x. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Tillakaratne NJ, Bigbee AJ, de Leon RD, Roy RR. Plasticity of the spinal neural circuitry after injury. Annu Rev Neurosci. 2004;27:145–167. doi: 10.1146/annurev.neuro.27.070203.144308. [DOI] [PubMed] [Google Scholar]
- Faden AI, O'Leary DM, Fan L, Bao W, Mullins PG, Movsesyan VA. Selective blockade of the mGluR1 receptor reduces traumatic neuronal injury in vitro and improvesoOutcome after brain trauma. Exp Neurol. 2001;167:435–444. doi: 10.1006/exnr.2000.7577. [DOI] [PubMed] [Google Scholar]
- Farhat MY, Lavigne MC, Ramwell PW. The vascular protective effects of estrogen. Faseb J. 1996;10:615–624. [PubMed] [Google Scholar]
- Farooque M, Hillered L, Holtz A, Olsson Y. Changes of extracellular levels of amino acids after graded compression trauma to the spinal cord: an experimental study in the rat using microdialysis. J Neurotrauma. 1996;13:537–548. doi: 10.1089/neu.1996.13.537. [DOI] [PubMed] [Google Scholar]
- Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehlings MG, Perrin RG. The role and timing of early decompression for cervical spinal cord injury: update with a review of recent clinical evidence. Injury. 2005;36 Suppl 2:B13–B26. doi: 10.1016/j.injury.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Filbin MT. Recapitulate development to promote axonal regeneration: good or bad approach? Philos Trans R Soc Lond B Biol Sci. 2006;361:1565–1574. doi: 10.1098/rstb.2006.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonnum F. Glutamate: a neurotransmitter in mammalian brain. J Neurochem. 1984;42:1–11. doi: 10.1111/j.1471-4159.1984.tb09689.x. [DOI] [PubMed] [Google Scholar]
- Friedman WJ. Cytokines regulate expression of the type 1 interleukin-1 receptor in rat hippocampal neurons and glia. Exp Neurol. 2001;168:23–31. doi: 10.1006/exnr.2000.7595. [DOI] [PubMed] [Google Scholar]
- Galani R, Hoffman SW, Stein DG. Effects of the duration of progesterone treatment on the resolution of cerebral edema induced by cortical contusions in rats. Restor Neurol Neurosci. 2001;18:161–166. [PubMed] [Google Scholar]
- Gary DS, Bruce-Keller AJ, Kindy MS, Mattson MP. Ischemic and excitotoxic brain injury is enhanced in mice lacking the p55 tumor necrosis factor receptor. J Cereb Blood Flow Metab. 1998;18:1283–1287. doi: 10.1097/00004647-199812000-00001. [DOI] [PubMed] [Google Scholar]
- Giaquinto S, Buzzelli S, Di Francesco L, Nolfe G. Evaluation of sexual changes after stroke. J Clin Psychiatry. 2003;64:302–307. doi: 10.4088/jcp.v64n0312. [DOI] [PubMed] [Google Scholar]
- Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7:437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- Gordon LB, Knopf PM, Cserr HF. Ovalbumin is more immunogenic when introduced into brain or cerebrospinal fluid than into extracerebral sites. J Neuroimmunol. 1992;40:81–87. doi: 10.1016/0165-5728(92)90215-7. [DOI] [PubMed] [Google Scholar]
- Grilli M, Barbieri I, Basudev H, Brusa R, Casati C, Lozza G, Ongini E. Interleukin-10 modulates neuronal threshold of vulnerability to ischaemic damage. Eur J Neurosci. 2000;12:2265–2272. doi: 10.1046/j.1460-9568.2000.00090.x. [DOI] [PubMed] [Google Scholar]
- Grossman KJ, Goss CW, Stein DG. Effects of progesterone on the inflammatory response to brain injury in the rat. Brain Res. 2004;1008:29–39. doi: 10.1016/j.brainres.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Gu W, Brannstrom T, Wester P. Cortical neurogenesis in adult rats after reversible photothrombotic stroke. J Cereb Blood Flow Metab. 2000;20:1166–1173. doi: 10.1097/00004647-200008000-00002. [DOI] [PubMed] [Google Scholar]
- Guttman L. Spinal cord injuries. Comprehensive managment and research. Philadelphia: Blackwell Publishing; 1976. [Google Scholar]
- Haas J, Erdo SL. Quisqualate-induced excitotoxic death of glial cells: transient vulnerability of cultured astrocytes. Glia. 1991;4:111–114. doi: 10.1002/glia.440040113. [DOI] [PubMed] [Google Scholar]
- Hansen AJ. Extracellular potassium concentration in juvenile and adult rat brain cortex during anoxia. Acta Physiol Scand. 1977;99:412–420. doi: 10.1111/j.1748-1716.1977.tb10394.x. [DOI] [PubMed] [Google Scholar]
- Harling-Berg CJ, Knopf PM, Cserr HF. Myelin basic protein infused into cerebrospinal fluid suppresses experimental autoimmune encephalomyelitis. J Neuroimmunol. 1991;35:45–51. doi: 10.1016/0165-5728(91)90160-9. [DOI] [PubMed] [Google Scholar]
- Hertz L, Code WE, Huang R, Juurlink BH, Peng L, Sochocka E, Zhong Z, Yu AC. Glutamate and anoxic-ischemic cell death in neurons and astrocytes. Clin Neuropharmacol. 1992;15(Pt A) Suppl 1:126A–127A. doi: 10.1097/00002826-199201001-00068. [DOI] [PubMed] [Google Scholar]
- Hlatky R, Valadka AB, Robertson CS. Intracranial hypertension and cerebral ischemia after severe traumatic brain injury. Neurosurg Focus. 2003;14:e2. doi: 10.3171/foc.2003.14.4.2. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R, Kerschensteiner M, Stadelmann C, Lassmann H, Wekerle H. The neuroprotective effect of inflammation: implications for the therapy of multiple sclerosis. J Neuroimmunol. 2000;107:161–166. doi: 10.1016/s0165-5728(00)00233-2. [DOI] [PubMed] [Google Scholar]
- Holliday R. Evolution of human longevity, population pressure and the origins of warfare. Biogerontology. 2005;6:363–368. doi: 10.1007/s10522-005-4811-5. [DOI] [PubMed] [Google Scholar]
- Jackson DC, Heisler N. Intracellular and extracellular acid-base and electrolyte status of submerged anoxic turtles at 3 degrees C. Respir Physiol. 1983;53:187–201. doi: 10.1016/0034-5687(83)90066-x. [DOI] [PubMed] [Google Scholar]
- Jiang N, Chopp M, Stein D, Feit H. Progesterone is neuroprotective after transient middle cerebral artery occlusion in male rats. Brain Res. 1996;735:101–107. doi: 10.1016/0006-8993(96)00605-1. [DOI] [PubMed] [Google Scholar]
- Jones TB, Basso DM, Sodhi A, Pan JZ, Hart RP, MacCallum RC, Lee S, Whitacre CC, Popovich PG. Pathological CNS autoimmune disease triggered by traumatic spinal cord injury: implications for autoimmune vaccine therapy. J Neurosci. 2002;22:2690–2700. doi: 10.1523/JNEUROSCI.22-07-02690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jover T, Tanaka H, Calderone A, Oguro K, Bennett MV, Etgen AM, Zukin RS. Estrogen protects against global ischemia-induced neuronal death and prevents activation of apoptotic signaling cascades in the hippocampal CA1. J Neurosci. 2002;22:2115–2124. doi: 10.1523/JNEUROSCI.22-06-02115.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajihara K, Kawanaga H, De la Torre JC, Mullan S. Dimethyl sulfoxide in the treatment of experimental acute spinal cord injury. Surg Neurol. 1973;1:16–22. [PubMed] [Google Scholar]
- Kaplan HJ, Streilein JW. Immune response to immunization via the anterior chamber of the eye. I. F. lymphocyte-induced immune deviation. J Immunol. 1977;118:809–814. [PubMed] [Google Scholar]
- Kass IS, Lipton P. Mechanisms involved in irreversible anoxic damage to the in vitro rat hippocampal slice. J Physiol. 1982;332:459–472. doi: 10.1113/jphysiol.1982.sp014424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsura K, Kristian T, Siesjo BK. Energy metabolism, ion homeostasis, and cell damage in the brain. Biochem Soc Trans. 1994;22:991–996. doi: 10.1042/bst0220991. [DOI] [PubMed] [Google Scholar]
- Kawahara N, Wang Y, Mukasa A, Furuya K, Shimizu T, Hamakubo T, Aburatani H, Kodama T, Kirino T. Genome-wide gene expression analysis for induced ischemic tolerance and delayed neuronal death following transient global ischemia in rats. J Cereb Blood Flow Metab. 2004;24:212–223. doi: 10.1097/01.WCB.0000106012.33322.A2. [DOI] [PubMed] [Google Scholar]
- Kermer P, Klocker N, Bahr M. Neuronal death after brain injury. Models, mechanisms, and therapeutic strategies in vivo. Cell Tissue Res. 1999;298:383–395. doi: 10.1007/s004410050061. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner M, Gallmeier E, Behrens L, Leal VV, Misgeld T, Klinkert WE, Kolbeck R, Hoppe E, Oropeza-Wekerle RL, Bartke I, Stadelmann C, Lassmann H, Wekerle H, Hohlfeld R. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med. 1999;189:865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Edsall L, Garcia M, Zhang H. The release of polyunsaturated fatty acids and their lipoxygenation in the brain. Adv Exp Med Biol. 1999;447:75–85. doi: 10.1007/978-1-4615-4861-4_7. [DOI] [PubMed] [Google Scholar]
- Knoblach SM, Faden AI. Interleukin-10 improves outcome and alters proinflammatory cytokine expression after experimental traumatic brain injury. Exp Neurol. 1998;153:143–151. doi: 10.1006/exnr.1998.6877. [DOI] [PubMed] [Google Scholar]
- Krause DN, Duckles SP, Pelligrino DA. The influence of sex steroid hormones on cerebrovascular function. J Appl Physiol. 2006 doi: 10.1152/japplphysiol.01095.2005. [DOI] [PubMed] [Google Scholar]
- Kwidzinski E, Mutlu LK, Kovac AD, Bunse J, Goldmann J, Mahlo J, Aktas O, Zipp F, Kamradt T, Nitsch R, Bechmann I. Self-tolerance in the immune privileged CNS: lessons from the entorhinal cortex lesion model. J Neural Transm Suppl. 2003:29–49. doi: 10.1007/978-3-7091-0643-3_2. [DOI] [PubMed] [Google Scholar]
- Labombarda F, Pianos A, Liere P, Eychenne B, Gonzalez S, Cambourg A, De Nicola AF, Schumacher M, Guennoun R. Injury elicited increase in spinal cord neurosteroid content analyzed by gas chromatography mass spectrometry. Endocrinology. 2006;147:1847–1859. doi: 10.1210/en.2005-0955. [DOI] [PubMed] [Google Scholar]
- Lampson LA, Fisher CA. Weak HLA and beta 2-microglobulin expression of neuronal cell lines can be modulated by interferon. Proc Natl Acad Sci U S A. 1984;81:6476–6480. doi: 10.1073/pnas.81.20.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RB, Devore I. Man the Hunter. Hawthorne, NY: Aldine Transaction; 1968. [Google Scholar]
- Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- Liu D, Thangnipon W, McAdoo DJ. Excitatory amino acids rise to toxic levels upon impact injury to the rat spinal cord. Brain Res. 1991;547:344–348. doi: 10.1016/0006-8993(91)90984-4. [DOI] [PubMed] [Google Scholar]
- Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998;18:7768–7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XZ, Xu XM, Hu R, Du C, Zhang SX, McDonald JW, Dong HX, Wu YJ, Fan GS, Jacquin MF, Hsu CY, Choi DW. Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci. 1997;17:5395–5406. doi: 10.1523/JNEUROSCI.17-14-05395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Loddick SA, Rothwell NJ. Neuroprotective effects of human recombinant interleukin-1 receptor antagonist in focal cerebral ischaemia in the rat. J Cereb Blood Flow Metab. 1996;16:932–940. doi: 10.1097/00004647-199609000-00017. [DOI] [PubMed] [Google Scholar]
- Lucas DR, Newhouse JP. The toxic effect of sodium L-glutamate on the inner layers of the retina. AMA Arch Ophthalmol. 1957;58:193–201. doi: 10.1001/archopht.1957.00940010205006. [DOI] [PubMed] [Google Scholar]
- Lutz PL. Mechanisms for anoxic survival in the vertebrate brain. Annu Rev Physiol. 1992;54:601–618. doi: 10.1146/annurev.ph.54.030192.003125. [DOI] [PubMed] [Google Scholar]
- Lutz PL, Milton SL. Negotiating brain anoxia survival in the turtle. J Exp Biol. 2004;207:3141–3147. doi: 10.1242/jeb.01056. [DOI] [PubMed] [Google Scholar]
- Lutz PL, Nilsson GE, Perez-Pinzon MA. Anoxia tolerant animals from a neurobiological perspective. Comp Biochem Physiol B Biochem Mol Biol. 1996;113:3–13. doi: 10.1016/0305-0491(95)02046-2. [DOI] [PubMed] [Google Scholar]
- Ma YL, Zhu X, Rivera PM, Toien O, Barnes BM, LaManna JC, Smith MA, Drew KL. Absence of cellular stress in brain after hypoxia induced by arousal from hibernation in Arctic ground squirrels. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1297–R1306. doi: 10.1152/ajpregu.00260.2005. [DOI] [PubMed] [Google Scholar]
- Mason JL, Suzuki K, Chaplin DD, Matsushima GK. Interleukin-1beta promotes repair of the CNS. J Neurosci. 2001;21:7046–7052. doi: 10.1523/JNEUROSCI.21-18-07046.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews RT, Kelly GM, Zerillo CA, Gray G, Tiemeyer M, Hockfield S. Aggrecan glycoforms contribute to the molecular heterogeneity of perineuronal nets. J Neurosci. 2002;22:7536–7547. doi: 10.1523/JNEUROSCI.22-17-07536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Chan SL. Calcium orchestrates apoptosis. Nat Cell Biol. 2003;5:1041–1043. doi: 10.1038/ncb1203-1041. [DOI] [PubMed] [Google Scholar]
- Matute C, Sanchez-Gomez MV, Martinez-Millan L, Miledi R. Glutamate receptor-mediated toxicity in optic nerve oligodendrocytes. Proc Natl Acad Sci U S A. 1997;94:8830–8835. doi: 10.1073/pnas.94.16.8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdoo DJ, Xu GY, Robak G, Hughes MG. Changes in amino acid concentrations over time and space around an impact injury and their diffusion through the rat spinal cord. Exp Neurol. 1999;159:538–544. doi: 10.1006/exnr.1999.7166. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Hurn PD. Estrogen and ischemic neuroprotection: an integrated view. Trends Endocrinol Metab. 2003;14:228–235. doi: 10.1016/s1043-2760(03)00076-6. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Althomsons SP, Hyrc KL, Choi DW, Goldberg MP. Oligodendrocytes from forebrain are highly vulnerable to AMPA/kainate receptor-mediated excitotoxicity. Nat Med. 1998;4:291–297. doi: 10.1038/nm0398-291. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Woolley CS. Estradiol and progesterone regulate neuronal structure and synaptic connectivity in adult as well as developing brain. Exp Gerontol. 1994;29:431–436. doi: 10.1016/0531-5565(94)90022-1. [DOI] [PubMed] [Google Scholar]
- McKinley W, Meade MA, Kirshblum S, Barnard B. Outcomes of early surgical management versus late or no surgical intervention after acute spinal cord injury. Arch Phys Med Rehabil. 2004;85:1818–1825. doi: 10.1016/j.apmr.2004.04.032. [DOI] [PubMed] [Google Scholar]
- Medawar PB. Immunity to homologous grafted skin. III. The fate of skin homografts transplanted to the brain, to subcutaneous tissue and to the anterior chamber of the eye. British Journal of Experimental Pathology. 1948:58–69. [PMC free article] [PubMed] [Google Scholar]
- Meindl R. Human populations before agriculture. In: Jones S, Martin R, Pilbeam D, editors. The Cambridge Encyclopedia of Human Evolution. Cambridge: Cambridge University Press; 1992. pp. 406–410. [Google Scholar]
- Mendonca MT, Tousignant AJ, Crews D. Seasonal changes and annual variability in daily plasma melatonin in the red-sided garter snake (Thamnophis sirtalis parietalis) Gen Comp Endocrinol. 1995;100:226–237. doi: 10.1006/gcen.1995.1152. [DOI] [PubMed] [Google Scholar]
- Mendonca MT, Tousignant AJ, Crews D. Pinealectomy, melatonin, and courtship behavior in male red-sided garter snakes (Thamnophis sirtalis parietalis) J Exp Zool. 1996;274:63–74. doi: 10.1002/(SICI)1097-010X(19960101)274:1<63::AID-JEZ7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Micu I, Jiang Q, Coderre E, Ridsdale A, Zhang L, Woulfe J, Yin X, Trapp BD, McRory JE, Rehak R, Zamponi GW, Wang W, Stys PK. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature. 2006;439:988–992. doi: 10.1038/nature04474. [DOI] [PubMed] [Google Scholar]
- Moosmann B, Behl C. The antioxidant neuroprotective effects of estrogens and phenolic compounds are independent from their estrogenic properties. Proc Natl Acad Sci U S A. 1999;96:8867–8872. doi: 10.1073/pnas.96.16.8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel S, Genius J, Heiland S, Horstmann S, Gardner H, Wagner S. Diphenyleneiodonium and dimethylsulfoxide for treatment of reperfusion injury in cerebral ischemia of the rat. Brain Res. 2007;1132:210–217. doi: 10.1016/j.brainres.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007) Brain Behav Immun. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawashiro H, Tasaki K, Ruetzler CA, Hallenbeck JM. TNF-alpha pretreatment induces protective effects against focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 1997;17:483–490. doi: 10.1097/00004647-199705000-00001. [DOI] [PubMed] [Google Scholar]
- Nesic O, Xu GY, McAdoo D, High KW, Hulsebosch C, Perez-Pol R. IL-1 receptor antagonist prevents apoptosis and caspase-3 activation after spinal cord injury. J Neurotrauma. 2001;18:947–956. doi: 10.1089/089771501750451857. [DOI] [PubMed] [Google Scholar]
- Nesse RM, Williams GC. Why We Get Sick. New York: Vintage Books; 1996. [Google Scholar]
- Niederkorn JY. See no evil, hear no evil, do no evil: the lessons of immune privilege. Nat Immunol. 2006;7:354–359. doi: 10.1038/ni1328. [DOI] [PubMed] [Google Scholar]
- Nilsson GE, Lutz PL. Release of inhibitory neurotransmitters in response to anoxia in turtle brain. Am J Physiol. 1991;261:R32–R37. doi: 10.1152/ajpregu.1991.261.1.R32. [DOI] [PubMed] [Google Scholar]
- Nwuga VC. A follow-up study of paraplegics and tetraplegics discharged from hospital. J Trop Med Hyg. 1979;82:30–33. [PubMed] [Google Scholar]
- Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- Park E, Velumian AA, Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma. 2004;21:754–774. doi: 10.1089/0897715041269641. [DOI] [PubMed] [Google Scholar]
- Payne KA, Huybrechts KF, Caro JJ, Craig Green TJ, Klittich WS. Long term cost-of-illness in stroke: an international review. Pharmacoeconomics. 2002;20:813–825. doi: 10.2165/00019053-200220120-00002. [DOI] [PubMed] [Google Scholar]
- Pera J, Zawadzka M, Kaminska B, Szczudlik A. Influence of chemical and ischemic preconditioning on cytokine expression after focal brain ischemia. J Neurosci Res. 2004;78:132–140. doi: 10.1002/jnr.20232. [DOI] [PubMed] [Google Scholar]
- Persengiev S, Kanchev L, Vezenkova G. Circadian patterns of melatonin, corticosterone, and progesterone in male rats subjected to chronic stress: effect of constant illumination. J Pineal Res. 1991;11:57–62. doi: 10.1111/j.1600-079x.1991.tb00456.x. [DOI] [PubMed] [Google Scholar]
- Pettus EH, Christman CW, Giebel ML, Povlishock JT. Traumatically induced altered membrane permeability: its relationship to traumatically induced reactive axonal change. J Neurotrauma. 1994;11:507–522. doi: 10.1089/neu.1994.11.507. [DOI] [PubMed] [Google Scholar]
- Piantadosi CA, Zhang J. Mitochondrial generation of reactive oxygen species after brain ischemia in the rat. Stroke. 1996a;27:327–331. doi: 10.1161/01.str.27.2.327. discussion 332. [DOI] [PubMed] [Google Scholar]
- Piantadosi CA, Zhang J. Mitochondrial Generation of Reactive Oxygen Species After Brain Ischemia in the Rat. Stroke. 1996b;27:327–332. doi: 10.1161/01.str.27.2.327. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Ralph CL. The pineal gland and geographical distribution of animals. Int J Biometeorol. 1975;19:289–303. doi: 10.1007/BF01451040. [DOI] [PubMed] [Google Scholar]
- Ramon y Cajal S. Degeneration and Regeneration of the Nervous System. London: Oxford University Press; 1928. [Google Scholar]
- Ransohoff RM, Kivisakk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3:569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- Rees PM, Fowler CJ, Maas CP. Sexual function in men and women with neurological disorders. Lancet. 2007;369:512–525. doi: 10.1016/S0140-6736(07)60238-4. [DOI] [PubMed] [Google Scholar]
- Rego AC, Oliveira CR. Mitochondrial dysfunction and reactive oxygen species in excitotoxicity and apoptosis: implications for the pathogenesis of neurodegenerative diseases. Neurochem Res. 2003;28:1563–1574. doi: 10.1023/a:1025682611389. [DOI] [PubMed] [Google Scholar]
- Relton JK, Rothwell NJ. Interleukin-1 receptor antagonist inhibits ischaemic and excitotoxic neuronal damage in the rat. Brain Res Bull. 1992;29:243–246. doi: 10.1016/0361-9230(92)90033-t. [DOI] [PubMed] [Google Scholar]
- Rhodes KE, Fawcett JW. Chondroitin sulphate proteoglycans: preventing plasticity or protecting the CNS? J Anat. 2004;204:33–48. doi: 10.1111/j.1469-7580.2004.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CL, Saraswati M, Fiskum G. Mitochondrial dysfunction early after traumatic brain injury in immature rats. J Neurochem. 2007;101:1248–1257. doi: 10.1111/j.1471-4159.2007.04489.x. [DOI] [PubMed] [Google Scholar]
- Roof RL, Duvdevani R, Stein DG. Gender influences outcome of brain injury: progesterone plays a protective role. Brain Res. 1993;607:333–336. doi: 10.1016/0006-8993(93)91526-x. [DOI] [PubMed] [Google Scholar]
- Roof RL, Hoffman SW, Stein DG. Progesterone protects against lipid peroxidation following traumatic brain injury in rats. Mol Chem Neuropathol. 1997;31:1–11. doi: 10.1007/BF02815156. [DOI] [PubMed] [Google Scholar]
- Ross AP, Christian SL, Zhao HW, Drew KL. Persistent tolerance to oxygen and nutrient deprivation and N-methyl-D-aspartate in cultured hippocampal slices from hibernating Arctic ground squirrel. J Cereb Blood Flow Metab. 2006;26:1148–1156. doi: 10.1038/sj.jcbfm.9600271. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Saito A, Maier CM, Narasimhan P, Nishi T, Song YS, Yu F, Liu J, Lee YS, Nito C, Kamada H, Dodd RL, Hsieh LB, Hassid B, Kim EE, Gonzalez M, Chan PH. Oxidative stress and neuronal death/survival signaling in cerebral ischemia. Mol Neurobiol. 2005;31:105–116. doi: 10.1385/MN:31:1-3:105. [DOI] [PubMed] [Google Scholar]
- Santizo RA, Anderson S, Ye S, Koenig HM, Pelligrino DA. Effects of estrogen on leukocyte adhesion after transient forebrain ischemia. Stroke. 2000;31:2231–2235. doi: 10.1161/01.str.31.9.2231. [DOI] [PubMed] [Google Scholar]
- Santos-Benito FF, Munoz-Quiles C, Ramon-Cueto A. Long-term care of paraplegic laboratory mammals. J Neurotrauma. 2006;23:521–536. doi: 10.1089/neu.2006.23.521. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. A mechanism for glucocorticoid toxicity in the hippocampus: increased neuronal vulnerability to metabolic insults. J Neurosci. 1985;5:1228–1232. doi: 10.1523/JNEUROSCI.05-05-01228.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Pulsinelli WA. Glucocorticoids potentiate ischemic injury to neurons: therapeutic implications. Science. 1985;229:1397–1400. doi: 10.1126/science.4035356. [DOI] [PubMed] [Google Scholar]
- Sayeed I, Wali B, Stein DG. Progesterone inhibits ischemic brain injury in a rat model of permanent middle cerebral artery occlusion. Restor Neurol Neurosci. 2007;25:151–159. [PubMed] [Google Scholar]
- Schnell L, Schwab ME. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature. 1990;343:269–272. doi: 10.1038/343269a0. [DOI] [PubMed] [Google Scholar]
- Shear DA, Galani R, Hoffman SW, Stein DG. Progesterone protects against necrotic damage and behavioral abnormalities caused by traumatic brain injury. Exp Neurol. 2002;178:59–67. doi: 10.1006/exnr.2002.8020. [DOI] [PubMed] [Google Scholar]
- Shohami E, Beit-Yannai E, Horowitz M, Kohen R. Oxidative stress in closed-head injury: brain antioxidant capacity as an indicator of functional outcome. J Cereb Blood Flow Metab. 1997;17:1007–1019. doi: 10.1097/00004647-199710000-00002. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Estrogen prevents the loss of CA1 hippocampal neurons in gerbils after ischemic injury. Neuroscience. 2003;116:851–861. doi: 10.1016/s0306-4522(02)00790-x. [DOI] [PubMed] [Google Scholar]
- Siesjo BK, Siesjo P. Mechanisms of secondary brain injury. Eur J Anaesthesiol. 1996;13:247–268. [PubMed] [Google Scholar]
- Singer D. Neonatal tolerance to hypoxia: a comparative-physiological approach. Comp Biochem Physiol A Mol Integr Physiol. 1999;123:221–234. doi: 10.1016/s1095-6433(99)00057-4. [DOI] [PubMed] [Google Scholar]
- Singh P, Mann KA, Mangat HK, Kaur G. Prolonged glutamate excitotoxicity: effects on mitochondrial antioxidants and antioxidant enzymes. Mol Cell Biochem. 2003;243:139–145. doi: 10.1023/a:1021668314070. [DOI] [PubMed] [Google Scholar]
- Slupphaug G, Kavli B, Krokan HE. The interacting pathways for prevention and repair of oxidative DNA damage. Mutat Res. 2003;531:231–251. doi: 10.1016/j.mrfmmm.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Kapoor R, Felts PA. Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol. 1999;9:69–92. doi: 10.1111/j.1750-3639.1999.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagliano NE, Perez-Pinzon MA, Moskowitz MA, Huang PL. Focal ischemic preconditioning induces rapid tolerance to middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 1999;19:757–761. doi: 10.1097/00004647-199907000-00005. [DOI] [PubMed] [Google Scholar]
- Stein DG, Hoffman SW. Concepts of CNS plasticity in the context of brain damage and repair. J Head Trauma Rehabil. 2003;18:317–341. doi: 10.1097/00001199-200307000-00004. [DOI] [PubMed] [Google Scholar]
- Stein DG, Wright DW, Kellermann AL. Does progesterone have neuroprotective properties? Ann Emerg Med. 2008;51:164–172. doi: 10.1016/j.annemergmed.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Stevens SL, Xiong Z, Lessov NS, Harrington CA, Mori M, Meller R, Rosenzweig HL, Tobar E, Shaw TE, Chu X, Simon RP. Effect of ischaemic preconditioning on genomic response to cerebral ischaemia: similarity to neuroprotective strategies in hibernation and hypoxiatolerant states. Lancet. 2003;362:1028–1037. doi: 10.1016/S0140-6736(03)14412-1. [DOI] [PubMed] [Google Scholar]
- Strasser R, Htun P, Schaper W. Salvage of jeopardized myocardium by ischemic preconditioning: is the quest over? Mol Cell Biochem. 1996;160–161:209–215. doi: 10.1007/BF00240051. [DOI] [PubMed] [Google Scholar]
- Stummer W, Baethmann A, Murr R, Schurer L, Kempski OS. Cerebral protection against ischemia by locomotor activity in gerbils. Underlying mechanisms. Stroke. 1995;26:1423–1429. doi: 10.1161/01.str.26.8.1423. discussion 1430. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Rabchevsky AG, Waldmeier PC, Springer JE. Mitochondrial permeability transition in CNS trauma: cause or effect of neuronal cell death? J Neurosci Res. 2005;79:231–239. doi: 10.1002/jnr.20292. [DOI] [PubMed] [Google Scholar]
- Svihla A, Bowman HR, Ritenour R. Prolongation of clotting time in dormant estivating mammals. Science. 1951;114:298–299. doi: 10.1126/science.114.2960.298. [DOI] [PubMed] [Google Scholar]
- Tan DX, Manchester LC, Sainz RM, Mayo JC, Leon J, Reiter RJ. Physiological ischemia/reperfusion phenomena and their relation to endogenous melatonin production: a hypothesis. Endocrine. 2005;27:149–158. doi: 10.1385/endo:27:2:149. [DOI] [PubMed] [Google Scholar]
- Tang Y, Pacary E, Freret T, Divoux D, Petit E, Schumann-Bard P, Bernaudin M. Effect of hypoxic preconditioning on brain genomic response before and following ischemia in the adult mouse: identification of potential neuroprotective candidates for stroke. Neurobiol Dis. 2006;21:18–28. doi: 10.1016/j.nbd.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Taylor AW. Neuroimmunomodulation in immune privilege: role of neuropeptides in ocular immunosuppression. Neuroimmunomodulation. 1996;3:195–204. doi: 10.1159/000097271. [DOI] [PubMed] [Google Scholar]
- Taylor AW, Streilein JW. Inhibition of antigen-stimulated effector T cells by human cerebrospinal fluid. Neuroimmunomodulation. 1996;3:112–118. doi: 10.1159/000097235. [DOI] [PubMed] [Google Scholar]
- Taylor AW, Streilein JW, Cousins SW. Alpha-melanocyte-stimulating hormone suppresses antigen-stimulated T cell production of gamma-interferon. Neuroimmunomodulation. 1994;1:188–194. doi: 10.1159/000097167. [DOI] [PubMed] [Google Scholar]
- Teitelbaum P, Cheng MF, Rozin P. Development of feeding parallels its recovery after hypothalamic damage. J Comp Physiol Psychol. 1969;67:430–441. doi: 10.1037/h0027288. [DOI] [PubMed] [Google Scholar]
- Toung TJ, Traystman RJ, Hurn PD. Estrogen-mediated neuroprotection after experimental stroke in male rats. Stroke. 1998;29:1666–1670. doi: 10.1161/01.str.29.8.1666. [DOI] [PubMed] [Google Scholar]
- Touzani O, Boutin H, LeFeuvre R, Parker L, Miller A, Luheshi G, Rothwell N. Interleukin-1 influences ischemic brain damage in the mouse independently of the interleukin-1 type I receptor. J Neurosci. 2002;22:38–43. doi: 10.1523/JNEUROSCI.22-01-00038.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- Tyurin VA, Tyurina YY, Borisenko GG, Sokolova TV, Ritov VB, Quinn PJ, Rose M, Kochanek P, Graham SH, Kagan VE. Oxidative stress following traumatic brain injury in rats: quantitation of biomarkers and detection of free radical intermediates. J Neurochem. 2000;75:2178–2189. doi: 10.1046/j.1471-4159.2000.0752178.x. [DOI] [PubMed] [Google Scholar]
- Ueda M, Nowak TS., Jr Protective preconditioning by transient global ischemia in the rat: components of delayed injury progression and lasting protection distinguished by comparisons of depolarization thresholds for cell loss at long survival times. J Cereb Blood Flow Metab. 2005;25:949–958. doi: 10.1038/sj.jcbfm.9600107. [DOI] [PubMed] [Google Scholar]
- Ultsch GR. Ecology and Physiology of Hibernation and Overwintering among Fresh-Water Fishes, Turtles, and Snakes. Biological Reviews of the Cambridge Philosophical Society. 1989;64:435–516. [Google Scholar]
- van der Goes A, Brouwer J, Hoekstra K, Roos D, van den Berg TK, Dijkstra CD. Reactive oxygen species are required for the phagocytosis of myelin by macrophages. J Neuroimmunol. 1998;92:67–75. doi: 10.1016/s0165-5728(98)00175-1. [DOI] [PubMed] [Google Scholar]
- van Dooremal JC. Die Entwicklung der in frenmden Grund vesetzten lebenden Geweba. Albrecht Von Graefes Archives of Opthalmology. 1873:358–373. [Google Scholar]
- Vanecek J, Jansky L, Illnerova H, Hoffmann K. Pineal melatonin in hibernating and aroused golden hamsters (Mesocricetus auratus) Comp Biochem Physiol A. 1984;77:759–762. doi: 10.1016/0300-9629(84)90197-x. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Isaacs E, Muter V. A review of cognitive outcome after unilateral lesions sustained during childhood. J Child Neurol. 1994;9 Suppl 2:67–73. [PubMed] [Google Scholar]
- Vegeto E, Belcredito S, Ghisletti S, Meda C, Etteri S, Maggi A. The endogenous estrogen status regulates microglia reactivity in animal models of neuroinflammation. Endocrinology. 2006;147:2263–2272. doi: 10.1210/en.2005-1330. [DOI] [PubMed] [Google Scholar]
- Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu Rev Immunol. 2002;20:125–163. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- Wenkel H, Streilein JW, Young MJ. Systemic immune deviation in the brain that does not depend on the integrity of the blood-brain barrier. J Immunol. 2000;164:5125–5131. doi: 10.4049/jimmunol.164.10.5125. [DOI] [PubMed] [Google Scholar]
- Wilson AM, Kriegstein AR. Turtle cortical neurons survive glutamate exposures that are lethal to mammalian neurons. Brain Res. 1991;540:297–301. doi: 10.1016/0006-8993(91)90523-x. [DOI] [PubMed] [Google Scholar]
- Wolgin DL, Hein A, Teitelbaum P. Recovery of forelimb placing after lateral hypothalamic lesions in the cat: parallels and contrasts with development. J Comp Physiol Psychol. 1980;94:795–807. doi: 10.1037/h0077836. [DOI] [PubMed] [Google Scholar]
- Wright DW, Kellermann AL, Hertzberg VS, Clark PL, Frankel M, Goldstein FC, Salomone JP, Dent LL, Harris OA, Ander DS, Lowery DW, Patel MM, Denson DD, Gordon AB, Wald MM, Gupta S, Hoffman SW, Stein DG. ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann Emerg Med. 2007;49:391–402. doi: 10.1016/j.annemergmed.2006.07.932. 402 e1-2. [DOI] [PubMed] [Google Scholar]
- Wuermser LA, Ho CH, Chiodo AE, Priebe MM, Kirshblum SC, Scelza WM. Spinal cord injury medicine. 2. Acute care management of traumatic and nontraumatic injury. Arch Phys Med Rehabil. 2007;88:S55–S61. doi: 10.1016/j.apmr.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Xu W, Chi L, Xu R, Ke Y, Luo C, Cai J, Qiu M, Gozal D, Liu R. Increased production of reactive oxygen species contributes to motor neuron death in a compression mouse model of spinal cord injury. Spinal Cord. 2005;43:204–213. doi: 10.1038/sj.sc.3101674. [DOI] [PubMed] [Google Scholar]
- Yeo JD, Walsh J, Rutkowski S, Soden R, Craven M, Middleton J. Mortality following spinal cord injury. Spinal Cord. 1998;36:329–336. doi: 10.1038/sj.sc.3100628. [DOI] [PubMed] [Google Scholar]
- Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoles E, Hauben E, Palgi O, Agranov E, Gothilf A, Cohen A, Kuchroo V, Cohen IR, Weiner H, Schwartz M. Protective autoimmunity is a physiological response to CNS trauma. J Neurosci. 2001;21:3740–3748. doi: 10.1523/JNEUROSCI.21-11-03740.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiner A, Holzer M, Sterz F, Behringer W, Schorkhuber W, Mullner M, Frass M, Siostrzonek P, Ratheiser K, Kaff A, Laggner AN. Mild resuscitative hypothermia to improve neurological outcome after cardiac arrest. A clinical feasibility trial. Hypothermia After Cardiac Arrest (HACA) Study Group. Stroke. 2000;31:86–94. doi: 10.1161/01.str.31.1.86. [DOI] [PubMed] [Google Scholar]
- Zhao HW, Ross AP, Christian SL, Buchholz JN, Drew KL. Decreased NR1 phosphorylation and decreased NMDAR function in hibernating Arctic ground squirrels. J Neurosci Res. 2006;84:291–298. doi: 10.1002/jnr.20893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Zhu X, Castellani RJ, Stimmelmayr R, Perry G, Smith MA, Drew KL. Hibernation, a model of neuroprotection. Am J Pathol. 2001;158:2145–2151. doi: 10.1016/S0002-9440(10)64686-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Smith MA, Perry G, Wang Y, Ross AP, Zhao HW, Lamanna JC, Drew KL. MAPKs are differentially modulated in arctic ground squirrels during hibernation. J Neurosci Res. 2005;80:862–868. doi: 10.1002/jnr.20526. [DOI] [PubMed] [Google Scholar]